Abstract
Severe hypoxemia refractory to pulmonary mechanical ventilation remains life-threatening in critically ill patients. Peritoneal ventilation has long been desired for extrapulmonary oxygenation owing to easy access of the peritoneal cavity for catheterization and the relative safety compared to an extracorporeal circuit. Unfortunately, prior attempts involving direct oxygen ventilation or aqueous perfusates of fluorocarbons or hemoglobin carriers have failed, leading many researchers to abandon the method. We attribute these prior failures to limited mass transfer of oxygen to the peritoneum and have designed an oxygen formulation that overcomes this limitation. Using phospholipid-coated oxygen microbubbles (OMBs), we demonstrate 100% survival for rats experiencing acute lung trauma to at least 2 h. In contrast, all untreated rats and rats treated with peritoneal oxygenated saline died within 30 min. For rats treated with OMBs, hemoglobin saturation and heart rate were at normal levels over the 2-h timeframe. Peritoneal oxygenation with OMBs was therefore shown to be safe and effective, and the method requires less equipment and technical expertise than initiating and maintaining an extracorporeal circuit. Further translation of peritoneal oxygenation with OMBs may provide therapy for acute respiratory distress syndrome arising from trauma, sepsis, pneumonia, aspiration, burns and other pulmonary diseases.
Keywords: Phospholipid monolayer, Oxygen absorption and transport, Acute lung injury, Hypoxemia, Acute respiratory distress syndrome
1. Introduction
Severe hypoxemia arising from lung injury is life-threatening in critically ill patients. Mechanical ventilation may be inadequate owing to limited mass transfer in the injured lung; overinflation, barotrauma and cyclic closing and reopening of the alveoli may further damage the lung and trigger a pulmonary and systemic inflammatory reaction that may lead to multiple system organ failure [1]. Researchers have long sought a safe and effective method for extrapulmonary oxygenation to treat these patients.
Currently, the last resort for treating pulmonary failure uses extracorporeal membrane oxygenation (ECMO), a temporary artificial extracorporeal support of the respiratory system and/or cardiac system [2]. ECMO was first used in an adult in 1972 to treat severe respiratory failure [3] and in 1974 on the first newborn [4]. Innovations in ECMO include the introduction of polymethylpentene hollow fibers with nonthrombogenic coatings and thin wire-reinforced cannula walls. ECMO use has historically centered on neonatal care [5]. Recently, the H1N1 flu pandemic led to wider use of ECMO, proving its utility in hypoxemic emergencies.
Alternative therapies are required, however, because ECMO is correlated with significant complications associated with the mechanically powered external blood circuit [2], [6]. Thrombosis and other deleterious factors are common results. To mitigate these problems, anticoagulants (heparin) are administered to the patient, often with additional adverse effects. Intracranial brain hemorrhage is an unpredictable and lethal complication of ECMO that occurs in ∼5% of patients [2], [7], [8]. Additionally, ECMO is expensive and complex to operate, limiting its accessibility for emergency care.
Thus, researchers have focused on other methods of extrapulmonary respiration, such as peritoneal oxygenation. Peritoneal oxygenation uses the large surface area of the peritoneum, the membrane that lines the abdominal cavity, as a gas exchanger. The main advantages of peritoneal oxygenation are easy access for catheterization; relative safety of circulating oxygen through the peritoneal cavity, in which the mesothelium acts as a gas-permeable barrier between the perfusate and the blood; and avoidance of extracting and reintroducing blood. The technique is analogous to peritoneal dialysis, a simple, low-tech form of renal replacement therapy that is less expensive and complex than conventional in-center hemodialysis [9].
The most obvious method is to mechanically ventilate the peritoneal cavity with pure oxygen gas. This method was first proposed almost a century ago [10]. Perhaps the most successful application of mechanical ventilation of the peritoneal cavity was reported by Barr et al. [11], [12], who showed an increase in arterial oxygen partial pressure (PaO2) and decrease in mortality rate for a lung injury model compared to pulmonary mechanical ventilation alone. The same group later showed improved outcome from peritoneal mechanical ventilation therapy in a hemorrhagic shock model [13]. In a subsequent large animal study, however, peritoneal mechanical ventilation showed no increase in mixed venous PO2 [14]. A more recent study in rabbits suffering a tracheal clamp showed only a moderate increase in survival time from 5.0 min for no treatment to 6.5 min for peritoneal mechanical ventilation [15], supporting claims of low clinical utility.
Prior peritoneal oxygenation studies introducing aqueous perfusates, such as perfluorocarbons (PFCs) and red blood cells (RBCs), into the intraperitoneal space have reported no effect or only a mild increase in PaO2 [15], [16], [17], [18], [19], [20]. Prior to our study, the most successful application of peritoneal oxygenation used liposomal synthetic hemoglobin carrier TRM-645, which produced a mean increase in rat cardiac arrest time following a right pneumothorax from 9 to 33 min [20].
Here we report on a new method of peritoneal oxygenation, in which we use OMBs designed for high oxygen carrying capacity, high oxygen delivery rate and sufficient stability for storage and transport. This follows a recent study by Kheir et al. [21], which showed a limited but significant therapeutic benefit from the intravenous injection of OMBs in rabbit hypoxic ventilation and tracheal clamp asphyxiation studies. The intravenous route appears to be a promising method for short-term rescue, but the prolonged continuous infusion of oxygen microbubbles into the bloodstream poses significant challenges for clinical translation, including the potential for embolism, thrombosis, immunogenicity and toxicities of lipid and saline load.
Our approach of direct systemic oxygenation by injecting OMBs into the peritoneal space is a radical change from existing oxygen delivery platforms. The procedure and apparatus for circulating OMBs through the peritoneal cavity is simple and straightforward, and the therapy precludes the need for an extracorporeal loop to circulate blood, thus potentially circumventing the complications from thrombosis and intracranial hemorrhage presented by ECMO. In this study, we examined the utility of peritoneal oxygenation with OMBs to increase mean survival time of rats experiencing a right pneumothorax lung injury, a common model for pulmonary failure that has been used in prior studies to examine the efficacy of peritoneal oxygenation.
