Abstract
Background
Although physical activity has many known health benefits, its association with lung function in childhood/adolescence remains unclear. We examined the association of physical-activity trajectories between 11 and 15 years with lung function at 15 years in 2266 adolescents.
Methods
A population-based cohort of 14 305 singleton births alive at 1 year was recruited in the UK population-based Avon Longitudinal Study of Parents and Children cohort. Physical activity (counts/minute and moderate-to-vigorous physical activity) was assessed for 7 days using an accelerometer at 11, 13 and 15 years. We identified sex-specific physical-activity trajectories applying K-means for longitudinal data in children with at least two accelerometer measurements (n = 3584). We then estimated the sex-specific associations of these trajectories with post-bronchodilation lung-function parameters using multivariable linear-regression models (n = 2266, 45% boys).
Results
Fewer than 7% of participants met the WHO physical-activity recommendations (i.e. daily average of at least 60 minutes of moderate-to-vigorous physical activity). Boys were substantially more active than girls. In both sexes, we identified three distinct physical-activity trajectories (‘low’: 39.8% boys, 45.8% girls; ‘moderate’: 42.9% boys, 41.4% girls; and ‘high’ physical activity: 17.3% boys, 12.8% girls). Girls in the moderate and high physical-activity trajectories had 0.11 L [95% confidence interval (CI): 0.04–0.19] and 0.15 L (95% CI: 0.03–0.26) higher forced vital capacity than their less-active peers. No association was observed in boys.
Conclusions
Higher childhood physical activity relates to higher lung-function levels in adolescent girls. A better understanding of the mechanisms underlying this association should be pursued.
Keywords: ALSPAC, children, moderate-to-vigorous physical activity, respiratory health
Key Messages
This study is the first to report an association of higher regular physical activity during childhood with higher lung-function measures in adolescent girls.
Reverse causation or confounding cannot explain these results, as they are based on a prospective cohort with multiple objective physical-activity measures (i.e. by accelerometer) and an analysis that includes relevant potential confounders, such as body-composition measures, pubertal status and lung function at baseline.
Strategies for promoting physical activity in childhood could benefit respiratory health.
Introduction
Lung function is a well-known objective marker of respiratory health and a predictor of cardiorespiratory morbidity and mortality along the life course.1 Since lung function tracks from childhood to adulthood, the identification of factors influencing the development of lung function in childhood is important. Lifestyle behaviours, and specifically physical activity, are particularly interesting because they are modifiable and track from childhood to adulthood.2 Regular physical activity in children and adolescents has both acute and long-term health benefits (e.g. reduced risk of obesity and metabolic syndrome, improved mental well-being).3 Despite these well-known health benefits, the amount and intensity of daily physical activity during childhood have decreased over the past decades.4 In most European countries, more than half of children and adolescents fail to achieve the WHO recommended daily average of at least 60 minutes of moderate-to-vigorous physical activity (MVPA).5
The evidence supporting a beneficial effect of physical activity on lung-function development is weak, since previous research is subject to important limitations. First, most previous studies had a cross-sectional design6–9 and therefore cannot establish temporality. Second, past studies have primarily relied on self-reported physical activity7–14 and thus are subject to misclassification from errors in recall, which most likely would dilute the strength of the association. Of note, the one existing study that did use a direct physical-activity measure (accelerometry) was cross-sectional and reported no association of physical activity with lung function.6 Finally, previous findings may be biased because they did not consider relevant confounders, such as body mass index (BMI) or pubertal status, both of which are associated with lung function15,16 and physical activity.17,18
This study aimed to assess the relation of physical activity, from childhood to adolescence, with lung function at adolescence in a large UK birth cohort [the Avon Longitudinal Study of Parents and Children (ALSPAC)] with longitudinal data, repeated objective measures of physical activity and a wide range of potential confounders.
Methods
Additional details are provided in the Supplementary Methods, available as Supplementary data at IJE online.
Study design and population
We used data from the ALSPAC study, which has been described previously.19,20 Briefly, ALSPAC recruited 15 247 pregnant women residents in Avon, UK, with expected dates of delivery between the 1 April 1991 and 31 December 1992. This recruitment resulted in a cohort of 14 305 singleton children alive at 1 year of age. Children were regularly followed up by postal questionnaires and health examinations. The ALSPAC Ethics and Law Committee and the Local Research Ethics Committees gave ethical approval. The ALSPAC study website contains all the data that are available through a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/).
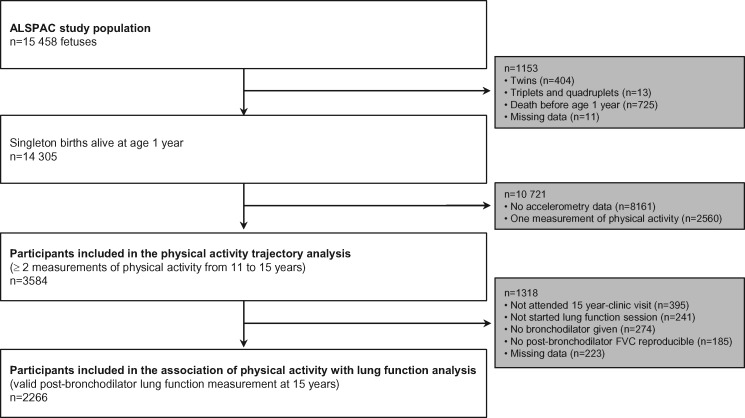
We used data from children with at least two accelerometer-based measures of physical activity between 11 and 15 years to identify the physical-activity trajectories. Children who additionally had lung-function measures at 15 years were included in the analysis that assessed associations of physical-activity trajectories with lung function (Figure 1).
