Highlights
► Flavone prescriptions composed with suitable compatibility could possess synergistical action of antiviral effect. ► EF-BSRF, EF-SF-WDFF-BSRF and EF-WDFF-BSRF could significantly inhibit the cellular infectivity of NDV in CEF. ► EF-SF-WDFF-BSRF could improve the protective effect of ND vaccine. ► EF-SF-WDFF-BSRF would be expected to exploit into a new-type antiviral drug.
Abbreviations: CHMs, Chinese herbal medicines; CHMIs, Chinese herb medicinal ingredients; ND, Newcastle disease; NDV, Newcastle disease virus; EF, epimedium flavone; BSRF, baical skullcap root flavone; WDFF, wild dendranthema flower flavone; SF, sanchi flavone; CEF, chicken embryo fibroblast; MEM, minimum essential medium; MM, maintenance medium; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; CMF, calcium and magnesium-free; PBS, phosphate-buffered saline; EF-WDFF, epimedium flavone plus wild dendranthema flower flavone; EF-SF, epimedium flavone plus sanchi flavone; EF-BSRF, epimedium flavone plus baical skullcap root flavone; WDFF-BSRF, wild dendranthema flower flavone plus baical skullcap root flavone; EF-WDFF-SF, epimedium flavone plus wild dendranthema flower flavone plus sanchi flavone; EF-BSRF-SF, epimedium flavone plus baical skullcap root flavone plus sanchi flavone; WDFF-BSRF-SF, wild dendranthema flower flavone plus baical skullcap root flavone plus sanchi flavone; EF-WDFF-BSRF, epimedium flavone plus wild dendranthema flower flavone plus baical skullcap root flavone; EF-SF-WDFF-BSRF, epimedium flavone plus sanchi flavone plus wild dendranthema flower flavone plus baical skullcap root flavone; DMSO, Dimethyl sulfoxide; BC, blank control; VC, vaccine control; HI, hemagglutination inhibition
Keywords: Flavone ingredients prescriptions, Antivirus, Highest virus inhibitory rate, Mortality, Morbidity, Protective rate
Abstract
In order to screen better flavone prescriptions of anti-Newcastle disease virus (NDV), four flavone ingredients of epimedium flavones (EF), baikal skullcap root flavones (BSRF), wild dendranthema flower flavones (WDFF), and sanchi flavones (SF) screened in previous experiments and their prescriptions were added into chicken embryo fibroblast monolayer with three drug-adding modes respectively. The cellular A570 values, the highest virus inhibitory rates and the score based on virus inhibitory rate were calculated to compare their antiviral activity. In immune protective test, the effects of three preparations (EF-BSRF, EF-SF-WDFF-BSRF and EF-WDFF-BSRF screened by the results in vitro experiment) on NDV infection were compared in chickens vaccinated with ND vaccine then challenged with NDV. Blood was regularly sampled for serum antibody titer determination. The pathogenic and dead statuses of chickens were clinically examined. The results indicated that the A570 values of the nine prescriptions, especially the foresaid three prescriptions at almost all concentrations in three drug-adding modes were significantly higher than that of the virus control group. The foresaid three prescriptions presented at the top five of the highest virus inhibitory rate, and located at the highest three of the score rank. The antibody titers and protective rates of the three prescriptions groups were higher than that of VC group, especially EF-SF-WDFF-BSRF group showed significant difference. These results indicated that flavone prescriptions composed with suitable compatibility could possess synergistical action of antiviral effect, ES-SF-WDFF-BSRF prescription could inhibit the cellular infectivity of NDV, improve the protective effect of ND vaccine and would be expected to exploit into a new-type antiviral drug.
1. Introduction
In the past few years, animal infectious diseases, especially the viral diseases, such as cow transmissible spongiform encephalopathy, severe acute respiratory syndrome, swine hyperpyrexia disease, untypical classical swine fever, Newcastle Disease (ND) and so on are worldwide concerned as they emerge continuously and take a stage of comeback with spread quickly, and usually cause a great loss in domestic animal and poultry industry [1]. Because virus has unique biological characteristics and pathogenesis, there are no effective treatment methods for viral diseases [2]. The virus disease has become one of the most serious threatens to human or animal health. Vaccination is the most common preventive method, but some infectious diseases are still difficult to control because the efficacies of some vaccines are severely decreased when a virus circulation does not have a good match with vaccine strains due to antigenic drift or inaccurate epidemiological predictions [3], or some vaccines with inferior quality, improper conservation and transportation problems [4]. A few chemical drugs have been used, but their clinical effects are not satisfied and there are obvious negative effects such as drug residues, drug tolerance, high recurrence rate, environmental pollution and so on [5]. Therefore, it becomes so urgent to study and develop new-type antiviral drugs with high efficiency and low toxicity, which will be an important topic to many scholars [6].
In recent years, it has been proved that many Chinese herbal medicines (CHMs) and their ingredients (CHMIs) possess antiviral effect [7]. Some CHMs or their extracts such as astragalus polysaccharides injection, extract of indigowoad root have been developed into antibacterial and antiviral agents [8]. On one hand, CHMs possess many biological activities, such as adjusting immunologic function, inhibiting viral processes (adsorption, transcription, replication and release) and so on, which showed reliable biological functions with little side effects and low toxicity. On the other hand, many researches demonstrated that CHMs possess many antiviral active materials. And many active materials had not been discovered yet [9]. Moreover, new virus or strains of virus emerge continually. So, antiviral compound screened from CHMs became a hotspot for many investigators.
Newcastle disease is a kind of violent infectious disease caused by Newcastle disease virus (NDV) and usually causes great loss in poultry industry. Up to now, the control of this disease is mainly by vaccination of ND vaccine. Although the strict immune program has been taken in the farms, the disease is still hard to control. Though some Chinese veterinary herbal medical preparations, such as injection of honeysuckle flower-baical skullcap root-forsythia suspense, and injection of astragalus polysaccharides have become one of principal weapons in veterinary clinical therapy and play an important role in clinical epidemic prevention. But these preparations have not displayed satisfactory clinical effects on this disease. Researching and developing effective clinical medicines is still one of the primary tasks to many veterinary workers. The pharmacological effects of CHMs are based on their chemical constituents.