2. Materials and methods
2.1. Synthesis of oxygen microbubbles
All solutions were prepared using filtered, 18 MΩ cm−1 deionized water (Direct-Q, Millipore, Billerica, MA). Glassware was cleaned with 70 vol% ethyl alcohol solution (Sigma–Aldrich; St. Louis, MO) and rinsed with deionized water. Phospholipid 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) was purchased from NOF (Tokyo, Japan), and polyoxyethylene-40 stearate (PEG-40S) was purchased from Sigma–Aldrich (St. Louis, MO). Phosphate buffered saline used for the control experiments was assumed to have neutral PaO2 tension relative to atmosphere, and thus was “oxygenated” considering the PaO2 in venous and arterial blood.
DSPC and PEG-40S were weighed in dry form, combined to a 9:1 m ratio, and dissolved into a 0.2-μm filtered solution of 0.15 m phosphate buffered saline (PBS) to a final lipid concentration of 12 mg/mL. The mixture was heated to 65 °C and dispersed using a Branson 450 sonifier (Danbury, CT) with an output power of 5/10 until the solution was translucent. The resulting solution was stored in a refrigerator prior to microbubble synthesis. Oxygen microfoam was prepared by adapting the process design developed by Swanson et al. [22] for synthesizing large volumes of oxygen microbubbles. The process consisted of an ultrasonic horn reactor enclosed in a water-cooled, continuous-flow chamber (Branson, Danbury, CT). The lipid suspension was fed at 5 °C and combined with room temperature oxygen gas in the reactor at roughly equal volumetric flow rates. The oxygen gas was emulsified at full sonication power and then fed to a flotation column to separate the oxygen microbubbles (bottom) from the macrofoam (top). Oxygen microbubbles were immediately collected from the bottom of the column using 60-mL syringes and centrifuged (Eppendorf, Hauppauge, NY) at 110 relative centrifugal force (RCF) for 4 min to form a ∼70 vol% concentrate. The concentrated oxygen microfoam was then transferred to 500-mL Wheaton serum bottles (Pasadena, TX), sealed under an oxygen atmosphere, and stored in the refrigerator. The infranatant lipid suspension from the centrifugal wash was collected and recycled into the sonication process to produce more microfoam. The process was repeated until the desired volume of 70 vol% oxygen microfoam was produced. Oxygen microbubble size distribution was determined using the electrozone sensing method (Coulter Multisizer III, Beckman Coulter, Opa Locka, FL). Oxygen gas volume fraction was determined by subtracting the weight of a fixed volume of microfoam from the weight of the same volume of aqueous medium (10 mg/mL lipid in PBS), and dividing this difference by the weight of the same volume of aqueous medium. Gas headspace purity in the serum bottle was determined using a model 6600 precision headspace gas analyzer (Illinois Instruments Inc, Johnsburg, IL).
2.2. Right pneumothorax lung injury model
The objective of this study was to test our hypothesis that oxygen microbubbles delivered by intraperitoneal infusion can significantly increase the mean survival time of an animal experiencing acute lung injury, in comparison to an intraperitoneal infusion of oxygenated saline solution. The research subjects were male Wistar rats (n = 10, mass = 430 ± 15 g, Charles River), housed 2 per cage and acclimated for 4 days with free access to food and water. We performed a controlled laboratory study to test the therapeutic benefit of an intraperitoneal infusion of oxygen microbubbles in rats experiencing acute lung injury. Right pneumothorax was chosen as an appropriate model for acute lung injury based on prior work reported in the literature [20]. Two treatments were applied: oxygen microbubbles or oxygenated saline control. Following induction of pneumothorax, we observed vital signs and measured intraperitoneal pressure, infused volume, rectal temperature, heart rate and oxygen saturation (SaO2). The animals were randomly assigned to cages by the caretakers, and the animals were randomly chosen for treatments. No additional blinding was done in the study. The number of animals used in this study was determined from work by Matsutani et al. [20] and a preliminary feasibility study. In Matsutani, a synthetic oxygen carrier was injected into the intraperitoneal (IP) space of rats for the purpose of systemic oxygenation. The mean standard deviation and mean effective size were 6.9 and 19.9, respectively [20]. We assumed the same standard deviation, but half the effective size (μ − μ 0 = 10). Using these values, a target Type I error of 0.05 and a sample size of 6 rats per group yielded a power of 0.81. In our preliminary feasibility study, a sample size of 4 rats was used in the oxygen microfoam group, and 3 rats were used in the control group. Using the Log-rank test for comparison of the survival distribution (α = 0.05), a significant difference was found between the two groups (Χ 2 = 6.624, P = 0.010). Based on these results, it was decided that a sample size of 5 rats per group would be adequate for the lung injury study. Data collection was stopped due to either cardiac arrest or achievement of the primary endpoint. Additional criteria were established to allow for the exclusion of samples where complications arose. Criteria for exclusion included the occurrence of unsuccessful pneumothorax or infrequent data points being collected by the veterinary monitor. One rat from the control group was excluded from the study because both exclusion criteria occurred in the trial. No other data were excluded. No test for outliers was used; all data were included in the study. A primary endpoint of 2 h following induction of pneumothorax was selected to avoid complications arising from animal recovery from anesthesia and the initial lung injury. We sometimes observed these complications in animals living more than 2 h in our preliminary feasibility study. We calculated that the 2-h endpoint would be sufficiently long to demonstrate a statistically significant increase in mean survival time between animal receiving our therapy and the control group. Prior to our study, the longest mean survival time for an intraperitoneal oxygenation therapy following pneumothorax reported in the literature was ∼33 min [20]; thus, a mean survival time exceeding 2 h was deemed a significant improvement over the current state of the art and of high clinical utility. No other endpoints were specified. No replicates were performed. Each animal received only one pneumothorax and one treatment.
