Abstract
This work describes purification and characterisation of a monocot mannose-specific lectin from Hyacinth bulbs. The purified lectin has a molecular mass of ∼30 kDa in reducing as well as in non-reducing SDS-PAGE. In hydrodynamic studies by Dynamic Light Scattering (DLS) showed that purified lectin was monomeric in nature with a molecular size of 2.38 ± 0.03 nm. Agglutination activity of purified lectin was confirmed by rabbit erythrocytes and its agglutination activity was inhibited by d-mannose and a glycoprotein (ovalbumin). Glycoprotein nature of purified lectin was confirmed by Periodic Acid Schiff’s (PAS) stain. Purified lectin showed moderate pH and thermal stability by retaining hemagglutination activity from pH 6–8 and temperature up to 60 °C. It also suppressed the growth of human colon cancer cells (Caco-2) and cervical cancer cells (HeLa) with IC50 values of 127 μg/mL and 158 μg/mL respectively, after 24-h treatment. Morphological studies of treated cells (Caco-2 and HeLa) with hyacinth lectin by AO/EB dual staining indicated that purified lectin is capable of inducing apoptosis.
1. Introduction
Lectins are non-immune origin glycoproteins having tremendous applications in analytical biochemistry as well as in pharmacology [1]. Initially, lectins were characterised as hemagglutinins due to their ability to agglutinate either human or rabbit erythrocytes [2]. They are found in all living organisms (bacteria, fungus, plant, and animal) [3] and are mainly involved in carbohydrate recognition with various applications in immunology, glycoproteins purification, virology, cytochemistry and cell biology [2], [4], [5], [6], [7]. In plants, they are expressed in all parts and are extracted from seeds, bulbs, leaves, fruits, roots, and flowers [8], [9], [10], [11]. Due to their diverse role in plants such as storage of proteins [12], protection from insects [13], cell wall propagation [14], a carrier for sugar moieties [15] and mitogenic stimulation [16], lectins have been explored more in detail over the last couple of years. Besides their biological activities plant lectins are also used for diagnostics purpose of various diseases, and widely used in drug delivery systems [17]. Several lectins also act as antiviral agents such as concavalin A, musa acuminate lectin, narcissus pseudonarcissusu lectin and many more [18], [19]. Mannose specific lectin isolated from plant showed anti-corona viral activity which is an acute respiratory syndrome caused by coronavirus [20].
Identification and biochemical characterisation of new lectins will certainly help in the development of new tools for disease control or to understand disease biology. Lectins have been characterised from various plant families like Solanaceae, Rosaceae, Malavaceae and Leguminceae [21]. However, lectin from Liliaceae, Asparagaceae, Iridiaceae and other plant families have not been studied much in details and therefore it is necessary to characterise new lectins from these classes of plants [22]. Hyacinth lily is a bulbous monocotyledon plant that belongs to the family of Asparagaceae and subfamily Scilloideae. In literature some reports on few small molecules like carbohydrate, alkaloids from this plant exist, however the functions of these molecules are not clear and there is strong evidence that these molecules may play significant role in plant defence system.
In this study we have made attempts to purify hyacinth lectin with significant application in disease biology. Many reports about lectins studies have been shown that lectins bind to specific carbohydrates that make it to distinguish between normal and malignant cells [23], [24]. It was reported that lectins such as ricin, mistletoe, many more can be inhibiting the growth of malignant cells in dosage dependent manner [25], [26]. Therefore, we have investigated anti-proliferative activity and apoptosis induced activity of hyacinth lectin on human cancer cells, colon cancer cell line (Caco-2) and cervical cancer cell line (HeLa).
2. Materials and methods
2.1. Extraction of plant material
Hyacinth plant bulbs were collected from New-Delhi, India during the months of Oct-Dec. The bulbs were washed with Milli Q water; air dried and kept in −80 °C until purification was carried out. Bulbs of hyacinth were cut into small pieces and pulverized in presence of liquid nitrogen in a ventilated hood. The pulverized plant tissues were grinded and homogenized for 24 h at 4 °C in 300 mL of extraction buffer (50 mM Tris buffer; pH 7.2) containing 2.5% of phenylmethylsulfonyl fluoride (PMSF). The homogenate obtained was centrifuged at 8000 rpm for 30 min at 4 °C; supernatant was further processed for protein purification.
2.2. Fractionation of protein by ammonium sulphate precipitation (NH4)2 SO4
The supernatant was subjected to fractionation by ammonium sulphate in order to remove non-proteinaceous contaminants and non-desired proteins. The hyacinth crude sample was first processed by 20% saturated ammonium sulphate precipitation. The precipitate solution was centrifuged at 8000 rpm for 30 min at 4 °C. The resultant supernatant was further processed with 80% saturated ammonium sulphate precipitation. After centrifugation at 8000 rpm for 30 min at 40 °C, precipitate was then re-suspended in 50 mM Tris buffer (pH 7.2) and dialyzed to remove (NH4)2 SO4 against 50 mM Tris buffer for 24 h at 4 °C under constant stirring on a magnetic stirrer. Dialyzed sample was then analyzed on Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE).
2.3. Ion exchange chromatography
Ion exchange chromatography was carried out using diethylaminoethyl (DEAE)- Sepharose Fastflow resin (GE Healthcare). Activated resin was loaded on polypropylene column of dimension 40 × 1.5 cm with an excess of Tris buffer pH 7.2. Dialyzed protein sample 50 mg/mL concentration was loaded on the column which was pre-equilibrated with equilibration buffer (50 mM tris buffer, pH 7.2). Unbound protein was washed with wash buffer (50 mM tris buffer, pH 7.2). Bound protein was then eluted with elution buffer (50 mM Tris buffer) containing increasing concentration of NaCl in the concentration range of 100, 200, 300 mM, and 1 M with an elution rate of 500 μL/min. All fractions were collected and individually evaluated for hemagglutination activity and protein content by Bradford protein assay [27]. The fraction that displayed hemagglutination activity was subjected to gel filtration chromatography (GFC).
