Abstract
Background
Evidence suggests a link between air pollution and dementia. Cardiovascular disease (CVD) may be a potential determinant of dementia. This motivated us to quantify the contribution of CVD to the association between air pollution and dementia.
Methods
A cohort of Canadian-born residents of Ontario, who participated in the 1996–2003 Canadian Community Health Surveys, was followed through 2013 or until dementia diagnosis. Exposure to nitrogen dioxide (NO2) and fine particulate matter (PM2.5) was estimated with a 3-year average and 5-year lag before dementia diagnosis. Incident CVD was evaluated as a mediator. We used multi-level Cox proportional and Aalen additive hazard regression models, adjusting for individual- and neighbourhood-level risk factors to estimate associations with NO2 and PM2.5. We estimated the total, direct and indirect effects of air pollution on dementia through cardiovascular disease.
Results
This study included 34 391 older adults. At baseline, the mean age of this cohort was 59 years. The risk of dementia was moderately higher among those more exposed to NO2 (hazard ratio (HR) 1.10, 95% confidence interval (CI) 0.99–1.19; and 100 additional cases per 100 000 [standard error (SE) <100x10-5]) and PM2.5 [(HR 1.29, 95% CI 0.99–1.64; 200 additional cases per 100 000] [SE 100x10-5]) after adjusting for covariates; however, these estimates are imprecise. A greater proportion of the relationship between PM2.5 and dementia was mediated through CVD than NO2 for both scales.
Conclusions
These results suggest some of the association between air pollution and dementia is mediated through CVD, indicating that improving cardiovascular health may prevent dementia in areas with higher exposure to air pollution.
Keywords: Air pollution, dementia, cardiovascular disease, mediation analysis
Key Messages
A causal mediation analysis is a valuable tool to disentangle complex relationships and provides insight toward understanding disease aetiology and identifying specific prevention efforts.
We applied a formal causal mediation analysis on both relative and absolute scales to examine the relative contribution of cardiovascular disease to the effect of air pollution on incident dementia.
This study underscores that cardiovascular disease plays an important role in the development of dementia and that it partially mediates the observed impact of air pollution on heightening dementia risk.
Focusing efforts on improving cardiovascular health may prevent dementia, especially in areas with higher levels of air pollution.
Introduction
Ambient air pollution has a range of acute and chronic health implications. In 2015, an estimated 4 million premature deaths globally were attributable to exposure to air pollution.1 Ambient air pollution is a complex mixture of particulate matter (PM), gaseous pollutants (e.g. nitrogen dioxide:NO2), persistent organic pollutants and heavy metals. Long-term exposure can result in oxidative stress and increased inflammation which can impact the pathophysiological processes of major chronic diseases and exacerbate existing health conditions.2
Recent evidence suggests air pollution affects diseases of the central nervous system, including dementia.3 Insight into the aetiology and prevention of these diseases and neurodegeneration is valuable, especially with increasing concern with ageing populations and the current absence of a cure. The worldwide prevalence of dementia is expected to increase sharply, from 44 million people with dementia in 2013 to an estimated 135 million in 2050.4
A systematic review of 18 epidemiological studies published through 2014 on air pollution and dementia and cognitive function found most studies identified at least one notable association between increased exposure to air pollution and worse cognitive outcome.5 All previous studies examining the relationship between long-term exposure to ambient air pollution and dementia suggest an association.6–13 For example, traffic-related air pollutants and close proximity to heavy traffic roads were found to be associated with increased risk of dementia in the UK, Sweden and Ontario, Canada.10,12–14 Evidence relating air pollution to cognitive performance is less consistent. Associations between air pollution and measures of cognitive function and decline were found in studies of older US adults15–17 and in selected urban cities in Germany,18 but not found in studies of older adults in Los Angeles19 or the UK.20
Despite growing evidence linking air pollution to dementia,6–13 the exact mechanism is not well understood. Emerging literature suggests oxidative stress, neuroinflammation and cardiovascular disease (CVD) to be contributing factors.3,21,22 Knowledge of the causal pathway can provide valuable insight into disease aetiology and pathophysiology and inform where medical and public health interventions can be most effectively applied to reduce disease burden. Identifying modifiable intermediates along a causal pathway are of particular interest in public health research because they offer opportunities to intervene at a population level and can have lasting effects.