Flavone is one of the most important active ingredients and can enhance immune function of organism and inhibit the virus infection [10]. In previous researches, it was confirmed that epimedium flavone (EF), baical skullcap root flavone (BSRF), wild dendranthema flower flavone (WDFF) and sanchi flavone (SF) could significantly inhibit the cellular infectivity of NDV in chicken embryo fibroblast (CEF) [11]. In present experiment, the antiviral effects of nine flavone ingredients prescriptions based on the above-mentioned four flavones ingredients were compared by determination of the effect on cellular infectivity of NDV. Then 3 better prescriptions were chosen and their protective effects of ND by artificial challenge were observed. The purpose of this experiment is to observe the antiviral action of these flavone ingredients prescriptions, search for optimal combination of flavone ingredients, select out the best prescription and offer theoretical evidences for development of new-type antiviral drugs.
2. Materials and methods
2.1. Reagents
Eagle's minimum essential medium (MEM) (Gibco) supplemented with penicillin 100 IU/mL, streptomycin 100 IU/mL and 5% fetal bovine serum was called growth medium and used for culturing the cells, 2% fetal bovine serum, maintenance medium (MM) for diluting flavone ingredients and maintaining the cells. Hanks’ solution was used for washing the chick embryo tissue shiver. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Amresco Co.) was dissolved into 5 mg/mL with calcium and magnesium-free (CMF) phosphate-buffered saline (PBS, pH 7.4). Trypsin (Amresco-0858) was dissolved with CMF-PBS (pH 7.4) to 0.25%. Dimethyl sulfoxide (DMSO) was produced by Zhengxing Institute of Chemical Engineering in Suzhou, China. These reagents were filtered through a 0.22 μm syringe filter. MEM and MM were stored at 4 °C, MTT solution at 4 °C in dark bottles. Other chemical used in the experiment were analytical grade.
2.2. Preparation of prescriptions
Epimedium flavone (EF, net content of 60.89%), baical skullcap root flavone (BSRF, net content of 79.40%), wild dendranthema flower flavone (WDFF, net content of 70.89%) and sanchi flavone (SF, net content of 61.13%) were extracted and refined with macroporous resin in our laboratory [11]. Nine flavone ingredients prescriptions including epimedium flavone plus wild dendranthema flower flavone (EF-WDFF), epimedium flavone plus sanchi flavone (EF-SF), epimedium flavone plus baical skullcap root flavone (EF-BSRF), wild dendranthema flower flavone plus baical skullcap root flavone (WDFF-BSRF), epimedium flavone plus wild dendranthema flower flavone plus sanchi flavone (EF-WDFF-SF), epimedium flavone plus baical skullcap root flavone plus sanchi flavone (EF-BSRF-SF), wild dendranthema flower flavone plus baical skullcap root flavone plus sanchi flavone (WDFF-BSRF-SF), epimedium flavone plus wild dendranthema flower flavone plus baical skullcap root flavone (EF-WDFF-BSRF), epimedium flavone plus sanchi flavone plus wild dendranthema flower flavone plus baical skullcap root flavone (EF-SF-WDFF-BSRF) were formed based on the above-mentioned four flavones ingredients. The composition proportion of each ingredient in the nine prescriptions is 1:1. The concentration of each flavone ingredient in the prescriptions was the maximal safe concentration on CEF (62.5 μg/mL) [11]. For test in vitro, four single flavone ingredients and nine flavone ingredient prescriptions were diluted into four concentrations (31.3, 15.6, 7.8 and 3.9 μg/mL) in two-fold serial dilutions with MM, sterilized and stored at 4 °C. According to the results of test in vitro, three ingredient prescriptions of EF-BSRF, EF-SF-WDFF-BSRF, EF-WDFF-BSRF were screened for test in vivo. The three ingredient prescriptions were diluted into 40 mg/mL (net content) with CMF-PBS. For test in vivo, the endotoxin amount was up to the standard of Chinese Veterinary Pharmacopoeia [12].
2.3. Cells and viruses
The CEF was prepared with 10-day-old specific pathogen free chicken embryo (Nanjing pharmaceutical and apparatus factory of China Animal Husbandry Industry Company). In brief, after the eggshell was disinfected and opened, the chick embryo was taken out, removed the head, extremities and viscera, washed with Hanks’ solution, cut into 1–2 mm3 pieces, and washed three times with Hanks’ solution. The trypsogen solution of 0.25% was added, trypsinized for 30 min at 37 °C and centrifugalized. The precipitation was washed three times with Hanks’ solution and filtered through a 4-tier gauze. The cells were counted and diluted into 1 × 106/mL with MEM and inoculated in 96-well or 12-well culture plates for 24 h at 38.5 °C in a humid atmosphere of 5% CO2. When CEF grew into monolayer, MEM was removed. The CEF monolayer was washed with calcium and magnesium-free phosphate-buffered saline (CMF-PBS, pH 7.4) once, and taken for standby after CMF-PBS removed again.
ND vaccine (Mukteswar strain) was purchased from Beijing Veterinary Bio-drug Company. TCID50 of the virus liquid was 1 × 10−8 by Reed–Mueech assay [13]. It was diluted into 10−6 (100 TCID50) with MM and used for antiviral assays. ND virus (NDV, F48E9 strain) used for challenge experiment was supplied by China Institute of Veterinary Drug Control and propagated with 10-day-old specific pathogen-free chicken embryo. The titer of NDV (F48E9 strain) stock solution was 9.2 × 106, by Reed–Mueech assay.
2.4. Antiviral assays
The CEF was prepared as above-mentioned method. When CEF grew into monolayer, the serial twofold dilutions of four single flavone ingredients and nine flavone ingredient prescriptions and NDV (Mukteswar strain, 100 TCID50) were added in three sample-adding modes respectively [14].
Simultaneous-adding drug and virus after mixed: Four single flavone ingredients and nine flavone ingredient prescriptions solution of different concentrations (31.3, 15.6, 7.8 and 3.9 μg/mL) were mixed with equal-volume of NDV solution respectively, incubated for 2 h at 4 °C, and added into the CEF 96-well plates, 200 μL per well, four wells for per concentration.
Pre-adding drug: Four single flavone ingredients and nine flavone ingredient prescriptions solution of different concentrations were added into CEF plate first, 100 μL per well, four wells for per concentration, incubated for 2 h at 4 °C. The virus solution was added, 100 μL per well.
Post-adding drug: The virus solution was added into CEF plates first, 100 μL per well. The plates were incubated in 5% CO2 incubator at 37 °C for 2 h, then four single flavone ingredients and nine flavone ingredient prescriptions solution of different concentrations were added into CEF plates, 100 μL per well, four wells for per concentration.
