Abstract
Background
The advent of very large cohort studies (n > 500 000) has given rise to prospective analyses of health outcomes being undertaken after short (<4 years) follow-up periods. However, these studies are potentially at risk of reverse causality bias. We investigated differences in the associations between self-reported physical activity and all-cause and cardiovascular disease (CVD) mortality, and incident CVD, using different follow-up time cut-offs and methods to account for reverse causality bias.
Methods
Data were from n = 452 933 UK Biobank participants, aged 38–73 years at baseline. Median available follow-up time was 7 years (for all-cause and CVD mortality) and 6.1 years (for incident CVD). We additionally analysed associations at 1-, 2- and 4-year cut-offs after baseline. We fit up to four models: (1) adjusting for prevalent CVD and cancer, (2) excluding prevalent disease, (3) and (4) Model 2 excluding incident cases in the first 12 and 24 months, respectively.
Results
The strength of associations decreased as follow-up time cut-off increased. For all-cause mortality, Model 1 hazard ratios were 0.73 (0.69–0.78) after 1 year and 0.86 (0.84–0.87) after 7 years. Associations were weaker with increasing control for possible reverse causality. After 7-years follow-up, the hazard ratios were 0.86 (0.84–0.87) and 0.88 (0.86–0.90) for Models 1 and 4, respectively. Associations with CVD outcomes followed similar trends.
Conclusions
As analyses with longer follow-up times and increased control for reverse causality showed weaker associations, there are implications for the decision about when to analyse a cohort study with ongoing data collection, the interpretation of study results and their contribution to meta-analyses.
Keywords: Exercise, physical activity, epidemiologic methods, prospective studies, follow-up studies, bias
Key Messages
Analyses with shorter follow-up times showed stronger associations between self-reported physical activity and all-cause mortality, cardiovascular disease mortality and incident cardiovascular disease.
Different methods to account for reverse causality bias also influenced the strength of the association: least controlled analyses showed stronger associations.
The magnitude of the differences between approaches varied by outcome.
This has implications for the decision about when to analyse a cohort study with ongoing data collection, as well as the interpretation of study results and their contribution to meta-analyses.
Introduction
Many prospective cohort studies have demonstrated prospective associations between lifestyle behaviours and the risk of mortality or morbidity.1–3 Typically, follow-up periods have been at least 5 years, and often >10 years, because it was typically necessary to wait for a sufficient number of events to occur before analyses could be undertaken.4 With the advent of very large cohort studies such as the Million Women Study (n > 1 000 000) and the UK and China Kadoorie Biobanks (both n > 500 000), the number of events rarely limits the possibility of early prospective analyses.5–8 However, there is little research to indicate if the length of follow-up time after which analyses are undertaken, combined with the analytical choices made to address issues of reverse causality, impacts on the estimated association between behavioural exposures and health outcomes.
In this study, we focus on physical activity behaviour. Previous work in this field has focussed on the potential biases introduced by using very long follow-up periods (>30 years). Longer durations have generally but not consistently attenuated the protective inverse association between physical activity and all-cause mortality,9–12 coronary heart disease incidence and mortality.12–15 Andersen9 attributed this to violation of the constant exposure assumption, i.e. real changes in physical activity behaviour during that time.
For substantially shorter follow-up periods, a major issue of concern is reverse causality, i.e. the potential that underlying (diagnosed or undiagnosed) illness impacts negatively on physical activity, so the observed association between physical activity and health outcomes is more driven by the causal link between the underlying disease state and subsequent outcome events, rather than the physical activity exposure per se.16 This will typically lead to an overestimation of the true association between physical activity and health outcomes. Common methods to account for this bias include removing individuals who have prevalent disease, or those who experience the event soon after baseline, who are presumed to represent those with undiagnosed illness at baseline. However, the latter is not always feasible when the study follow-up period is short or the number of events is low.7,8
The aim of this study was to investigate whether the strength of the associations between physical activity and all-cause mortality and cardiovascular disease (CVD) outcomes varies over different follow-up times in the range 1–7 years. A secondary aim was to examine how different approaches to account for reverse causality impact on the strength of the association.
Methods
Data source
We used data from UK Biobank, a study involving over 500 000 individuals aged 37–73 years at baseline. Participants undertook an assessment at one of 22 centres across Great Britain between 2006 and 2010. This included a touchscreen self-administered questionnaire and anthropometric measurements.17,18 Consent to link these data to the national death registries and to hospital episode inpatient data was also obtained. Questionnaire and mortality data were downloaded on 31 August 2018, containing information from 502 543 participants after withdrawals. The hospital episode data were downloaded on 9 April 2018.
Physical activity exposure
The questionnaire covered the frequency and duration of individuals’ participation in four types of discretionary physical activity (home and leisure domains) which are considered to be in the moderate-to-vigorous physical activity intensity range:19 (i) walking for pleasure, not as a means of transport, (ii) other exercises, e.g. swimming, cycling, keep-fit, bowling, (iii) strenuous sports, and (iv) heavy do-it-yourself (DIY) home maintenance, e.g. weeding, lawn mowing, carpentry, digging. The response options for frequency were: none, once in the last 4 weeks, 2–3 times in last 4 weeks, once per week, 2–3 times per week, 4–5 times per week, or every day. The response options for duration were: <15 min, 15–30 min, 30–60 min, 1–1½ h, 1½–2 h, 2–3 h or >3 h. Responses were scaled to a total weekly duration using the median values (maximum duration of 3½ h), and truncated at 2000 min (n = 544 affected). We excluded those missing both frequency and duration for an activity type (n = 19 643) and those who completed the different pilot questionnaire (n = 3798). We imputed the frequency or duration from the median of all others reporting participation in that activity type (n = 5994) if only one was missing.
