Abstract
Epidemiologists are often confronted with datasets to analyse which contain measurement error due to, for instance, mistaken data entries, inaccurate recordings and measurement instrument or procedural errors. If the effect of measurement error is misjudged, the data analyses are hampered and the validity of the study’s inferences may be affected. In this paper, we describe five myths that contribute to misjudgments about measurement error, regarding expected structure, impact and solutions to mitigate the problems resulting from mismeasurements. The aim is to clarify these measurement error misconceptions. We show that the influence of measurement error in an epidemiological data analysis can play out in ways that go beyond simple heuristics, such as heuristics about whether or not to expect attenuation of the effect estimates. Whereas we encourage epidemiologists to deliberate about the structure and potential impact of measurement error in their analyses, we also recommend exercising restraint when making claims about the magnitude or even direction of effect of measurement error if not accompanied by statistical measurement error corrections or quantitative bias analysis. Suggestions for alleviating the problems or investigating the structure and magnitude of measurement error are given.
Keywords: Measurement error, misclassification, bias, bias corrections, misconceptions
Introduction
Epidemiologists are often confronted with datasets to analyse which contain data errors. Settings where such errors occur include, but are not limited to, measurements of dietary intake,1–3 blood pressure,4–6 physical activity,7–9 exposure to air pollutants,10–12 medical treatments received13–15 and diagnostic coding.16–18 Mismeasurements and misclassifications, hereinafter collectively referred to as measurement error, are mentioned as a potential study limitation in approximately half of all the original research articles published in highly ranked epidemiology journals.19,20 The actual burden of measurement error in all of epidemiological research is likely to be even higher.19
Despite the attention given to measurement error in the discussion and limitation sections of many published articles, empirical investigations of measurement error in epidemiological research remain rare.19–21 Notably, statistical methods that aim to investigate the impact of measurement error or alleviate their consequences for the epidemiological data analyses at hand continue to be rarely used. Authors instead appear to rely on simple heuristics about measurement error structure, e.g. whether or not measurement error is expected to be nondifferential, and impact on epidemiological data analyses, e.g. whether or not the measurement error creates bias towards null effects, despite ample warnings that such heuristics are oversimplified and often wrong.22–27 As we will illustrate, the impact of measurement error often plays out in a way that counters common conceptions.
In this paper we describe and reply to five myths about measurement error which we perceive to exist in epidemiology. It is our intention to clarify misconceptions about mechanisms and bias introduced by measurement error in epidemiological data analyses, and to encourage researchers to use analytical approaches to investigate measurement error. We first briefly characterize measurement error variants before discussing the five measurement error myths.
Measurement error: settings and terminology
Throughout this article (except for myth 5) we assume that measurement error is to occur in a non-experimental epidemiological study designed to estimate an exposure effect, that is the relationship between a single exposure (denoted by , e.g. adherence versus non-adherence to a 30-day physical exercise programme) and a single outcome (denoted by e.g. post-programme body weight in kg), statistically controlled for one or more confounding variables (e.g. age, sex and pre-programme body weight). Some simplifying assumptions are made for brevity of this presentation.
It is assumed that the confounders are adequately controlled for by conventional multivariable linear, risk or rate regression (e.g. ordinary least squares, logistic regression, Cox or Poisson regression), or by an exposure model (e.g. propensity score analysis).28 Besides measurement error, other sources that could affect inferences about the exposure effect are assumed not to play an important role, e.g. no selection bias.29 Unless otherwise specified, we assume that the measurement error has the classical additive form: Observation = Truth + Error, shortened as O = T + E, where the mean of E is assumed to be zero, meaning that the Observations do not systematically differ from the Truth. Alternative and more complex models for measurement error relevant to epidemiological research, such as systematic and Berkson error models,30 are not considered here. We also assume that there is an agreed underlying reality (T) of the phenomenon that one aims to measure and an imperfectly measured representation of that reality (O) subject to measurement error (E). This identifiable measurement error assumption is often reasonable in epidemiological research but may be less so in some circumstances, for instance with the measurement of complex diseases. For in-depth discussion on the theories of measurement we refer to the work by Hand.31
In the simplest setting, we may assume (or in rare cases, know) that the measurement error is univariate, that is to say that measurement error occurs only in a single variable. Measurement error in an exposure variable () is further commonly classified as nondifferential if error in the measurement error is independent of the true value of the outcome (i.e. ) and differential otherwise. Likewise, error in the measurement of the outcome () is said to be nondifferential only if the error is independent of the true value of the exposure (i.e. ),32–35 or in an alternative notation if for each possible outcome status y of , Pr( = y| = y, is a constant for all possible values a of . (Note that nondifferential error sometimes refers to a broader definition that includes covariates; for a single covariate L with true values, the assumption can then be specified by Pr( = y| = y, .)