Simultaneously, virus control group (only adding NDV), cell control group (only adding MM) and blank group (no cell) were designed. All groups were keeping at 37 °C 5% CO2 incubator. When the NDV control group appeared obviously cytopathic effect (72 h), the CEF livingness was measured by the MTT colorimetric assay [15]. 30 μL of MTT was added into each well, re-incubated for another 4 h. The plates were centrifuged at 1000 × g for 10 min at room temperature. The supernatant was removed carefully and 100 μL of DMSO was added into each well to dissolve the formazan crystals [16], [17]. The plates were shaken for 5 min to dissolve the crystals completely. The cellular optical absorbance at a wave length of 570 nm (A570 value) in each well was measured by microliter enzyme-linked immunosorbent assay reader (Model RT-6000, Leidu Bioscience Corporation, Shenzhen City). The virus inhibitory rate was calculated based on the formula [18]: Virus inhibitory rate = (Ā drug+virus − Ā virus control)/(Ā cell control − Ā virus control) × 100%. In order to compare antiviral activity among different ingredients easily, especially between signal flavones and prescriptions, the score based on virus inhibitory rate was analyzed. Drug with the highest virus inhibitory rate was scored 13 (13 kinds drugs in all), next 12, analogized one by one, the lowest scored 1, and then the score was ranked. The A570 values and virus inhibitory rate and the score were considered as the indicator of antiviral activity.
2.5. Determination of test in vivo
2.5.1. Animals
One-day-old White Roman chickens (male) purchased from Tangquan Poultry Farm were housed in wire cages (60 cm × 100 cm) in air-conditioned rooms at 37 °C and lighted for 24 h at the beginning of pretrial period. The temperature was gradually declined to the room temperature and the light time to 12 h per day, which were kept constant in the following days. Chickens were fed with the commercial starter diet provided by the feed factory of Jiangsu Academy of Agricultural Science.
2.5.2. Immune protection test
Two hundred and fifty 14-day-old chickens were randomly divided into five groups and vaccinated with Newcastle disease vaccine except for blank control (BC) group, re-vaccinated at 28 days of age. Before 24 h of the vaccination, the chickens in three flavone prescriptions groups (EF-BSRF, EF-SF-WDFF-BSRF, EF-WDFF-BSRF, screened according to the results in antiviral assays experiment) were treated by aqueous EF-BSRF, EF-SF-WDFF-BSRF and EF-WDFF-BSRF solution at the dosage of 20 mg per feather, once a day for three days respectively. Vaccine control (VC) and BC groups were replaced with the same volume of CMF-PBS, once a day for three days. On day 28 after the first vaccination, the chickens except for BC group were challenged with 0.5 mL of NDV at 10 LD50 by intramuscular injection. Before vaccination (D 0) and on days 7, 14, 21 and 28 after the first vaccination, and day 12 after the challenge (D c12), blood was sampled for determination of serum hemagglutination inhibition (HI) antibody titer. The pathogenic and dead statuses of chickens were clinically examined daily for 12 successive days after the challenge (D c12). The mortality, morbidity and protective rate in every groups were calculated according to the formula: Mortality (%) = The number of dead chicken on D c12/the number of sample × 100%, Morbidity (%) = The number of chickens dead and showing clinical symptoms on D c12/the number of sample × 100%, Protective rate (%) = The number of chickens without clinical symptoms during the experiment/the number of sample × 100%.
2.5.3. Serum HI antibody assay
One milliliter of blood sample per chick were collected from the brachial vein, put into 1.5 mL Eppendorf tubes and allowed to clot at 37 °C for 2 h. The serum was separated and stored at −20 °C for HI antibody assay. Briefly, after inactivating complements at 56 °C for 30 min, two-fold serial dilutions were made, dilutions ranged from 1:2 to 1:2048 in a 96-well V-shaped bottom microtiter plate containing 50 μL of CMF-PBS in each well, then 50 μL of NDV antigen (4 HA units) was added into all wells except for the last row as controls. The antigen serum mixture was incubated for 10 min at 37 °C, then 50 μL of 1% rooster erythrocytes suspension was added into each well and continued to incubate for 30 min. A immune positive serum, negative serum, erythrocytes and antigens were also included as controls. The highest dilution of serum causing complete inhibition was considered as the endpoint. The geometric mean titer was expressed as reciprocal log2 values of the highest dilution that displayed HI.
2.6. Statistical analysis
The data of A570 value and antibody titer were expressed as the mean ± S.D. Duncan's multiple range test was used to analyze the difference among groups with the software SPSS 16.0. x 2-test was used to analyze the difference of the mortality, morbidity and protective rate. Significant differences were considered as p < 0.05.
3. Results
3.1. Antiviral activity of different drugs in CEF
3.1.1. Antiviral activity of different drugs in pre-adding drug
The A570 values of every group in pre-adding drug were listed in Table 1 . The A570 values of the two prescriptions of EF-WDFF-BSRF and EF-BSRF-SF at all concentrations were significantly larger than those of the corresponding virus control group (p < 0.05). The values of the prescriptions of EF-BSRF at 31.3–7.8 μg/mL, EF-SF-WDFF-BSRF at 31.3–15.6 μg/mL, WDFF-BSRF-SF at 15.6–7.8 μg/mL, EF-WDFF-SF at 15.6 and 3.9 μg/mL, EF-SF and EF-WDFF at 31.3 μg/mL, were significantly larger than those of the corresponding virus control group (p < 0.05). The A570 values of flavone ingredients of EF, BSRF and SF at all concentrations, and WDFF at 31.3 μg/mL groups were also significantly larger than those of the corresponding virus control group (p < 0.05).
Table 1.
The A570 values of every group in pre-adding drug n = 4.