Outcomes
The three outcomes of interest were all-cause mortality, CVD mortality and CVD incidence (fatal and non-fatal). CVD was defined as a primary or secondary diagnosis with International Classification of Disease 10th revision codes I20–25 for ischaemic heart disease and I60–69 for cerebrovascular disease. The censor dates differed for the mortality and hospital episode data for different home nations. Participants attending an assessment centre in England and Wales were followed-up for vital status until 31 January 2016 and those attending one in Scotland until 30 November 2015. For total incident CVD (based on mortality records and hospital episode data), censor dates were 31 March 2015 in England, 31 October 2015 in Scotland and 29 February 2016 in Wales.
Eleven individuals were excluded due to inconsistencies in their mortality data (death date prior to interview, diagnosis but no death date, death date but no diagnosis).
Covariates
Potential confounders were chosen a priori, based on previous literature. Demographic characteristics included age, sex, ethnicity (white/non-white), highest educational level achieved (degree or above/any other qualification/no qualification) and Townsend indicator of deprivation (a continuous index derived from the respondent’s post code, with higher scores indicating higher deprivation). Lifestyle behaviours included smoking status (never/previous/current); alcohol consumption (never/previous drinker/current drinker); addition of salt to food (never or rarely/sometimes/usually or always); consumption of oily fish (never/less than once per week/at least once per week); fruit and vegetable intake (a score of 0–4 was computed from questions asking the frequency of raw and cooked vegetable, fresh and dried fruit intake; respondents were given 1 point if they reported more than 2 portions of each type); consumption of processed or red meat (average days per week derived from questions on the frequency of processed, beef, lamb and pork intake); leisure screen time (combined total duration of reported TV and computer time during leisure time, categorized to <4 h per day/≥4 h per day in line with current estimates of where the risk of all-cause mortality and CVD mortality increases non-linearly3); and usual sleep per 24-h period (<7 h/7–8 h/>8 h). We also derived a five-category variable that combined the responses from questions on employment status (unemployed/in paid or self-employment), walk or cycle to work (yes/no) and heavy manual/physical job (yes/no) because the latter two were only applicable to those in work.
Variables indicating self-reported health status included: current prescription of blood pressure medication (yes/no); current prescription of cholesterol medication (yes/no); diabetes (insulin prescription or self-reported diagnosis/neither); paternal or maternal history of heart attack, angina or stroke (yes to either parent/neither); and paternal or maternal history of cancer (yes to either parent/neither). Body mass index (BMI) was derived from height and weight measured at baseline and categorized into under/normal weight (<25 kg/m2), overweight (≥25–<30 kg/m2) and obese (≥30 kg/m2). Individuals with prevalent CVD were identified from their self-reported previous diagnosis of a heart attack, angina or stroke, or a hospital episode with a previous relevant diagnosis. Individuals with prevalent cancer were also identified via self-report or hospital episode data (International Classification of Disease 10th revision codes C00-C99). Missing data in these covariates led to the exclusion of n = 26 104 individuals.
Statistical analyses
Cox regression with age as the underlying timescale was used to estimate the association between physical activity at baseline and all-cause and CVD mortality, and CVD incidence. We used cubic spline regression to examine the nature of the dose–response relationships and identify a transformation of the physical activity variable which would enable us to include this in the model as a continuous variable (i.e. assuming a log–linear association with the hazard), and hence simplify the presentation of the results from different models (see Supplementary Figure 1, available as Supplementary data at IJE online). Based on the findings, the physical activity variable (in min/week) was transformed by adding 1 and taking the natural log. We subsequently standardized this variable by subtracting the sample mean and dividing by its standard deviation.
The median available follow-up time was 6.1 years (for incident CVD) and 7 years (for all-cause and CVD mortality). We also used cut-offs at 1, 2 and 4 years of follow-up after baseline. Where possible within each outcome/follow-up time combination, four models were fitted using different methods to account for reverse causality bias: Model 1 = adjustment for prevalent CVD and cancer; Model 2 = exclusion of individuals with prevalent CVD and cancer; Model 3 = Model 2 plus excluding incident cases that occurred within the first year; Model 4 = Model 2 plus excluding incident cases that occurred within the first 2 years. All models were adjusted for potential confounders.
Proportional hazard assumptions for the exposure and all covariates were assessed using log–log plots for each outcome based on Model 4 with the maximum follow-up time. Assessment centre, ethnicity, alcohol consumption and the combined variable for employment status/active commuting/manual work were accounted for by stratification of the baseline hazard function, rather than as covariates in the linear predictor, because they did not always meet the proportional hazard assumptions.
We quantified the potential degree of regression dilution bias using data from two sub-samples that undertook repeat exposure assessment at one of two follow-up visits; these were conducted a median of 4.4 years (n = 18 213) and 7.6 years (n = 21 205) after baseline. We also created a further sub-sample of those who undertook the first repeat visit <3 years after baseline (n = 2122; minimum follow-up 2 years). We standardized the natural log of the minutes of reported physical activity +1 at these visits to the baseline scale (subtracting the baseline mean and dividing by the baseline standard deviation). The coefficient from a linear regression of the follow-up visit variable on the baseline variable (also transformed and standardized as above) was estimated to indicate the degree of stability in the measured exposure variable over time.
We also performed the models without adjustment for BMI, diabetes diagnosis or insulin prescription, blood pressure medication or cholesterol medication as sensitivity analyses, as these covariates could plausibly be on the causal pathway.
Results
Sample sizes ranged between 384 615 and 452 993 across the different analyses. Table 1 summarizes the baseline characteristics of the cases and non-cases in the analysis samples used to fit Model 1 for each outcome, with the maximum available follow-up time. Cases for all outcomes were less active, older, of higher education level and more overweight than non-cases. They were also more likely to be unemployed, a current smoker, a previous drinker, report higher leisure screen time, take medication and have a history of disease.
Table 1.