In this study, we hypothesized that CVD is on the causal pathway between air pollution and dementia. Air pollution has been consistently related to increased risk of incidence, complications and mortality from CVD.23–26 Furthermore, growing evidence has linked CVD and its risk factors to impaired cognitive function and often to co-occurrence with dementia.27,28
To date, no study has investigated the extent to which CVD plays an intermediate role in the association between air pollution, specifically NO2 and PM2.5, and incident dementia. We aimed to disentangle this relationship on both relative and absolute scales to describe the strength of the effect and quantify the potential public health benefits, respectively.29 Dissecting this relationship can provide important insight into the causal mechanism of dementia, identify vulnerable populations and prioritize public health efforts to reduce the burden of dementia. We thus performed a causal mediation analysis in a large population-based cohort in Ontario, Canada.
Methods
Study design and population
We conducted a population-based cohort study of older adults in Ontario, Canada. Eligible participants included Canadian-born Ontario residents who participated in the 1996–97 cycle of the National Population Health Survey (NPHS) and the 2000/01, 2003 and 2005 cycles of the Canadian Community Health Survey (CCHS). NPHS and CCHS are population-based surveys administered across Canada, which collect information about health status, health care use and health determinants, covering approximately 98% of the Canadian population aged 12 years or older, with a response rate of about 80%.30 Data from these surveys have been widely used for public health surveillance and research.
Participants were included in this study if they lived in Ontario for at least 5 years and were 45 years or older at the date of survey (i.e. study baseline). This allowed us to measure previous cumulative exposure to air pollution in Ontario and capture older individuals who are at higher risk of developing dementia.
To study the potential mediating role of CVD, the exposure had to accumulate before the mediator, which must have subsequently occurred before the outcome. We achieved this by using the year of survey completion to define time periods, to ensure proper temporality. Briefly, we estimated chronic exposure to air pollution before baseline survey completion. Then, CVD was assessed during a window after the baseline survey. Finally, to ascertain dementia status, individuals were followed from the end of CVD follow-up through 2013 or until incident dementia diagnosis. We a priori considered this cohort structure to incorporate a hypothesized lag before the effects of air pollution can present itself in neurocognitive outcomes. The Research Ethics Board of Sunnybrook Health Sciences Centre, Toronto, Canada, approved the study.
Exposure assessment
Chronic exposure to ambient air pollutants, specifically NO2 and fine particulate matter (PM2.5), was the exposure of interest for this study. We used previously estimated mean measurements of NO2 and PM2.5 at a spatial resolution of about 1 x 1km for each year between 1993 and 2013, to calculate these exposures. Details are included in the supplementary material, available as Supplementary data at IJE online.
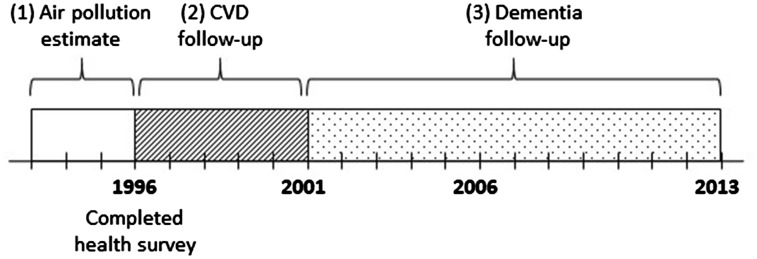
To estimate chronic exposure to ambient air pollutants, running averages of pollutant measurements over the 3 years leading up to the time of baseline survey completion were calculated. For example, if a participant completed the survey at the end of 1996, the participant’s chronic exposure was estimated as an average of pollutant measurement from 1994, 1995 and 1996 (Figure 1). This 3-year running average was estimated for both NO2 and PM2.5 separately. These estimates were updated as the follow-up continued through the study period.
