Abstract
Background
Metabolomics profiling has shown promise in elucidating the biological pathways underpinning mortality, but there are limited data in female populations.
Methods
We applied a liquid chromatography-tandem mass spectrometry metabolomics platform to EDTA-plasma to measure 470 metabolites at baseline in a discovery set of 943 postmenopausal women (including 417 incident deaths, median time to death of 10.6 years) with validation in an independent set of 1355 postmenopausal women (including 685 deaths, median time to death of 9.1 years) in the Women’s Health Initiative.
Results
Eight new metabolites were discovered to be associated with all-cause mortality. Findings included protective effects of increased levels of three amino acids (asparagine, homoarginine and tryptophan) and docosatrienoic acid; and detrimental effects of increased levels of C4-OH-carnitine, hexadecanedioate and two purine/pyrimidines (N2, N2-dimethylguanosine and N4-acetylcytidine). In addition, a set of nine previously published metabolite associations were replicated. A metabolite score comprising 17 metabolites was associated with mortality (P < 10–8) after adjustment for risk factors, with a hazard ratio of 1.95 (95% CI: 1.46–2.62) for women in the highest quartile compared with the lowest quartile of metabolite score. The score was robust among younger women and older women, for both cardiovascular and non-cardiovascular mortality, and associated with both early deaths (within the first 10 years of baseline) and later deaths.
Conclusions
Our study fills a gap in the literature by identifying eight novel metabolite associations with all-cause mortality in women, using a robust study design involving independent discovery and validation datasets.
Keywords: All-cause mortality, longevity, metabolomics, women’s health
Key Messages
We discovered eight novel metabolites that were associated with all-cause mortality in a robust study using two independent datasets of postmenopausal women.
In addition, a previously published set of nie metabolite associations with all-cause mortality were replicated in our study.
A metabolite score comprising 17 metabolites was associated with all-cause mortality, after adjustment for risk factors. The metabolite score was robust for association with all-cause mortality in younger and older women, with both cardiovascular and non-cardiovascular mortality and with both early (within the first 10 years following blood specimen) and later deaths.
Our findings in comparison with the literature indicated the possibility of sex-specific metabolite associations with all-cause mortality. More studies confirming these observations are needed in other populations.
Introduction
The mechanisms underpinning mortality are multifactorial but many involve metabolic processes such as inflammation and the generation of reactive oxygen species. For example, caloric deprivation has been shown to have dramatic effects on enhancing longevity in animal models.1,2 Metabolomic profiling, an emerging technology, has shown promise in identifying small molecule metabolites associated with mortality and longevity in animal models and human populations.3
Previous studies have evaluated the association between metabolic pathways and the risk of mortality in human populations and in animal models.4–16 There is accruing evidence of the associations of small molecule metabolites with mortality and associated ageing processes, but the data in human populations are limited both by sample size and the number of metabolites profiled.
Recent literature documents substantial gender differences in the metabolome, perhaps reflecting well-established sex differences in disease susceptibility and clinical manifestation.17,18 Women also have relatively longer life expectancies relative to men.19 Despite growing evidence of the role of the metabolome with mortality and related processes, as well as evidence of significant sex differences in both the metabolome and disease pathways, data in women are lacking.
We measured metabolomic profiles in the Women’s Health Initiative (WHI)20 to replicate previous findings and to identify novel metabolic signatures for mortality, with independent discovery and validation sets derived from the Observational Study (WHI-OS)21 and the Hormone Trials (WHI-HT),22 respectively.
Methods
The WHI recruited 161 808 postmenopausal women aged 50–79 at 40 clinical centres across the USA, from 1993 to 1998 with ongoing follow-up.23,24 The women were recruited into an observational study (WHI-OS, n = 93 676) and into three randomized controlled clinical trials (n = 68 132).21 The two trials of hormone therapy (WHI-HT) included a randomized, placebo-controlled trial of conjugated equine estrogens plus medroxyprogesterone acetate (CEE+MPA) in 16 608 postmenopausal women aged 50–79 years with an intact uterus,22 and a randomized, double-blind, placebo-controlled disease prevention trial of 0.625 mg/day of conjugated equine estrogens (CEE) in 10 739 postmenopausal women, aged 50–79 years, with previous hysterectomy.25
This nested case/control study of coronary heart disease (CHD) within the WHI included 2306 participants.26 Of these, 1362 participants were selected from each of the placebo and active arms of the CEE and CEE+MPA trials, with roughly equal numbers of CHD cases and healthy controls frequency-matched on 5-year age, race/ethnicity, hysterectomy status and 2-year enrolment groups. The remaining 944 participants were selected from the WHI-OS with equal numbers of CHD cases frequency-matched to healthy controls, as in the WHI-HT.