Figure 1.
Flow chart of participants in this study from the ALSPAC population.
Measures
Physical activity
Physical-activity measurement has been described previously.21,22 Children who attended the research visits at 11, 13 and 15 years were asked to wear an Actigraph AM7164 2.2 accelerometer (Actigraph LLC, Fort Walton Beach, FL, USA) around their waist, at the right hip, for 7 days. A valid day was defined as providing data for at least 10 hours per day (excluding sequences of 10 or more minutes with consecutive zero counts) and children were only included in the analyses if they provided at least 3 valid days of recording. We derived the total volume of physical activity [counts per minute (CPM)] and time spent in MVPA (≥3600 CPM).
Lung function
Lung function was measured by spirometry at 8 and 15 years of age (Vitalograph 2120; Vitalograph, Maids Moreton, UK), according to the American Thoracic Society standards.23 At 15 years, lung function was assessed before and after bronchodilation with salbutamol. The best three technically acceptable flow-volume curves repeatable within 200 mL of forced vital capacity (FVC) were retained (according to the criteria at that time). FVC, forced expiratory volume in 1 second (FEV1) and forced expiratory flow at 25 and 75% of FVC (FEF25–75) were obtained and the FEV1/FVC ratio was calculated.
Covariates
Socio-demographic, anthropometric, psychological and other lifestyle factors were collected at different time points. Questionnaires during the antenatal period provided information on: maternal education; maternal and paternal occupational social class; maternal anxiety; and maternal smoking during pregnancy. At birth, sex, birthweight, pre-term delivery, age of mother at delivery and parity were extracted from medical records. Postnatal questionnaires provided data on exposure to tobacco smoke, breastfeeding and presence of furry pets at home. At 10 years, we obtained dietary energy-intake data based on a 3-day diet report. At the 11-year clinic visit, body weight and body composition (assessed using a dual-energy x-ray absorptiometry scanner) were measured. We calculated BMI (kg/m2), lean BMI (kg/m2) and fat mass index (kg/m2). We defined weight status using BMI Z-scores relative to UK 1990 population reference data: healthy weight (Z-score <1.04), overweight (Z-score ≥1.04–1.63) and obese (Z-score ≥1.64).24–26 From the 14-year questionnaire, we obtained personal smoking information. At 15 years, lifetime doctor-diagnosed asthma was reported. Finally, pubertal status, based on changes in voice for boys and menarche status for girls, was collected in the puberty questionnaires administered from 8 to 15 years. We categorized early menarche as ≤11.3 years [≤1 standard deviation (SD) below the sample mean], average menarche as >11.3–13.5 years (within 1 SD of the sample mean) and late menarche as >13.5 years (≥1 SD above the sample mean).27
Statistical analysis
We described participant characteristics using absolute (n) and relative frequencies (%), as well as the mean (SD) and median (25th and 75th percentiles, P25–P75). We compared the characteristics of children included in this analysis to those excluded using t-tests for normal distributions, Mann–Whitney tests for non-normal distributions and chi-squared tests for categorical variables. Given the sex differences in physical-activity levels and lung function, we stratified all analyses by sex.
Identification of physical-activity trajectories
We used the three-dimensional version of the K-means for longitudinal data (R Package kml3d, R Foundation for Statistical Computing, Vienna, Austria)28 to identify and categorize participants based on patterns of longitudinal change in physical activity (considering simultaneously CPM and time spent in MVPA) from 11 to 15 years. We built the models for 3 to 20 trajectories and kept the number of relevant trajectories with the best data separation according to the cluster validity index of Davies–Bouldin.29
Associations of physical activity with lung function
We examined the associations of physical-activity trajectories with post-bronchodilation FVC, FEV1, FEF25–75 and FEV1/FVC ratio at 15 years using linear-regression analyses. We considered as confounders: (i) factors related to both the exposure and the outcome in bivariable analyses (P < 0.20), (ii) factors that modified (>10% change in regression coefficient) the estimate of the exposure variable or (iii) factors deemed relevant according to the scientific literature. Then we combined forward and backward strategies to build the most parsimonious model that still explains the data.30 Final multivariable models included: parental social class, pre-term delivery, baseline weight status, smoking status, pubertal status, as well as age and height at 15 years. We additionally adjusted the models for lung function at 8 years to reduce potential reverse causation. We assessed the goodness-of-fit of the final models by means of normality of residuals, heteroscedasticity, linearity, multicollinearity and identification of influential data.
Additional analyses
As a post hoc analysis, we considered a larger number of trajectories in boys so that the lowest physical-activity trajectory in boys was equivalent to the lowest trajectory in girls. Sensitivity analyses were also conducted: (i) repeating the analyses using standardized and percent predicted spirometry measures31; (ii) repeating the analyses using percentage of wear time spent in MVPA instead of MVPA; (iii) excluding children with extreme spirometry measures; (iv) excluding children with any lifetime history of doctor-diagnosed asthma; (v) adjusting the models for body composition (lean and fat BMIs) instead of BMI; (vi) using cumulative physical-activity measures instead of trajectories.
Regression analyses were fitted using Stata/SE 14.0 (StataCorp, College Station, TX, USA) and results were expressed as regression coefficients with 95% confidence intervals (95% CIs).