Figure 1.
Schematic of cohort follow-up periods. Example exposure, mediator and outcome follow-up periods for an individual who completed a health survey in 1996: (1) 3-year air pollution measurement (exposure); (2) 5-year follow-up for CVD (mediator); (3) dementia follow-up through 2013 (outcome).
Outcome assessment
Cases of incident dementia were defined as having at least one of the following three criteria (see supplementary material, available as Supplementary data at IJE online for further details):
at least one hospital admission with a diagnosis of dementia; or
at least three physician claims over a 2-year period; or
a prescription relating to dementia.
We ascertained this information using data linkage to population-based health administrative databases. The databases included: hospital discharge records from the Canadian Institute for Health Information’s Discharge Abstracts Database, physician claims from the Ontario Health Insurance Plan database and prescription claims from the Ontario Drug Benefits database. These datasets were linked using unique encoded identifiers, and analysed at ICES. The province of Ontario has a single-payer, universal health care system offered through the provincial government; virtually all Ontario residents are covered through this system and are included in these registries. The algorithm for identifying dementia cases has been validated with medical chart review, and has a sensitivity of 79% and specificity of 99%.31
Cohort members were followed for incident dementia from 5 years after completion of the baseline health survey through 2013; this took into account a hypothesized 5-year lag period for air pollution to have an effect on dementia. For example, the same individual who completed the survey at the end of 1996 would be followed for dementia from 2001 through 2013. We chose 5 years as the lag period because this was the greatest length we could statistically account for, given the size of this cohort. Individuals diagnosed with dementia during the lag period were not included in the analysis.
Mediator assessment
We examined incident cardiovascular events as a potential mediator between exposure to air pollution and dementia. After excluding individuals with prevalent cardiovascular events at the time of survey completion, cohort members were followed for first cardiovascular event for up to 5 years, beginning at the year of baseline health survey. Cardiovascular events were defined as hospital admissions or medical procedures for: coronary heart disease, stroke, arrhythmia and congestive heart failure. We obtained information about these events from hospital discharge abstracts, medical procedure codes and the Ontario Congestive Heart Failure Database (see supplementary material, available as Supplementary data at IJE online).32
The 5-year mediator follow-up occurred immediately after the interval during which air pollution exposure was measured; it coincided with the 5-year lag between air pollution and dementia follow-up. For instance, if the cohort member completed the health survey in 1996, he/she would be followed for cardiovascular events from 1996 to 2001 and then followed for dementia. Figure 1 describes the timing of outcome, exposure and mediator follow-up periods.
Covariates
We selected a priori potential confounders to include as covariates in the model. We ascertained demographic and health behaviour information from the health surveys. The details of all covariates can be found in the supplementary material, available as Supplementary data at IJE online.
Statistical analysis
The study cohort was described with means (standard deviation: SD) and frequencies (%) for all variables of interest. We then estimated the association between air pollution and dementia and air pollution and cardiovascular events, separately. We generated a Cox proportional hazards model and an Aalen additive hazards model for each of these relationships, to estimate hazard ratios (HR) and 95% confidence intervals (CI), and parameter estimates (β) and standard errors (SE) for every 5-ppb and 10-μg/m3 increase in NO2 and PM2.5, respectively. In these eight models, we accounted for spatial clustering by incorporating two-level clustering with census neighbourhood nested within census division as a random effect. We additionally ran minimally adjusted models for each pollutant as sensitivity analyses. Final adjusted models included covariates for individual age, sex, education, marital status, income quintile, smoking status, body-mass index, physical activity, rural residence and northern region, and neighbourhood-level percentages of recent immigrants, income quintile, unemployment and less than high school education.