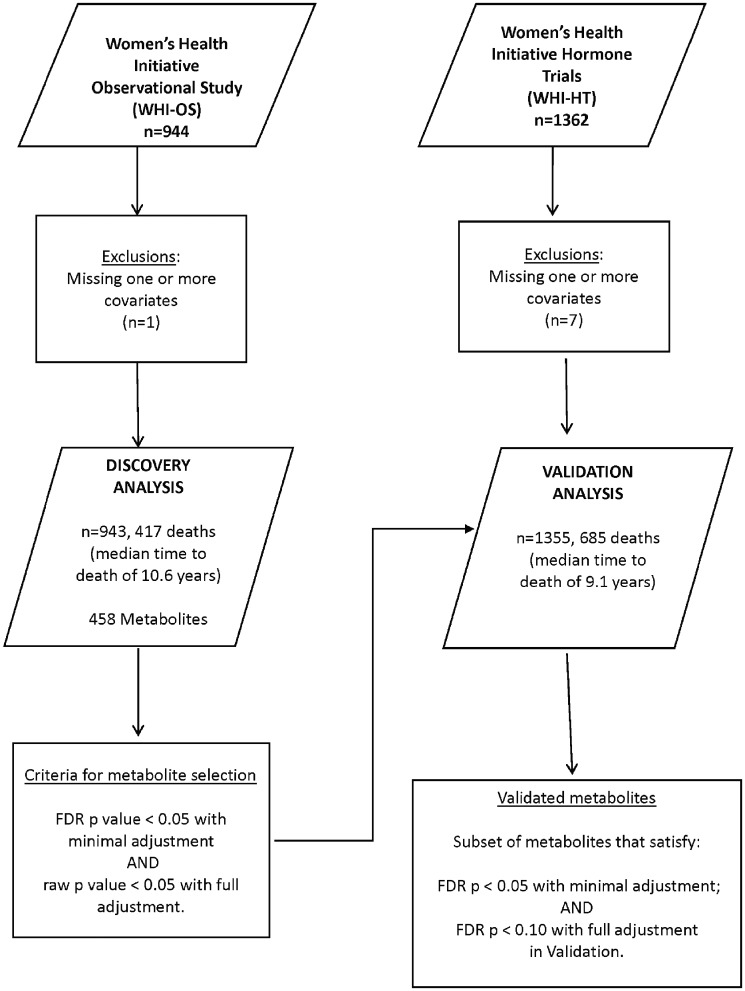
Figure 1 shows the scheme for the discovery of metabolite associations with all-cause mortality in this study. Of the 2306 subjects, eight subjects were excluded due to missing data on one or more covariates. The dataset of 2298 subjects was divided into a ‘Discovery’ dataset of 943 subjects (including 417 deaths) who were enrolled in the WHI-OS cohort and a ‘Validation’ dataset of 1355 subjects (including 685 deaths) who were enrolled in the WHI-HT cohort. A sensitivity analysis was conducted in a subset of 643 participants in the WHI-HT dataset, who were randomized to placebo therapy to confirm findings.
Figure 1.
Discovery and validation of novel metabolite associations with all-cause mortality in the Women’s Health Initiative (n = 2298). FDR denotes ‘false-discovery rate’.
Supplementary Figure 1, available as Supplementary data at IJE online, shows the schema for the discovery of metabolite associations with longevity in this study. Longevity was defined as living to age 85. Of the 2306 participants, 1382 were included in analysis after exclusions for ineligibility (based on age at entry and duration of follow up) and censoring.
Metabolomic profiling
Metabolomic measurements were made using four complementary LC-MS methods in EDTA-plasma samples, resulting in 470 metabolites for the WHI participants. Details on the metabolomics assays can be found in the Supplementary data, available at IJE online, and in a previous publication.26
Statistical analysis
The association of individual metabolites with all-cause mortality was based on Cox proportional hazards (PH) models; the outcome was the time from baseline until death (observed) or last contact (censored), and the primary exposure variable was the log-transformed and standardized levels of each metabolite (Figure 1). To account for the case-control design of the WHI ancillary study, a sensitivity analysis was conducted by weighting the Cox PH model with inverse probability of sampling weights (details in the Supplementary data, available at IJE online). The association of individual metabolites with longevity (living to age 85 years) was based on logistic regression models (Supplementary Figure 1, available as Supplementary data at IJE online). Effect estimates are given in terms of hazard ratios (HR) from the Cox models or odds ratios (OR) from the logistic regression models corresponding to one standard deviation (SD) change in metabolite levels.
The 470 metabolites were considered in two sets: (i) 12 metabolites previously reported as associated with all-cause mortality were analysed separately in the combined WHI-OS and WHI-HT dataset; and (ii) the remaining 458 metabolites were included in the two-stage procedure for discovery (WHI-OS) and validation (WHI-HT) to discover novel metabolite associations (Figure 1). To account for the multiplicity of hypothesis tests, false-discovery rate (FDR)-adjusted P-values were calculated using the two-stage Benjamini and Hochberg (2006) step-up FDR-controlling procedure. Each model was fit with: Model A) (minimal adjustment) for age, WHI arm and CHD case/control status; and Model B) (full adjustment)— with additional covariates including body mass index (BMI), systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and high-density lipoprotein (HDL) cholesterol. Age, BMI, systolic blood pressure and total and HDL cholesterol were included as continuous variables. Criteria for prioritizing metabolites for associations with all-cause mortality (longevity) are noted in Figure 1 (and Supplementary Figure 1, available as Supplementary data at IJE online).
The set of validated metabolites associated with all-cause mortality were translated into a single metabolite score [Mortality (M)-metabo-score] estimated in a LASSO penalized Cox PH model.27 Similarly, the set of metabolites associated with longevity were translated into a single metabolite score, namely the Longevity (L)-metabo-score (see Supplementary data, available at IJE online for details).
Results
The WHI-OS and WHI-HT cohorts were similar with regard to mean systolic blood pressure but differed according to other characteristics (Table 1). In the WHI-OS, 417 of the 943 women died during the course of follow-up, due to any cause, with a median time to death of 10.6 years (First quartile (Q1) = 6.6 years; Third quartile (Q3) = 14.0 years). In the WHI-HT, 685 of 1355 women died during the course of follow-up, due to any cause, with a median time to death of 9.1 years (Q1 = 5.2 years; Q3 = 14.5 years, P = 0.003).
Table 1.