Results
Characteristics of study sample
We included 3584 children in the analysis of physical-activity trajectories (Figure 1). Compared with the children excluded from this analysis, those included were more frequently girls, less exposed to maternal smoking during pregnancy, had higher parental social class, maternal age and breastfeeding duration, and the boys were less physically active at 11 years (Supplementary Table 1, available as Supplementary data at IJE online). Among them, 2266 children had lung-function data and were therefore included in the analysis of physical activity with lung function (Figure 1). The only additional difference of this subgroup compared with the excluded children was that the latter were from a lower parental social class (Supplementary Table 2, available as Supplementary data at IJE online). Table 1 shows the main characteristics of the study sample. The boy/girl sex ratio was equal to 0.8 and half of the children came from high socio-economic backgrounds. At 11 years, 27.3% of children were overweight or obese. At 14 years, 1.4% of participants were regular smokers. The mean (SD) age at menarche was 12.4 (1.1) years and the voice of half of the boys had completely broken by 15 years. Boys had higher FVC, FEV1 and FEF25–75 than girls at all age periods, whereas girls had higher FEV1/FVC ratios.
Table 1.
Descriptive statistics of the study sample from the ALSPAC by sex
| Characteristics, median (P25–P75), mean (SD) or na (%) | Boys (n = 1030) | Girls (n = 1236) | P |
|---|---|---|---|
| Potential confounders | |||
| Maternal educational achievement (n = 2124) | |||
| Low level (none, CSE and vocational) | 155 (16.0) | 191 (16.5) | |
| Intermediate level (O-level) | 330 (34.0) | 410 (35.5) | 0.659 |
| High level (A-level, university degree) | 484 (50.0) | 554 (48.0) | |
| Parental social classb (n = 2059) | |||
| Professional and intermediate (class I and class II) | 636 (68.0) | 722 (64.3) | |
| Skilled non-manual (class III non-manual) | 197 (21.0) | 264 (23.5) | 0.219 |
| Skilled manual, partly and unskilled (class III manual, class IV and class V) | 103 (11.0) | 137 (12.2) | |
| Maternal age (years) (n = 2095) | 29.5 (4.4) | 29.2 (4.4) | 0.225 |
| Maternal anxiety during pregnancy (n = 2064) | 164 (17.4) | 193 (17.4) | 0.949 |
| Maternal smoking during pregnancy (n = 1976) | 148 (16.5) | 186 (17.3) | 0.633 |
| Parity (n = 2117) | 459 (47.4) | 571 (49.7) | 0.277 |
| Pre-term delivery (<37 weeks of gestation) (n = 2168) | 52 (5.3) | 40 (3.4) | 0.031 |
| Birthweight (grams) (n = 2138) | 3500 (3140–3860) | 3400 (3100–3680) | <0.001 |
| Breastfeeding for 3 months or more (n = 2064) | 582 (61.0) | 700 (63.1) | 0.337 |
| Family furry pets (cat/dog) during first year (n = 2040) | 385 (41.4) | 484 (43.6) | 0.334 |
| Dietary energy intake at 10 years (kcal/day) (n = 2175) | 1927 (1701–2178) | 1770 (1561–2000) | <0.001 |
| Body mass index at 11 years (kg/m2) (n = 2226) | 18.0 (16.5–20.2) | 18.5 (16.8–21.0) | <0.001 |
| Weight statusc (n = 2226) | |||
| Healthy weight (Z-score <1.04) | 729 (71.9) | 890 (73.4) | |
| Overweight (Z-score ≥1.04–1.63) | 139 (13.7) | 155 (12.8) | 0.710 |
| Obese (Z-score ≥1.64) | 146 (14.4) | 167 (13.8) | |
| Lean body mass index at 11 years (kg/m2) (n = 2210) | 13.2 (12.6–13.9) | 12.6 (11.9–13.5) | <0.001 |
| Fat mass index at 11 years (kg/m2) (n = 2210) | 3.7 (2.6–5.8) | 5.0 (3.5–7.0) | <0.001 |
| Smoking status at 14 years (n = 1916) | 8 (1.0) | 18 (1.7) | 0.173 |
| Pubertal status | |||
| Age of menarche (in years) (n = 1195) | – | 12.4 (1.1) | |
| Early age (<11.3 years) | 238 (19.9) | ||
| Average age (≥11.3–13.5 years) | 774 (64.8) | ||
| Late age (>13.5 years) | 183 (15.3) | ||
| Voice-breaking status (n = 1013) | |||
| Completely broken | 577 (57.0) | – | |
| Starting to break | 329 (32.5) | ||
| Not yet started | 107 (10.5) | ||
| Height at 15 years (cm) (n = 2246) | 174.3 (169.0–179.0) | 164.6 (160.6–168.9) | <0.001 |
| Lifetime doctor-diagnosed asthma (n = 2265) | 259 (25.2) | 267 (21.6) | 0.045 |
| Spirometry measures | |||
| 8 years | |||
| FVC (L) (n = 1995) | 2.0 (0.3) | 1.8 (0.3) | <0.001 |
| FEV1 (L) (n = 1972) | 1.7 (0.3) | 1.6 (0.3) | <0.001 |
| FEF25–75 (L/s) (n = 1995) | 2.0 (0.5) | 2.1 (0.5) | 0.032 |
| FEV1/FVC (%) (n = 1972) | 87.2 (6.7) | 89.4 (6.0) | <0.001 |
| Z-score of FVC (n = 1831) | –0.05 (1.00) | –0.06 (0.99) | 0.943 |
| Z-score of FEV1 (n = 1809) | –0.05 (1.02) | –0.01 (0.98) | 0.299 |
| Z-score of FEF25–75 (n = 1831) | –0.13 (1.06) | –0.14 (1.00) | 0.929 |
| Z-score of FEV1/FVC (n = 1809) | 0.01 (1.09) | 0.07 (1.05) | 0.230 |
| 15 years (post-bronchodilation measures) | |||
| FVC (L) (n = 2265) | 4.2 (0.9) | 3.3 (0.6) | <0.001 |
| FEV1 (L) (n = 2177) | 3.8 (0.8) | 3.1 (0.6) | <0.001 |
| FEF25–75 (L/s) (n = 2265) | 4.6 (1.2) | 4.0 (1.0) | <0.001 |
| FEV1/FVC (%) (n = 2177) | 91.1 (6.7) | 93.0 (6.3) | <0.001 |
| Z-score of FVC (n = 2080) | –0.90 (1.28) | –0.98 (1.30) | 0.186 |
| Z-score of FEV1 (n = 2001) | –0.38 (1.28) | –0.59 (1.36) | <0.001 |
| Z-score of FEF25–75 (n = 2080) | 0.14 (1.11) | 0.09 (1.20) | 0.300 |
| Z-score of FEV1/FVC (n = 2001) | 0.91 (1.13) | 0.77 (1.11) | 0.006 |
FEF25–75, forced expiratory flow at 25 and 75% of FVC; FEV1, volume expired in the first second; FVC, forced vital capacity; MVPA, moderate-to-vigorous physical activity, P25–P75, 25th and 75th percentiles; SD, standard deviation; Z-scores according to GLI-equations; –, not relevant.