2.4. Gel-filtration chromatography
GFC was carried out using Sephadex G-75 (GE healthcare). Activated G-75 swelled beads were loaded on a polypropylene column with a dimension 150 × 1.6 cm. 100 mM DEAE fraction was loaded in Sephadex G-75 column and eluted with an excess of 50 mM Tris buffer, pH 7.2 with at flow rate of 250 μL/min.
2.5. SDS-PAGE and silver staining
The average molecular weight of the purified protein from Hyacinth bulb was determined by SDS-PAGE. 10 μL of purified lectin was loaded on SDS-PAGE (12.5%) with standard broad range protein ladder (Bio-Rad) as a marker. Coomassie brilliant blue G-250 staining was used to visualize protein bands on the gel. Further, purity of the purified lectin was determined by silver staining [28].
2.6. Hemagglutination assay
Hemagglutination assay of purified lectin was performed by using rabbit and human (A, B and O) erythrocytes both in 96-well microtiter U-plate. The purified hyacinth lectin solution of 100 μL (1 mg/mL) was added to the first well and from that 50 μL was serially diluted into the consecutive wells with phosphate buffer saline (PBS), pH 7.2 containing 2% rabbit red blood cell suspension. The plate was incubated for 30 min at 37 °C and hemagglutination activity was observed by the presence of agglutinated red blood cell in the well relative to negative control. One hemagglutination titer was defined as the reciprocal of the maximum dilution of sample inducing complete agglutination of RBC [29].
2.7. Inhibition of hemagglutination in presence of different sugars
The inhibition of hemagglutination by sugars was examined by 50 μL of 50 mM monosaccharides (Glucose, fructose, arabinose, mannose, galactose), disaccharides (Cellobiose, lactose, sucrose,) and glycoprotein (Ovalbumin). The sugar solutions were placed in well and serially diluted with PBS (pH 7.2) in a microtiter U-plate followed by addition of 50 μL of 1 mg/mL protein solution into each well. Plates were then incubated for 30 min at 37 °C. After incubation 2% rabbit erythrocytes were added to each well and further incubated for 30 min at 37 °C. Plates were subsequently observed for hemagglutination activity and hemagglutination inhibition titers were observed according to Benevidies et al. [30].
2.8. Total carbohydrate estimation and glycosylation observation using periodic acid Schiff’s (PAS) stain
Total carbohydrate was estimated as described by Hedge et al. [31]. 1 mg of lyophilized hyacinth protein was hydrolyzed using 250 μL of 2N HCl in boiling water bath for 3 h. Excess of HCl was neutralized by addition of Na2CO3 and diluted to 1 mL. 50 μL of protein hydrolysate was used for total carbohydrate estimation using anthrone reagent. Standard graph was prepared using 1 mg/mL glucose stock solution as standard.
Glycosylation of protein was detected according to the method described by Zacharius et al. Briefly, protein band on 12.5% SDS-PAGE was fixed in 12% tri-chloro acetic acid for 30 min and washed 2–3 times with water for 10 min. After washing, gel was placed in 1% periodic acid solution for 1 h. Subsequently, gel was washed thoroughly with distilled water for 90 min. The gel was then immersed in Fuchsin sulphate reagent (Schiff’s) for 1 h in dark followed by washing with freshly prepared sodium meta bisulphite for 30 min. The gel was then kept in 7% acetic acid for 1 h and dried for development of glycosylated protein bands.
2.9. Effect of temperature, pH and denaturant on stability of lectin
To investigate the thermal stability of purified hyacinth lectin, 1 mg/mL of purified lectin was dissolved in 50 mM PBS (pH 7.2). Thermal stability was examined by incubating the lectin at temperatures ranging from 30 °C to 80 °C for 1 h and samples were set aside to cool down to room temperature. Hemagglutination activity was carried out by adding 2% rabbit erythrocytes and further incubated for 30 min at 37 °C.
To investigate the pH stability of purified hyacinth lectin, 1 mg/mL of protein was dissolved in different buffer solutions; phosphate buffer (pH 4 and 6), Tris-HCl buffer (pH 8) and glycine NaOH buffer (pH10 and12) and incubated for 24 h at 37 °C. Hemagglutination activity was carried out by adding 2% rabbit erythrocytes and further incubated for 30 min at 37 °C.
Purified hyacinth lectin (1 mg/mL) in PBS (pH 7.2) was incubated with different concentrations of denaturant (Urea 1 M, 2 M, 3 M and 4 M) in microtiter plate for 0, 2, 4 and 24 h. Hemagglutination activity was carried out by adding 2% rabbit erythrocytes and further incubated for 30 min at 37 °C.
2.10. Protein size estimation by dynamic light scattering measurements
To measure the hydrodynamic radius of purified protein, Dynamic light scattering measurements were performed. Hyacinth lectin was dissolved in millipore water at a concentration of 1 mg/mL. The samples were mixed thoroughly using a vortex mixer. The prepared protein solutions were filtered through 0.22 μm millipore syringe filter followed by centrifuge for removal of dust from the samples. Only the supernatant was considered for all the DLS measurements. Dynamic light scattering measurements were performed using Photocor Complex (Photocor, Russia) equipped with a 25 mW diode LASER operating at a wavelength of 654 nm, a multiple tau correlator card and an Avalanche photo diode as a detector. This measures the normalized intensity – intensity autocorrelation function (ICF), , was at scattering vector, q given by,
Where the scattering vector, , with, λ, θ, and n are the wavelength of incident light, scattering angle and solution refractive index respectively.
The measured normalized ICF, is related to the normalized field – field autocorrelation function, , by the Siegert’s relation given by;
where, ‘β’ is the instrumental constant. From the field correlation function, information about decay constant, Γ, is extracted which is related to translational diffusion coefficient, D, by the relation, Γ = Dq 2.
From the measured diffusion coefficient, the protein size estimated using the Stokes – Einstein relation given by,
| D = kBT/6πηrH |
where, rH is the hydrodynamic radius of the proteins, kB is the Boltzmann constant, T is the absolute temperature, and η is the solution viscosity.