Baseline characteristics of 2298 participants in the Women’s Health Initiative
| Characteristic | WHI-OS | WHI-HT | P-value |
|---|---|---|---|
| (n = 943) | (n = 1355) | ||
| Age (mean years) (SD) | 67.4 (7.0) | 66.7 (7.0) | 0.020 |
| Age (minimum–maximum) | 50.0–79.0 | 50.0–79.0 | |
| BMI (kg/m2), mean (SD) | 27.9 (6.0) | 29.3 (6.0) | 0.001 |
| Systolic blood pressure, mean (SD) | 132.0 (19.0) | 134.0 (18.0) | 0.100 |
| Race (%) | <0.001 | ||
| • White | 74.0% | 84.0% | |
| • Black | 15.0% | 12.0% | |
| • Hispanic | 3.0% | 3.0% | |
| • Asian | 3.0% | 1.0% | |
| Current smoking (%) | 8.1% | 14.2% | <0.001 |
| Diabetes (%) | 10.9% | 14.8% | 0.009 |
| Hypertension (%) | 42.0% | 47.5% | <0.001 |
| All-cause deaths (%) | 44.2% | 50.6% | 0.003 |
| Cardiovascular disease- related deaths (%) | 24.3% | 27.5% | 0.095 |
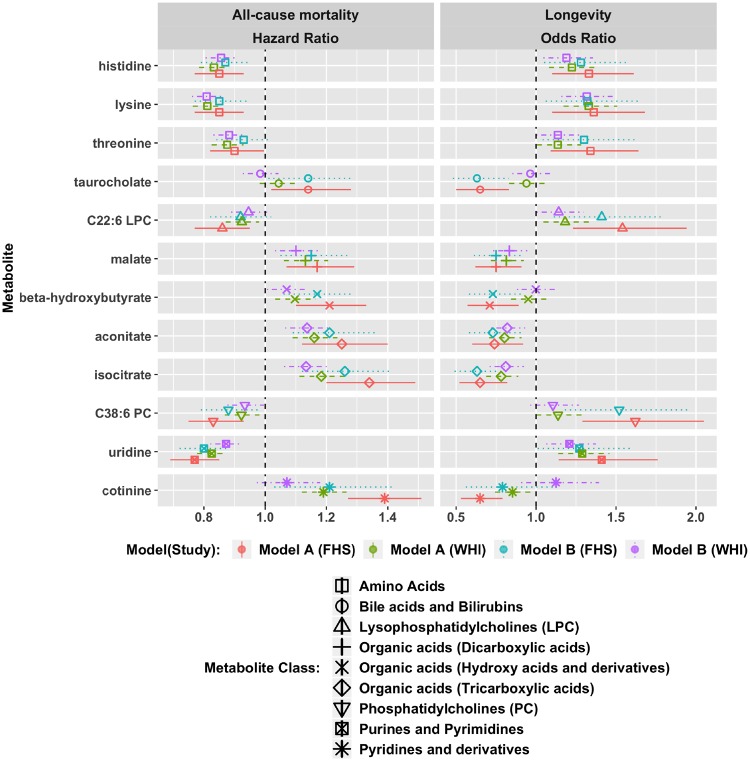
We replicated the associations of a set of 12 metabolites originally observed in the Framingham Heart Study (FHS) with all-cause mortality in models adjusted for age, sex and other risk factors.12 Protective effects were reported in FHS for cotinine, aconitate, β-hydroxybutyrate, isocitrate, malate and taurocholate; harmful effects were reported for histidine, lysine, threonine, uridine, C22: 6 lysophoshatidylcholine (LPC) and C38: 6 phoshatidylcholine (PC).12
We replicated these findings in our study using both minimally adjusted (age, WHI arm, CHD case control status) and fully adjusted (including BMI, systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and HDL cholesterol) models, consistent with the previous report (Figure 2, Supplementary Table 1, available as Supplementary data at IJE online). An FDR-adjusted P-value threshold of 0.05 was satisfied in models with both minimal and full adjustment for all but 3=three metabolites, namely taurocholate, cotinine and C22: 6 LPC. Cotinine was associated with all-cause mortality with minimal adjustment, with HR = 1.19 (95% CI: 1.12–1.27, P < 10–07). However, this association was attenuated after additional adjustments including smoking status in the fully adjusted model (HR = 1.07, 95% CI: 0.97–1.18, P = 0.17). The association of C22: 6 LPC was similarly weakened after full adjustment. Taurocholate was not associated with all-cause mortality, even with minimal adjustment (HR = 1.04, 95% CI: 0.98–1.11, P = 0.17).
Figure 2.
Replication of 12 metabolite associations discovered in the Framingham Heart Study (FHS). Model (A): adjusted for age, WHI arm, CHD case/control status. Model (B): model (A) factors + BMI, systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and HDL cholesterol.
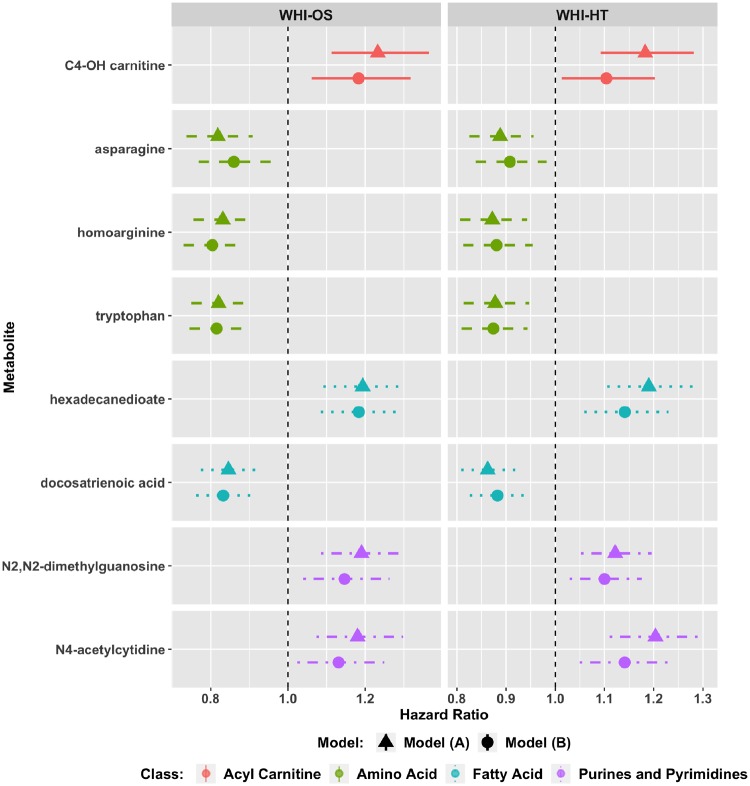
Each of the remaining 458 metabolites was individually assessed for its association with all-cause mortality in both minimally and fully adjusted models (Figure 1). In the WHI-OS dataset, 36 metabolites met the threshold of FDR-adjusted P < 0.05 with minimal, and raw P < 0.05 with full, adjustment; of these metabolites, eight associations were validated with FDR-adjusted P < 0.05 with minimal, and FDR-adjusted P < 0.10 with full, adjustment (Figure 1) in the independent WHI-HT dataset (Figure 3; Supplementary Table 2, available as Supplementary data at IJE online). After full adjustment, these included protective effects of docosatrienoic acid [HR = 0.83 (0.76–0.91) in the WHI-OS; HR = 0.88 (0.83–0.94) in WHI-HT], asparagine [HR = 0.86 (0.77–0.96) in WHI-OS; HR = 0.91 (0.84–0.98) in WHI-HT], and similar effects for amino