Baseline participant characteristics from the UK Biobank by health status after a median of 7 years of follow-upa
| All-cause mortality |
CVD mortality |
CVD incidence |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 12 277) | Non-cases (n = 440 716) | Cases (n = 2643) | Non-cases (n = 450 350) | Cases (n = 30 146) | Non-cases (n = 422 847) | |||||||
| Minutes of discretionary PA, median (IQR) | 101.3 | (14.1–262.5) | 134.5 | (45.0–311.3) | 76.9 | (1.9–243.8) | 133.1 | (45.0–309.4) | 112.5 | (18.8–287.8) | 135.0 | (45.0–311.3) |
| Age at baseline interview, n (%) | ||||||||||||
| <55 years | 1965 | (16.0) | 174 751 | (39.7) | 320 | (12.1) | 176 396 | (39.2) | 4544 | (15.1) | 172 172 | (40.7) |
| 55–<60 years | 1870 | (15.2) | 80 756 | (18.3) | 361 | (13.7) | 82 265 | (18.3) | 4696 | (15.6) | 77 930 | (18.4) |
| 60–<65 years | 3691 | (30.1) | 105 406 | (23.9) | 806 | (30.5) | 108 291 | (24.0) | 9366 | (31.1) | 99 731 | (23.6) |
| ≥65 years | 4751 | (38.7) | 79 803 | (18.1) | 1156 | (43.7) | 83 398 | (18.5) | 11 540 | (38.3) | 73 014 | (17.3) |
| Sex, n (%) | ||||||||||||
| Women | 4803 | (39.1) | 240 473 | (54.6) | 636 | (24.1) | 244 640 | (54.3) | 9935 | (33.0) | 235 341 | (55.7) |
| Men | 7474 | (60.9) | 200 243 | (45.4) | 2007 | (75.9) | 205 710 | (45.7) | 20 211 | (67.0) | 187 506 | (44.3) |
| Ethnicity, n (%) | ||||||||||||
| White | 11 939 | (97.2) | 418 938 | (95.1) | 2544 | (96.3) | 428 333 | (95.1) | 28 709 | (95.2) | 402 168 | (95.1) |
| Non-white | 338 | (2.8) | 21 778 | (4.9) | 99 | (3.7) | 22 017 | (4.9) | 1437 | (4.8) | 20 679 | (4.9) |
| Highest educational level achieved, n (%) | ||||||||||||
| Degree or above | 3668 | (29.9) | 70 646 | (16.0) | 958 | (36.2) | 73356 | (16.3) | 9268 | (30.7) | 65046 | (15.4) |
| Any other qualification | 5600 | (45.6) | 221 281 | (50.2) | 1125 | (42.6) | 225 756 | (50.1) | 14 186 | (47.1) | 212 695 | (50.3) |
| No qualification | 3009 | (24.5) | 148 789 | (33.8) | 560 | (21.2) | 151 238 | (33.6) | 6692 | (22.2) | 145 106 | (34.3) |
| Townsend indicator of multiple deprivation, median (IQR) | −1.7 | (−3.4–1.5) | −2.2 | (−3.7–0.4) | −1.0 | (−3.0–2.2) | −2.2 | (−3.7–0.4) | −1.8 | (−3.4–1.4) | −2.2 | (−3.7–0.4) |
| Employment and commuting status, n (%) | ||||||||||||
| Unemployed | 8222 | (67.0) | 179 900 | (40.8) | 1959 | (74.1) | 186 163 | (41.3) | 19 542 | (64.8) | 168 580 | (39.9) |
| Employed, non-manual work, non-active commute | 2858 | (23.3) | 179 549 | (40.7) | 496 | (18.8) | 181 911 | (40.4) | 7437 | (24.7) | 174 970 | (41.4) |
| Employed, manual work, non-active commute | 523 | (4.3) | 27 709 | (6.3) | 106 | (4.0) | 28 126 | (6.2) | 1528 | (5.1) | 26 704 | (6.3) |
| Employed, non-manual work, active commute | 566 | (4.6) | 47 205 | (10.7) | 63 | (2.4) | 47 708 | (10.6) | 1352 | (4.5) | 46 419 | (11.0) |
| Employed, manual work, active commute | 108 | (0.9) | 6353 | (1.4) | 19 | (0.7) | 6442 | (1.4) | 287 | (1.0) | 6174 | (1.5) |
| Smoking, n (%) | ||||||||||||
| Never | 4633 | (37.7) | 244 195 | (55.4) | 818 | (30.9) | 248 010 | (55.1) | 12 073 | (40.0) | 236 755 | (56.0) |
| Previous | 5218 | (42.5) | 152 214 | (34.5) | 1218 | (46.1) | 156 214 | (34.7) | 13 658 | (45.3) | 143 774 | (34.0) |
| Current | 2426 | (19.8) | 44 307 | (10.1) | 607 | (23.0) | 46 126 | (10.2) | 4415 | (14.6) | 42 318 | (10.0) |
| Alcohol consumption, n (%) | ||||||||||||
| Never | 519 | (4.2) | 18 122 | (4.1) | 121 | (4.6) | 18 520 | (4.1) | 1628 | (5.4) | 17 013 | (4.0) |
| Previous drinker | 846 | (6.9) | 14 814 | (3.4) | 227 | (8.6) | 15 433 | (3.4) | 1833 | (6.1) | 13 827 | (3.3) |
| Current drinker | 10 912 | (88.9) | 407 780 | (92.5) | 2295 | (86.8) | 416 397 | (92.5) | 26 685 | (88.5) | 392 007 | (92.7) |
| Addition of salt to food, n (%) | ||||||||||||
| Never or rarely | 6238 | (50.8) | 246 184 | (55.9) | 1325 | (50.1) | 251 097 | (55.8) | 15 718 | (52.1) | 236 704 | (56.0) |
| Sometimes | 5180 | (42.2) | 174 182 | (39.5) | 1112 | (42.1) | 178 250 | (39.6) | 12 463 | (41.3) | 166 899 | (39.5) |