Values are medians (25th–75th percentiles), means (SD) or absolute numbers (percentages).
P-value from the chi-squared test, Student’s t-test or the Mann–Whitney test comparing distributions across sexes.
Values in bold have P-values <0.05.
Number of children may vary because of missing information.
Highest parental social class.
Weight status defined according to body mass index Z-scores relative to UK 1990 population reference data.
Physical activity from 11 to 15 years
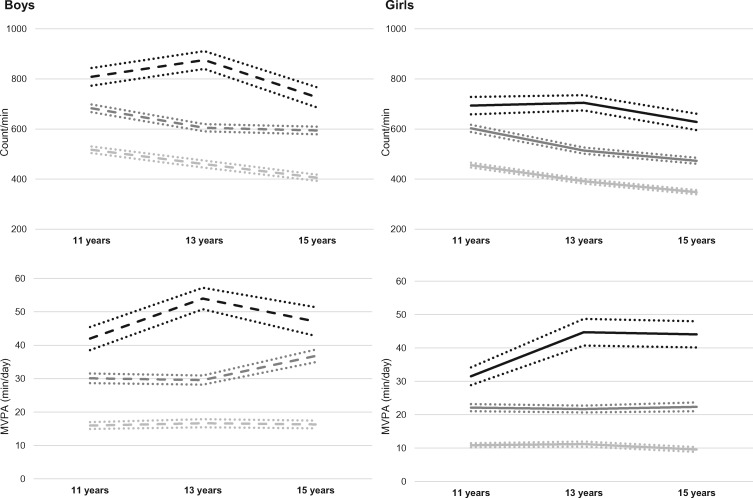
The physical-activity characteristics of the children are presented in Table 2. Fewer than 7% of the children achieved the recommended level of physical activity (i.e. 60 minutes in MVPA per day) at any time period. At baseline (11 years), the median MVPA was 24.0 minutes in boys and 15.7 minutes in girls (P < 0.001). At all periods and for all physical-activity variables, boys were more active than girls. We identified three physical-activity trajectories in both sexes (Figure 2 and Supplementary Figure 1, available as Supplementary data at IJE online). For boys, the first trajectory (labelled ‘low physical activity’) captured 39.8% of children and had a median overall physical activity (CPM) and time spent in MVPA of 511 CPM and 14.9 minutes/day, respectively, at 11 years (Supplementary Table 3, available as Supplementary data at IJE online). The second (‘moderate physical activity’) and third (‘high physical activity’) trajectories grouped 42.9 and 17.3% of boys, respectively. These trajectories did not cross the first group. The medians of overall physical activity and time spent in MVPA were: 680 CPM, 29.5 minutes/day and 841 CPM, 44.5 minutes/day at 11 years for the moderate and high trajectories, respectively. Whereas overall physical activity decreased over time, the time spent in MVPA increased.
Table 2.