acids homoarginine and tryptophan (Supplementary Table 2, available as Supplementary data at IJE online). Higher levels were associated with increased risk of all-cause mortality for C4-OH carnitine [HR = 1.18 (1.06–1.32) in WHI-OS; HR = 1.10 (1.01–1.20) in WHI-HT], hexadecanedioate [HR = 1.18(1.08–1.29) in WHI-OS; HR = 1.14(1.06–1.23) in WHI-HT] and N2, N2 dimethylguanosine [HR = 1.15 (1.04–1.26) in WHI-OS; HR = 1.10 (1.03–1.18) in WHI-HT] and N4 acetylcytidine [HR = 1.13 (1.02–1.25) in WHI-OS; HR = 1.14 (1.05–1.24]. The full set of analysis results for the WHI-OS and WHI-HT are in Supplementary Files 1 and 2, available as Supplementary data at IJE online.
Figure 3.
Eight novel metabolite associations with all-cause mortality in the Women’s Health Initiative Observational Study (WHI-OS) and the Women’s Health Initiative Hormone Trials (WHI-HT). Model (A): adjusted for age, WHI arm, CHD case/control status. Model (B): model (A) factors + BMI, systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and HDL cholesterol .
Since some of these metabolites may change due to active hormone therapy, a sensitivity analysis was conducted in a subset of 643 participants randomized to the placebo arms of the WHI-HT. All eight metabolites remained associated with all-cause mortality in the minimally adjusted model (Supplementary Table 7, available as Supplementary data at IJE online). Five of the eight metabolites also remained associated with all-cause mortality after adjustment for sampling weights to account for the case-control nature of the design (Supplementary Table 8, available as Supplementary data at IJE online).
A metabolite score associated with all-cause mortality (M-metabo-score) was estimated as a linear combination of the eight metabolites in Figure 3 and the nine replicated FHS metabolites. The coefficients associated with each of 17 metabolites in the score estimated in a LASSO algorithm are shown in Table 2.
Table 2.
Regression coefficients corresponding to 17 metabolites, used in a linear combination to obtain the M-metabo-score
| Metabolite | Class | Coefficient |
|---|---|---|
| C4-OH carnitine | Acyl carnitines | 0.11 |
| Asparagine | Amino acids | −0.05 |
| Histidine | Amino acids | −0.05 |
| Homoarginine | Amino acids | −0.09 |
| Lysine | Amino acids | −0.02 |
| Threonine | Amino acids | −0.05 |
| Tryptophan | Amino acids | −0.07 |
| Docosatrienoic acid | Fatty acids | −0.16 |
| Hexadecanedioate | Fatty acids | 0.13 |
| Malate | Organic acids (dicarboxylic acids) | 0.05 |
| β-hydroxybutyrate | Organic acids (hydroxy acids) | −0.14 |
| Aconitate | Organic acids (tricarboxylic acids) | 0.02 |
| Isocitrate | Organic acids (tricarboxylic acids) | 0.09 |
| C38: 6 PC | PC | 0.05 |
| N2, N2- dimethylguanosine | Purines and pyrimidines | 0.11 |
| N4-acetylcytidine | Purines and pyrimidines | 0.09 |
| Uridine | Purines and pyrimidines | −0.20 |
PC, phosphatidylcholine.
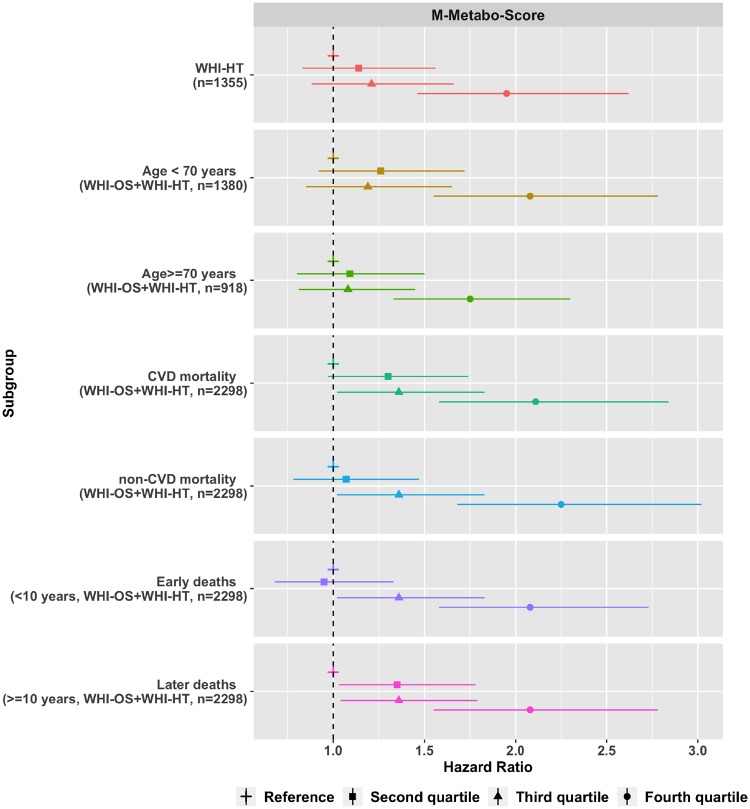
The M-metabo-score was associated with all-cause mortality after full adjustment (P < 10–8) with HR = 1.95 (95% CI: 1.46-2.62) for women in the fourth quartile compared with the first quartile in the WHI-HT (Figure 4, Supplementary Table 3, available as Supplementary data at IJE online). Kaplan-Meier survival curves estimated within M-metabo-score quartiles are shown in Supplementary Figure 2, available as Supplementary data at IJE online, showing clear visual separation of the fourth quartile from the first (lowest) quartile.