| Concentration (μg/mL) | EF | BSRF | WDFF | SF | EF-SF |
|---|---|---|---|---|---|
| 31.3 | 0.458 ± 0.009a | 0.319 ± 0.015a | 0.294 ± 0.045a | 0.348 ± 0.015b | 0.297 ± 0.010b |
| 15.6 | 0.435 ± 0.001a | 0.318 ± 0.012a | 0.272 ± 0.016abc | 0.360 ± 0.012b | 0.166 ± 0.010d |
| 7.8 | 0.426 ± 0.009a | 0.329 ± 0.005a | 0.278 ± 0.007ab | 0.346 ± 0.014b | 0.183 ± 0.007d |
| 3.9 | 0.344 ± 0.013b | 0.325 ± 0.006a | 0.220 ± 0.008c | 0.357 ± 0.012b | 0.186 ± 0.006d |
| Virus control | 0.133 ± 0.009c | 0.235 ± 0.009b | 0.235 ± 0.009bc | 0.232 ± 0.012c | 0.230 ± 0.006c |
| Cell control | 0.319 ± 0.011b | 0.316 ± 0.008a | 0.315 ± 0.008ab | 0.402 ± 0.006a | 0.375 ± 0.007a |
| Concentration (μg/mL) | EF-WDFF | WDFF-BSRF | EF-BSRF | EF-WDFF-SF | WDFF-BSRF-SF |
|---|---|---|---|---|---|
| 31.3 | 0.324 ± 0.024b | 0.269 ± 0.019b | 0.391 ± 0.005b | 0.253 ± 0.021c | 0.257 ± 0.012cd |
| 15.6 | 0.232 ± 0.014c | 0.266 ± 0.015b | 0.426 ± 0.009a | 0.350 ± 0.015a | 0.288 ± 0.003bc |
| 7.8 | 0.235 ± 0.003c | 0.173 ± 0.123c | 0.453 ± 0.013a | 0.280 ± 0.027bc | 0.340 ± 0.009a |
| 3.9 | 0.203 ± 0.014c | 0.180 ± 0.011c | 0.235 ± 0.006c | 0.322 ± 0.006ab | 0.240 ± 0.012d |
| Virus control | 0.230 ± 0.006c | 0.230 ± 0.006b | 0.247 ± 0.005c | 0.235 ± 0.009c | 0.235 ± 0.009d |
| Cell control | 0.375 ± 0.007a | 0.375 ± 0.007a | 0.397 ± 0.006b | 0.315 ± 0.008ab | 0.315 ± 0.008ab |
| Concentration (μg/mL) | EF-BSRF-SF | EF-WDFF-BSRF | EF-SF-WDFF-BSRF | ||
|---|---|---|---|---|---|
| 31.3 | 0.305 ± 0.012b | 0.371 ± 0.014ab | 0.472 ± 0.011a | ||
| 15.6 | 0.278 ± 0.022b | 0.361 ± 0.020bc | 0.414 ± 0.010b | ||
| 7.8 | 0.286 ± 0.007b | 0.371 ± 0.007ab | 0.273 ± 0.004c | ||
| 3.9 | 0.272 ± 0.021b | 0.330 ± 0.004c | 0.269 ± 0.008c | ||
| Virus control | 0.230 ± 0.006c | 0.247 ± 0.005d | 0.247 ± 0.005c | ||
| Cell control | 0.375 ± 0.007a | 0.397 ± 0.006a | 0.397 ± 0.006b |
Note: a–dData within a column without the same superscripts differ significantly (p < 0.05).
The A570 values of EF at 31.3–7.8 μg/mL groups, and the prescriptions of EF-BSRF at 15.6–7.8 μg/mL groups and EF-SF-WDFF-BSRF at 31.3 μg/mL group were not only significantly larger than those of the corresponding virus control group (p < 0.05), but also significantly larger than those of the corresponding cell control group (p < 0.05).
The highest virus inhibitory rates were illustrated in Table 2 . The highest virus inhibitory rate of EF was the highest (174.7%), the next one was EF-SF-WDFF-BSRF. The top five rates from high to low in pre-adding drug mode were EF, EF-SF-WDFF-BSRF, EF-WDFF-SF, EF-BSRF and WDFF-BSRF-SF by turn.
Table 2.
The highest virus inhibition rates of drugs in three drug-adding modes (%).
| Drug | Drug-adding method | Concentration (μg/mL) |
|||
|---|---|---|---|---|---|
| 31.3 | 15.6 | 7.8 | 3.9 | ||
| EF | 1 | 174.7 | 162.4 | 157.5 | 113.4 |
| 2 | 186.2 | 204.3 | 197.4 | 99.1 | |
| 3 | 73.3 | 87.9 | 70.7 | 30.9 | |
| BSRF | 1 | 103.7 | 102.5 | 116.0 | 111.1 |
| 2 | 125.9 | 154.3 | 139.7 | 134.5 | |
| 3 | 126.3 | 96.5 | 27.8 | 19.7 | |
| WDFF | 1 | 73.7 | 46.2 | 53.7 | -18.8 |
| 2 | 58.3 | 95.6 | 81.7 | 61.7 | |
| 3 | 113.6 | 82.3 | 67.2 | 49.0 | |
| SF | 1 | 68.2 | 75.3 | 67.1 | 73.5 |
| 2 | 34.6 | 54.3 | 82.4 | 6.5 | |
| 3 | 45.6 | 74.8 | 55.2 | 51.9 | |
| EF-SF | 1 | 46.2 | -44.1 | -32.4 | -30.3 |
| 2 | 91.7 | 88.2 | 97.2 | 64.6 | |
| 3 | 96.1 | 81.5 | 54.5 | -10.1 | |
| EF-WDFF | 1 | 64.9 | 1.4 | 3.5 | -18.6 |
| 2 | 161.1 | 163.9 | 123.6 | 101.4 | |
| 3 | 82.0 | 121.9 | 68.5 | -0.6 | |
| WDFF-BSRF | 1 | 26.9 | 24.8 | -39.3 | -34.5 |
| 2 | 127.8 | 122.9 | 98.6 | 72.9 | |
| 3 | 97.2 | 117.9 | 96.1 | 55.0 | |
| EF-BSRF | 1 | 96.0 | 119.3 | 137.3 | -8.0 |
| 2 | 177.7 | 246.6 | 249.5 | 215.5 | |
| 3 | 164.1 | 138.9 | 140.5 | 44.3 | |
| EF-WDFF-SF | 1 | 22.5 | 143.8 | 56.3 | 108.8 |
| 2 | 33.3 | 63.9 | 238.9 | 219.4 | |
| 3 | 13.6 | 48.0 | 58.1 | 63.6 | |
| WDFF-BSRF-SF | 1 | 27.5 | 66.3 | 131.3 | 6.3 |
| 2 | 138.5 | 247.9 | 209.4 | 177.8 | |
| 3 | 50.5 | 55.6 | 48.5 | 51.5 | |
| EF-BSRF-SF | 1 | 51.7 | 33.1 | 38.6 | 29.0 |
| 2 | 43.8 | 102.1 | 101.4 | 81.3 | |
| 3 | 23.6 | 33.7 | -6.2 | 97.8 | |
| EF-WDFF-BSRF | 1 | 82.7 | 76.0 | 82.7 | 55.3 |
| 2 | 113.6 | 228.2 | 207.8 | 184.5 | |
| 3 | 86.3 | 155.7 | 106.9 | 80.2 | |
| EF-SF-WDFF-BSRF | 1 | 150.0 | 111.3 | 17.3 | 14.7 |
| 2 | 159.2 | 275.8 | 241.7 | 229.1 | |
| 3 | 140.5 | 127.5 | 130.5 | 83.2 | |
Note: 1, 2, 3 means respectively pre-adding drug, post-adding drug and simultaneous-adding drug and virus.