| Usually or always | 859 | (7.0) | 20 350 | (4.6) | 206 | (7.8) | 21 003 | (4.7) | 1965 | (6.5) | 19 244 | (4.6) |
| Consumption of oily fish, n (%) | ||||||||||||
| Never | 1435 | (11.7) | 47 516 | (10.8) | 326 | (12.3) | 48 625 | (10.8) | 3505 | (11.6) | 45 446 | (10.7) |
| Less than once per week | 3818 | (31.1) | 147 304 | (33.4) | 806 | (30.5) | 150 316 | (33.4) | 8999 | (29.9) | 142 123 | (33.6) |
| At least once per week | 7024 | (57.2) | 245 896 | (55.8) | 1511 | (57.2) | 251 409 | (55.8) | 17 642 | (58.5) | 235 278 | (55.6) |
| Fruit and vegetable intake score, median (IQR) | 1.0 | (1.0–2.0) | 2.0 | (1.0–2.0) | 1.0 | (1.0–2.0) | 2.0 | (1.0–2.0) | 1.0 | (1.0–2.0) | 2.0 | (1.0–2.0) |
| Weekly frequency of red or processed meat intake, median (IQR) | 0.9 | (0.6–1.4) | 0.8 | (0.5–1.3) | 1.0 | (0.6–1.4) | 0.8 | (0.5–1.3) | 0.9 | (0.6–1.3) | 0.8 | (0.5–1.3) |
| Leisure screen time, n (%) | ||||||||||||
| <4 h | 4944 | (40.3) | 227 610 | (51.6) | 949 | (35.9) | 231 605 | (51.4) | 11 496 | (38.1) | 221 058 | (52.3) |
| ≥4 h | 7333 | (59.7) | 213 106 | (48.4) | 1694 | (64.1) | 218 745 | (48.6) | 18 650 | (61.9) | 201 789 | (47.7) |
| Typical sleep per 24-h period, n (%) | ||||||||||||
| <7 h | 3225 | (26.3) | 107 233 | (24.3) | 729 | (27.6) | 109 729 | (24.4) | 8460 | (28.1) | 101 998 | (24.1) |
| 7–8 h | 7449 | (60.7) | 300 810 | (68.3) | 1502 | (56.8) | 306 757 | (68.1) | 18 107 | (60.1) | 290 152 | (68.6) |
| >8 h | 1603 | (13.1) | 32 673 | (7.4) | 412 | (15.6) | 33 864 | (7.5) | 3579 | (11.9) | 30 697 | (7.3) |
| BMI, n (%) | ||||||||||||
| Normal/underweight <25 kg/m2 | 3564 | (29.0) | 147 232 | (33.4) | 608 | (23.0) | 150 188 | (33.3) | 6072 | (20.1) | 144 724 | (34.2) |
| Overweight 25–<30 kg/m2 | 5026 | (40.9) | 187 827 | (42.6) | 1078 | (40.8) | 191 775 | (42.6) | 13 189 | (43.8) | 179 664 | (42.5) |
| Obese ≥30 kg/m2 | 3687 | (30.0) | 105 657 | (24.0) | 957 | (36.2) | 108 387 | (24.1) | 10 885 | (36.1) | 98 459 | (23.3) |
| Current prescription of blood pressure medicine, n (%) | ||||||||||||
| 4422 | (36.0) | 88 579 | (20.1) | 1389 | (52.6) | 91 612 | (20.3) | 15 464 | (51.3) | 77 537 | (18.3) | |
| Current prescription of cholesterol medicine, n (%) | ||||||||||||
| 3837 | (31.3) | 73 838 | (16.8) | 1328 | (50.2) | 76 347 | (17.0) | 16 203 | (53.7) | 61 472 | (14.5) | |
| Diagnosis of diabetes or insulin prescription, n (%) | ||||||||||||
| 1563 | (12.7) | 21 428 | (4.9) | 602 | (22.8) | 22 389 | (5.0) | 4574 | (15.2) | 18 417 | (4.4) | |
| Parental history of CVD, n (%) | ||||||||||||
| 6939 | (56.5) | 240 351 | (54.5) | 1592 | (60.2) | 245 698 | (54.6) | 19 632 | (65.1) | 227 658 | (53.8) | |
| Parental history of cancer, n (%) | ||||||||||||
| 3989 | (32.5) | 136 065 | (30.9) | 791 | (29.9) | 139 263 | (30.9) | 9019 | (29.9) | 131 035 | (31.0) | |
| Previous diagnosis (self-report or hospital episode) of CVD or cancer, n (%) | ||||||||||||
| 4692 | (38.2) | 59 884 | (13.6) | 1261 | (47.7) | 63 315 | (14.1) | 15 707 | (52.1) | 48 869 | (11.6) | |
Sample includes all those with prevalent disease and does not exclude early cases. The follow-up time for cardiovascular disease incidence was median of 6.1 years.
Figure 1 and Table 2 show the hazard ratios (HRs) for a one standard deviation difference in the transformed physical activity variable, for each combination of outcome, model and follow-up time cut-off. With a few exceptions, for any given model the strength of the association decreased as follow-up time increased (Table 2). The greatest difference was seen for all-cause mortality. For example, for Model 1, the hazard ratio was 0.86 (0.84–0.87) after 7 years of follow-up compared with 0.73 (0.69–0.78) after 1 year, i.e. a 2-fold difference in magnitude (log hazard ratios −0.32 vs −0.15). The equivalent estimates for CVD mortality and incidence were approximately 30–70% higher. There were some exceptions to this in the models that excluded those with early events (Models 3 and 4) after the longer follow-up times (4 and 7 years), but the percentage differences were of smaller magnitudes (<25%).
Figure 1.