Causal mediation analysis
The primary objective of this paper was to perform a formal causal mediation analysis to decompose the total effect of air pollution on incident dementia into its natural direct and natural indirect effect through cardiovascular events. We used the two-stage regression method for mediation analysis for survival data under (i) Cox proportional and (ii) Aalen additive hazard models.33–35 Survival analysis in epidemiology most frequently employs Cox proportional hazard models. A newly developed, alternative and more flexible approach to mediation analysis with survival data involves Aalen additive hazard models.36 This approach offers additional flexibility by not requiring the proportional hazards assumption, and is a straightforward and intuitive way of interpreting effect sizes extended to absolute number of events.35
We made the following assumptions on both models: no unmeasured confounding and no mediator-outcome confounder affected by the exposure itself. These were tested with sensitivity analyses (see details below). We also a priori assumed no exposure-mediator interaction in our main analysis; thus, we expect the controlled direct effect (CDE) to coincide with the natural direct effect (NDE) and interpreted them interchangeably.34 Sensitivity analyses accounting for potential exposure-mediator interaction are described and presented in Supplemental material. For multiplicative and additive scales, the NDE compares the dementia incident risk or additional cases for each unit increase in exposure to air pollution, controlling for the CVD pathway and all other covariates. The natural indirect effect (NIE) represents the change in dementia risk or additional cases when exposure to ambient air pollution is held constant while CVD risk changes in response to one unit increase in air pollution exposure.
For each hazard model, we fitted two multilevel regression models to estimate the total effect (TE), NDE and NIE. Further details about estimating these quantities are described in the supplementary material, available as Supplementary data at IJE online. With these estimates we calculated the estimated proportion of the total effect of ambient air pollution on dementia mediated through CVD. TEs, NDEs and NIEs and their 95% CIs were computed using bootstrapping procedures (250 replications). We conducted various sensitivity analyses to assess the robustness of our findings (see details in the supplementary material, available as Supplementary data at IJE online). All data management and statistical analyses were conducted using RStudio Version 1.1.423 with the extension packages coxme and timereg.29,37
Results
This study included 34 391 older adults from Ontario, Canada, who contributed a total of 366 208 person-years. Approximately 7% (n = 2559) of individuals developed dementia during this period. At baseline, the mean age of this cohort was ∼60 years; 58% were female and about half of the population (55%) attended some or completed college. About one-third of the study population had no history of smoking (Table 1).
Table 1.
Baseline characteristics of study population in Ontario, Canada (n = 34 391)
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Demographics | |
| Age at entry (years) | 60.19 (10.56) |
| Sex | |
| Male | 14 555 (42) |
| Female | 19 836 (58) |
| Education | |
| Less than high school | 9055 (26) |
| High school diploma | 6462 (19) |
| Some college or more | 18 874 (55) |
| Income | |
| Lowest | 758 (2) |
| Low-middle | 1856 (5) |
| Middle | 5117 (15) |
| Middle-high | 8083 (24) |
| Highest | 6645 (19) |
| Unknown | 11 932 (35) |
| Marital status | |
| Married | 21 175 (62) |
| Single | 2827 (8) |
| Separated, widowed, divorced | 10 373 (30) |
| Unknown | 16 (<1) |
| Health information | |
| Physical activity | |
| Active | 7346 (21) |
| Moderate | 8792 (26) |
| Not active | 18 253 (53) |
| Smoking status | |
| Never smoker | 9976 (29) |
| Former smoker | 16 441 (48) |
| Current smoker | 7974 (23) |
| Weight status | |
| Underweight | 500 (2) |
| Normal | 13 505 (39) |
| Overweight | 13 455 (39) |
| Obese | 6931 (20) |
| Pre-existing comorbidity | |
| Diabetes | 3262 (10) |
| Hypertension | 12 026 (35) |
| Traumatic brain injury | 1279 (4) |
| Physician density per 1000 | 1.49 (1.17) |
| Geography | |
| Northern latitude | 5989 (17) |
| Missing | 6 (<1) |
| Rural residence | 11 243 (33) |
| Area-level risk factors | |
| Percentage of recent immigrants | 1.87 (2.61) |
| Percentage unemployed | 6.71 (1.93) |
| Percentage under high school | 28.50 (5.74) |
The 3-year cumulative exposures to NO2 and PM2.5 5 years before dementia follow-up were 10.4 ppb [range: 2.2–54.4 ppb; interquartile range (IQR): 7.6 ppb] for NO2 and 8.6 µg/m3 (range: 0.8–35.2; IQR: 4.7 µg/m3) for PM2.5.