Figure 4.
M-Metabo-score comprising 17 metabolites associated with all-cause mortality in the Women’s Health Initiative Observational Study (WHI-OS) and the Women’s Health Initiative Hormone Trials (WHI-HT). Models adjusted for age, WHI arm, CHD case/control status, BMI, systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and HDL cholesterol .
In a stratified analysis by age at baseline (70 years) in the combined WHI-OS and WHI-CT dataset, the M-metabo-score was associated with all-cause mortality in both the younger women (HR = 2.08, 95% CI: 1.55–2.78 for the highest quartile) and the older women with (HR = 1.75, 95% CI: 1.33–2.30). The M-metabo-score was also associated with both deaths within 10 years of baseline (HR = 2.08, 95% CI: 1.58–2.73 for the highest quartile) and later deaths (HR = 2.08, 95% CI: 1.55–2.78 for the highest quartile).
As these data were profiled in a CHD nested case/control study, the datasets were enriched for cardiovascular disease-related adverse events and deaths. Therefore, the results were tested by specific cause of mortality in order to assess the robustness to mortality type. Of the 1102 total deaths, we observed 601 CHD-related deaths (54.5%) and 501 non-CHD deaths (45.5%) in the combined WHI-OS and WHI-HT dataset (n = 2298). The M-metabo-score was robust for both CVD mortality (P < 10–7) and non-CVD mortality (P < 10–9), with HRs for women in the highest score quartile relative to the lowest of 2.11 (95% CI: 1.58—2.84) for CVD mortality and 2.25 (95% CI: 1.68—3.02) for non-CVD mortality.
We separately examined metabolite associations with longevity, defined as attaining 85 years of age. Eight of the twelve metabolites associated with longevity in the FHS12 were also observed to be significantly associated with longevity in our study, after full adjustment. These replicated metabolites included protective associations (i.e. increased longevity with higher levels) for lysine, uridine, histidine, C22: 6 LPC and threonine and negative associations for isocitrate, aconitate and malate (Figure 2; Supplementary Table 4, available as Supplementary data at IJE online). C38: 6 PC, β-hydroxybutyrate and taurocholate were not associated with longevity in our study even in the minimally adjusted model. The association of cotinine with longevity was diminished after full adjustment including for smoking status.
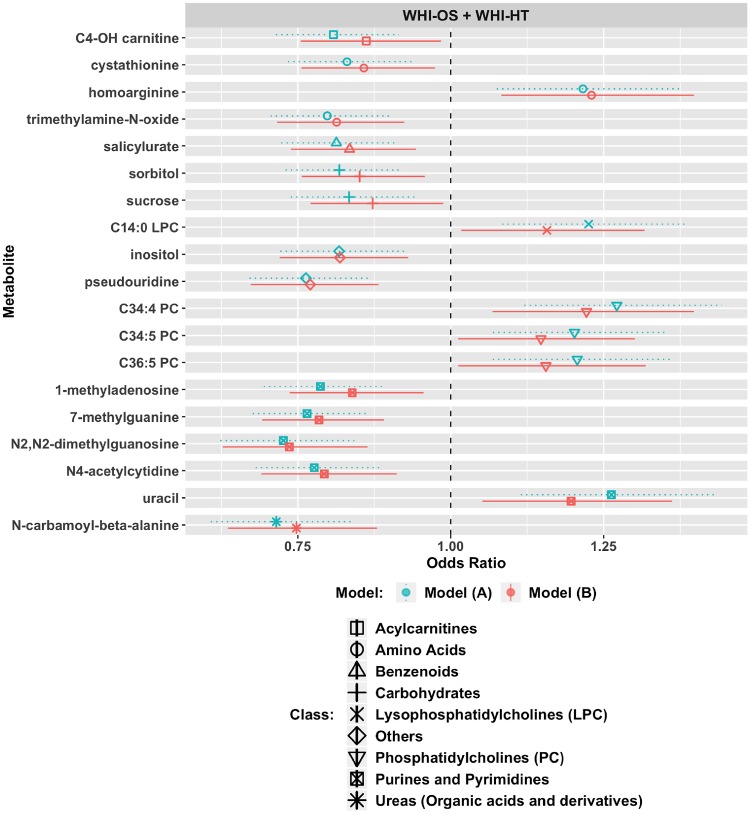
In a longevity discovery analysis of 458 metabolites, 19 metabolites met an FDR P-value <0.05 in the model with minimal adjustment and a raw P-value <0.05 with full adjustment (Figure 5; Supplementary Table 5, available as Supplementary data at IJE online). These included harmful associations, where high levels correspond to reduced likelihood of longevity, in the case of C4-OH-carnitine, amino acids (cystathionine, trimethylamine-N-oxide), salicylurate, carbohydrates (sorbitol, sucrose), inositol, pseudouridine, four purines/pyrimidines (1-methyladenosine, 7-methylguanine, N2, N2-dimethylguanosine, N4-acetylcytidine) and N-carbamoyl-beta-alanine. Protective associations included homoarginine, C14: 0 LPC, uracil and 3 PCs (C34: 4, C34: 5, C36: 5). The full set of analysis results is in Supplementary File 3, available at IJE online.
Figure 5.