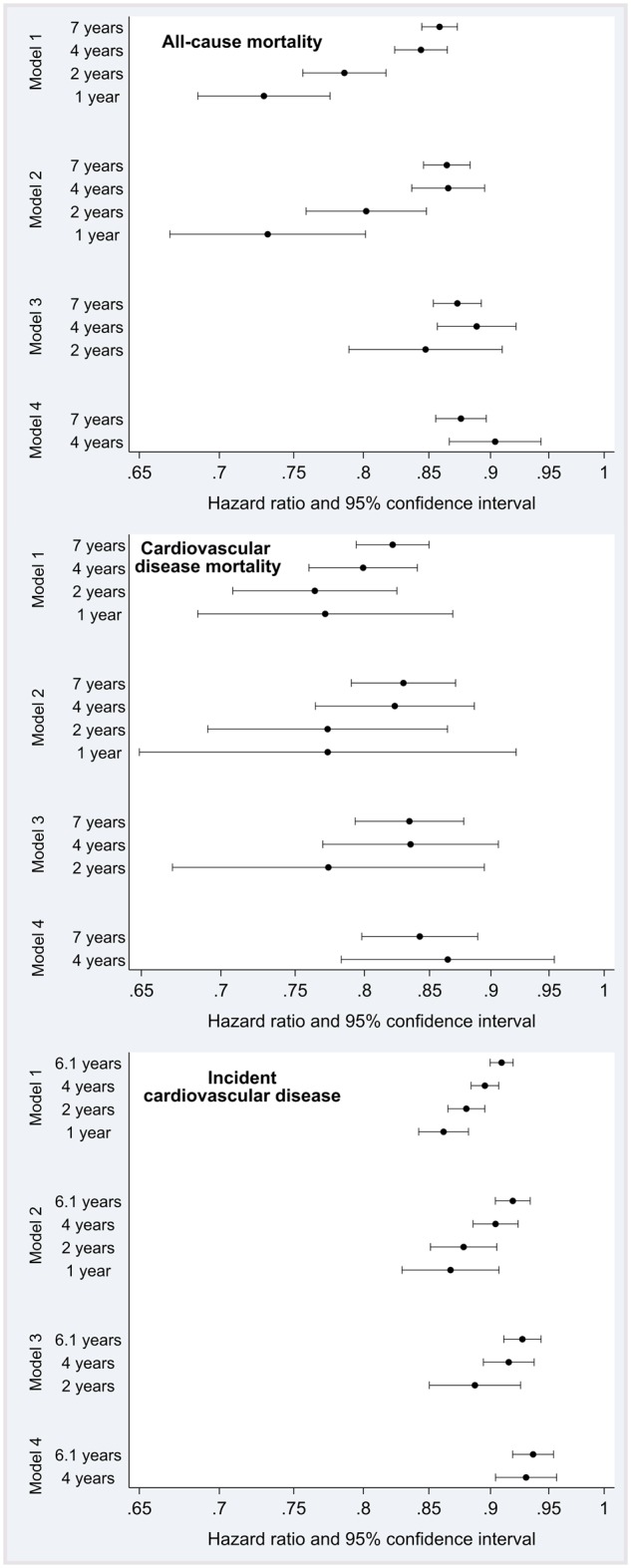
Prospective associations between physical activity and health outcomes by modelling approach and follow-up time, in the UK Biobank study (2006–2010 to 2015–2016). 1, 2 and 4 years of follow-up time are cut-off values; 6.1 and 7 years are median values. The log-hazard ratios estimate the increase in risk of the outcome for an increase of 1 standard deviation in the log(min of MVPA + 1). All analyses were adjusted for covariates listed in the text. Model 1: also adjusted for prevalent disease (CVD and cancer); Model 2: excluded those with prevalent disease; Model 3: Model 2 + excluded cases occurring in first year of follow-up; Model 4: Model 2 + excluded cases occurring in first 2 years of follow-up.
Table 2.
Prospective associations between physical activity and health outcomes by modelling approach and follow-up time, in the UK Biobank study (2006–2010 to 2015–2016)
| Model (n) | Follow-up timea | Cases | Person-years | Hazard ratio (95% CI) | % Difference in log hazard ratio from longest follow up time |
|---|---|---|---|---|---|
| All- cause mortality | |||||
| Model 1 (452 993) | 7 years | 12 277 | 3 130 875 | 0.86 (0.84–0.87) | – |
| 4 years | 5509 | 1 802 723 | 0.84 (0.82–0.86) | 11 | |
| 2 years | 2102 | 904 227 | 0.79 (0.76–0.82) | 58 | |
| 1 year | 806 | 452 658 | 0.73 (0.69–0.78) | 107 | |
| Model 2 (388 417) | 7 years | 7585 | 2 695 399 | 0.86 (0.85–0.88) | – |
| 4 years | 3093 | 1 548 749 | 0.87 (0.84–0.90) | −1 | |
| 2 years | 1062 | 775 980 | 0.80 (0.76–0.85) | 51 | |
| 1 year | 381 | 388 262 | 0.73 (0.67–0.80) | 114 | |
| Model 3 (388 036) | 7 years | 7204 | 2 695 173 | 0.87 (0.85–0.89) | – |
| 4 years | 2712 | 1 548 523 | 0.89 (0.86–0.92) | −13 | |
| 2 years | 681 | 775 754 | 0.85 (0.79–0.91) | 22 | |
| Model 4 (387 355) | 7 years | 6523 | 2 694 129 | 0.88 (0.86–0.90) | – |
| 4 years | 2031 | 1 547 479 | 0.90 (0.87–0.94) | −24 | |
| Cardiovascular disease mortality | |||||
| Model 1 (452 993) | 7 years | 2643 | 3 130 875 | 0.82 (0.79–0.85) | – |
| 4 years | 1177 | 1 802 723 | 0.80 (0.76–0.84) | 14 | |
| 2 years | 505 | 904 227 | 0.76 (0.71–0.82) | 37 | |
| 1 year | 210 | 452 658 | 0.77 (0.69–0.87) | 32 | |
| Model 2 (388 417) | 7 years | 1382 | 2 695 399 | 0.83 (0.79–0.87) | – |
| 4 years | 601 | 1 548 749 | 0.82 (0.76–0.89) | 4 | |
| 2 years | 249 | 775 980 | 0.77 (0.69–0.86) | 38 | |
| 1 year | 103 | 388 262 | 0.77 (0.65–0.92) | 38 | |
| Model 3 (388 314) | 7 years | 1279 | 2 695 342 | 0.83 (0.79–0.88) | – |
| 4 years | 498 | 1 548 692 | 0.84 (0.77–0.91) | −1 | |
| 2 years | 146 | 775 923 | 0.77 (0.67–0.89) | 42 | |
| Model 4 (388 168) | 7 years | 1133 | 2 695 128 | 0.84 (0.80–0.89) | – |
| 4 years | 352 | 1 548 478 | 0.86 (0.78–0.95) | −15 | |
| Incident cardiovascular disease | |||||
| Model 1 (452 993) | 6.1 years | 30 146 | 2 709 543 | 0.91 (0.90–0.92) | – |
| 4 years | 20 479 | 1 762 204 | 0.90 (0.88–0.91) | 16 | |