All animals were housed and underwent procedures approved by the University of Nebraska, Lincoln, Institutional Animal Care and Use Committee. Male Wistar rats (n = 10, mass = 430 ± 15 g, Charles River) were anesthetized in an induction chamber with 5% isoflurane to effect and maintained on 2% isoflurane. Each rat was weighed, given an IP injection of sodium pentobarbital solution (50 mg/kg dose), and then placed in the supine position on a warming pad (T/pump Classic, Gaymar) set at 38 °C to maintain body temperature. After the rat was fully sedated and unresponsive to pain delivered by paw pinches, a small incision into the skin was made to expose the fascia of the abdominal wall. A 12-gauge indwelling catheter was then inserted into the IP cavity and fitted to tubing (3.2 mm inner diameter, Tygon) by a Luer lock for subsequent infusions. A 22-gauge indwelling catheter was also inserted into the IP cavity and connected to two pressure transducers (4426-005G, Measurement Specialties Inc.) for measuring intra-abdominal pressure via a data acquisition system (myDAQ, LabVIEW, National Instruments). A veterinary monitor (SurgiVet Advisor, Smith's Medical) was used to monitor vitals. A pulse oximetry sensor (V3078, Smith's Medical) was clipped to the hind leg of the rat to measure the pulse rate and arterial oxygen hemoglobin saturation (SaO2). A temperature probe (WWV3418, Smith's Medical) was placed rectally for monitoring body temperature. A bolus of perfusate was pumped through a fluid warmer (iWarm, Midmark) set at 40.4 °C and into the IP space with a peristaltic pump (Thermo Scientific, FH100M) at 40 mL/min for 1 min, and then at 8 mL/min thereafter. At this time, the rats experienced a right pneumothorax by perforating the pleura of the pulmonary cavity with a scalpel. Infusion lasted until distension of the abdomen was noticeable, and the total infused volume of perfusate was recorded. The fluids used as a perfusate were saline as the control and oxygen microfoam (70 vol% O2 gas volume fraction).
During the experiment, temperature, pulse rate, and oxygen saturation (SaO2) of the blood was monitored in 8-s averages and recorded every 30 s for each rat. The intra-abdominal pressure was monitored and recorded at a frequency of 10 samples per second. Survival time was measured as the elapsed time between right pneumothorax and cardiac arrest. If still living, the rat was given a second dose of sodium pentobarbital 1 h following the administration of the previous dose. The endpoint of data collection occurred when the rat had suffered functional cardiac arrest or when the predefined endpoint of 2 h of survival time was reached. At 2 h plus 5 min, any surviving rats were euthanized by isoflurane overdose.
2.3. Statistical analysis
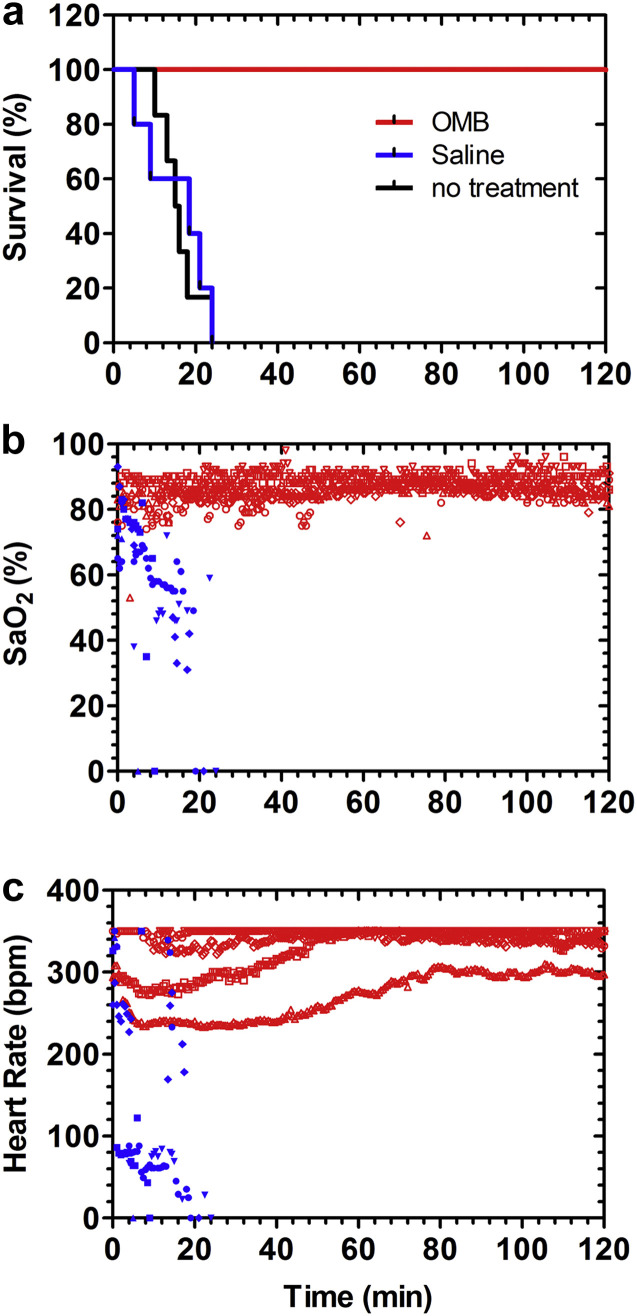
Kaplan–Meier survival curves (Fig. 3a ) were created with a sample size of 5 rats per group for a representation of the survival rate for the control and oxygen microfoam groups. A Log-rank (Mandel-Cox) test was done to compare the survival distribution of the oxygen microfoam group to the control group for a significant difference (α = 0.01). It was found that there was in fact a significant difference between the two groups (Χ 2 = 9.701, P = 0.002).
Fig. 3.