Nineteen metabolites associated with longevity in the Women’s Health Initiative Observational Study (WHI-OS) and the Women’s Health Initiative Hormone Trials (WHI-HT). Longevity is defined as living to age 85 years. Model (A): adjusted for age, WHI arm, CHD case/control status. Model (B): model (A) factors + BMI, systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and HDL cholesterol.
A metabolite score associated with all-cause mortality [Longevity (L)-metabo-score] was estimated as a linear combination of the 19 metabolites in Figure 5 and the eight replicated FHS metabolites. The coefficients associated with each of 27 metabolites in the score estimated in a LASSO algorithm are shown in Table 3; the final score included 17 metabolites. Details of the LASSO model are included in the Supplementary data available at IJE online.
Table 3.
Regression coefficients corresponding to 27 metabolites, used in a linear combination to obtain the L-metabo-score
| Metabolite | Metabolite class | Coefficient |
|---|---|---|
| C4-OH carnitine | Acyl carnitines | 0 |
| Cystathionine | Amino acids | −0.06 |
| Histidine | Amino acids | 0.11 |
| Homoarginine | Amino acids | 0.02 |
| Lysine | Amino acids | 0.1 |
| Threonine | Amino acids | 0.06 |
| Trimethylamine- N-oxide | Amino acids | −0.11 |
| Salicylurate | Benzenoids | −0.12 |
| Sorbitol | Carbohydrates | −0.04 |
| Sucrose | Carbohydrates | −0.12 |
| C14: 0 LPC | LPC | 0 |
| C22: 6 LPC | LPC | 0.09 |
| Malate | Organic acids (dicarboxylic acids) | −0.06 |
| Aconitate | Organic acids (tricarboxylic acids) | 0 |
| Isocitrate | Organic acids (tricarboxylic acids) | −0.02 |
| N-carbamoyl-beta- alanine | Organic acids (ureas) | −0.08 |
| Inositol | Others | 0 |
| Pseudouridine | Others | 0 |
| C34: 4 PC | PC | 0.12 |
| C34: 5 PC | PC | 0 |
| C36: 5 PC | PC | 0 |
| N2, N2- dimethylguanosine | Purines and pyrimidines | 0 |
| N4-acetylcytidine | Purines and pyrimidines | −0.05 |
| Uracil | Purines and pyrimidines | 0.16 |
| Uridine | Purines and pyrimidines | 0 |
| 1-methyladenosine | Purines and pyrimidines | 0 |
| 7-methylguanine | Purines and pyrimidines | −0.1 |
LPC, lysophosphatidylcholine; PC, phosphatidylcholine.
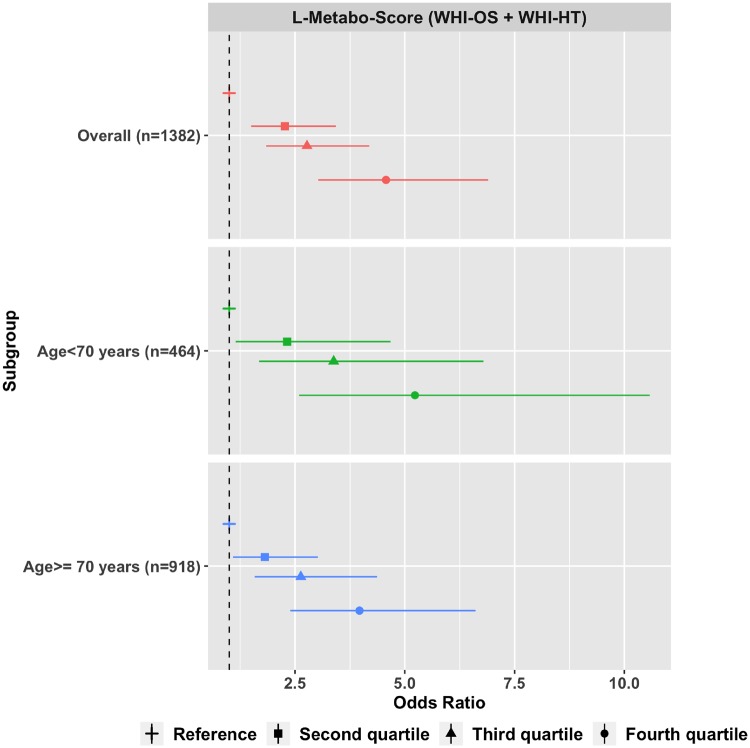
The L-metabo-score was associated with longevity (P < 10–11) in the fully adjusted model with an OR of 4.57 (95% CI: 3.03-6.90) for women with scores in the fourth quartile when compared with those in the lowest quartile (Figure 6;Supplementary Table 6, available as Supplementary data at IJE online). In a stratified analysis by age at baseline (70 years), the L-metabo-score was associated with longevity in both younger and older women (Figure 6). Correlation plots of the sets of metabolites associated with all-cause mortality and longevity are shown in Supplementary Figures 3 and 4, available as Supplementary data at IJE online. Coefficient of variation (CV) metrics associated with each metabolite associated with all-cause mortality and longevity are in Supplementary data File 4, are available at IJE online.
Figure 6.
L-Metabo-score comprising 17 metabolites associated with longevity in the Women’s Health Initiative Observational Study (WHI-OS) and the Women’s Health Initiative Hormone Trials (WHI-HT). Models adjusted for age, WHI arm, CHD case/control status, BMI, systolic blood pressure, hypertension treatment, diabetes, smoking status, total cholesterol and HDL cholesterol.