3.1.2. Antiviral activity of different drugs in post-adding drug
The A570 values of every group in post-adding drug were listed in Table 3 . The A570 values of the nine prescriptions (except EF-WDFF-SF at 31.3 μg/mL) and the four flavone ingredients (except SF at 3.9 μg/mL) at all concentrations were significantly larger than those of the corresponding virus control group (p < 0.05).
Table 3.
The A570 values of every group in post-adding drug n = 4.
| Concentration (μg/mL) | EF | BSRF | WDFF | SF | EF-SF |
|---|---|---|---|---|---|
| 31.3 | 0.481 ± 0.005a | 0.411 ± 0.008b | 0.262 ± 0.007c | 0.221 ± 0.010c | 0.346 ± 0.012a |
| 15.6 | 0.502 ± 0.018a | 0.444 ± 0.002a | 0.329 ± 0.008ab | 0.251 ± 0.007b | 0.341 ± 0.008a |
| 7.8 | 0.494 ± 0.012a | 0.427 ± 0.007ab | 0.304 ± 0.007b | 0.294 ± 0.002a | 0.354 ± 0.008a |
| 3.9 | 0.380 ± 0.010b | 0.421 ± 0.005ab | 0.268 ± 0.008c | 0.178 ± 0.009d | 0.307 ± 0.009b |
| Virus control | 0.265 ± 0.009c | 0.265 ± 0.009d | 0.157 ± 0.012d | 0.168 ± 0.003d | 0.214 ± 0.004c |
| Cell control | 0.381 ± 0.008b | 0.381 ± 0.008c | 0.337 ± 0.012a | 0.321 ± 0.013a | 0.358 ± 0.011a |
| Concentration (μg/mL) | EF-WDFF | WDFF-BSRF | EF-BSRF | EF-WDFF-SF | WDFF-BSRF-SF |
|---|---|---|---|---|---|
| 31.3 | 0.446 ± 0.012a | 0.398 ± 0.022a | 0.291 ± 0.010b | 0.184 ± 0.005cd | 0.427 ± 0.010c |
| 15.6 | 0.450 ± 0.007a | 0.391 ± 0.007ab | 0.362 ± 0.020a | 0.206 ± 0.029bc | 0.555 ± 0.001a |
| 7.8 | 0.392 ± 0.003b | 0.356 ± 0.006b | 0.365 ± 0.015a | 0.332 ± 0.003a | 0.510 ± 0.009b |
| 3.9 | 0.360 ± 0.018c | 0.319 ± 0.005c | 0.330 ± 0.013a | 0.318 ± 0.009a | 0.473 ± 0.027b |
| Virus control | 0.214 ± 0.004d | 0.214 ± 0.004d | 0.108 ± 0.005d | 0.160 ± 0.015d | 0.265 ± 0.009e |
| Cell control | 0.358 ± 0.011c | 0.358 ± 0.011b | 0.211 ± 0.010c | 0.232 ± 0.009b | 0.382 ± 0.008d |
| Concentration (μg/mL) | EF-BSRF-SF | EF-WDFF-BSRF | EF-SF-WDFF-BSRF | ||
|---|---|---|---|---|---|
| 31.3 | 0.277 ± 0.011b | 0.225 ± 0.018c | 0.272 ± 0.015c | ||
| 15.6 | 0.361 ± 0.008a | 0.343 ± 0.012a | 0.392 ± 0.002a | ||
| 7.8 | 0.360 ± 0.022a | 0.322 ± 0.003ab | 0.357 ± 0.009ab | ||
| 3.9 | 0.331 ± 0.012a | 0.298 ± 0.005b | 0.344 ± 0.042b | ||
| Virus control | 0.214 ± 0.004c | 0.108 ± 0.005d | 0.108 ± 0.005e | ||
| Cell control | 0.358 ± 0.011a | 0.211 ± 0.010c | 0.211 ± 0.010d |
Note: a–eData within a column without the same superscripts differ significantly (p < 0.05).
The A570 values of flavone ingredients of EF and BSRF at 31.3–7.8 μg/mL groups, and of these prescriptions of EF-WDFF and WDFF-BSRF-SF and EF-SF-WDFF-BSRF at all concentrations, EF-WDFF at 31.3–7.8 μg/mL groups, EF-WDFF-BSRF at 15.6–3.9 μg/mL groups, EF-WDFF-SF at 7.8–3.9 μg/mL groups and WDFF-BSRF at 31.3 μg/mL group were not only significantly larger than those of the corresponding virus control group (p < 0.05), but also significantly larger than those of the corresponding cell control group (p < 0.05).
The top five rates from high to low in post-adding drug mode were EF-SF-WDFF-BSRF, EF-BSRF, WDFF-BSRF-SF, EF-WDFF-SF, EF-WDFF-BSRF by turn. The highest virus inhibitory rate of EF-SF-WDFF-BSRF was the highest (275.7%) (Table 2).
3.1.3. Antiviral activity of different drugs in simultaneous-adding drug and virus after mixed
The A570 values of every group in simultaneous-adding drug and virus mode were listed in Table 4 . The A570 values of the five prescriptions of WDFF-BSRF, EF-BSRF, WDFF-BSRF-SF, EF-WDFF-BSRF, EF-SF-WDFF-BSRF and the four flavone ingredients at all concentrations were significantly larger than those of the corresponding virus control group (p < 0.05).
Table 4.
The A570 values of every group in simultaneous-adding drug and virus n = 4.