Reconsider the hypothetical example of the relation between the exposure physical exercise programme adherence and post-programme body weight. Differential exposure measurement error would mean that mismeasurement of programme adherence occurs more frequently or more infrequently in individuals with a higher (or lower) post-programme body weight. For the binary exposure programme adherence, nondifferential error simplifies to assuming that the sensitivity and specificity of measured programme adherence are the same for all possible true values of post-programme body weight.
If two or more variables in the analysis are subject to measurement error, we may speak of multivariate (or joint) measurement error. When two variables are measured with error, measurement error (which may be differential or nondifferential for either variable) is said to be independent if the errors in the one error-prone variable are statistically unrelated to the errors in the other error-prone variable and dependent otherwise, i.e. multivariate measurement error in A and Y is said to be independent if 32–34 Dependent measurement error may for instance occur in an exposure variable when error on exposure becomes more (or less) likely for units that are misclassified on the outcome variable. In the hypothetical example, if both adherence to a physical activity programme and post-programme body weight were self-reported, we may expect error in both exposure and outcome measurements. Further, we may also anticipate that respondents who misreport adherence to the exercise programme also misreport their post-programme body weight, which would result in multivariate dependent measurement error.
Five myths about measurement error
In this section we discuss five myths about measurement in epidemiological research, in particular as regards the impact of measurement error on study results (myths 2 and 5), solutions to mitigate the impact (myths 1 and 4) and the mechanisms of measurement error (myth 3). Each myth is accompanied by a short reply that is substantiated in a more detailed explanation.
Myth 1: measurement error can be compensated for by large numbers of observations
Reply: no, a large number of observations does not resolve the most serious consequences of measurement error in epidemiological data analyses. These remain regardless of the sample size.
Explanation: one intuition is that measurement error distorts the true existing statistical relationships between variables, analogous to noise (the measurement error) lowering the ability to detect a signal (the true statistical relationships) that can be picked up from the data. Continuing on this thought, increasing the sample size would amplify the signal to become better distinguishable from the noise, thereby compensating for the measurement error. Unfortunately, this signal to noise analogy rarely applies to epidemiological studies.
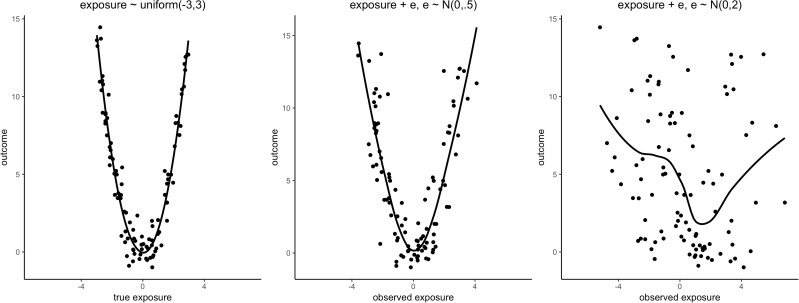
Measurement error can have impact on epidemiological data analyses in at least three ways, as summarized by the Triple Whammy of Measurement Error.30 First, measurement error can create a bias in the measures of the exposure effect estimate. Second, measurement error affects the precision of the exposure effect estimate, often by reducing it, reflected in larger standard errors and widening of confidence intervals for the exposure effect estimates, and a lower statistical power of the significance test for the null exposure effect. Biased exposure effect estimates may, however, be accompanied by smaller rather than larger expected standard errors and conserved statistical power.36 Third, measurement error can mask the features of data, such as non-linear functional relationships between the exposure and outcome variables. Figure 1 illustrates feature masking by univariate nondifferential measurement error.