Descriptive statistics of the physical-activity measures by sex
| Median (P25–P75) or na (%) | Boys (n = 1030) | Girls (n = 1236) | P |
|---|---|---|---|
| Physical activity at 11 years (n = 2146) | |||
| Overall activity (CPM) | 620 (514–750) | 525 (444–628) | <0.001 |
| MVPA (minutes) | 24.0 (15.0–35.6) | 15.7 (9.6–24.2) | <0.001 |
| Percentage of time spent in MVPA (%) | 3.0 (1.9–4.6) | 2.0 (1.2–3.1) | <0.001 |
| Met guidelines of 60 minutes of MVPA | 38 (3.8) | 3 (0.3) | <0.001 |
| Physical activity at 13 years (n = 2054) | |||
| Overall activity (CPM) | 566 (449–703) | 461 (377–570) | <0.001 |
| MVPA (minutes) | 24.7 (14.5–38.4) | 16.8 (9.6–26.8) | <0.001 |
| Percentage of time spent in MVPA (%) | 3.1 (1.9–4.8) | 2.2 (1.2–3.4) | <0.001 |
| Met guidelines of 60 minutes of MVPA | 62 (6.6) | 23 (2.1) | <0.001 |
| Physical activity at 15 years (n = 1329) | |||
| Overall activity (CPM) | 510 (406–643) | 414 (341–506) | <0.001 |
| MVPA (minutes) | 26.6 (16.6–41.6) | 15.0 (8.0–26.0) | <0.001 |
| Percentage of time spent in MVPA (%) | 3.3 (2.1–5.2) | 1.9 (1.0–3.2) | <0.001 |
| Met guidelines of 60 minutes of MVPA | 41 (7.0) | 14 (1.9) | <0.001 |
| Averaged physical activity from 11 to 15 years (n = 2266) | |||
| Averaged overall activity (CPM) | 586 (490–692) | 486 (418–570) | <0.001 |
| Averaged time in MVPA (minutes) | 26.1 (18.1–36.6) | 16.9 (11.2–24.5) | <0.001 |
| Averaged percentage of time spent in MVPA (%) | 3.3 (2.3–4.6) | 2.1 (1.4–3.1) | <0.001 |
CPM, counts per minute; MVPA, moderate-to-vigorous physical activity; P25–P75, 25th and 75th percentiles.
Values are medians (25th–75th percentiles) or absolute numbers (percentages).
P-value from the chi-squared test or the Mann–Whitney test comparing distributions across sexes.
Values in bold have P-values <0.05.
Number of children may vary because of missing information.
Figure 2.
Sex-specific physical-activity trajectories based on overall physical activity (counts/minute) and time spent in moderate-to-vigorous physical activity (MVPA) from 11 to 15 years. Mean values with 95% confidence intervals are presented for each trajectory. The results for boys are in dotted lines and the results for girls are in solid lines. Count/min, counts per minute (overall physical activity); MVPA, moderate-to-vigorous physical activity.
Three trajectories were also identified in girls but the physical-activity levels were lower in all trajectories compared with those found in boys. The low, moderate and high physical-activity trajectories grouped 45.8, 41.4 and 12.8% of girls, and the medians of overall physical activity and time spent in MVPA at 11 years were 449 CPM, 10.1 minutes/day, 595 CPM, 21.2 minutes/day and 664 CPM, 27.9 minutes/day, respectively (Figure 2, Supplementary Figure 1 and Table 3, available as Supplementary data at IJE online).
Associations of physical-activity trajectories from 11 to 15 years with lung function at 15 years
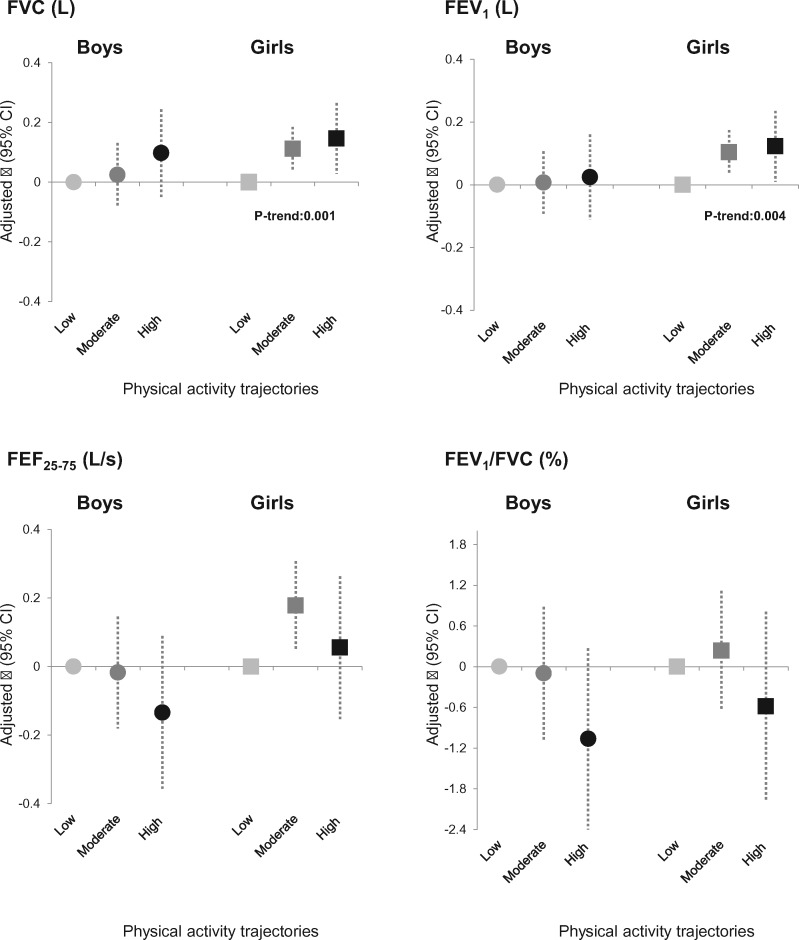
After adjustment, girls in the moderate and high physical-activity trajectories had 0.11 L (95% CI: 0.04–0.19) and 0.15 L (95% CI: 0.03–0.26) higher post-bronchodilation FVC than girls in the low physical-activity trajectory (P-trend = 0.001) (Figure 3 and Supplementary Table 4, available as Supplementary data at IJE online). Similarly, girls in the moderate and high physical-activity trajectories had 0.10 L (95% CI: 0.03–0.17) and 0.12 L (95% CI: 0.01–0.24) higher post-bronchodilation FEV1 than girls in the low physical-activity trajectory (P-trend = 0.004). For FEF25–75, a positive association was only observed for girls in the moderate (compared with low) physical-activity trajectory. No association was observed with the FEV1/FVC ratio. No interaction between lung function at 8 years and the physical-activity trajectories was identified.