| Concentration (μg/mL) | EF | BSRF | WDFF | SF | EF-SF |
|---|---|---|---|---|---|
| 31.3 | 0.456 ± 0.009b | 0.459 ± 0.009a | 0.434 ± 0.004a | 0.341 ± 0.004c | 0.421 ± 0.016a |
| 15.6 | 0.484 ± 0.011a | 0.400 ± 0.008b | 0.372 ± 0.027bc | 0.420 ± 0.011b | 0.395 ± 0.008a |
| 7.8 | 0.451 ± 0.014b | 0.264 ± 0.012c | 0.342 ± 0.015cd | 0.367 ± 0.005c | 0.347 ± 0.012b |
| 3.9 | 0.375 ± 0.007c | 0.248 ± 0.012c | 0.306 ± 0.012d | 0.358 ± 0.019c | 0.232 ± 0.009c |
| Virus control | 0.316 ± 0.008d | 0.209 ± 0.004d | 0.209 ± 0.004e | 0.218 ± 0.006d | 0.250 ± 0.013c |
| Cell control | 0.507 ± 0.003a | 0.407 ± 0.009b | 0.407 ± 0.009ab | 0.488 ± 0.010a | 0.428 ± 0.007a |
| Concentration (μg/mL) | EF-WDFF | WDFF-BSRF | EF-BSRF | EF-WDFF-SF | WDFF-BSRF-SF |
|---|---|---|---|---|---|
| 31.3 | 0.396 ± 0.010bc | 0.423 ± 0.015ab | 0.481 ± 0.004a | 0.236 ± 0.007d | 0.309 ± 0.010b |
| 15.6 | 0.467 ± 0.013a | 0.460 ± 0.003a | 0.448 ± 0.017b | 0.304 ± 0.019c | 0.319 ± 0.019b |
| 7.8 | 0.372 ± 0.023c | 0.421 ± 0.011b | 0.450 ± 0.009b | 0.324 ± 0.008bc | 0.305 ± 0.007b |
| 3.9 | 0.249 ± 0.019d | 0.348 ± 0.015c | 0.324 ± 0.015d | 0.335 ± 0.001b | 0.311 ± 0.004b |
| Virus control | 0.250 ± 0.013d | 0.250 ± 0.013d | 0.266 ± 0.007e | 0.209 ± 0.004d | 0.209 ± 0.004c |
| Cell control | 0.428 ± 0.007ab | 0.428 ± 0.007ab | 0.397 ± 0.008c | 0.407 ± 0.009a | 0.407 ± 0.009a |
| Concentration (μg/mL) | EF-BSRF-SF | EF-WDFF-BSRF | EF-SF-WDFF-BSRF | ||
|---|---|---|---|---|---|
| 31.3 | 0.292 ± 0.009b | 0.379 ± 0.045b | 0.450 ± 0.013a | ||
| 15.6 | 0.310 ± 0.007b | 0.470 ± 0.015a | 0.433 ± 0.013a | ||
| 7.8 | 0.239 ± 0.009c | 0.406 ± 0.016b | 0.437 ± 0.017a | ||
| 3.9 | 0.424 ± 0.013a | 0.371 ± 0.021b | 0.375 ± 0.012b | ||
| Virus control | 0.250 ± 0.013c | 0.266 ± 0.007c | 0.266 ± 0.007c | ||
| Cell control | 0.428 ± 0.007a | 0.397 ± 0.008b | 0.397 ± 0.008b |
Note: a–dData within a column without the same superscripts differ significantly (p < 0.05).
The A570 values of flavone ingredient of BSRF at 31.3 μg/mL groups, and of the prescriptions of EF-BSRF and EF-SF-WDFF-BSRF at 31.3–7.8 μg/mL groups and EF-WDFF-BSRF at 15.6 μg/mL groups were not only significantly larger than those of the corresponding virus control group (p < 0.05), but also significantly larger than those of the corresponding cell control group (p < 0.05).
The top five rates from high to low in simultaneous-adding drug and virus mode were EF-BSRF, EF-WDFF-BSRF, BSRF, EF-SF-WDFF-BSRF, EF-WDFF by turn. The highest virus inhibitory rate of EF-BSRF was the highest (164.1%) (Table 2).
3.1.4. Comparison of antiviral activity between single flavone ingredients with their prescriptions
The scores and rank of antiviral activity of flavone ingredients and their prescriptions were listed in Table 5 . The top two total scores from high to low were respectively EF-SF-WDFF-BSRF, EF-BSRF, and the next was EF-WDFF-BSRF. The total scores of the three prescriptions were larger than the others. The total scores of SF and EF-SF were the lowest. When comparing within different adding drug modes, the scores of EF, SF, EF-WDFF-SF and WDFF-BSRF-SF in pre-adding drug mode was larger than that of other adding drug modes, the scores of BSRF, WDFF-BSRF and EF-WDFF-BSRF in simultaneous-adding virus and drug were larger than that of other adding drug modes. However, the scores of prescriptions of EF-SF-WDFF-BSRF and EF-BSRF in three adding drug modes approached to each other, and all presented at front row.
Table 5.
The scores and rank of antiviral activity of flavone ingredients and their prescriptions.
| Drug | Scores of different adding drug methods |
Total scores | Rank | ||
|---|---|---|---|---|---|
| Pre-adding drug | Post-adding drug | Simultaneous-adding drug and virus | |||
| EF | 13 | 8 | 4 | 25 | 4 |
| BSRF | 8 | 6 | 10 | 24 | 5 |
| WDFF | 5 | 2 | 7 | 14 | 9 |
| SF | 6 | 1 | 3 | 10 | 12 |
| EF-SF | 2 | 3 | 5 | 10 | 12 |
| EF-WDFF | 4 | 7 | 9 | 20 | 8 |
| WDFF-BSRF | 1 | 5 | 8 | 14 | 9 |
| EF-BSRF | 10 | 12 | 13 | 35 | 2 |
| EF-WDFF-SF | 11 | 10 | 2 | 23 | 6 |
| WDFF-BSRF-SF | 9 | 11 | 1 | 21 | 7 |
| EF-BSRF-SF | 3 | 4 | 6 | 13 | 11 |
| EF-WDFF-BSRF | 7 | 9 | 12 | 28 | 3 |
3.2. Results of test in vivo
3.2.1. The changes of antibody titer
The antibody titers of every group were illustrated in Fig. 1 . Before vaccination (D 0) and on day 7 after the first vaccination, there were not significant differences among the titers of five groups (p > 0.05). On days 14–40 after the first vaccination, the antibody titers in BC group were significantly lower than those in other four groups (p < 0.05), and in VC group were significantly lower than those in three flavone prescriptions groups (except EF-WDFF-BSRF group on D 14) (p < 0.05). On days 14–28 after the first vaccination, there was no significant difference among the titers of three flavone prescriptions groups (p > 0.05). On days 21–28 after the first vaccination, the antibody titers in EF-SF-WDFF-BSRF group were higher than those in the other two flavone prescriptions groups (p > 0.05), and on day 12 after challenge (D c12) was higher than that in EF-WDFF-BSRF group (p > 0.05) and significantly higher than that in EF-BSRF group (p < 0.05).
Fig. 1.
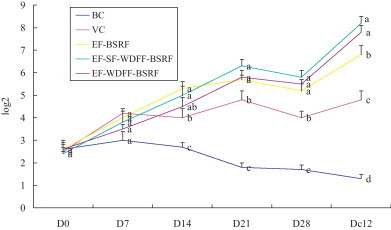
The change of antibody titer in immune protective test.