2.11. Cell cultures
The human cancer cell lines Caco-2 and HeLa were obtained from NCCS, Pune (India). Cells were propagated and maintained in culture Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 1% glutamine, 1% (v/v) penicillin (10,000 IU/mL) and streptomycin (100 mg/L) in a humidified 5% CO2 incubator at 37 °C.
2.12. Cell growth inhibition assay by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
In vitro inhibition effects of hyacinth lectin on human cancer cell lines Caco-2 and HeLa were demonstrated using MTT assay. The cells in logarithmic growth phase were digested with 1% trypsin and adjusted to 5 × 104 cells/mL using DMEM complete medium. 150 μL of the cells sample were pipetted into each well of 96-well plates and cultured for 24 h at 37 °C in 5% CO2. Then cells were cultured with different concentrations of hyacinth lectin (varying from 23 to 185 μg/mL) and incubated for 24 h at 37 °C in 5% CO2. After incubation, the culture medium was removed and adhered cells were washed with PBS. Subsequently, 100 μL of MTT reagent diluted in culture medium to the final concentration of 0.5 mg/mL was added and incubated for 4 h. MTT reagent was then removed and 150 μL of Dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The absorbance of the solution was recorded at 570 nm and cell viability was determined for each assay including control wells that did not contain lectin. All measurements were performed in triplicates. The inhibition rate was calculated according to the formula as following:
2.13. Cell morphological changes by acridine orange/ethidium bromide (AO/EB) dual staining
Caco-2 and HeLa cells (5 × 104 cells/well) were seeded into 24-well culture plate and incubated in presence of different concentrations of lectin varying from 23 μg/mL to 185 μg/mL for 24 h. Two controls, negative control that did not contain lectin and positive control that contained doxorubicin were also included in the experimental set. After incubation, media was removed and cells were washed with PBS. Cells were then fixed with 4% paraformaldehyde for 15 min and washed twice with PBS followed by staining with 50 μM AO/EB dye solution for 10 min at room temperature in dark and washed twice with PBS. Cellular morphology was observed under an inverted fluorescence microscope.
3. Results
3.1. Purification of hyacinth lectin
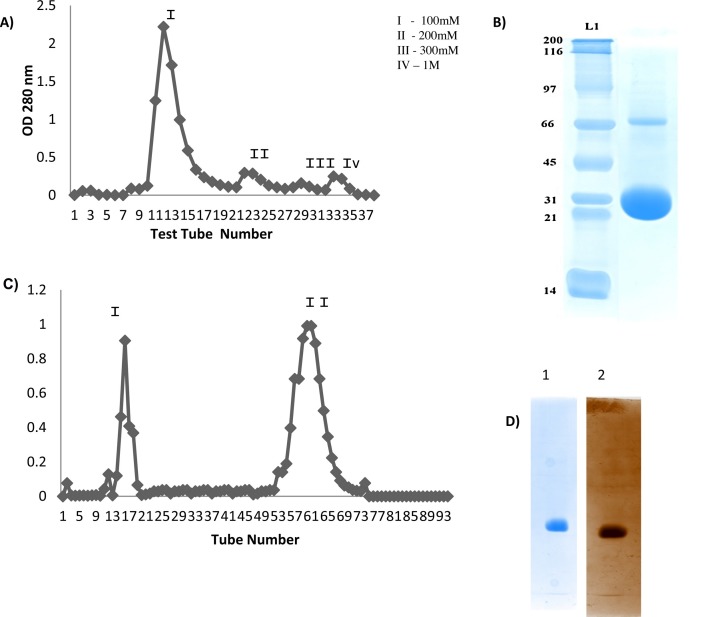
Lectin was purified using ammonium sulphate precipitation method [32] followed by anion exchange and gel filtration chromatography. Hemagglutination activity showed in 80% of saturated ammonium sulphate precipitation towards rabbit blood RBC was taken for the lectin purification. The DEAE (anion exchange) elution profile of 80% saturated ammonium sulphate precipitate of hyacinth bulbs showed four peaks (I–IV; Fig. 1 A) with hemagglutination activity being observed only in the elute of the 1st peak (Fig. 1A). On resolving the elute obtained in 1 st peak by SDS-PAGE we obtained two protein bands with molecular masses of approximately 30 kDa and 60 kDa (Fig. 1B). For further purification of the sample 1 st peak eluate was concentrated and loaded on Sephadex G-75 column with a fixed fraction collection volume of 1 mL. The elution profile showed two distinct peaks (I and II; Fig. 1C). We further tested the eluate peaks for hemagglutination activity and observed that only peak II showed hemagglutination activity. We further performed SDS-PAGE in a non-reducing (without treatment with β-mercaptoethanol) and reducing (treatment with β-mercaptoethanol) conditions with peak II eluate. Staining with Coomassie brilliant blue as well as silver staining of gel showed that hyacinth lectin is a monomeric protein with the molecular mass of ∼30 kDa (Fig. 1D). The data obtained during the extraction and purification process are shown in Table 1 .
Fig. 1.
Purification of protein using DEAE-Sepharose. (A) Chromatogram showing peaks for; (I) Elution with 100 mM NaCl, (II) 200 mM NaCl, (III) 300 mM NaCl and 1 M (IV). (B) SDS-PAGE analysis of I peaks obtained from chromatographic procedure showing; Commercial marker (L1) and Elution with 100 mM NaCl (L2). (C) Gel filtration chromatography using G-75, chromatogram showing two peaks, (D) SDS-PAGE analysis of peak (II) shows 30 kDa of protein (1)silver staining of polyacrylamide gel (2).
Table 1.