Peritoneal perfusion with OMBs provides life-sustaining oxygenation. (a) All 5 animals treated with OMBs survived to the 2-h endpoint and were subsequently euthanized as directed by the IACUC protocol. The median survival times for untreated animals and those treated with oxygenated saline were 15.5 (n = 6) and 18.5 min (n = 5), respectively. Thus, OMB infusion significantly increased survival time compared to saline control (P = 0.0039, unpaired two-tailed t-test using the 125 min endpoint for OMBs). (b) Animals treated with OMBs had an SaO2 between 80% and 90%, as measured by a paw-cuff pulse oximeter. SaO2 rapidly declined for animals treated with saline control. (c) The heart rate for animals treated with OMBs was 240–350 bpm immediately following the right pneumothorax and increased to 300–350 bpm throughout the experiment. Animals treated with saline control experienced a rapidly declining heart rate. The pulse oximeter could read up to a maximum heart rate of 350 bpm.
3. Results
3.1. Oxygen microbubble synthesis
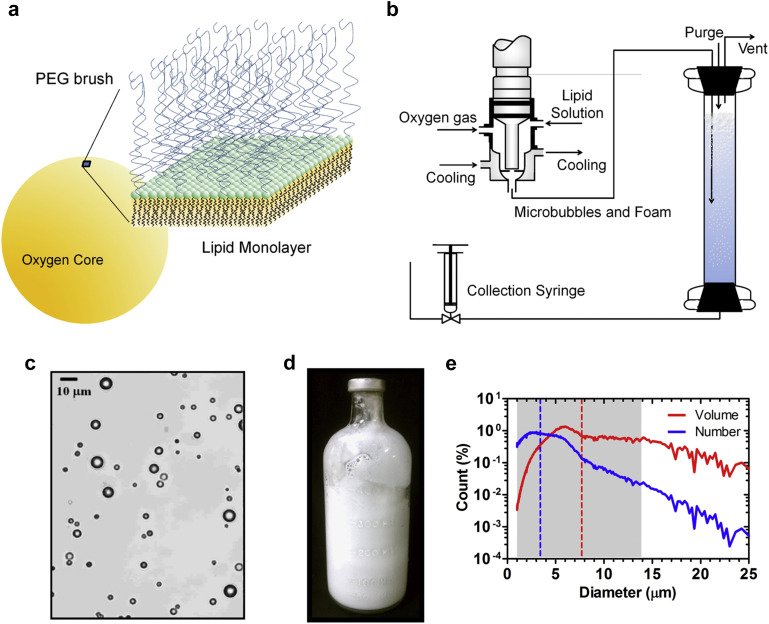
We designed oxygen microbubbles to comprise a phospholipid coating stabilizing a microscopic bubble of oxygen gas (Fig. 1a). Our design was chosen to mimic the naturally occurring surfactant that lines the lung alveolus [23], which is primarily composed of diacyl phosphatidyl-choline (PC). Saturated PC lipids self-assemble at the gas/water interface to form a 3-nm thick monomolecular layer that can be compressed to achieve very low interfacial tensions [24], which is necessary to avoid premature microbubble dissolution [25]. We chose distearoyl PC (DSPC) as the main lipid component because it provides an optimal tradeoff between in-plane rigidity to stabilize microbubbles for long durations in storage [26] and relatively low gas permeation resistance [27]. The lipid monolayer is permeable to oxygen and other gases, so the bubble is in continuous diffusive exchange with surrounding dissolved gases [28]. While highly permeable, the lipid shell modulates gas exchange by resisting monolayer expansion and compression beyond the close-packed lipid configuration at the microbubble resting radius [29]. We also chose a poly(ethylene glycol) (PEG) conjugated lipopolymer with a matching stearate lipid anchor as an emulsifying agent to improve yield by promoting lipid adsorption onto newly formed oxygen microbubbles and inhibiting microbubble coalescence. Self-consistent field theory calculations indicate that under these conditions the PEG chains form a ∼10-nm thick hydrated brush layer [30], which can lead to very high repulsive pressures between closely approaching microbubbles [31]. The PC/PEG shell comprises only ∼1% of the total microbubble volume, but is essential for stabilizing the microbubbles against coalescence and dissolution.
Fig. 1.
Lipid-coated oxygen microbubbles. (a) Oxygen gas comprises 99% of the microbubble volume. The oxygen core is stabilized by a thin (∼3 nm) phospholipid monolayer membrane, which reduces surface tension and provides in-plane rigidity. A hydrated PEG brush (∼10 nm height) grafted to the lipid surface provides steric repulsion to prevent microbubble coalescence. (b) A scaled-up OMB manufacturing process is used to generate up to 2 L of OMBs (∼70 vol%) per day. (c) Bright field microscopy of diluted OMBs showed discrete, spherical microbubbles. (d) OMBs have a milky white appearance and can be stored and transported in 500-mL serum bottles. (e) Shown are the volume and number-weighted size distributions for freshly generated OMBs. The dashed lines are the number (blue) and volume (red) mean diameters. The shaded area covers the size range for 90% of the total OMB gas volume. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Oxygen microbubbles were prepared by adapting the process design developed by Swanson et al. [22] for synthesizing large volumes of concentrated (∼70 vol%) microfoam (Fig. 1b–c). OMBs were synthesized and stored in sealed 500-mL serum bottles for storage and transport (Fig. 1d). The microbubbles were stable to several days of storage and interstate transport for animal studies. The OMBs were polydisperse in size (Fig. 1e), with a number-weighted mean diameter of 3.4 ± 1.9 μm (mean ± SD) and a volume-weighted mean diameter of 7.7 ± 4.2 μm. 90% of the total gas volume was contained in microbubbles with diameters less than 14 μm.