3.2.2. The immune protective effects
The results of the morbidity, mortality and protective rate in immune protective test were listed in Table 6 . The mortality and morbidity in three flavone prescriptions groups were lower than that in VC group, in EF-SF-WDFF-BSRF group was the lowest and significantly lower than that in VC group (p < 0.05). There were no significant differences of the morbidity and mortality among the three flavone prescriptions groups (p > 0.05). The protective rates in three flavone prescriptions groups were higher than that in VC group, in EF-SF-WDFF-BSRF group was the highest and significantly higher than that in VC group (p < 0.05), and higher than those in the other two flavone prescriptions groups (p > 0.05).
Table 6.
The morbidity, mortality and protective rate in immune protective test.
| Groups | Morbidity (%) | Mortality (%) | Protective rate (%) |
|---|---|---|---|
| BC | 0c | 0c | – |
| VC | 64.0a | 42.0a | 36.0b |
| EF-BSRF | 48.0ab | 34.0ab | 52.0ab |
| EF-SF-WDFF-BSRF | 40.0b | 20.0b | 60.0a |
| EF-WDFF-BSRF | 48.0ab | 28.0ab | 52.0ab |
Note: a–bData within a column without the same superscripts differ significantly (p < 0.05).
4. Discussion
4.1. Antiviral effects of flavone prescriptions in vitro
The cell absorbance value (A570 value) is an index to reflect the number of living cells and related to cell growth, the larger of the A570 value, the more live cells are [19], [20]. This experiment in vitro designed three drug-adding modes. They are respectively corresponding to prevention, sterilization and treatment of three clinical administrations. In the present experiment, the A570 values of the nine prescriptions at all concentrations in post- and simultaneous-adding modes, and of most of the prescriptions at proper dose in pre-adding mode were significantly higher than those of the corresponding virus control group. The results suggested that these prescriptions could effectively inhibit NDV from infecting in vitro, and the sterilization and treatment effects were better than the preventative effect.
The virus inhibitory rate directly reflects the antiviral intensity of drug [14]. The scores and rank of antiviral activity is of benefit to compare antiviral activity. In three drug-adding modes, the prescriptions of EF-SF-WDFF-BSRF, EF-BSRF and EF-WDFF-BSRF presented at the top five of the highest virus inhibitory rate. Furthermore, their total scores located the highest three of the score rank. Moreover, the A570 values of prescriptions of EF-SF-WDFF-BSRF (pre-, post-, simultaneous-adding), EF-BSRF (pre-, simultaneous-adding) and EF-WDFF-BSRF (post-, simultaneous-adding) were not only significantly larger than those of the corresponding virus control group (p < 0.05), but also significantly larger than those of the corresponding cell control group (p < 0.05). These results suggested that the prescriptions of EF-SF-WDFF-BSRF, EF-BSRF and EF-WDFF-BSRF possessed the best antiviral effect to inhibit the cellular infectivity of NDV in vitro than the other prescriptions and flavone ingredients. Thus, the three prescriptions of EF-SF-WDFF-BSRF, EF-BSRF and EF-WDFF-BSRF were chosen as the objective medicines in the vivo experiment.
4.2. The protective effect of flavone prescriptions on ND in chicken
In order to validate whether the antiviral effect of prescriptions in vitro and in vivo were consistent or not, the experiment in vivo was designed. Humoral immunity mediated by B lymphocytes is one of the main factors to resist infectious diseases as an important specific immune response. The peripheral antibody level in poultry is the marker reflecting humoral immune function in bird species [21], and plays an important role in the host's defense against infectious diseases. If the antibody titer is higher, the infection degree of chicken to NDV will be less serious [22]. The results of immune protection test showed that on days 14, 21 and 28 after the first vaccination and day 12 after challenge (D 40), the antibody titers in three flavone prescriptions groups, especially in ES-SF-WDFF-BSRF group were significantly higher than those in VC group and BC group. This confirmed that the three flavone prescriptions could accelerate the antibody production in vaccinated and challenged chickens, the efficacy of ES-SF-WDFF-BSRF was stronger than those of the other two prescriptions. The results were consistent with that of antiviral effect of prescriptions in vitro, which confirmed that three flavone prescriptions, especially ES-SF-WDFF-BSRF had the best efficacy in enhancing humoral immunity thus improving the immune effect of ND vaccine.
The effect of Chinese herbal medicine on enhancing immune function is probably due to its direct action on immune system or indirect effect on other systems [23]. Since the three flavone prescriptions could improve the immune effect of ND vaccine, the challenge test was designed to evaluate its immunity protective effect from infection of NDV. The result showed that in ES-SF-WDFF-BSRF group, the mortality and mortality were the lowest; the protective rate was the highest, which proved that ES-SF-WDFF-BSRF possessed best immunity protective effect from infection of ND, and the results in vitro and in vivo were consistent.
In this experiment, the chickens were vaccinated with ND vaccine before challenge, therefore the challenge time was selected on day 28 after the first vaccination when their average titer of maternal antibody in VC group was over 4.0 log2. Because the NDV used for challenge was a virulent strain, its lethal dose was difficult to control. Though the mortality of experimental chicken was on the low side, the experimental data could still distinguish the protective effect of all groups.
4.3. Comparisons of antiviral action between single flavone ingredients and their prescriptions
Antiviral effects between 4 single flavones and their 9 prescriptions were compared by three adding drug modes. The highest virus inhibitory rate of EF was the highest in pre-adding drug mode (174.73%). Thus, during pre-adding drug mode, combining with any other single flavone will weaken the antiviral effect of EF. EF-SF-WDFF-BSRF possessed the highest virus inhibitory rate in nine prescriptions, just 150%. The highest virus inhibitory rate of WDFF-BSRF was lower than any other, which indicated that there was a mutual inhibition between WDFF and BSRF. However, the mutual inhibition will be weakened when SF or EF was added into WDFF-BSRF prescription. For this reason, the highest virus inhibitory rates of EF-WDFF-BSRF and WDFF-BSRF-SF and EF-SF-WDFF-BSRF located in front rank.
The highest virus inhibitory rates of the prescriptions of EF-SF-WDFF-BSRF and EF-BSRF presented the highest rank both in post- and simultaneous-adding modes. These results suggested that the combinations of the four ingredients (EF, SF, WDFF and BSRF) or of the two ingredients of EF and BSRF in the two foresaid modes showed synergistic effect. In addition, the combinations of EF-BSRF, EF-WDFF-BSRF and WDFF-BSRF-SF in post-adding drug, EF-WDFF-BSRF in simultaneous-adding virus and drug demonstrated the same effect. However, during in simultaneous-adding mode the combinations of EF-WDFF-SF and WDFF-BSRF-SF showed significantly mutual inhibition.