For every 5-ppb and 10-µg/m3 unit increase in cumulative exposure to NO2 and PM2.5, there was a positive association with the incidence of dementia, with fully adjusted HRs of 1.10 (95% CI 0.99–1.19) and 1.29 (95% CI 0.99–1.64), respectively (Table 2). Additive models indicate that for each unit increase, 100 (SE <100 x 10-5) and 200 (SE 100 x 10-5) additional cases of dementia per 100 000 per year are diagnosed for NO2 and PM2.5, respectively (Table 2). See Supplementary Table 1, available as Supplementary data at IJE online for minimally adjusted estimates.
Table 2.
Associations between air pollutant and dementia and cardiovascular disease
| Cox PH model | Aalen model | |
|---|---|---|
| HR a (95% CI) | Estimate a (SE) | |
| NO2b | ||
| Dementia c | 1.10 (0.99–1.19) | 100 x 10-5 (<100 x 10-5) |
| Cardiovascular disease | 1.01 (0.96–1.06) | 100 x 10-5 (<100 x 10-5) |
| PM2.5b | ||
| Dementia c | 1.29 (0.99–1.64) | 200 x 10-5 (100 x 10-5) |
| Cardiovascular disease | 1.08 (0.94–1.24) | 300 x 10-5 (100 x 10-5) |
PH, proportional hazards.
Adjusted for age, sex, education, marital status, income quintile, smoking status, body mass index, physical activity, rural residence and northern region; area level: recent immigrants, unemployment and education.
NO2 per 5 ppb, PM2.5 per 10 µg/m3.
Total effect obtained from product method.
Associations between exposure to NO2 and PM2.5 and CVD were also detected, with HRs of 1.01 (95% CI 0.96–1.06) and 1.08 (95% CI 0.94–1.24), although these estimates have wide confidence intervals and are imprecise (Table 2). For each unit increase, 100 (SE <100 x 10-5) and 300 (SE 100 x 10-5) additional CVD cases per 100 000 individuals per year were diagnosed for NO2 and PM2.5, respectively (Table 2).
Our mediation analysis showed that the effect of air pollution on dementia may be partially mediated through cardiovascular events for both scales (Tables 3 and 4). On the multiplicative scale, we observed an indirect effect HR of 1.01 (95% CI 0.98–1.03) for NO2 and 1.06 (95% CI 0.99–1.12) for PM2.5 mediated through CVD. These translate to approximately 9% of the effect of NO2 on dementia and 21% of the effect of PM2.5 on dementia being mediated through cardiovascular events in this study population. In additive models, for NO2 we observe that approximately 1.5 (95% CI 1.0–2.6) additional cases per 100 000 per year can be attributed to the pathway through CVD. For PM2.5, approximately 4.2 (95% CI 2.9–6.9) additional cases per 100 000 per year can be attributed to the pathway through CVD. These translate to approximately 2% and 4% of the pathway from NO2 and PM2.5, respectively, can be attributed to the pathway through CVD. For both relative and absolute scales, a greater proportion of the effect of PM2.5 on dementia may be mediated through CVD than for NO2. It is important to note that measures of proportion mediated should be interpreted as a qualitative measure and are imprecise due to the wide confidence intervals of our indirect effects. Our conclusions did not change appreciably with sensitivity analyses (see supplementary material, available as Supplementary data at IJE online).
Table 3.