% Recovery of lectin at different purification steps.
| Procedure/step | Fraction volume (mL) | Protein (mg/mL) | Total protein (mg) | Recovery (%) | Hemagglutination unit | Total hemagglutination activity | Specific activity | Fold purification |
|---|---|---|---|---|---|---|---|---|
| Crude | 180 | 06.48 | 1167.12 | 100.00 | 1 | 180 | 0.15 | 1 |
| (NH4)2SO4 precipitation | 50 | 17.80 | 0890.15 | 076.26 | 4 | 200 | 0.22 | 1.45 |
| Ion exchange | 50 | 06.00 | 300.01 | 033.70 | 4 | 200 | 0.66 | 4.28 |
| Gel filtration | 15 | 04.33 | 065.00 | 021.66 | 16 | 240 | 3.69 | 25.71 |
3.2. Hemagglutination and hemagglutination inhibition assays
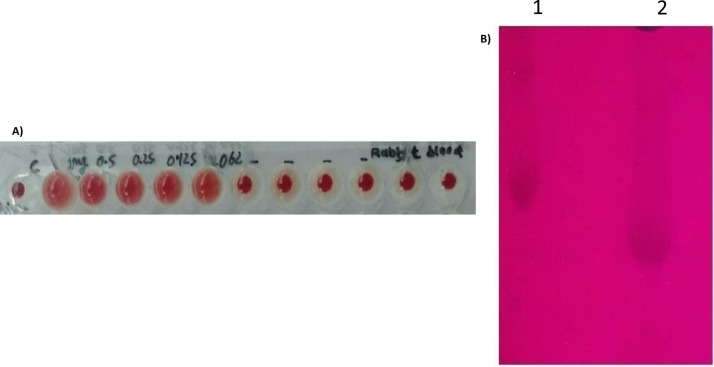
Purified lectin showed hemagglutination activity with rabbit erythrocytes only (Fig. 2 A) whereas in case of human (A, B and O) erythrocytes purified lectin was not shown any agglutination activity. Titer value of hemagglutination was observed at minimum concentration up to 0.06 mg/mL of hyacinth lectin. Hemagglutination assay of hyacinth lectin in presence of carbohydrates showed that lectin is highly specific for mannose as inhibition was not observed in case of any other monosaccharides or disaccharides tested (Table 2 ). Hemagglutination activity of hyacinth lectin was also inhibited by ovalbumin which is a glycoprotein containing mannose as glycan moieties.
Fig. 2.
(A) Hemagglutination activity of purified lectin with rabbit blood cells. (B) Periodic acid–Schiff (PAS) staining of ovalbumin as positive control (1)and purified lectin (2).
Table 2.
Minimum Inhibitory Concentration (MIC) values of different sugars.
| Sugars | Minimum inhibitory concentration (mM) |
|---|---|
| Mannose | 3.00 |
| Glucose | No inhibition |
| Fructose | No inhibition |
| Arabinose | No inhibition |
| Galactose | No inhibition |
| Lactose | No inhibition |
| Sucrose | No inhibition |
| Cellobiose | No inhibition |
| Ovalbumin | 5.00 |
3.3. Carbohydrate content and glycosylation nature of hyacinth lectin
Carbohydrate content and glycosylation nature of Hyacinth bulb lectin was determined by anthrone assay and PAS staining respectively. The carbohydrate content of purified lectin was found to be 5.2% and according to literature carbohydrate content of lectin vary from 1.5 to 16% [33], [34]. PAS stain result indicated that hyacinth lectin was glycoprotein in nature (Fig. 2B).
3.4. Hyacinth lectin stability at different temperatures, pH and denaturant
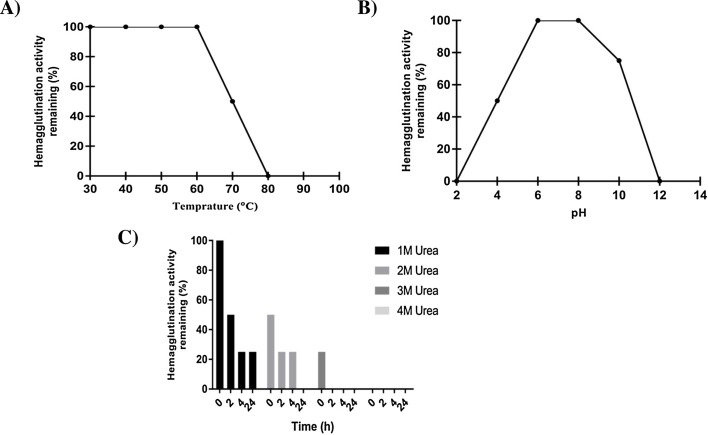
Hemagglutination activity of hyacinth lectin was completely retained in temperature range of 30 °C to 60 °C. Lectin activity decreased gradually at temperatures beyond 60 °C with no activity exhibited at 80 °C (Fig. 3 A).
Fig. 3.
Biophysical properties of hyacinth lectin. (A) Themal stability of lectin activity. (B) Effect of pH on lectin activity. (C) Impact of urea on lectin activity.
In case of variable pH range hyacinth lectin showed moderate stability. At pH 6–8, complete hemagglutination activity was retained whereas at low pH 4 hemagglutination activity was decreased to half and at pH 2 it is totally inactivated. Moreover hyacinth lectin showed residual activity even at pH 10 but has complete loss of activity at pH 12. It is important to note that hyacinth lectin maintained its hemagglutination activity from pH 4–10 even after 24 h of incubation. (Fig. 3B).