Figure 3.
Sex-specific associations of the physical-activity trajectories with post-bronchodilation lung-function measures at 15 years. 95% CI, confidence interval at 95%; FEF25–75, forced expiratory flow at 25 and 75% of FVC; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity. Adjustment for lung function at 8 years, parental social class, pre-term delivery, weight status at 11 years, smoking status, pubertal status (menarche age for girls and voice-breaking for boys), as well as age and height at 15 years.
No association was observed in boys between any physical-activity trajectory and any lung-function parameter. This was true even when a higher number of trajectories was considered (Supplementary Table 5, available as Supplementary data at IJE online).
Standardized outcomes (Z-scores or percent predicted values) produced the same results (Supplementary Table 6, available as Supplementary data at IJE online). Results from other sensitivity analyses also confirmed the associations found (Supplementary Tables 7–11, available as Supplementary data at IJE online).
Discussion
This study showed a positive association of physical-activity trajectories, defined using repeated accelerometry measurements from 11 to 15 years, with lung function measured at 15 years in girls from a large well-defined birth cohort. No associations were found among boys.
Our finding that physical activity may influence lung-function development in girls is consistent with previous longitudinal studies.10,14 Ji et al. showed that Chinese girls (9–11 years old, followed for 18 months) who reported being physically active at either or both follow-up surveys had higher growth rates of lung function than inactive girls.10 Menezes et al. reported that adolescent girls were more likely to have better lung function at 15 years if they reported being active at both ages 11 and 15 years.14 Whereas the comparison of our estimates with previous studies is challenging due to the use of different methodologies (e.g. study design, assessment of physical activity, definition of being active), the magnitude of the association we found between physical activity and lung function (about 100–150 mL higher in medium and high physical-activity trajectories than in the low one) is in line with previous estimates. First, Smith et al. suggested 3% higher FVC (∼100 mL) in children grouped in last MVPA quintile measured with accelerometry than peers in the lowest quintile (from pairwise comparison), although the association became non-significant after Bonferroni correction.6 Second, Holmen et al. documented a mean difference of 119 and 122 mL in FEV1 and FVC between girls in the highest and lowest level of self-reported exercise.32 Finally, Berntsen et al. reported an increase of 90 and 114 mL in FEV1 and FVC among girls who were physically active at least four times a week as compared with those who reported being physically active less than once a week.7 In our study, we overcome some limitations of previous studies by objectively measuring physical activity, by reducing potential reverse causation (by adjusting for baseline lung function) and by taking into account relevant confounders (e.g. body weight/composition, pubertal status). The lack of association with the FEV1/FVC ratio should be interpreted with substantial caution, as it may be driven by the different magnitude of the effect of physical activity on FVC compared with FEV1.
There is biological plausibility underlying our associations. First, regular physical activity may be producing an anti-inflammatory effect.33 An inverse relationship between regular physical activity and C-reactive protein (CRP, a systemic inflammatory marker) levels has been reported34 and CRP levels were associated with reduced lung function.35 Second, physical activity may lead to higher lung function through its beneficial effects on body-composition changes and fat distribution, which can affect lung mechanics36 and be linked to low-grade systemic inflammation.33 Our findings were robust to adjustments for BMI as well as lean and fat mass indices, indicating that other pathways may be involved. Third, physical activity can improve pulmonary function by increasing muscle strength. Regular and forceful inspiration and expiration during exercise strengthen the respiratory muscles,37 which could improve lung function. Our results are in line with this hypothesis, as associations were slightly attenuated after adjustment for lean body mass. Future studies should investigate the potential underlying mechanisms in the relation of physical activity with lung function.
We did not observe an association of the physical-activity trajectories with lung function in boys, which is similar to previous studies.10,14 Possible explanations for this observed sex difference could be related to biological or behavioural factors, as well as to a combination of both. Among them, the growth spurt occurs earlier in girls than in boys, so any effect of physical activity on lung function may be more easily observed at an earlier age in girls. Results from the Brazilian cohort (Pelotas) are in line with this hypothesis: only active girls at ages 11 and 15 years had larger lung-function measures at 15 years.14 However, when the authors examined lung-function gains from 15 to 18 years, they found a positive association with physical activity in boys.13 Holmen et al.32 also reported an association in boys but they were older than in our study. Another explanation for the observed sex differences may be the differences in the type of physical activity practised by boys and girls. Some sports improve lung function better than others (e.g. swimming, rowing, basketball).38 Unfortunately, detailed information on the type of physical activity the participants were doing was not available in our study. Further studies with longer follow-up periods (specifically in boys), a greater range of physical-activity levels and detailed information on the types of activities conducted are needed to replicate the associations in both sexes and clarify whether sex differences truly exist.