3.2. Peritoneal treatment of lung injury with oxygen microbubbles
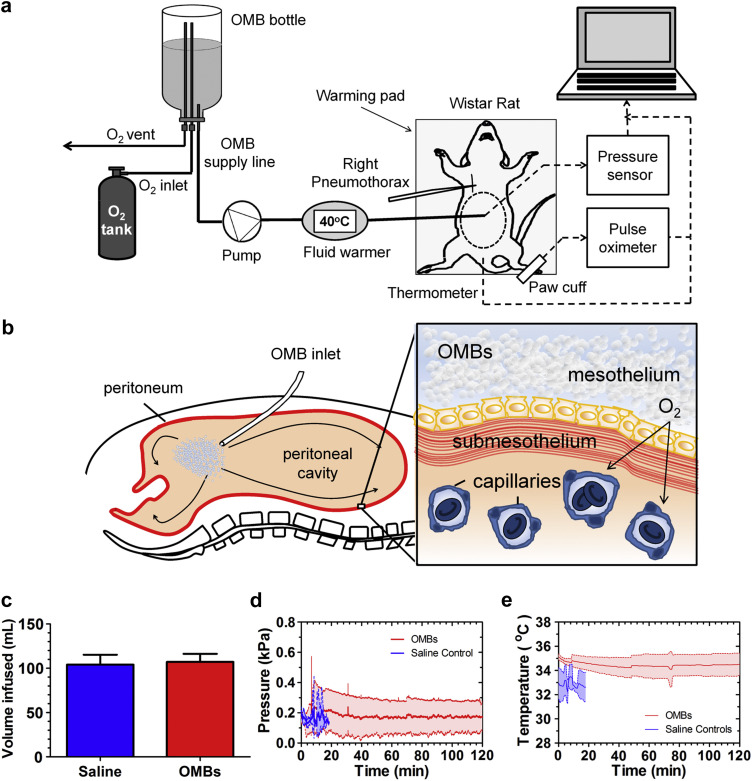
Pneumothorax to the right lung of the rat was chosen as an appropriate model for in vivo lung trauma based on prior studies. The right lung of the rat is composed of four lobes, whereas the left lung consists of a single lobe [32]. Thus, damage to the right lung can cause a drastic reduction in oxygen intake and respiratory function. Previous studies in rats have shown that the volume of the right lung is approximately 60% of the total lung volume [33], and collapse of the right lung by pneumothorax is fatal [20]. In this study, male Wistar rats (n = 10, mass = 430 ± 15 g) were anesthetized and placed in the supine position on a warming pad (Fig 2a). After the rat was fully sedated and unresponsive to pain delivered by paw pinches, perfusate and pressure sensor catheters were surgically inserted into the peritoneal cavity. A pulse oximeter sensor was clipped to the hind leg to measure pulse rate and arterial oxygen hemoglobin saturation (SaO2), and a temperature probe was placed rectally to monitor body temperature. A bolus of perfusate was pumped through a fluid warmer and into the peritoneal cavity with a peristaltic pump at 40 mL/min for 1 min, and then at 8 mL/min thereafter. The perfusion fluids were oxygenated saline as the control and OMBs (70 vol% O2 gas volume fraction). At this time, the pleura of the pulmonary cavity was perforated with a scalpel to give the rat a right pneumothorax, and an examination was performed to determine whether oxygenation through peritoneal infusion of OMBs could avoid the onset of hypoxemia and extend life.
Fig. 2.
Peritoneal oxygenation with OMBs. (a) The experimental setup is shown for treatment of a right pneumothorax model for acute lung injury. (b) OMBs infused into the peritoneal cavity deliver oxygen through the parietal peritoneum and visceral peritoneum to adjacent blood and tissue. (c) The total infused volume per rat was equivalent between the OMBs and saline control. Plots show the (d) pressure (mean ± SD) in the peritoneal cavity and (e) rectal temperature (mean ± SD) versus time for OMBs and saline.
Oxygen was delivered from OMBs by diffusive transport through the peritoneum into the adjacent blood and tissue of the abdominal wall and internal organs (Fig. 2b). Infusion lasted until distension of the abdomen was noticeable. The total infused volume of OMBs was 107 ± 9 mL, which was not statistically different from the saline control (Fig. 2c). The intra-abdominal pressure was maintained at less than 0.6 kPa gauge throughout the experiment for animals treated with both saline and OMBs (Fig. 2d). Following pneumothorax, the mean temperature was 34.5 ± 0.8 °C for animals receiving OMBs and 33.0 ± 1.0 °C for those receiving saline (Fig. 2e). These temperatures were below the normal temperature range of rats (35.9–37.5 °C) [32], but were unlikely a significant factor in causing death [34], [35], [36].
Peritoneal infusions of OMBs produced 100% survival to the predetermined 2-h endpoint in rats experiencing a right pneumothorax, compared to a median survival time of only 18.5 min for oxygenated saline and 15.5 min for untreated controls (Fig. 3a). Oxygen saturations were observed to be in the normal range throughout the 2-h timeframe for animals treated with OMBs, while they dropped precipitously within minutes for those treated with saline control (Fig. 3b). Furthermore, heart rate was also observed to be in the normal range over the 2 h for animals treated with OMBs, while it dropped rapidly within minutes for the controls treated with saline (Fig. 3c).
4. Discussion
The OMBs used in this study were designed for high oxygen carrying capacity and rapid oxygen delivery to the peritoneum. A 70 vol% suspension of OMBs at 1.0 bar and 37 °C contains an estimated 0.88 mg-O2 mL−1, compared to 0.31, 0.62 and 0.57 mg-O2 mL−1 for blood, liposomal hemoglobin and pure PFC, respectively [18], [20]. Furthermore, OMBs provide a greater fraction of oxygen availability to the body. The entire mass of oxygen can be released to low-oxygen surroundings because complete microbubble dissolution is thermodynamically favorable, whereas the finite solubility of oxygen in PFC, or the oxygen affinity of hemoglobin, restricts complete oxygen release from these formulations.