In case of 1 M urea hyacinth lectin retained hemagglutination activity 100% at zero h and almost half after 2 h of incubation while after 4 h and 24 h hemagglutination activity was retained up to 20%. Where as in case of 2 M urea lectin retained its hemagglutination activity up to 50% at zero h while after 2 h and 4 h hemagglutination activity was retained up to 20% and after 24 h lectin was denatured completely no activity was observed. In case of 3 M urea only at zero h of incubation lectin activity was observed up to 20% after that lectin was denatured completely no activity was observed while in case of 4 M of urea no activity was observed in any incubation time (Fig. 3C)
3.5. Molecular size determination of hyacinth lectin
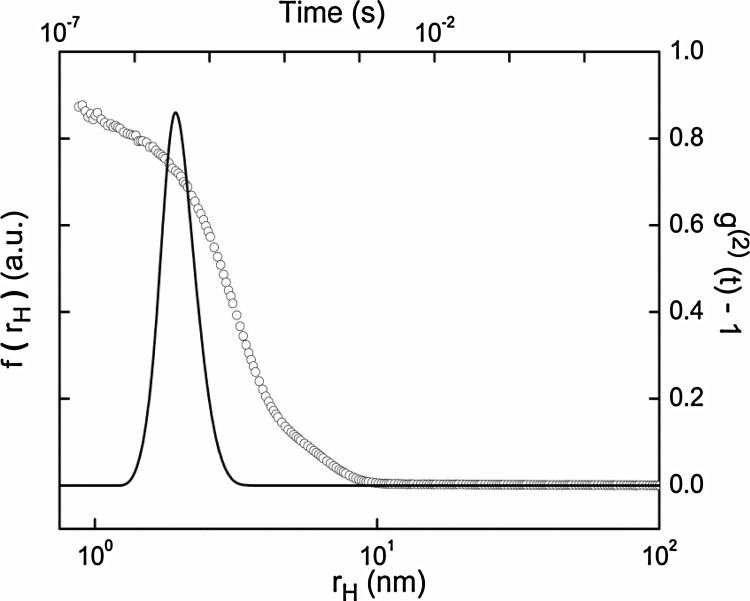
The autocorrelation function shown in (Fig. 4 ) was fitted using DynaLS software to determine decay constant, Γ of the particles. The diffusion constant of protein molecules is related to decay constant by the relation Γ = Dq^2. From the translational diffusion coefficient the hydrodynamic size, rH of purified hyacinth lectin was estimated to be, 2.38 ± 0.03 nm using the equation D = (k_B T)/(6πhr_H). The reported, rH, values are average of multiple runs carried out on the protein samples. All the measurements were carried out at a constant temperature of 25.0 ± 0.1 °C.
Fig. 4.
The above figure is shows auto-correlation and the corresponding hydrodynamic radius distribution function for purified lectin as measured by dynamic light scattering. The left axis of the figure corresponds to the hydrodynamic radius, where as the right axis corresponds to the auto correlation function.
3.6. Effect of hyacinth lectin on Caco-2 and HeLa cells proliferation
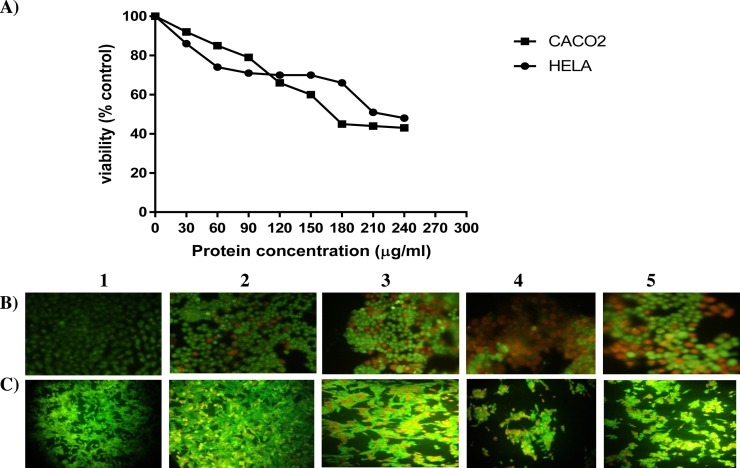
The anti-proliferative activity of hyacinth lectin was checked by MTT assay [35], [36]. Human cancer cells Caco-2 and HeLa when treated with different concentrations of hyacinth lectin ranging from 23 μg/mL to 185 μg/mL for 24 h, showed significant change in cell viability of both the cells when compared to untreated cells with IC50 of 127 ± 5 μg/mL and 158 ± 5 μg/mL respectively (Fig. 5 a). Anti-proliferative activity of various plant lectins against different cancer cell lines have been compared with hyacinth lectin in Table 3 .
Fig. 5.
(A) Cell viability of Caco-2 and HeLa Cell lines treated with varying concentrations (23–185 μg/mL)of Hyacinth lectin. (B) and (C) Morphological observation of treated human colon cancer cell line (CaCo2) and human cervical cancer cell line (HeLa) with different lectin concentrations (1) Control (2) 46 μg/mL (3) 69 μg/mL and (4) 92 μg/mL (5) Positive control treated with Doxorubicin further stained with AO/EB.
Table 3.
Reported plant lectins with their IC50 values.
| Sr. no. | Plant source | Cell line | IC50 (μg/mL) | Exposure time (h) | Reference |
|---|---|---|---|---|---|
| 1. | Caladium bicolor lectin | SW-620 | 100 | 48 | [43] |
| Colo-205 | 100 | 48 | |||
| 2. | Dioscorea bulbifera lectin | HT29 | 110 | 72 | [44] |
| 3. | Arisaema utile lectin | SW 620 | 9.8 | 72 | [45] |
| HepG2 | 40 | 72 | |||
| SW-620 | 38 | 72 | |||
| 4. | Allium chinense lectin | HCT-15 | 42 | 72 | [46] |
| SK-N-SH | 43 | 72 | |||
| IMR-32 | 49 | 72 | |||
| Colo-205 | 50 | 72 | |||
| HT-29 | 89 | 72 | |||
| HEP-3B | 60 | 48 | |||
| 5. | Pouteria torta lectin | Hela | 5 | 24 | [36] |
| 6. | Bauhinia ungulate lectin | HT-29 | 6 | 24 | [47] |
| HEP-2 | 6.8 | 24 | |||
| HT29 | 130 | 24 | |||
| 7. | Lotus corniculatus lectin | THP-1 | 39 | 24 | [48] |
| 8. | Phaseolus vulgaris lectin | HOP62 | 50 | 24 | [49] |
| HCT116 | 60 | 24 | |||
| HONE-1 | 1000 | 24 | |||
| 9. | Hyacinth lectin | HELA | 158 | 24 | – |
| CACO-2 | 127 | 24 |
3.7. Hyacinth lectin promotes apoptosis in Caco-2 and HeLa cells (observation of morphological changes by AO/EB staining)
Morphological changes of human cancer cells Caco-2 and HeLa with dual stain AO/EB clearly indicated that hyacinth lectin induce apoptosis in cancer cells. Treatment of cells with lower concentration (46 μg/mL) of hyacinth lectin induced early apoptosis that produced apoptotic bodies. These apoptotic bodies absorbed acridine orange and were observed as doted green colour (Fig. 5B2 & C2). We have observed orange colour stained cells which could be cells which were in late apoptosis after treatment with slightly higher concentration of lectin (69 μg/mL) (Fig. 5B3 & C3) [37]. Further, with higher concentration of hyacinth lectin (92 μg/mL) the cells were dead (due to loss of their membrane integrity) which incorporated only ethidium bromide stain and are observed as red (Fig. 5B4 & C4), whereas control cells without lectin showed uniformly green colour. In case of HeLa cells after treatment with lectin with higher concentration the dead cells were detached during the staining processe.