| 2 years | 11 124 | 893 077 | 0.88 (0.87–0.90) | 34 | |
| 1 year | 6005 | 449 640 | 0.86 (0.84–0.88) | 57 | |
| Model 2 (388 417) | 6.1 years | 14 439 | 2 373 103 | 0.92 (0.90–0.93) | – |
| 4 years | 8524 | 1 534 185 | 0.90 (0.89–0.92) | 19 | |
| 2 years | 3802 | 772 609 | 0.88 (0.85–0.91) | 54 | |
| 1 year | 1781 | 387 470 | 0.87 (0.83–0.91) | 68 | |
| Model 3 (386 636) | 6.1 years | 12 658 | 2 372 164 | 0.93 (0.91–0.94) | – |
| 4 years | 6743 | 1 533 246 | 0.92 (0.89–0.94) | 17 | |
| 2 years | 2021 | 771 669 | 0.89 (0.85–0.93) | 58 | |
| Model 4 (384 615) | 6.1 years | 10 637 | 2 369 116 | 0.94 (0.92–0.95) | – |
| 4 years | 4722 | 1 530 198 | 0.93 (0.90–0.96) | 10 | |
1, 2, and 4 years of follow-up time are cut-off values; 6.1 and 7 years are median values.
The log-hazard ratios estimate the increase in risk of the outcome for an increase of 1 standard deviation in the log(min of MVPA + 1). All analyses were adjusted for age, sex, BMI, smoking status, education, deprivation, sleep, leisure screen time, salt intake, oily fish intake, fruit and vegetable intake, processed/red meat intake, blood pressure medication, cholesterol medication, diabetes and/or insulin medication, parental history of cardiovascular disease and parental history of cancer. The baseline hazards were stratified by assessment centre, ethnicity, alcohol intake, employment/active commuting/manual work status. Model 1: adjusted for prevalent disease (cardiovascular disease and cancer); Model 2: excluded those with prevalent disease; Model 3: Model 2 + excluded cases occurring in first year of follow-up; Model 4: Model 2 + excluded cases occurring in first 2 years of follow-up.
Supplementary Table 1 and Figure 2, available as Supplementary data at IJE, online display these same estimates, re-arranged to allow direct comparison between models for the same follow-up time cut-off. With the maximum available follow-up time, the estimates from Model 4 (excluding those with prevalent disease and those experiencing the outcome within the first 2 years of follow-up) were attenuated compared with those from Model 1 (adjustment for prevalent disease only). The relative differences between models were greatest in analyses using 4-years of follow-up. The relative differences between models were greatest for incident CVD, although the absolute differences were similar for the other outcomes.
Figure 2.
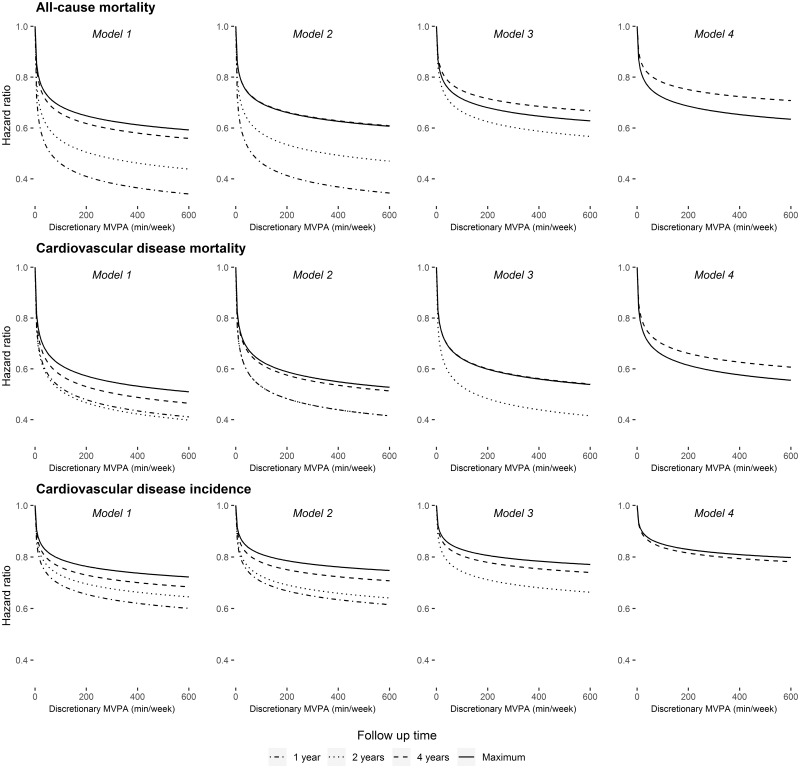
Dose–response relationships between physical activity and health outcomes at 1, 2, 4 and 7 years of follow-up (6.1 years for CVD incidence) using four modelling approaches in the UK Biobank study (2006–2010 to 2015–2016). All analyses were adjusted for covariates listed in the text. Model 1: also adjusted for prevalent disease (CVD and cancer); Model 2: excluded those with prevalent disease; Model 3: Model 2 + excluded cases occurring in first year of follow-up; Model 4: Model 2 + excluded cases occurring in first 2 years of follow-up.