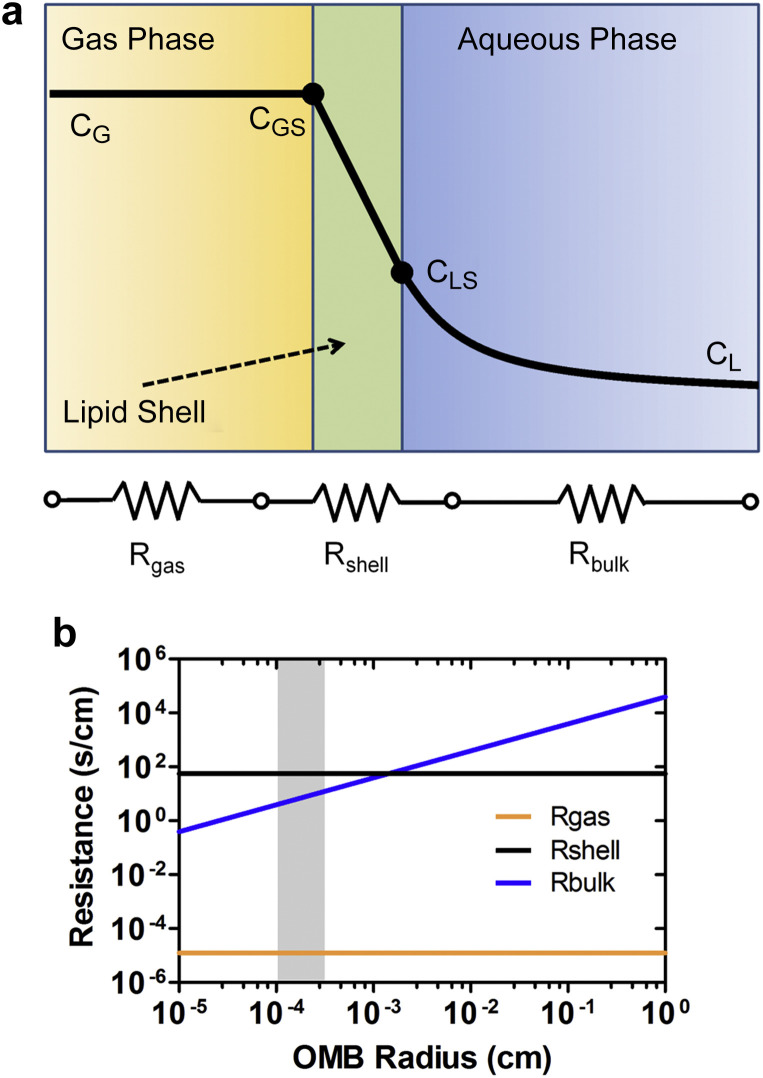
However, if available oxygen carrying capacity was the only criterion for successful peritoneal oxygenation, then ventilation with pure oxygen (1.26 mg-O2 mL−1) should not have failed to provide clinically relevant oxygenation, as reported by Giffin et al. [14]. We attribute the limited uptake for pure oxygen ventilation to the problem with injecting a bolus of free gas into the peritoneal cavity. Surface area is minimized owing to the high surface free energy (∼73 mJ/m2) of the gas/water interface. Thus, one may compare the transport of a centimeter-scale bubble for pure oxygen ventilation to hundreds of billions of micrometer-scale bubbles for OMBs. The transport process from the bubble to the bulk fluid occurs via three steps (Fig. 4a): (i) diffusion from the bulk gas to the inner surface of the lipid monolayer shell, (ii) diffusion through the lipid monolayer shell and absorption into the liquid phase and (iii) diffusion in the liquid from the bubble surface to the bulk. The mass transport resistance for diffusion from the gas to the surface (R gas) is estimated from kinetic theory to be approximately 10−5 s/cm [37] and is independent of microbubble size assuming zero tension in the lipid shell [24]. The shell resistance to oxygen permeation (R shell) has been measured to be approximately 102 s/cm [27], which is larger than 1.2 s/cm predicted by a statistical thermodynamics calculation for absorption at a clean oxygen gas/liquid interface [38]. The mass transfer resistance for diffusion in the liquid (R bulk) for a purely diffusing sphere is given as [39]:
| (1) |
where R is the bubble radius and D is the diffusivity of oxygen in water. A plot of the three transport resistances shows that diffusion in the liquid dominates the transport process for larger bubbles, but begins to approach that of the shell for microbubbles (Fig. 4b). Thus, the oxygen flux from microbubbles to the peritoneum approaches the physical upper limit for gas absorption through a lipid monolayer. In addition to increasing the oxygen transfer coefficient (k L), OMBs provide an increase in interfacial area per unit volume (a = 2.10 × 104 cm2 mL−1 for 70 vol% of 8-μm diameter OMBs) compared to a 1-cm diameter ventilation bubble (a = 4.20 cm2 mL−1). This gives an estimated volumetric transport coefficient (k L a) of 45 s−1 for OMBs compared to 2.1 × 10−4 s−1 for a ventilation bubble. The total oxygen transport rate is equal to the product of the volumetric transport coefficient and the concentration gradient between the gas phase and the peritoneum. Thus, OMBs provide a much higher overall oxygen delivery rate to the peritoneum, providing a plausible explanation as to why OMBs are successful in peritoneal oxygenation where pure oxygen ventilation has failed.
Fig. 4.
Oxygen transport from a microbubble. (a) Oxygen gas molecules must pass through a series of three resistances to enter the bulk fluid phase of the peritoneal cavity, including diffusion from the gas to the shell (Rgas), diffusion through the lipid shell and absorption into the liquid (Rshell) and diffusion to the bulk liquid (Rbulk). The diagram shows the relative concentrations. (b) A quantitative comparison of the three mass transfer resistances versus bubble radius at 25 °C and 1 bar. Rgas and Rshell are independent of bubble size, and Rgas is approximately six orders of magnitude less than Rshell. Rbulk is computed for a purely diffusing sphere; it dominates for large bubbles but approaches Rshell for microbubbles. The shaded region represents the OMB size range comprising 90% of the total gas volume, showing that the main mass transport limitation for these microbubbles is diffusion through the lipid shell.
The achievement of 100% survival to 2 h for our lung injury model, in which the median survival time for saline controls was only 18.5 min, indicates a potential clinical utility for patients suffering mild to severe hypoxemia where mechanical ventilation is inadequate or injurious. In neonates, for example, the method could be used to treat hypoxemia arising from meconium aspiration syndrome, pulmonary hypertension, congenital diaphragmatic hernia, sepsis and cardiac anomalies [5]. More research will be needed to determine if microbubble peritoneal oxygenation can also be used to treat patients suffering from lung diseases, such as acute respiratory distress syndrome (ARDS), avian flu and severe acute respiratory syndrome (SARS) or breathing complications, such as chronic obstructive pulmonary disease (COPD).