4. Discussion and conclusion
In the present study, lectin was successfully purified from hyacinth lily bulbs which belong to the family Asparagaceae. This is the first report on lectin from hyacinth plant. Lectin was purified using a combination of an ion exchange chromatography DEAE and gel filtration chromatography sephadex G-75. Purified hyacinth lectin showed hemagglutination activity towards rabbit erythrocytes with titter value of hemagglutination observed up to 0.06 mg/mL. Purified lectin was found to be a glycoprotein as determined by PAS staining whereas carbohydrate content of lectin was observed up to 5.2%. Similar carbohydrate contents of lectin have been reported in case of lectin purified from Artocarpus integerifolia and Clathrotropis nitida [34] Hemagglutination activity of hyacinth lectin was inhibited by mannose and ovalbumin which suggested that its manose binding lectin which is one of the significant properties of lectin purified from monocotyledon plants. Hyacinth lectin showed moderate temperature and pH stability. It is stable up to 60 °C and started to lose hemagglutination activity after heating above 60 °C. It maintained 50% hemagglutination activity upto 70 °C while above 70 °C it did not showed any activity. Similar pattern of stability of lectin was shown in case of Colocasia esculenta, Crinum latifolium [38]. Hyacinth lectin is stable over wide a range of pH from 4 to 12. Hemagglutination activity of hyacinth lectin is maximum in the range of pH 6–8 while in lower pH 4 its hemagglutination activity decreased upto 50% in higher pH 10 it is reduced upto 70% and above pH 10 it did not showed any hemagglutination activity. Dynamic scattering experiment showed that hyacinth lectin was monomeric in nature with no aggregation in solution. The hydrodynamic redii of hyacinth lectin showed 2.38 ± 0.03 nm which corresponds to a molecular weight of ∼30 kDa [39]. Antiproliferative and apoptosis activity have been shown in case of various lectins isolated from plant bulbs such as Sauromatum venosum, Arisaema jacquemontii [40], [41] We have demonstrated dose dependent antiproliferative nature of hyacinth lectin against human cancer cell lines Caco-2 and HeLa. Among the two cell lines we observe higher sensitivity of lectin mediated apoptotic response in Caco-2 cells with an IC50 value of 127 ± 5 μg/mL. This is the first report from hyacinth lectin on antiproliferative activity although other lectins from different plant sources have been reported for their anti-proliferative activity [42].
To demonstrate apoptotic cell death induced by hyacinth lectin, we treated Caco-2 and HeLa tumor cell lines with hyacinth lectin. Apoptotic activity was checked by Acridine orange/ethidium bromide dual staining and morphological changes were observed using fluorescence microscopy. We know that live cells absorb acridine orange and appear bright green whereas apoptosized cell shows orange colour. Upon treatment with hyacinth lectin we observe an increase in orange fluorescence in both Caco-2 and HeLa cells in higher concentrations indicating induction of apoptosis by hyacinth lectin.
The present study describes the purification and characterisation of lectin from Indian hyacinth plant bulbs having potential biological function in cancer biology.
Acknowledgements
We acknowledge the financial assistance from the Department of Science & Technology (DST), Ministry of Science and Technology. S.K and S Naik thank S.E.R.B for financial assistance under the Fast Track Scheme (SB/FT/LS-190/2012). We thank Dr. Kali Kishore Reddy Tetala and Debasish Roy for their valuable and critical comments on the work.