Figure 1.
Illustration of Whammy 3: Measurement error may mask a functional relation. True model: outcome = 3/2*exposure^2 + e, e∼N(0, 1). Measurement error model: observed exposure value = true exposure value + exposure error. Line is a LOESS curve.
Key Messages
The strength and direction of effect of measurement error on any given epidemiological data analysis is generally difficult to conceive without appropriate quantitative investigations.
Frequently used heuristics about measurement error structure (e.g. nondifferential error) and impact (e.g. bias towards null) are often wrong and encourage a tolerant attitude towards neglecting measurement error in epidemiological research.
Statistical approaches to mitigate the effects of unavoidable measurement error should be more widely adopted.
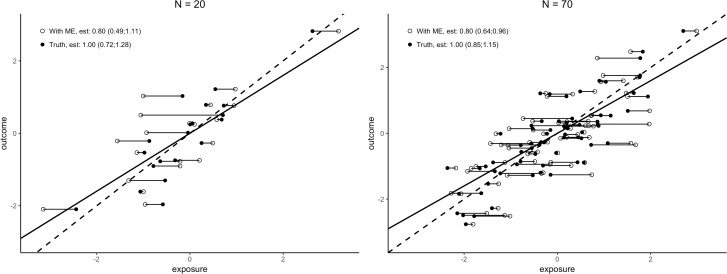
With sample size increasing and assuming all else remains equal, exposure effect estimates will on average be closer to their limiting expected values, while not necessarily closer in distance to their respective true values. A large sample size thus improves the assurance that the exposure effect estimate comports to the expected value under the measurement error mechanism, affecting the second Whammy of Measurement Error (precision) but not directly the first (bias) (Figure 2). A larger sample size may thus compensate for the loss in precision and power which is due to the presence of measurement error. The compensation needed for studies with data that contain measurement error can be a 50-fold or more increase of the sample size when the reliability of measurement is low.37,38 In consequence, even a dataset of a spectacularly large size containing measurement errors may or may not yield more precise estimates and more powerful testing than a much smaller dataset without measurement error.
Figure 2.
Illustration of Whammy 1 (bias) and Whammy 2 (precision, see width confidence intervals) of measurement error. Dashed is regression line without measurement error (Truth), solid line is regression line with measurement error (With ME). N = sample size. Lines, point estimates and confidence intervals based on 5000 replicate Monte Carlo simulations (Truth: outcome = exposure + e, e∼N(0, 0.6), exposure∼N(0, 1), With ME: Truth + er, er∼N(0, 0.5)). Plotted points the first single simulation replicate.
Myth 2: the exposure effect is underestimated when variables are measured with error
Reply: no, an exposure effect can be over- or underestimated in the presence of measurement error depending on which variables are affected, how measurement error is structured and the expression of other biasing and data sampling factors. In contrast to common understanding, even univariate nondifferential exposure measurement error, which is often expected to bias towards the null, may yield a bias away from null.
Explanation: more than a century ago, Spearman39 derived his measurement error attenuation formula for a pairwise correlation coefficient between two variables wherein at least one of the variables was measured with error. Spearman identified that this correlation coefficient would on average be underestimated by a predictable amount if the reliability of the measurements was known. This systematic bias towards the null value, also known as regression dilution bias, attenuation to the null and Hausman’s iron law, is now known to apply beyond simple correlations to other types of data and analyses.25,40–42
It is, however, an overstatement to say that—by iron law—the exposure estimates are underestimated in any given epidemiological study analysing data with measurement error. For instance, selective filtering of statistically significant exposure effects in measurement error-contaminated data estimated with low precision is likely to lead to substantial overestimation of the exposure effect estimates for the variables that withstand the significance test.26 Even if one is willing to assume that measurement error is the only biasing factor, a simplifying assumption that we make in this article for illustration purposes only, statistical estimation is subject to sampling variability. The distance of the exposure effect estimate relative to its true value varies from dataset to dataset. In a particular dataset with measurement error, exposure effects may be overestimated only due to sampling variability, illustrations of which are found in Hutcheon et al. and Jurek et al.22,43 Hence, a defining characteristic of the iron law is that it applies to averages of exposure effect estimates, e.g. after many hypothetical replications of a study with the same measures.