There were some limitations to our study. First, there was some concern that the pneumothorax lung injury model would cause death, not from gas exchange failure, but from increased oxygen demand from work of breathing, cardiogenic failure from altered venous return in a depressurized thorax, or from another unanticipated complication due to the wound. The success of microbubble peritoneal oxygenation in prolonging life in all five animals suggests that these complications are secondary to the need for rapid oxygen delivery.
A general limitation of peritoneal oxygenation is that in humans only about 20–30% of the systemic blood supply circulates through the portal and hepatic circuits and therefore only a fraction of the blood would be oxygenated [40]. More research must be done to investigate microbubble peritoneal oxygenation in larger animals where the ratio of the peritoneal area to total body mass is closer to humans. To mitigate this limitation, other serous membranes could be perfused to increase the surface area for O2 transfer. For example, it may be possible to circulate OMBs through the intrapleural space. This limitation underscores the necessity of efficient O2 transfer between the perfusate and the serous membrane, and as shown in this work, OMBs provide high O2 carrying capacity and rapid diffusion capability.
Another limitation to our study was that we only employed a one-way delivery of oxygen, but did not simultaneously remove carbon dioxide, which may ultimately result in complications such as hypercapnia and acidosis. Further work must be done to extend the technology for circulation, removal and replenishment of the OMBs in the peritoneal cavity. We do not view these engineering challenges as major obstacles for ultimate clinical translation given the precedent set by methods of peritoneal dialysis. Prior to translation, however, the technology must be examined in other acute lung injury models, such as inhalation injury and aspiration injury.
5. Conclusions
Oxygen microbubbles were designed for stability in storage and transport, high oxygen carrying capacity (0.88 mg-O2 mL−1) and rapid diffusion capability (k L a = 45 s−1). Peritoneal oxygenation with oxygen microbubbles produced 100% survival to the 2-h endpoint for rats suffering a right pneumothorax lung injury, whereas untreated and saline-treated controls had a median survival time of only 18.5 min and 0% of these animals survived to the 2-h endpoint. These results suggest a potential clinical utility for patients suffering mild to severe hypoxemia where mechanical ventilation is inadequate or injurious.
Acknowledgments
A portion of these research results were presented at the 2013 ASME Summer Bioengineering Conference in Sunriver, OR, and the 2013 BMES annual meeting in Seattle, WA. We wish to thank UNL's Attending Veterinarian, Dr. Kelly Heath and Veterinary Technician, Holly Reiling, as well as the Institutional Animal Care staff who helped us establish expertise in performing the procedures and for procuring, housing and caring for the animals used in this study. Funding was provided by NSF grant CBET 1059726 to M.B. and University of Nebraska new faculty startup funds to B.T.
Contributor Information
Mark A. Borden, Email: mark.borden@colorado.edu.
Benjamin S. Terry, Email: bterry2@unl.edu.
References
- 1.Ragaller M., Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 2010;3:43–51. doi: 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L., Carlesso E., Langer T. Clinical review: extracorporeal membrane oxygenation. Crit Care. 2011;15 doi: 10.1186/cc10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill J.D., Bramson M.L., Gerbode F., Osborn J.J., Obrien T.G., Dontigny L. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome) – use of the bramson membrane lung. N Engl J Med. 1972;286:629. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett R.H., Gazzaniga A.B., Jefferies M.R., Huxtable R.F., Haiduc N.J., Fong S.W. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Tran Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 5.Kim E.S., Stolar C.J. ECMO in the newborn. Am J Perinatol. 2000;17:345–356. doi: 10.1055/s-2000-13449. [DOI] [PubMed] [Google Scholar]
- 6.Houmes R.J., Wildschut E., Pokorna P., Vobruba V., Kraemer U., Reiss I. Challenges in non-neonatal extracorporeal membrane oxygenation. Minerva Pediatr. 2012;64:439–445. [PubMed] [Google Scholar]
- 7.Barrett C.S., Bratton S.L., Salvin J.W., Laussen P.C., Rycus P.T., Thiagarajan R.R. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 8.Factora F.N.F., Bustamante S., Spiotta A., Avitsian R. Intracranial hemorrhage surgery on patients on mechanical circulatory support: a case series. J Neurosurg Anesthesiol. 2011;23:30–34. doi: 10.1097/ANA.0b013e3181eee55e. [DOI] [PubMed] [Google Scholar]
- 9.Davies S.J. Peritoneal dialysis-current status and future challenges. Nat Rev Nephrol. 2013;9:399–408. doi: 10.1038/nrneph.2013.100. [DOI] [PubMed] [Google Scholar]
- 10.Singh I. Absorption of oxygen from the peritoneal cavity and the stomach. Q J Exp Physiol. 1935;24:45–54. [Google Scholar]