Although our study requires replication, our observed effect together with the high prevalence of physical inactivity (fewer than 7% of the ALSPAC children achieved the WHO recommendation) may result in a considerable impact on lung function at the population level.39 Furthermore, given the tracking of physical activity over time,2 the promotion of physical activity in childhood could help better respiratory health in adult life.40,41
This study had several strengths. It was carried out in a large population, which limits random variation in the findings. We used data from a prospective cohort, which allowed the assessment of temporality between physical activity and lung function. Moreover, we minimized potential reverse causation by including the levels of lung function at 8 years (measured prior to the first physical-activity assessment) as a covariate. Furthermore, ALSPAC is a well-defined cohort with detailed information on a wide range of potential confounders (e.g. pubertal status, body weight or body composition), which limits potential residual confounding. Another strength is the use of standardized repeated objective measures of physical activity, which limits exposure misclassification. Finally, by considering physical-activity trajectories instead of fixed baseline levels, we were able to take into account potential changes in physical activity over time, although, in our study sample, few children changed their activity levels over time. Indeed, we observed parallel trajectories reflecting a similar changes of physical activity over time between groups and a small change in physical activity within groups. Among the several available approaches to building trajectories, we used K-means for longitudinal data28 because we aimed to capture complementary accelerometer information including simultaneously both CPM (a marker of amount) and MVPA (a marker of intensity). Besides the co-evolution of variable trajectories, this non-parametric approach does not require any normality or parametric assumptions, which is of interest for physical-activity measures that have highly positively skewed distributions and does not require any assumption regarding the shape of the trajectory. More repeated physical-activity measures over a longer time window and/or other age periods would allow the identification of potential changes in physical activity over time.
The present study also had several limitations. Study participants were drawn from a regional sample of babies born in Avon (UK) and thus the generalization of the study results to other geographic areas, particularly those with more ethnic and racial variability, should be performed with caution. The average low physical-activity levels in this population may have precluded the assessment of the effects of ‘real’ high physical-activity levels. The observed effects might thus underestimate the true potential benefits of physical activity. Additionally, our results may be subject to selection bias. About one-third of the children who had physical-activity trajectories had no lung-function data and were therefore not included in the final analysis. The only difference between those included and excluded was that those excluded came from lower socio-economic backgrounds (there were no differences in the distribution of the physical-activity trajectories). Since low socio-economic status was associated with reduced lung function at 15 years, it is thus likely that any bias from sample attrition has led to an under-estimation of our associations. Finally, we cannot rule out residual confounding, e.g. by built environment factors or air pollution. Further studies are needed to examine how these benefits of physical activity in childhood are moderated by the environment.
In summary, higher physical activity during childhood relates to higher lung-function levels at adolescence in girls. A better understanding of the mechanisms underlying this association should be pursued.
Supplementary Material
Acknowledgments
Funding
The present analyses are part of the Ageing Lungs in European Cohorts (ALEC) Study (www.alecstudy.org), which has received funding from the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 633212). The UK Medical Research Council, the Wellcome Trust (grant: 102215/2/13/2) and the University of Bristol provide core support for the Avon Longitudinal Study of Parents and Children (ALSPAC). Elaine Fuertes is supported by a Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2015; proposal number 704268). Célina Roda is the recipient of a European Respiratory Society Fellowship (RESPIRE3-201703–00127, under H2020—Marie Skłodowska-Curie actions COFUND). These funders did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgements
We are grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We thank Amanda Hill for acting as our ALSPAC data buddy. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.
Conflict of interest: Judith Garcia-Aymerich’s institution has received consulting and lecture fees from AstraZeneca (not related to this study). Judith Garcia-Aymerich has received lecture fees from Esteve and Chiesi (not related to this study). The other authors declare no conflict of interest.
References
- 1. Sin DD, Wu L, Man S.. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005;127:1952–59. [DOI] [PubMed] [Google Scholar]
- 2. Telama R. Tracking of physical activity from childhood to adulthood: a review. Obes Facts 2009;2:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (World Health Organization). Global Recommendations on Physical Activity for Health. Geneva: WHO, 2010; 1–60. [PubMed] [Google Scholar]
- 4.IOM (Institute of Medicine). Status and Trends of Physical Activity Behaviors and Related School Policies. In: Educating the Student Body: Taking Physical Activity and Physical Education to School. Washington, DC: The National Academies Press, 2013; pp. 35–95. [PubMed] [Google Scholar]
- 5. Van Hecke L, Loyen A, Verloigne M. et al. Variation in population levels of physical activity in European children and adolescents according to cross-European studies: a systematic literature review within DEDIPAC. Int J Behav Nutr Phys Act 2016;13:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith MP, Berg A, von Berdel D. et al. Physical activity is not associated with spirometric indices in lung-healthy German youth. Eur Respir J 2016;48:428–40. [DOI] [PubMed] [Google Scholar]