Total, controlled direct and natural indirect effects of ambient air pollutant through cardiovascular disease (Cox proportional hazards model)
| Pollutant | Total effect | Natural direct effect | Natural indirect effect |
|---|---|---|---|
| HRa (95% CI) | HRb (95% CI) | HRb (95% CI) | |
| NO2c | 1.10 (0.99–1.19) | 1.09 (1.00–1.18) | 1.01 (0.98–1.03) |
| PM2.5c | 1.29 (0.99–1.64) | 1.22 (0.95–1.56) | 1.06 (0.99–1.12) |
Total effect obtained from product method.
Adjusted for age, sex, education, marital status, income quintile, smoking status, body mass index, physical activity, rural residence and northern region; area level: recent immigrants, unemployment and education.
NO2 per 5 ppb, PM2.5 per 10 µg/m3.
Table 4.
Total, controlled direct and natural indirect effects of ambient air pollutant through cardiovascular disease (Aalen additive hazards model)
| Pollutant | Total effect | Natural direct effect | Natural indirect effect |
|---|---|---|---|
| Estimatea (95% CI) | Estimateb (95% CI) | Estimateb (95% CI) | |
| NO2c | 100 x 10−5 (1.20 x 10−5−100 x 10−5) | 100 x 10−5 (<100 x 10−5−100 x 10−5) | 1.45 x 10−5 (1.00 x 10−5–2.56 x 10−5) |
| PM2.5c | 100 x 10−5 (3.60 x 10−5–300 x 10−5) | 100 x 10−5 (<100 x 10−5 –300 x 10−5) | 4.20 x 10−5 (2.85 x 10−5–6.91 x 10−5) |
Total effect obtained from product method.
Adjusted for age, sex, education, marital status, income quintile, smoking status, body mass index, physical activity, rural residence and northern region; area level: recent immigrants, unemployment and education.
NO2 per 5 ppb, PM2.5 per 10 µg/m3.
Discussion
Using a population-based cohort of 34 391 individuals in Ontario, Canada, we decomposed the total effect of exposure to ambient air pollutants, specifically NO2 and PM2.5, on incident dementia into its respective direct and indirect effects through CVD, on multiplicative and additive scales. We found an increased risk of dementia among those with higher exposure to NO2 (HR 1.10, β 100 x 10-5) and PM2.5 (HR 1.15, β 200 x 10-5). We found some evidence of an indirect effect through CVD for both pollutants, with incident CVD mediating more of the relationship between PM2.5 and dementia than the relationship between NO2 and dementia. These effects are observed in a region with pollutant concentrations that are among the lowest in the world.
This total effect is in line with previous studies. Two recent systematic reviews of air pollution and cognitive functioning and dementia reported that the majority of reviewed studies found positive associations between higher exposure to air pollution (PM2.5 or living in a high traffic area) and worse cognitive ageing and dementia.5,38
Such findings are biologically plausible and are supported by neuroimaging and biological studies. Pathology studies have shown that dementia is the result of a combination of neurodegenerative and vascular lesions, suggesting commonalities in the mechanisms of dementia and vascular disease.33,34 Taking this into account, with the substantial literature linking air pollution with cardiovascular and cerebrovascular risk factors and disease, provides motivation to investigate CVD as an intermediate on the pathway.23–26
Understanding dementia disease aetiology and the role of CVD in dementia can provide invaluable insight toward prevention strategies. Since well-defined interventions to prevent CVD exist, targeted efforts to improve cardiovascular health may be beneficial to dementia prevention in areas with increased exposure to air pollution. For instance, cardiovascular health programmes, screening and access to CVD health care can be prioritized in highly polluted areas to not only improve CVD outcomes but also potentially reduce the risk of dementia.
Many methodological challenges exist when studying dementia at the population level.33 First, our findings are subject to selective attrition due to mortality. Whereas this would likely result in underestimating the effect sizes, we acknowledge the possibility that a portion of study participants died from air pollution-related causes (e.g. CVD, respiratory conditions) before living long enough to develop dementia. Thus, those who developed dementia could be a healthier group of participants who were less vulnerable to detrimental air pollution effects. We attempted to address this concern by examining the influence of potential risk factors for competing risks in our models.