- 11.Barr J., Livne A., Lushkov G., Vinograd I., Efrati Y., Ballin A. Peritoneal ventilation – an animal-model of extrapulmonary ventilation in experimental adult-respiratory-distress-syndrome. Pediatr Res. 1994;35:682–684. doi: 10.1203/00006450-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Barr J., Lushkov G., Strauss S., Gurevitch S., Lahat E., Bistritzer T. Peritoneal ventilation in rabbits: augmentation of gas exchange with cisapride. Thorax. 1996;51:82–86. doi: 10.1136/thx.51.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr J., Prueckner S., Safar P., Tisherman S.A., Radovsky A., Stezoski J. Peritoneal ventilation with oxygen improves outcome after hemorrhagic shock in rats. Crit Care Med. 2000;28:3896–3901. doi: 10.1097/00003246-200012000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Giffin D.M., Gow K.W., Warriner C.B., Walley K.R., Phang P.T. Oxygen uptake during peritoneal ventilation in a porcine model of hypoxemia. Crit Care Med. 1998;26:1564–1568. doi: 10.1097/00003246-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J.Y., Wang X.H., Wang L.J., Xu B., Zheng M. Effect of oxygenation of transperitoneal ventilation on the death time after asphyxiation in rabbits. Minerva Anestesiol. 2010;76:913–918. [PubMed] [Google Scholar]
- 16.Klein J., Faithfull N.S., Salt P.J., Trouwborst A. Transperitoneal oxygenation with fluorocarbons. Anesth Analg. 1986;65:734–738. [PubMed] [Google Scholar]
- 17.Schmidt J.A., Bilge F.H., Colacino J.M., von Recum A.F. Peritoneal oxygenation of normoxic and hypoxic dogs. ASAIO J. 1989;35:35–39. [PubMed] [Google Scholar]
- 18.Carr S.R., Cantor J.P., Rao A.S., Lakshman T.V., Collins J.E., Friedberg J.S. Peritoneal perfusion with oxygenated perfluorocarbon augments systemic oxygenation. Chest. 2006;130:402–411. doi: 10.1378/chest.130.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsutani N., Takase B., Nogami Y., Ozeki Y., Ishihara M., Maehara T. The peritoneum as a novel oxygenation organ: revitalization of intraperitoneal oxygenation. Shock. 2008;30:250–253. doi: 10.1097/shk.0b013e318162be0a. [DOI] [PubMed] [Google Scholar]
- 20.Matsutani N., Takase B., Nogami Y., Ozeki Y., Kaneda S., Maehara T. Efficacy of peritoneal oxygenation using a novel artificial oxygen carrier (TRM-645) in a rat respiratory insufficiency model. Surg Today. 2010;40:451–455. doi: 10.1007/s00595-009-4104-8. [DOI] [PubMed] [Google Scholar]
- 21.Kheir J.N., Scharp L.A., Borden M.A., Swanson E.J., Loxley A., Reese J.H. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci Trans Med. 2012;4 doi: 10.1126/scitranslmed.3003679. [DOI] [PubMed] [Google Scholar]
- 22.Swanson E.J., Mohan V., Kheir J., Borden M.A. Phospholipid-stabilized microbubble foam for injectable oxygen delivery. Langmuir. 2010;26:15726–15729. doi: 10.1021/la1029432. [DOI] [PubMed] [Google Scholar]
- 23.Notter R.H., Wang Z.D. Pulmonary surfactant: physical chemistry, physiology, and replacement. Rev Chem Eng. 1997;13:1–118. [Google Scholar]
- 24.Duncan P.B., Needham D. Test of the epstein-plesset model for gas microparticle dissolution in aqueous media: effect of surface tension and gas undersaturation in solution. Langmuir. 2004;20:2567–2578. doi: 10.1021/la034930i. [DOI] [PubMed] [Google Scholar]
- 25.Epstein P.S., Plesset M.S. On the stability of gas bubbles in liquid-gas solutions. J Chem Phys. 1950;18:1505–1509. [Google Scholar]
- 26.Garg S., Thomas A., Borden M.A. The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence. Biomaterials. 2013;34:6862–6870. doi: 10.1016/j.biomaterials.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borden M.A., Longo M.L. Oxygen permeability of fully condensed lipid monolayers. J Phys Chem B. 2004;108:6009–6016. [Google Scholar]
- 28.Kwan J.J., Borden M.A. Microbubble dissolution in a multi-gas environment. Langmuir. 2010;26:6542–6548. doi: 10.1021/la904088p. [DOI] [PubMed] [Google Scholar]
- 29.Kwan J.J., Borden M.A. Lipid monolayer dilatational mechanics during microbubble gas exchange. Soft Matter. 2012;8:4756–4766. [Google Scholar]
- 30.Chen C.C., Borden M.A. Ligand conjugation to bimodal poly(ethylene glycol) brush layers on microbubbles. Langmuir. 2010;26:13183–13194. doi: 10.1021/la101796p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhl T.L., Leckband D.E., Lasic D.D., Israelachvili J.N. Modulation of interaction forces between bilayers exposing short-chained ethylene-oxide headgroups. Biophys J. 1994;66:1479–1488. doi: 10.1016/S0006-3495(94)80938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suckow M.A., Weisbroth S.H., Franklin C.L. Academic Press; 2005. The laboratory rat. [Google Scholar]
- 33.Wagner E.M., Jenkins J., Perino M.G., Sukkar A., Mitzner W. Lung and vascular function during chronic severe pulmonary ischemia. J Appl Physiol. 2011;110:538–544. doi: 10.1152/japplphysiol.01308.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanamoto H., Nagata I., Niitsu Y., Zhang Z., Xue J.H., Sakai N. Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke. 2001;32:232–239. doi: 10.1161/01.str.32.1.232. [DOI] [PubMed] [Google Scholar]
- 35.Lagina A.T., Calo L., Deogracias M., Sanderson T., Kumar R., Wider J. Combination therapy with insulin-like growth factor-1 and hypothermia synergistically improves outcome after transient global brain ischemia in the rat. Acad Emerg Med. 2013;20:344–351. doi: 10.1111/acem.12104. [DOI] [PubMed] [Google Scholar]
- 36.Logue E.S., McMichael M.J., Callaway C.W. Comparison of the effects of hypothermia at 33 °C or 35 °C after cardiac arrest in rats. Acad Emerg Med. 2007;14:293–300. doi: 10.1197/j.aem.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 37.Ward C.A., Findlay R.D., Rizk M. Statistical rate theory of interfacial transport .1. Theoretical development. J Chem Phys. 1982;76:5599–5605. [Google Scholar]
- 38.Ward C.A. Rate of gas absorption at a liquid interface. J Chem Phys. 1977;67:229–235. [Google Scholar]
- 39.Dean W.M. Oxford University Press; 2011. Analysis of transport phenomena. [Google Scholar]
- 40.Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50–58. doi: 10.1093/bja/77.1.50. [DOI] [PubMed] [Google Scholar]