References
- 1.Nascimento K.S., Cunha A.I., Nascimento K.S., Cavada B.S., Azevedo A.M., Aires-Barros M.R. An overview of lectins purification strategies. J. Mol. Recognit. 2012;25(11):527–541. doi: 10.1002/jmr.2200. [DOI] [PubMed] [Google Scholar]
- 2.Garderes J., Bourguet-Kondracki M.L., Hamer B., Batel R., Schroder H.C., Muller W.E. Porifera lectins: diversity, physiological roles and biotechnological potential. Mar. Drugs. 2015;13(8):5059–5101. doi: 10.3390/md13085059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albores S., Mora P., Bustamante M.J., Cerdeiras M.P., Franco Fraguas L. Purification and applications of a lectin from the mushroom Gymnopilus spectabilis. Appl. Biochem. Biotechnol. 2014;172(4):2081–2090. doi: 10.1007/s12010-013-0665-5. [DOI] [PubMed] [Google Scholar]
- 4.Mason C.P., Tarr A.W. Human lectins and their roles in viral infections. Molecules. 2015;20(2):2229–2271. doi: 10.3390/molecules20022229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole A.Z., Kitchen S.A., Weis V.M. The role of complement in cnidarian-dinoflagellate symbiosis and immune challenge in the sea anemone aiptasia pallida. Front. Microbiol. 2016;7:519. doi: 10.3389/fmicb.2016.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeze H.H. Special considerations for glycoproteins and their purification. Curr. Protoc. Mol. Biol. 2001;(Unit 17.1) doi: 10.1002/0471142727.mb1701s22. [DOI] [PubMed] [Google Scholar]
- 7.Bonilla E., Schotland D.L., Wakayama Y. Application of lectin cytochemistry to the study of human neuromuscular disease. Muscle Nerve. 1980;3(1):28–35. doi: 10.1002/mus.880030105. [DOI] [PubMed] [Google Scholar]
- 8.Delatorre P., Rocha B.A., Souza E.P., Oliveira T.M., Bezerra G.A., Moreno F.B., Freitas B.T., Santi-Gadelha T., Sampaio A.H., Azevedo W.F., Jr., Cavada B.S. Structure of a lectin from Canavalia gladiata seeds: new structural insights for old molecules. BMC Struct. Biol. 2007;7:52. doi: 10.1186/1472-6807-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar M.A., Timm D.E., Neet K.E., Owen W.G., Peumans W.J., Rao A.G. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J. Biol. Chem. 1993;268(33):25176–25183. [PubMed] [Google Scholar]
- 10.Smeets K., Van Damme E.J., Verhaert P., Barre A., Rouge P., Van Leuven F., Peumans W.J. Isolation, characterization and molecular cloning of the mannose-binding lectins from leaves and roots of garlic (Allium sativum L.) Plant Mol. Biol. 1997;33(2):223–234. doi: 10.1023/a:1005717020021. [DOI] [PubMed] [Google Scholar]
- 11.Peumans W.J., Smeets K., Van Nerum K., Van Leuven F., Van Damme E.J. Lectin and alliinase are the predominant proteins in nectar from leek (Allium porrum L.) flowers. Planta. 1997;201(3):298–302. doi: 10.1007/s004250050070. [DOI] [PubMed] [Google Scholar]
- 12.Chrispeels M.J., Raikhel N.V. Lectins, lectin genes, and their role in plant defense. Plant Cell. 1991;3(1):1–9. doi: 10.1105/tpc.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyay S.K., Singh P.K. Receptors of garlic (Allium sativum) lectins and their role in insecticidal action. Protein J. 2012;31(6):439–446. doi: 10.1007/s10930-012-9423-8. [DOI] [PubMed] [Google Scholar]
- 14.Malinovsky F.G., Fangel J.U., Willats W.G. The role of the cell wall in plant immunity. Front. Plant Sci. 2014;5:178. doi: 10.3389/fpls.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabius H.J., Andre S., Jimenez-Barbero J., Romero A., Solis D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem. Sci. 2011;36(6):298–313. doi: 10.1016/j.tibs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Larsson E.L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- 17.Konska G., Wojtowicz U., Pituch-Noworolska A. Possible application of lectins in diagnostics and therapy. Part I. Diagnostic application. Przegl. Lek. 2008;65(4):189–194. [PubMed] [Google Scholar]
- 18.Hopper J.T.S., Ambrose S., Grant O.C., Krumm S.A., Allison T.M., Degiacomi M.T., Tully M.D., Pritchard L.K., Ozorowski G., Ward A.B., Crispin M., Doores K.J., Woods R.J., Benesch J.L.P., Robinson C.V., Struwe W.B. The tetrameric plant lectin BanLec neutralizes HIV through bidentate binding to specific viral glycans. Structure. 2017;25(5):773–782. doi: 10.1016/j.str.2017.03.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkouh O., Ng T.B., Singh S.S., Yin C., Dan X., Chan Y.S., Pan W., Cheung R.C. Lectins with anti-HIV activity: a review. Molecules. 2015;20(1):648–668. doi: 10.3390/molecules20010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balding P., Gold E.R. Observations on the reaction of en(a-)cells with sophora japonica haemagglutinin. Z. Immunitatsforsch. Exp. Klin. Immunol. 1975;145(2):156–165. [PubMed] [Google Scholar]
- 22.Peumans W.J., Barre A., Bras J., Rougé P., Proost P., Van Damme E.J.M. The liverwort contains a lectin that is structurally and evolutionary related to the monocot mannose-binding lectins. Plant Physiol. 2002;129(3):1054–1065. doi: 10.1104/pp.010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007;282(5):2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 24.Tang H., Hsueh P., Kletter D., Bern M., Haab B. The detection and discovery of glycan motifs in biological samples using lectins and antibodies: new methods and opportunities. Adv. Cancer Res. 2015;126:167–202. doi: 10.1016/bs.acr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung M.L., Baudino S., Ribereau-Gayon G., Beck J.P. Characterization of cytotoxic proteins from mistletoe (Viscum album L.) Cancer Lett. 1990;51(2):103–108. doi: 10.1016/0304-3835(90)90044-x. [DOI] [PubMed] [Google Scholar]
- 26.Pohleven J., Obermajer N., Sabotic J., Anzlovar S., Sepcic K., Kos J., Kralj B., Strukelj B., Brzin J. Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim. Biophys. Acta. 2009;1790(3):173–181. doi: 10.1016/j.bbagen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Chevallet M., Luche S., Rabilloud T. Silver staining of proteins in polyacrylamide gels. Nat. Protoc. 2006;1(4):1852–1858. doi: 10.1038/nprot.2006.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi F., Iwaya T., Haraguchi T., Goldstein I.J. The lectin from leaves of Japanese cycad, Cycas revoluta Thunb. (gymnosperm) is a member of the jacalin-related family. Eur. J. Biochem. 2002;269(17):4335–4341. doi: 10.1046/j.1432-1033.2002.03127.x. [DOI] [PubMed] [Google Scholar]