Second, our classification of dementia is limited to diagnosed cases. This can be a concern when cases are ascertained from a data source that does not definitively capture dementia information. For example, using the hospital discharge data alone underestimates dementia cases because the diagnosis and management of dementia do not require hospital admission. We addressed this by using three sources (i.e. hospital discharge records, physician claims and prescription claims) to identify cases in a population with universal access to health care. It is also possible that there might be inconsistencies in diagnoses, because the diagnosis often depends on caregiver’s concern and access to care.34 Additionally, individuals with CVD conditions may interact more frequently with the health care system and therefore increase their probability of being diagnosed earlier for dementia. However, since our study population is restricted to individuals who have completed a health survey, individuals in our study are more likely to be health-conscious and are likely of higher socioeconomic status. This may limit the generalizability of our findings, but we believe that studying dementia in this population minimizes potential outcome misclassification, and thus improves the validity of our findings. Similarly, we were unable to account for undiagnosed CVD cases.
Next, the results from this mediation analysis rely on the assumptions of no unmeasured confounding and no mediator-outcome confounder affected by the exposure. To check this assumption, we conducted sensitivity analyses to assess the extent of confounding by measured risk factors and health status variables (e.g. diabetes, hypertension, traumatic brain injury) that may violate the assumption of no unmeasured mediator-outcome confounding, and found no appreciable change in our observed indirect effect sizes. We also assumed no interaction between air pollution and CVD in our main analysis, allowing the CDE and NDE to be interpreted similarly. To check this assumption, we accounted for exposure-mediator interaction in Cox proportional hazard models and found slightly attenuated natural indirect effect estimates.
This study has major strengths including its large size, ability to ascertain incident cardiovascular events and incident dementia from validated sources, availability of individual socioeconomic and health behaviour information and analytical approach. We also used two survival models to account for potential violations in either model assumptions. We encourage future studies to replicate our methods in established cohort studies where participants have routine evaluations for dementia and cognitive decline.
A formal causal mediation analysis is a valuable tool to disentangle complex relationships and provides insight toward understanding disease aetiology and identifying specific prevention efforts. Our results suggest an increased risk of dementia among individuals with higher cumulative exposure to air pollution, which was partially mediated through CVD. Our study identified and highlighted two modifiable risk factors, ambient air pollution and CVD, for dementia. Intervening on one or both has the potential to significantly reduce the burden of dementia.
Funding
This work was supported in part by Health Canada (MOA-4500314182), Public Health Ontario (PHO) and ICES, which are funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC) and the Alzheimer’s Disease Resource Center for advancing Minority Aging Research at the University of California San Diego (P30AG059299 National Institute on Aging). Parts of this material are based on data and information compiled and provided by the Canadian Information Health Institute (CIHI). K.T. and J.K. are supported by Research Scholar Awards from the Department of Family and Community Medicine at the University of Toronto. The opinions, results and conclusions reported in this article do not necessarily represent the views of PHO, ICES, MOHLTC or CIHI.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Cohen AJ, Brauer M, Burnett R. et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017;389:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kampa M, Castanas E.. Human health effects of air pollution. Environ Pollut 2008;151:362–67. [DOI] [PubMed] [Google Scholar]
- 3. Block ML, Calderón-Garcidueñas L.. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 2009;32:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prince M, Guerchet M, Prina M.. Policy Brief: The Global Impact of Dementia 2013–2050 2013. https://www.alz.co.uk/research/GlobalImpactDementia2013.pdf (9 July 2019, date last accessed).