In a word, antiviral effect of Chinese herbal ingredient was not only intimately correlated with compatibility of drugs but also with their modes of action. That to say, the same prescription could emerge a different biological activity in different mode of action. It also demonstrated that anti-NDV modes of Chinese herbal flavone ingredients and their prescriptions were multi-link. Yan and Tan [24] also confirmed that the antiviral effects of Chinese herbal flavone ingredients were related to directly killing virus, inhibiting the procedures of virus replication, or activating and strengthening the immunity. Therefore, they could exert antiviral efficacy in different ways. Generally speaking, it is complicated mechanisms between the compatibility of the traditional Chinese herbal medicine and its bioactivities (synergistic effect or mutual inhibition), which needs further study. Compatibility Theory of Traditional Chinese Medicine Prescription is valuable reference for this purpose.
In conclusion, flavone prescriptions composed of suitable compatibility could possess synergistical action of antiviral effect in vitro and in vivo, ES-SF-WDFF-BSRF prescription could significantly inhibit the cellular infectivity of NDV, improve the protective effect of ND vaccine in chicken and would be expected to exploit into a new-type antiviral drug and immunopotentiator.
Acknowledgements
The project was supported by National Natural Science Foundation of China (Grant No. 31172355), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are grateful to all other staff in the Institute of Traditional Chinese Veterinary Medicine of Nanjing Agricultural University for their assistances in the experiments.
References
- 1.Kong X.F., Hu Y.L., Rui R., Wang D.Y., Li X.R. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. International Immunopharmacology. 2004;4:975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Sun J., Zhang Z.W., Wu G.J. Application and mechanism of antiviral medicine in clinical veterinary. Chinese Journal of Veterinary Medicine. 2009;45:63–64. [Google Scholar]
- 3.Kang S.M., Song J.M., Quan F.S., Compans R.W. Influenza vaccines based on virus-like particles. Virus Research. 2009;143:140–146. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng M., Chen H.S. The progress of Chinese herbalmedicine and active ingredients on antivirus. Chinese Traditional and Herbal Drugs. 1998;29:632–635. [Google Scholar]
- 5.Guo Z.X., Zhao L.B., Jiang J.L. Current situation and strategy of Chinese materia medica internationalization. Chinese Traditional and Herbal Drugs. 2003;34:97–100. [Google Scholar]
- 6.Vidal M., Endoh H. Prospects for drug screening using the reverse two-hybrid system. Trends in Biotechnology. 1999;17:374–381. doi: 10.1016/s0167-7799(99)01338-4. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y.B. People's Health Press; Beijing: 2003. Chemistry and Pharmacology of Chinese Herbal Medicine Compound. pp. 18–35. [Google Scholar]
- 8.Schnitzler P., Schuhmacher A., Astani A., Jurgen R. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine. 2008;15:734–740. doi: 10.1016/j.phymed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Q.W. Zhang, C.Y. Zhang, The latest research progress of the traditional Chinese medicine on antiviral, Traditional Chinese Patent Medicine 1(2005) 116–119.
- 10.Kong X.F., Hu Y.L., Yin Y.L., Wu G.Y., Rui R., Wang D.Y. Chinese herbal ingredients are effective immune stimulators for chickens infected with the Newcastle disease virus. Poultry Science. 2006;85:2169–2175. doi: 10.1093/ps/85.12.2169. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y.Q., Wang S., Jing J., Wang G.C., Jin C.C., Liu J.G. Anti-virus function of 5 kinds of total flavone ingredients in vitro. Journal of Nanjing Agricultural University. 2012;3:95–99. [Google Scholar]
- 12.Veterinary Pharmacopoeia Commission of the People's Republic of China, Veterinary Pharmacopoeia of the People's Republic of China, Part 1, Chemical Industrial Press, Beijing, 2000, pp.72–73.
- 13.Yin Z., Liu J.H. Science Press; Beijing: 1997. Animal Virology. p. 87. [Google Scholar]
- 14.Fan Y.P., Liu J.G., Wang D.Y., Hu Y.L., Yang S.J., Wang J.M., Guo L.W., Zhao X.N., Wang H.L., Jiang Y. Epimedium polysaccharide and propolis flavone can synergistically inhibit the cellular infectivity of NDV and improve the curative effect of ND in chicken. International Journal of Biological Macromolecules. 2011;48:439–444. doi: 10.1016/j.ijbiomac.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang D.Y., Hu Y.L., Sun J.L., Kong X.F., Zhang B.K., Liu J.G. Comparative study on adjuvanticity of compound Chinese herbal medicinal ingredients. Vaccine. 2005;23:3704–3708. doi: 10.1016/j.vaccine.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Tomohiro M., Toshihiko T., Toshio I. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydrate Research. 2003;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 17.Huang X.Y., Wang D.Y., Hu Y.L., Lu Y., Guo Z.H., Kong X.F., Sun J.L. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. International Journal of Biological Macromolecules. 2008;42:166–171. doi: 10.1016/j.ijbiomac.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Histoshi T., Masanori B., Shiro S. An application of tetra zolim (MTT) colorimetric assay for the screening of anti-herpes simplex virus compounds. Viral Methods. 1991;33:61. doi: 10.1016/0166-0934(91)90008-n. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y., Wang D.Y., Hu Y.L., Huang X.Y., Wang J.M. Sulfated modification of epimedium polysaccharide and effects of the modifiers on cellular infectivity of IBDV. Carbohydrate Polymers. 2008;71:180–186. [Google Scholar]
- 20.Hu Y.L., Kong X.F., Li X.R., Wang D.Y., Liu J.G., Zhang B.K. Effects of ten Chinese herbal medicinal ingredients on proliferation and resisting NDV infection of CEF. Acta Veterinaria et Zootechnica Sinica. 2004;35:301–305. [Google Scholar]
- 21.Qiu Y., Hu Y.L., Cui B.A., Zhang H.Y., Wang Y.G. Effects of achyranthes bidentata polysaccharide on immune efficacy of vaccine in chickens. Acta Veterinaria et Zootechnica Sinica. 2007;38:723–727. [Google Scholar]
- 22.Xue F., Wu S.L., Tang Y.H., Zhang P.H., Ding H., Gao S., Liu X.F. Correlation between the immune protection and the antibody level of Newcastle disease and avian influenza in chicken vaccinated with different immune procedures. Chinese Journal of Veterinary Medicine. 2005;9:23–25. [Google Scholar]
- 23.He X.H. People's Medical Press; Beijing: 2002. Traditional Chinese Medicine Immunology. pp. 408–409. [Google Scholar]
- 24.Yan M., Tang X.L. Research overview of flavonoids about antiviral effects. Asia-Pacific Traditional Medicine. 2009;9:149–150. [Google Scholar]