- 7. Berntsen S, Wisløff T, Nafstad P, Nystad W.. Lung function increases with increasing level of physical activity in school children. Pediatr Exerc Sci 2008;20:402–10. [DOI] [PubMed] [Google Scholar]
- 8. Jones PR, Baber FM, Heywood C, Cotes JE.. Ventilatory capacity if healthy Chinese children: relation to habitual activity. Ann Hum Biol 1977;4:155–61. [DOI] [PubMed] [Google Scholar]
- 9. Eisenmann JC, Katzmarzyk P, Theriault G, Song T, Malina RM, Bouchard C.. Physical activity and pulmonary function in youth: the Quebec family study. Pediatr Exerc Sci 1999;11:208–17. [Google Scholar]
- 10. Ji J, Wang S, Liu Y, He Q.. Physical activity and lung function growth in a cohort of Chinese school children: a prospective study. PloS One 2013;8:e66098.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trabelsi Y, Pariès J, Harrabi I. et al. Factors affecting the development of lung function in Tunisian children. Am J Hum Biol 2008;20:716–25. [DOI] [PubMed] [Google Scholar]
- 12. Twisk JW, Staal BJ, Brinkman MN, Kemper HC, van Mechelen W.. Tracking of lung function parameters and the longitudinal relationship with lifestyle. Eur Respir J 1998;12:627–34. [DOI] [PubMed] [Google Scholar]
- 13. da Silva BGC, Wehrmeister FC, Quanjer PH. et al. Physical activity in early adolescence and pulmonary function gain from 15 to 18 years of age in a birth cohort in Brazil. J Phys Act Health 2016;13:1164–73. [DOI] [PubMed] [Google Scholar]
- 14. Menezes AMB, Wehrmeister FC, Muniz LC. et al. Physical activity and lung function in adolescents: the 1993 Pelotas (Brazil) birth cohort study. J Adolesc Health 2012;51:S27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung KP, Lau SP, Chow OK, Lee J, Wong TW.. Effects of overweight on lung function. Arch Dis Child 1990;65:512–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hopper JL, Hibbert ME, Macaskill GT, Phelan PD, Landau LI.. Longitudinal analysis of lung function growth in healthy children and adolescents. J Appl Physiol 1991;70:770–77. [DOI] [PubMed] [Google Scholar]
- 17. Chung AE, Skinner AC, Steiner MJ, Perrin EM.. Physical activity and BMI in a nationally representative sample of children and adolescents. Clin Pediatr 2012;51:122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker BL, Birch LL, Trost SG, Davison KK.. Advanced pubertal status at age 11 and lower physical activity in adolescent girls. J Pediatr 2007;151:488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyd A, Golding J, Macleod J. et al. Cohort Profile: the ’children of the 90s’: the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mattocks C, Leary S, Ness A. et al. Calibration of an accelerometer during free-living activities in children. Int J Pediatr Obes 2007;2:218–26. [DOI] [PubMed] [Google Scholar]
- 22. Mattocks C, Ness A, Leary S. et al. Use of accelerometers in a large field-based study of children: protocols, design issues, and effects on precision. J Phys Act Health 2008;5(Suppl 1):S98–111. [DOI] [PubMed] [Google Scholar]
- 23.ATS. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- 24. Booth JN, Tomporowski PD, Boyle JME. et al. Obesity impairs academic attainment in adolescence: findings from ALSPAC, a UK cohort. Int J Obes 2014;38:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole TJ, Freeman JV, Preece MA.. Body mass index reference curves for the UK, 1990. Arch Dis Child 1995;73:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reilly JJ, Dorosty AR, Emmett PM; Avon Longitudinal Study of Pregnancy and Childhood Study Team. Identification of the obese child: adequacy of the body mass index for clinical practice and epidemiology. Int J Obes 2000;24:1623–27. [DOI] [PubMed] [Google Scholar]
- 27. Adair LS. Size at birth predicts age at menarche. Pediatrics 2001;107:E59. [DOI] [PubMed] [Google Scholar]
- 28. Genolini C, Alacoque X, Sentenac M, Arnaud C.. kml and kml3d: R packages to cluster longitudinal data. J Stat Softw 2015;65:1–34. [Google Scholar]
- 29. Davies DL, Bouldin DW.. A cluster separation measure. IEEE Trans Pattern Anal Mach Intell 1979;1:224–27. [PubMed] [Google Scholar]
- 30. Hosmer DW, Lemeshow S.. Model-building strategies and methods for logistic regression In: Shewhart WA, Wilks SS (eds). Applied Logistic Regression. Hoboken, NJ: John Wiley & Sons, Inc, 2003, pp. 89–151. [Google Scholar]
- 31. Quanjer PH, Stanojevic S, Cole TJ. et al. Multi-ethnic reference values for spirometry for the 3-95 year age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holmen T, Barrett-Connor E, Clausen J, Holmen J, Bjermer L.. Physical exercise, sports, and lung function in smoking versus nonsmoking adolescents. Eur Respir J 2002;19:8–15. [DOI] [PubMed] [Google Scholar]
- 33. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA.. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607–15. [DOI] [PubMed] [Google Scholar]
- 34. Isasi CR, Deckelbaum RJ, Tracy RP, Starc TJ, Berglund L, Shea S.. Physical fitness and C-reactive protein level in children and young adults: the Columbia University BioMarkers Study. Pediatrics 2003;111:332–38. [DOI] [PubMed] [Google Scholar]
- 35. Hancox RJ, Poulton R, Greene JM. et al. Systemic inflammation and lung function in young adults. Thorax 2007;62:1064–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salome CM, King GG, Berend N.. Physiology of obesity and effects on lung function. J Appl Physiol 2010;108:206–11. [DOI] [PubMed] [Google Scholar]
- 37. Puente-Maestu L, Stringer WW.. Physical activity to improve health: do not forget that the lungs benefit too. Eur Respir J 2018;51:1702468.. [DOI] [PubMed] [Google Scholar]
- 38. Lazovic B, Mazic S, Suzic-Lazic J. et al. Respiratory adaptations in different types of sport. Eur Rev Med Pharmacol Sci 2015;19:2269–74. [PubMed] [Google Scholar]
- 39. Rose G. Sick individuals and sick populations. Int J Epidemiol 1985;14:32–38. [DOI] [PubMed] [Google Scholar]
- 40. Hancox RJ, Rasmussen F.. Does physical fitness enhance lung function in children and young adults? Eur Respir J 2018;51:1701374. [DOI] [PubMed] [Google Scholar]
- 41. Agustí A, Noell G, Brugada J, Faner R.. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017;5:935–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.