This is not to argue that measurement error in itself cannot, in principle, produce a bias in a predictable direction. The iron law does come with many exceptions. For instance, the law does not apply uniformly to univariate differential measurement error in any variable (which may produce bias in the exposure effect estimate away or towards null27,44–46), nor to univariate nondifferential error in the outcome variable (which may not affect bias in the exposure effect in case the outcome is continuous25) nor to univariate nondifferential measurement error in one of the confounding variables (which may create a bias in the exposure effect estimate away or towards null due to residual confounding27,44,47). For multivariate measurement error in any combination of exposure, outcome and confounders, bias in the exposure can be in either direction, with the exception of (strictly) independent and nondifferential measurement error in dichotomous exposure and outcome.34
There are also exceptions to the iron law in cases of univariate nondifferential exposure measurement error. Particularly, nondifferential misclassification of a polytomous exposure variable (i.e. with more than two categories) may create bias that is away from null for some of the exposure categories and towards null for others.29,48,49 Measurement error of any kind, including nondifferential exposure measurement error, also hampers the evaluation of exposure effect modification,44,50 interaction51,52 and mediation,53,54 creating bias away or towards null.
Myth 3: exposure measurement error is nondifferential if measurements are taken without knowledge of the outcome
Reply: no, exposure measurement error can be differential even if the measurement is taken without knowledge of the outcome.
Explanation: differential exposure measurement error is of particular concern because of its potentially strong biasing effects on exposure effect estimates.29,35,55 Differential error is a common suspect in retrospective studies where knowledge of the outcome status can influence the accuracy of measurement of the exposure. For instance, in a case-control study with self-reported exposure data, cases may recall or report their exposure status differently from controls, creating an expectation of differential exposure measurement error. Differential exposure measurement error may also arise due to bias by interviewers or care providers who are not blinded to the outcome status, or by use of different methods of exposure measurement for cases and controls.
Differential exposure measurement error is often not suspected in prospective data collection settings where the measurement of exposure precedes measurement of the outcome. Measurement of exposure before the outcome is nonetheless insufficient to guarantee that exposure measurement error is nondifferential. For example, as White56 noted: in a prospective design, differential measurement error in the exposure ‘family history of disease’ may be due to a more accurate recollection of family history among individuals with strong family history who are at a higher risk of the disease outcome. The nondifferential exposure measurement assumption is violated, as the measurement error in exposure is not independent of the true value of the outcome (i.e. is violated) despite that the exposure was measured before the outcome could have been observed.
Measurement error structures are also not invariant to discretization and collapsing of categories. For instance, discretization of a continuous exposure variable measured with nondifferential error into a categorical exposure variable can create differential exposure measurement error within the discrete categories.48,57,58 A clear numerical example of this is given in Wacholder et al., their second table.59 Although discretization in broader categories may be perceived as more robust to measurement error-induced fluctuations, the possible change in the mechanism of the error may do more harm than good for the estimation of the exposure effect.
Myth 4: measurement error can be prevented but not mitigated in epidemiological data analyses
Reply: no, statistical methods for measurement error bias-corrections can be used in the presence of measurement error provided that data are available on the structure and magnitude of measurement error from an internal or external source. This often requires planning of a measurement error correction approach or quantitative bias analysis, which may require additional data to be collected.
Explanation: a number of approaches have been proposed which examine and correct for the bias due to measurement error in analysis of an epidemiological study, typically focusing on adjusting for the bias in the exposure effect estimator and corresponding confidence intervals. These approaches include: likelihood based approaches60; score function methods61; method-of-moments corrections62; simulation extrapolation63; regression calibration64; latent class modelling65; structural equation models with latent variables66; multiple imputation for measurement error correction67; inverse probability weighing approaches68 and Bayesian analyses.69 Comprehensive text books30,62,69–71 and a measurement error corrections tutorial72 are available. Some applied examples are given in Table 1.
Table 1.