To assist with interpretation, Figure 2 shows the HRs for the different follow-up times by model across the range of 0–600 min/week of discretionary MVPA. Supplementary Figure 3, available as Supplementary data at IJE online, displays these estimates re-arranged to facilitate comparisons between models across the different follow-up times.
The coefficients from regression of standardized physical activity at follow-up on baseline were 0.2 (SE 0.020) for those who undertook the first re-visit <3 years after baseline, 0.49 (SE 0.007) for whole first re-visit sample (median 4.4 years after baseline) and 0.39 (SE 0.006) second re-visit (median 7.6 years after baseline).
The results of the sensitivity analyses without adjustment for covariates potentially on the causal pathway were almost identical to the main analyses (data not shown).
Discussion
With increasing availability of large datasets (e.g. biobanks), researchers face important decisions alongside the unique opportunities offered by these resources. This study is the first to quantify the combined impact of choice of follow-up time cut-off and method to address reverse causality bias on estimates of association between self-reported physical activity and these outcomes. We found that choice of cut-off for follow-up time within a range of 1–7 years can strongly influence the magnitude of the prospective association. When all-cause mortality was the outcome, the log HRs based on a maximum follow-up of 1 year were over double the magnitude of those obtained using a median 7-year follow-up. There may be a number of explanations for the attenuation of the association with increasing follow-up time, further to the reduced influence of reverse causality bias. For example, we would expect individual variation in physical activity levels, resulting in the baseline exposure measurement not perfectly reflecting exposure over the entire follow-up period. As Skogstad et al. showed, some health benefits of physical activity may not persist if levels are not maintained: after an 8 week physical activity intervention, both physical activity levels and various biomarkers of CVD risk returned to baseline levels 15 months later.20 Therefore, one should expect some attenuation as follow-up time increases. However, since the magnitude of the regression dilution over the 3–7 years after baseline were estimated to be fairly similar in this study, it is unlikely that changes in physical activity behaviour fully explain these differences. Excluding individuals with prevalent disease attenuated the estimated associations compared with adjusting for it, as did excluding early incident cases to address the issue of undiagnosed underlying disease impacting physical activity levels. However, the impact of different analytical approaches for dealing with reverse causality was smaller as follow-up time increased. Our results are comparable with the findings of Andersen et al. who observed attenuation in the associations of physical activity levels with all-cause mortality over a 10-year period in Danish adults.9 The HR comparing the risk of all-cause mortality for least active individuals with that of the highly active decreased from 2.6 to 1.9 when follow-up lengthened from 2 to 10 years, representing ∼30% difference on the log scale. The most comparable result in the present study was the 55% attenuation of the Model 2 estimates between 2- and 7-year follow-up. Different exposure measures and modelling choices, sample sizes and event rates, and sample characteristics are likely to influence the level of attenuation.21
One other study investigated differences in the association between physical activity and health outcomes by different cut-offs for follow-up time up to 7 years. De Bruijn et al. found attenuation in the association between physical activity and dementia over a 2–6 year period, to the extent that there was no evidence of an association after 5 years.22 In response to this finding, the authors discussed the need to consider the interplay between physical activity and the disease pathway when choosing the follow-up time. However for this outcome, there are further complexities to consider, as the disease state (even at a pre-diagnostic stage) may also negatively impact on the subjective recall of physical activity. Therefore, despite their rigorous screening methods at baseline, correlated measurement error may also explain the associations.
A major strength of the current work is its relevance to the decisions faced by researchers today. We used a large sample of middle-aged adults where event rates may in principle be high enough to undertake association analyses soon after baseline. By quantifying the difference in the estimates, we have assisted those who meta-analyse results from studies with different follow-up periods in the <10 year range. Our results are particularly timely as the follow-up time for the UK Biobank subsample with accelerometry measures (undertaken ∼3 years after baseline interview23) will soon be sufficient for prospective analyses. However, it remains unclear whether our findings would also apply to a situation where physical activity is measured objectively. This will be important to investigate to aid interpretation of the results of two recent studies8,24 reporting on the associations between accelerometer-derived physical activity and mortality after 1–2 years of follow-up. Accelerometer-derived metrics may be more precise at differentiating between levels of physical activity than self-report methods,25 which may change the strength of the association with health outcomes and cross-sectionally with underlying disease. If so, it is possible that there may be a different pattern of variation in the estimates based on different lengths of follow-up and ways of accounting for reverse causality. This study is also the first to present associations of this particular physical activity exposure summary measure and all-cause and CVD mortality and CVD incidence in the UK Biobank sample. Previous work has either reported associations by domain26 or has used summary measures from the International Physical Activity Questionnaire Short Form.7
There are also some limitations of our work. The UK Biobank sample is non-representative of the general population (5.5% response rate) and has been shown to be healthier than the UK population.27 This may affect the generalizability of the results. Potentially, the associations we observe in this study would likely be greater still in older or less healthy populations who have an increased prevalence of underlying disease, or for whom the timeline of disease progression may be different. Therefore, similar work in other population samples is needed. We have also only investigated one behavioural exposure; the pattern of associations may also be present for other exposures, for example specific sedentary behaviours or food intake. Lastly, ∼30% of the UK Biobank participants have been identified as having at least one third-degree or closer relative in the study. We have not accounted for this relatedness in our analyses, thus the certainty of individual HRs may be slightly overestimated. However, this should not impact on our main conclusions, as we focus here on the relative difference between models rather than absolute associations.