- 30.Benevides N.M.B., Holanda M.L., Melo F.R., Freitas A.L.P., Sampaio A.H. Purification and partial characterisation of the lectin from the marine red alga Enantiocladia duperreyi (C. Agardh) Falkenberg. Bot. Mar. 1998;41:521. [Google Scholar]
- 31.Ostergaard J.A., Thiel S., Hoffmann-Petersen I.T., Hovind P., Parving H.H., Tarnow L., Rossing P., Hansen T.K. Incident microalbuminuria and complement factor mannan-binding lectin-associated protein 19 in people with newly diagnosed type 1 diabetes. Diabetes Metab. Res. Rev. 2017;33 doi: 10.1002/dmrr.2895. [DOI] [PubMed] [Google Scholar]
- 32.Wingfield P.T. Protein precipitation using ammonium sulfate. Curr. Protoc. Protein Sci. 2001 doi: 10.1002/0471140864.psa03fs13. Appendix-3F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasconcelos M.A., Alves A.C., Carneiro R.F., Dias A.H., Martins F.W., Cajazeiras J.B., Nagano C.S., Teixeira E.H., Nascimento K.S., Cavada B.S. Purification and primary structure of a novel mannose-specific lectin from Centrolobium microchaete Mart seeds. Int. J. Biol. Macromol. 2015;81:600–607. doi: 10.1016/j.ijbiomac.2015.08.059. [DOI] [PubMed] [Google Scholar]
- 34.Alves A.C., Vasconcelos M.A., Santiago M.Q., Pinto-Junior V.R., Silva Osterne V.J., Lossio C.F., Bringel P.H., Castro R.R., Nagano C.S., Delatorre P., Souza L.A., Nascimento K.S., Assreuy A.M., Cavada B.S. A novel vasorelaxant lectin purified from seeds of Clathrotropis nitida: partial characterization and immobilization in chitosan beads. Arch. Biochem. Biophys. 2015;588:33–40. doi: 10.1016/j.abb.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 36.Boleti A.P., Ventura C.A., Justo G.Z., Silva R.A., de Sousa A.C., Ferreira C.V., Yano T., Macedo M.L. Pouterin, a novel potential cytotoxic lectin-like protein with apoptosis-inducing activity in tumorigenic mammalian cells. Toxicon. 2008;51(8):1321–1330. doi: 10.1016/j.toxicon.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Ponraj T., Paulpandi M., Vivek R., Vimala K., Kannan S. Protein regulation and Apoptotic induction in human breast carcinoma cells (MCF-7) through lectin from G. beauts. Int. J. Biol. Macromol. 2017;95:1235–1245. doi: 10.1016/j.ijbiomac.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Pereira P.R., Winter H.C., Vericimo M.A., Meagher J.L., Stuckey J.A., Goldstein I.J., Paschoalin V.M., Silva J.T. Structural analysis and binding properties of isoforms of tarin, the GNA-related lectin from Colocasia esculenta. Biochim. Biophys. Acta. 2015;1854:20–30. doi: 10.1016/j.bbapap.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Erickson H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online. 2009;11:32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh Bains J., Singh J., Kamboj S.S., Nijjar K.K., Agrewala J.N., Kumar V., Kumar A., Saxena A.K. Mitogenic and anti-proliferative activity of a lectin from the tubers of Voodoo lily (Sauromatum venosum) Biochim. Biophys. Acta. 2005;1723(1–3):163–174. doi: 10.1016/j.bbagen.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Kaur M., Singh K., Rup P.J., Kamboj S.S., Saxena A.K., Sharma M., Bhagat M., Sood S.K., Singh J. A tuber lectin from Arisaema jacquemontii Blume with anti-insect and anti-proliferative properties. J. Biochem. Mol. Biol. 2006;39:432–440. doi: 10.5483/bmbrep.2006.39.4.432. [DOI] [PubMed] [Google Scholar]
- 42.Ooi L.S., Ho W.S., Ngai K.L., Tian L., Chan P.K., Sun S.S., Ooi V.E. Narcissus tazetta lectin shows strong inhibitory effects against respiratory syncytial virus, influenza A (H1N1, H3N2, H5N1) and B viruses. J. Biosci. 2010;35:95–103. doi: 10.1007/s12038-010-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur M., Singh J., Singh S., Saxena K.A.K. A lectin with anti-proliferative, mitogenic and anti-insect potential from the tubers of caladium bicolor vent. J. Environ. Biol. 2011;36:1–9. [Google Scholar]
- 44.Sharma M., Hotpet V., BR S., AS K., Swamy B.M., Inamdar S.R. Purification, characterization and biological significance of mannose binding lectin from Dioscorea bulbifera bulbils. Int. J. Biol. Macromol. 2017;102:1146–1155. doi: 10.1016/j.ijbiomac.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 45.Dhuna V., Dhuna K., Singh J., Saxena A.K., Agrawal S.K., Kamboj S.S. Isolation, purification and characterization of an N-acetyl-D-lactosamine binding mitogenic and anti-proliferative lectin from tubers of a cobra lily Arisaema utile Schott. Adv. Biosci. Biotechnol. 2010;1(2):79–90. [Google Scholar]
- 46.Xiao X., He H., Ding X., Yang Q., Liu X., Liu S., Rang J., Wang T., Zuo M., Xia L. Purification and cloning of lectin that induce cell apoptosis from Allium chinense. Phytomedicine. 2015;22:238–244. doi: 10.1016/j.phymed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Silva H.C., d. Pinto L.S., Teixeira E.H., Nascimento K.S., Cavada B.S., Silva A.L.C. BUL: a novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process Biochem. 2014;49(2):203–209. [Google Scholar]
- 48.Rafiq S., Majeed R., Qazi A.K., Ganai B.A., Wani I., Rakhshanda S., Qurishi Y., Sharma P.R., Hamid A., Masood A., Hamid R. Isolation and antiproliferative activity of Lotus corniculatus lectin towards human tumour cell lines. Phytomedicine. 2013;21(1):30–38. doi: 10.1016/j.phymed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Ang A.S., Cheung R.C., Dan X., Chan Y.S., Pan W., Ng T.B. Purification and characterization of a glucosamine-binding antifungal lectin from Phaseolus vulgaris cv. Chinese pinto beans with antiproliferative activity towards nasopharyngeal carcinoma cells. Appl. Biochem. Biotechnol. 2014;172(2):672–686. doi: 10.1007/s12010-013-0542-2. [DOI] [PubMed] [Google Scholar]