Examples of measurement error corrections, models and bias analyses
| Measure with error | Methods | Applied example | Ref. |
|---|---|---|---|
| Serum measurement of Vitamin D | Regression calibration | To account for measurement error, serum measurements were calibrated to assay measurements (the preferred reference standard) using data from an earlier study containing measurements of both assay and serum of Vitamin D | 73 |
| Smoking status reported by health care providers | Multiple imputation | Clinical assessments of smoking status were available only for an internal validation subgroup. Multiple imputation was used to account for the potential measurement error in health care provider-reported smoking status for the remaining patients | 74 |
| Low-density lipoprotein cholesterol (LDL-c) measurement | SIMEX | Effect estimate of LDL-c on coronary artery disease was corrected for bias in the error contaminated LDL-c measurements using the Simulation Extrapolation (SIMEX) method | 75 |
| Self-reported dietary fibre intake | Regression calibration | Repeated measurement of error-prone self-reported dietary feedback was used to estimate within-person variation to correct for measurement error via regression calibration | 76 |
| Diagnostic tests for pulmonary tuberculosis (PTB) | Latent class analysis | Results from six diagnostic tests for PTB were available which were considered error-contaminated measurements of PTB infection. A latent class model was developed to estimate diagnostic accuracy in the absence of a gold standard | 77 |
| Self-reported influenza vaccination status | Quantitative bias analysis | Monte Carlo simulations were performed to evaluate the impact of measurement error in the relation between vaccination status of pregnant women and preterm birth, assuming a range of plausible accuracy values for self-reported influenza vaccination | 78 |
Measurement error correction methods79 require information to be gathered about the structure and magnitude of measurement error. These data may come from within the study data that are currently analysed (i.e. internal validation data) or from other data (i.e. external validation data). If an accurate measurement procedure exists (i.e. a gold standard), either external or on an internal subset, a measurement error validation study can be conducted to extract information about the structure and magnitude of measurement error. If no such gold standard measurement procedure exists, a reliability study can replicate measurements of the same imperfect measure (e.g. multiple blood pressure measurements within the same unit), or alternatively, observations on multiple measures that aim to measure the same phenomenon (e.g. different diagnostic tests for the same disease within the same unit). Different measurement error correction methods, and the ability of the bias correction to return an estimate nearer to the truth, may be more or less applicable depending on the data sources available for the measurement error correction.
The impact of measurement error on study results can also be investigated by a quantitative bias analysis,79,80 even in the absence of reliable information about structure and magnitude. In brief, a quantitative bias analysis is a sensitivity analysis that simulates the effect of measurement error assuming a certain structure and magnitude of that error. Since some degree of uncertainty about measurement error generally remains, in particular about error structure, sensitivity analyses can also be useful following the application of measurement error correction methods mentioned above.
Myth 5: certain types of epidemiological research are unaffected by measurement error
Reply: no, measurement error can affect all types of epidemiological research.
Explanation: measurement error affects epidemiology undoubtedly beyond the settings we have discussed thus far, i.e. studies of a single exposure and outcome variable statistically controlled for confounding. For instance, measurement error has also been linked to issues with data analyses in the context of record linkage,81,82 time series analyses,10,11 Mendelian randomization studies,83 genome-wide association studies,84 environmental epidemiology,85 negative control studies,86 diagnostic accuracy studies,87,88 disease prevalence studies,89 prediction modelling studies90,91 and randomized trials.92
It is worth noting that types of data and analyses can be differently affected by measurement error. This means that even when measurement error is similar in structure and magnitude, the error can have a different impact depending on the analyses conducted. For example, consider a two-arm randomized trial with univariate differential measurement error in the outcome variable. If it is not possible to blind patients and providers to the treatment assignment, then the accuracy of assessment of some outcomes may depend on the assigned treatment arm. In this setting, bias in the exposure (treatment) effect estimate can be in either direction and inflate or deflate both Type I and Type II error for the null hypothesis significance test of no effect of treatment.92 The impact the measurement error has on the inferences made from the trial’s results depends on whether the trial is a superiority, equivalence or non-inferiority trial.93
Concluding remarks
Our discussion of five measurement error-related myths adds to an already extensive literature that has warned against the detrimental effects of neglected measurement error, a problem that is widely acknowledged to be ubiquitous in epidemiology. We suspect that these persistent myths have contributed to the tolerant attitude towards neglecting measurement error found in most of the applied epidemiological literature, as evidenced by the slow uptake of quantitative approaches that mitigate or investigate measurement error.