- 5. Power MC, Adar SD, Yanosky JD, Weuve J.. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 2016;56:235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang KH, Chang MY, Muo CH, Wu TN, Chen CY, Kao CH.. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS One 2014;9:e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y-C, Lin Y-C, Yu H-L.. Association between air pollutants and dementia risk in the elderly. Alzheimer’s Dement 2015;1:220–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung CR, Lin YT, Hwang BF.. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis 2015;44:573–84. [DOI] [PubMed] [Google Scholar]
- 9. Kioumourtzoglou MA, Schwartz JD, Weisskopf MG. et al. Long-term PM2.5exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect 2016;124:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oudin A, Forsberg B, Adolfsson AN. et al. Traffic-related air pollution and dementia incidence in Northern Sweden: a longitudinal study. Environ Health Perspect 2016;124:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cacciottolo M, Wang X, Driscoll I. et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 2017;7:e1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Kwong JC, Copes R. et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 2017;108:271–77. [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Kwong JC, Copes R. et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 2017;389:718–26. [DOI] [PubMed] [Google Scholar]
- 14. Carey IM, Anderson R, Atkinson RW. et al. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 2018;8:e022404.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ailshire JA, Clarke P.. Fine particulate matter air pollution and cognitive function among U.S. older adults. J Gerontol B Psychol Sci Soc Sci 2015;70:322–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ailshire JA, Crimmins EM.. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 2014;180:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F.. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 2012;172:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tzivian L, Dlugaj M, Winkler A. et al. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf recall study. Environ Health Perspect 2016;124:1361.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gatto NM, Henderson VW, Hodis HN. et al. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 2014;40:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cullen B, Newby D, Lee D. et al. Cross-sectional and longitudinal analyses of outdoor air pollution exposure and cognitive function in UK Biobank. Sci Rep 2018;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayaraj RL, Rodriguez EA, Wang Y, Block ML.. Outdoor ambient air pollution and neurodegenerative diseases: the neuroinflammation hypothesis. Curr Environ Health Rep 2017;4:166–79. [DOI] [PubMed] [Google Scholar]
- 22. Weuve J. Invited commentary: how exposure to air pollution may shape dementia risk, and what epidemiology can say about it. Am J Epidemiol 2014;180:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brook RD, Rajagopalan S, Pope CA. et al. Particulate matter air pollution and cardiovascular disease. Circulation 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 24. Hoek G, Krishnan RM, Beelen R. et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health 2013;12:43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinelli N, Olivieri O, Girelli D.. Air particulate matter and cardiovascular disease: A narrative review. Eur J Intern Med 2013;24:295–302. [DOI] [PubMed] [Google Scholar]
- 26. Pope CA, Burnett RT, Thurston GD. et al. Cardiovascular mortality and long-term exposure to particulate air pollution. Circulation 2004;109:71–77. [DOI] [PubMed] [Google Scholar]
- 27.Alzheimer’s Association. Alzheimer’s disease facts and figures includes a special report on the financial and personal benefits of early diagnosis. Alzheimers Dement 2018;14:367–429. [Google Scholar]
- 28. Harrison SL, Ding J, Tang EYH. et al. Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS One 2014;9:e114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas Scheike M. Title Flexible Regression Models for Survival Data 2019. https://github.com/scheike/timereg.git (24 May 2019, date last accessed).
- 30. Béland Y. Canadian community health survey - methodological overview. Health Rep 2002;13:9–14. [PubMed] [Google Scholar]
- 31. Liisa Jaakkimainen R, Bronskill SE, Tierney MC. et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Affect Disord 2016;54:337–49. [DOI] [PubMed] [Google Scholar]
- 32. Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV.. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ 2012;184:E765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 34. VanderWeele T. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford, UK: Oxford University Press, 2015. [Google Scholar]
- 35. Lange T, Hansen JV.. Direct and indirect effects in a survival context. Epidemiology 2011;22:575–81. [DOI] [PubMed] [Google Scholar]
- 36. Aalen O. A Model for Nonparametric Regression Analysis of Counting Processes. New York, NY: Springer, 1980. [Google Scholar]
- 37. Therneau T, Mixed Effects Cox Models 2018. https://cran.r-project.org/web/packages/coxme/vignettes/coxme.pdf (23 May 2019, date last accessed).
- 38. Clifford A, Lang L, Chen R, Anstey KJ, Seaton A.. Exposure to air pollution and cognitive functioning across the life course: a systematic literature review. Environ Res 2016;147:383–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.