Discussion
In this study of the association of the EDTA-plasma metabolome with all-cause mortality in postmenopausal women, we identified eight novel metabolites and confirmed associations for nine metabolites originally reported in the FHS.12 We used a robust study design that involved metabolite discovery among 943 women in the WHI-OS, with independent validation in a dataset of 1355 women in the WHI-HT. The new findings included protective associations of docosatrienoic acid, asparagine, homoarginine and tryptophan where higher levels were associated with reduced risk, and harmful associations of C4-OH-carnitine, hexadecanedioate, N2, N2 dimethylguanosine and N4 acetylcytidine where higher levels were associated with increased risk. Moreover, an M-metabo-score comprising 17 metabolites was associated with a 95% increased risk of mortality for women in the highest quartile, which was robust for both younger and older women, as well as for both cardiovascular and non-cardiovascular mortality. The M-metabo-score was also associated with deaths in the first 10 years of follow-up as well as with deaths occurring more than 10 years after baseline, suggesting that this score captures long-term risk. Our study also identified 19 metabolite associations with longevity, attaining age 85 or older, including protective associations of 3 PCs (C34: 4, C34: 5, C36: 5), C14: 0 LPC, homoarginine and uracil and harmful associations of C4-OH-carnitine, amino acids (cystathionine, trimethylamine-N-oxide [TMAO]), salicylurate, carbohydrates (sorbitol, sucrose), inositol, pseudouridine, four purines/pyrimidines (1-methyladenosine, 7-methylguanine, N2, N2-dimethylguanosine, N4-acetylcytidine) and N-carbamoyl-beta-alanine.
Our study corroborates previous findings for mortality from the FHS for histidine, lysine, threonine, malate, β-hydroxybutyrate, C38: 6 PC, uridine, aconitate and isocitrate.12 Several previous studies lend support to our findings.12–14,28–31
Uridine was also associated with lower mortality in the Atherosclerosis Risk In Communities (ARIC) study.14 Increased levels of C 22: 6 LPC, a docosatrienoic acid containing phospholipid, was associated with decreased risk of all-cause mortality in the FHS and in our study. Showing a consistent trend, we also observed that increasing levels of docosatrienoic acid are associated with decreased risk of all-cause mortality. Increased levels of dihydroxy docosatrienoic acid were reported to be protective against heart failure in the ARIC study.28 We observed protective associations with respect to mortality risk with higher levels of asparagine and tryptophan. Tryptophan was also inversely associated with all-cause mortality (HR = 0.87, 95% CI: 0.83-0.92) in a community-based, Norwegian study (7015 participants, 55.6% female).13 Asparagine was also positively correlated with cardiovascular health score in the Framingham Heart Study.12
Our study did not corroborate the previous findings in the FHS that increasing levels of taurocholate are associated with increased risk of all-cause mortality and decreased longevity. Taurocholate was also associated with increased risk of all-cause mortality in an analysis of 620 men at high risk for cancer.16 In our analysis, even with minimal adjustment, taurocholate was not associated with all-cause mortality (HR = 1.04, 95% CI: 0.98–1.11, P = 0.17) nor with longevity (OR = 0.94, 95%CI: 0.83–1.06, P = 0.32). Sex differences in taurocholate have been previously reported, with lower taurocholate uptake into hepatocytes and higher renal clearance in female compared with male rats.32–34 Thus, our inability to replicate the associations of taurocholate with all-cause mortality could reflect sex differences in bile acid metabolism and its effects on mortality and longevity. We also did not confirm previously reported associations in the FHS with mortality for C22: 6 LPC and cotinine and associations with longevity for C38: 6 PC, β-hydroxybutyrate and cotinine (Figure 2, Supplementary Tables 1, 4, available as Supplementary data at IJE online).
In addition to the seven previously reported metabolites in the FHS (histidine, lysine, threonine, malate, aconitate, isocitrate and uridine), we found that four additional metabolites, C4-OH carnitine, homoarginine, N2, N2-dimethylguanosine and N4-acetylcytidine, were associated with both longevity and mortality, in opposite directions. Higher levels of C4-OH-carnitine were associated with increased risk of mortality and a corresponding decreased likelihood of longevity. C4-OH-carnitine is a carnitine ester of 3-hydroxybutyrate and has been implicated in pathways conferring increased risk of insulin resistance and obesity.31 Higher levels of acylcarnitines are associated with fatty acid oxidation impairment.30,31 Two markers associated with increased mortality and decreased longevity are related to tRNA modification. Higher levels of N4-acetylcytidine were associated with higher dietary insulinaemic potential in a recent study in the WHI.35 Supporting our observations, a recent study found that higher levels of N4-acetylcytidine were found among older individuals with higher inflammatory activation, oxidative stress and nucleotide metabolic dysfunction.36 N2, N2-demthylguanosine is post-transcriptional modification of tRNA.37 Consistent with our findings, increased levels of N2, N2-demthylguanosine were associated with mortality among patients with pulmonary artery hypertension.38 In contrast, higher homoarginine levels were associated with decreased mortality and increased longevity. Homoarginine is an analogue of L-arginine, the substrate for the synthesis of nitric oxide, a messenger involved in vascular resistance and oxidative function.39 In a recent meta-analysis of observational studies conducted in mostly male populations with cardiovascular or other chronic diseases, an inverse association between homoarginine and all cause-mortality (HR 0.64, 95% CI: 0.57–0.73) was observed.40 Our observations extend these early findings to women and average-risk individuals.
We found a detrimental association with longevity for trimethylamine N-oxide (TMAO), a metabolite produced by the gut microbiome, which has previously been associated with an increased risk of heart disease and stroke. High levels of TMAO were found to be associated with an increased risk of all-cause mortality in an analysis of 5469 participants in the PREVEND study in The Netherlands.41 However, TMAO was not a significant predictor of longevity (OR = 1.00, 95% CI: 0.80, 1.25) in the FHS.12 Sex-based differences in TMAO concentrations have been previously reported in animal models.42 Thus, gender composition should be considered in evaluating the consistency of the association.
Our study has several strengths including a well-validated metabolomics platform, detailed covariate information, a large number of carefully adjudicated endpoints and a robust methodology. In addition, the study provides a detailed evaluation of metabolomics and all-cause mortality in women, which has previously been lacking in the literature. In summary, multiple metabolites were associated with decreased mortality and increased longevity, highlighting the importance of metabolism in ageing and disease.