In conclusion, we have shown important differences in the associations between self-reported physical activity and all-cause mortality and CVD outcomes as follow-up time increases over a 7 year period. We have also shown that analytical approaches to account for reverse causality can affect estimates, particularly with shorter follow-up times. The expected time course of disease progression is critical to these decisions, and, as such, just because analyses can be undertaken, it does not mean that they should be.
Funding
This work was supported by the Medical Research Council [grant numbers MC_UU_12015/1 and MC_UU_12015/3] (T.S., K.W., S.J.S., P.C.D., N.W., S.B.) and a National Health and Medical Research Council of Australia research fellowship [No. 1142685] (P.C.D.).
Supplementary Material
Acknowledgements
We would like to thank the UK Biobank participants and all those who collect and manage the data. This study was undertaken under application 20684.
Conflict of interest: None declared.
References
- 1.Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 2. Li F, Hou LN, Chen W. et al. Associations of dietary patterns with the risk of all-cause, CVD and stroke mortality: a meta-analysis of prospective cohort studies. Br J Nutr 2015;113:16–24. [DOI] [PubMed] [Google Scholar]
- 3. Patterson R, McNamara E, Tainio M. et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodcock J, Franco OH, Orsini N, Roberts I.. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol 2011;40:121–38. [DOI] [PubMed] [Google Scholar]
- 5. Bennett DA, Du H, Clarke R. et al. Association of physical activity with risk of major cardiovascular diseases in Chinese men and women. JAMA Cardiol 2017;2:1349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Celis-Morales CA, Lyall DM, Anderson J. et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J 2017;38:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celis-Morales CA, Lyall DM, Welsh P. et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. BMJ-Brit Med J 2017;357:j1456. [DOI] [PubMed] [Google Scholar]
- 8. Tikkanen E, Gustafsson S, Ingelsson E.. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: Longitudinal analyses in the UK Biobank study. Circulation 2018;137:2583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersen LB. Relative risk of mortality in the physically inactive is underestimated because of real changes in exposure level during follow-up. Am J Epidemiol 2004;160:189–95. [DOI] [PubMed] [Google Scholar]
- 10. Gulsvik AK, Thelle DS, Samuelsen SO, Myrstad M, Mowé M, Wyller TB.. Ageing, physical activity and mortality: a 42-year follow-up study. Int J Epidemiol 2012;41:521–30. [DOI] [PubMed] [Google Scholar]
- 11. Holme I, Tonstad S.. Increased predictive ability of BMI but not other risk factors with time in men: 39-year follow-up of total mortality in the Oslo Study. Obes Facts 2014;7:311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Talbot LA, Morrell CH, Fleg JL, Metter EJ.. Changes in leisure time physical activity and risk of all-cause mortality in men and women: The Baltimore Longitudinal Study of Aging. Prev Med 2007;45:169–76. [DOI] [PubMed] [Google Scholar]
- 13. Wannamethee SG, Shaper AG, Whincup PH, Walker M.. Role of risk factors for major coronary heart disease events with increasing length of follow up. Heart 1999;81:374–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menotti A, Lanti M.. Coronary risk factors predicting early and late coronary deaths. Heart 2003;89:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosengren A, Wilhelmsen L.. Physical activity protects against coronary death and deaths from all causes in middle-aged men: evidence from a 20-year follow-up of the primary prevention study in Göteborg. Ann Epidemiol 1997;7:69–75. [DOI] [PubMed] [Google Scholar]
- 16. Sattar N, Preiss D.. Reverse causality in cardiovascular epidemiological research: More common than imagined? Circulation 2017;135:2369–372. [DOI] [PubMed] [Google Scholar]
- 17.UK Biobank. UK Biobank Anthropometry, 2014. http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi? id=146620; http://www.webcitation.org/73YUnEH8C.
- 18.UK Biobank. UKB Touchscreen Questionnaire: Resource 113241, 2014. http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi? id=113241; http://www.webcitation.org/73YUg6nOD.
- 19. Ainsworth BE, Haskell WL, Herrmann SD. et al. 2011 compendium of physical activities. Med Sci Sports Exerc 2011;43:1575–581. [DOI] [PubMed] [Google Scholar]
- 20. Skogstad M, Lunde LK, Ulvestad B. et al. The effect of a leisure time physical activity intervention delivered via a workplace: 15-Month follow-up study. Int J Environ Res Public Health 2018;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meinow B, Kareholt I, Parker MG, Thorslund M.. The effect of the duration of follow-up in mortality analysis: the temporal pattern of different predictors. J Gerontol B Psychol Sci Soc Sci 2004;59:S181–189. [DOI] [PubMed] [Google Scholar]
- 22. De Bruijn RF, Schrijvers EM, de Groot KA. et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur J Epidemiol 2013;28:277–83. [DOI] [PubMed] [Google Scholar]
- 23. Doherty A, Jackson D, Hammerla N. et al. Large scale population assessment of physical activity using wrist worn accelerometers: The UK Biobank study. Plos One 2017;12:e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee IM, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE.. Accelerometer-measured physical activity and sedentary behavior in relation to all-cause mortality: The Women's Health Study. Circulation 2018;137:203–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steene-Johannessen J, Anderssen SA, Van der Ploeg HP. et al. Are self-report measures able to define individuals as physically active or inactive? Med Sci Sports Exerc 2016;48:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wijndaele K, Sharp SJ, Wareham NJ, Brage S.. Mortality risk reductions from substituting screen time by discretionary activities. Med Sci Sports Exerc 2017;49:1111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fry A, Littlejohns TJ, Sudlow C. et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–034. [PMC][10.1093/aje/kwx246] [28641372] 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.