Supplementary data
Supplementary data are available at IJE online.
Funding
Metabolomic analysis in the WHI was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contract HHSN268201300008C. This work was also supported by NHLBI R01 HL122241. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. A list of WHI investigators is available online at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Weindruch R, Walford RL.. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL; C C Thomas, 1988. [Google Scholar]
- 2. Fishbein L. International Life Sciences Institute, National Center for Toxicological Research, National Institute on Aging Biological Effects of Dietary Restriction. Berlin, New York: Springer, 1991. [Google Scholar]
- 3. Ala-Korpela M, Kangas AJ, Soininen P.. Quantitative high-throughput metabolomics: a new era in epidemiology and genetics. Genome Med 2012;4:36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G.. The hallmarks of aging. Cell 2013;153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sebastiani P, Perls TT.. The genetics of extreme longevity: lessons from the New England centenarian study. Front Genet 2012;3:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogina B, Helfand SL, Frankel S.. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science 2002;298:1745.. [DOI] [PubMed] [Google Scholar]
- 7. Ogg S, Paradis S, Gottlieb S. et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997;389:994–99. [DOI] [PubMed] [Google Scholar]
- 8. Montoliu I, Scherer M, Beguelin F. et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging 2014;6:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collino S, Montoliu I, Martin FP. et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One 2013;8:e56564.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Covarrubias V, Beekman M, Uh HW. et al. Lipidomics of familial longevity. Aging Cell 2013;12:426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auro K, Joensuu A, Fischer K. et al. A metabolic view on menopause and ageing. Nat Commun 2014;5:4708.. [DOI] [PubMed] [Google Scholar]
- 12. Cheng S, Larson MG, McCabe EL. et al. Distinct metabolomic signatures are associated with longevity in humans. Nat Commun 2015;6:6791.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zuo H, Ueland PM, Ulvik A. et al. Plasma biomarkers of inflammation, the Kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland Health Study. Am J Epidemiol 2016;183:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu B, Heiss G, Alexander D, Grams ME, Boerwinkle E.. Associations between the serum metabolome and all-cause mortality among African Americans in the Atherosclerosis Risk In Communities (ARIC) study. Am J Epidemiol 2016;183:650–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer K, Kettunen J, Wurtz P. et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med 2014;11:e1001606.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang J, Weinstein SJ, Moore SC. et al. Serum metabolomic profiling of all-cause mortality: A prospective analysis in the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study cohort. Am J Epidemiol 2018;187:1721-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krumsiek J, Mittelstrass K, Do KT. et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015;11:1815–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mittelstrass K, Ried JS, Yu Z. et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 2011;7:e1002215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barford A, Dorling D, Davey Smith G, Shaw M.. Life expectancy: women now on top everywhere. BMJ 2006;332:808.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Women’s Health Initiative Study Writing Group. Design of the Women’s Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 21. Pradhan AD, Manson JE, Rossouw JE. et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. JAMA 2002;288:980–87. [DOI] [PubMed] [Google Scholar]
- 22. Rossouw JE, Anderson GL, Prentice RL. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 23. Vaughan L, Espeland MA, Snively B. et al. The rationale, design, and baseline characteristics of the Women's Health Initiative Memory Study of Younger Women (WHIMS-Y). Brain Res 2013;1514:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The Women's Health Initiative Study Group. Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 25. Anderson GL, Limacher M, Assaf AR. et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291:1701–12. [DOI] [PubMed] [Google Scholar]
- 26. Paynter NP, Balasubramanian R, Giulianini F. et al. Metabolic predictors of incident coronary heart disease in women. Circulation 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman JaH T, Tibshirani R.. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1. [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng Y, Yu B, Alexander D. et al. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC Study. Am J Epidemiol 2013;178:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chyan YJ, Poeggeler B, Omar RA. et al. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem 1999;274:21937–42. [DOI] [PubMed] [Google Scholar]
- 30. Rinaldo P, Cowan TM, Matern D.. Acylcarnitine profile analysis. Genet Med 2008;10:151–56. [DOI] [PubMed] [Google Scholar]
- 31. Schooneman MG, Vaz FM, Houten SM, Soeters MR.. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simon FR, Fortune J, Iwahashi M, Bowman S, Wolkoff A, Sutherland E.. Characterization of the mechanisms involved in the gender differences in hepatic taurocholate uptake. Am J Physiol 1999;276:G556–65. [DOI] [PubMed] [Google Scholar]
- 33. Brock WJ, Vore M.. Characterization of uptake of steroid glucuronides into isolated male and female rat hepatocytes. J Pharmacol Exp Ther 1984;229:175–81. [PubMed] [Google Scholar]
- 34. Schlattjan JH, Biggemann F, Greven J.. Gender differences in renal tubular taurocholate transport. Naunyn Schmiedebergs Arch Pharmacol 2005;371:449–56. [DOI] [PubMed] [Google Scholar]
- 35. Tabung FK, Balasubramanian R, Liang L. et al. Identifying metabolomic profiles of insulinemic dietary patterns. Metabolites 2019;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furman D, Chang J, Lartigue L. et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 2017;23:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirchner S, Ignatova Z.. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet 2015;16:98–112. [DOI] [PubMed] [Google Scholar]
- 38. Rhodes CJ, Ghataorhe P, Wharton J. et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017;135:460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsikas D, Wu G.. Homoarginine, arginine, and relatives: analysis, metabolism, transport, physiology, and pathology. Amino Acids 2015;47:1697–702. [DOI] [PubMed] [Google Scholar]
- 40. Zinellu A, Paliogiannis P, Carru C, Mangoni AA.. Homoarginine and all-cause mortality: a systematic review and meta-analysis. Eur J Clin Invest 2018;48:e12960.. [DOI] [PubMed] [Google Scholar]
- 41. Gruppen EG, Garcia E, Connelly MA. et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep 2017;7:13781.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bennett BJ, de Aguiar Vallim TQ, Wang Z. et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.