We have shown in this paper that the effect that measurement error can have on a data analysis is often counter-intuitive. Whereas we encourage epidemiologists to deliberate about the structure and potential impact of measurement error in their analyses, for instance via graphical approaches such as causal diagrams,33,45,94 we also recommend exercising restraint when making claims about the magnitude or even direction of bias of measurement error if not accompanied by analytical investigations. With the increase of collection and use of epidemiological data that are primarily collected without a specific research question in mind, such as routine care data,95 we anticipate that attention to measurement error and approaches to mitigate it will only become more important.
Funding
R.H.H.G. was funded by the Netherlands Organization for Scientific Research (NWO-Vidi project 917.16.430). T.L.L. was supported, in part, by R01LM013049 from the US National Library of Medicine.
Acknowledgements
We are most grateful for the comments received on our initial draft offered by Dr Katherine Ahrens, Dr Kathryn Snow, the Berlin Epidemiological Methods Colloquium journal club and the anonymous reviewers commissioned by the journal.
Author Contributions
M.S. conceptualized the manuscript. T.L. and R.G. provided inputs and revised the manuscript. All authors read and approved the final manuscript.
Conflict of interest: None declared.
References
- 1. Thiébaut ACM, Kipnis V, Schatzkin A, Freedman LS.. The role of dietary measurement error in investigating the hypothesized link between dietary fat intake and breast cancer—a story with twists and turns. Cancer Invest 2008;26:68–73. [DOI] [PubMed] [Google Scholar]
- 2. Freedman LS, Schatzkin A, Midthune D, Kipnis V.. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freedman LS, Commins JM, Willett W. et al. Evaluation of the 24-hour recall as a reference instrument for calibrating other self-report instruments in nutritional cohort studies: evidence from the validation studies pooling project. Am J Epidemiol 2017;186:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauldry S, Bollen KA, Adair LS.. Evaluating measurement error in readings of blood pressure for adolescents and young adults. Blood Press 2015;24:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Wel MC, Buunk IE, van Weel C, Thien T, Bakx JC.. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med 2011;9:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nitzan M, Slotki I, Shavit L.. More accurate systolic blood pressure measurement is required for improved hypertension management: a perspective. Med Devices 2017;10:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welk G. Physical Activity Assessments for Health-Related Research. Champaign, IL: Human Kinetics, 2002. [Google Scholar]
- 8. Ferrari P, Friedenreich C, Matthews CE.. The role of measurement error in estimating levels of physical activity. Am J Epidemiol 2007;166:832–40. [DOI] [PubMed] [Google Scholar]
- 9. Lim S, Wyker B, Bartley K, Eisenhower D.. Measurement error of self-reported physical activity levels in New York City: assessment and correction. Am J Epidemiol 2015;181:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeger SL, Thomas D, Dominici F. et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect 2000;108:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldman GT, Mulholland JA, Russell AG. et al. Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health 2011;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheppard L, Burnett RT, Szpiro AA. et al. Confounding and exposure measurement error in air pollution epidemiology. Air Qual Atmos Health 2012;5:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boudreau DM, Daling JR, Malone KE, Gardner JS, Blough DK, Heckbert SR.. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am J Epidemiol 2004;159:308–17. [DOI] [PubMed] [Google Scholar]
- 14. Schneeweiss S, Avorn J.. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol 2005;58:323–37. [DOI] [PubMed] [Google Scholar]
- 15. De Smedt T, Merrall E, Macina D, Perez-Vilar S, Andrews N, Bollaerts K.. Bias due to differential and non-differential disease- and exposure misclassification in studies of vaccine effectiveness. PLoS One 2018;13:e0199180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delate T, Jones AE, Clark NP, Witt DM.. Assessment of the coding accuracy of warfarin-related bleeding events. Thromb Res 2017;159:86–90. [DOI] [PubMed] [Google Scholar]
- 17. Yu AYX, Quan H, McRae AD, Wagner GO, Hill MD, Coutts SB.. A cohort study on physician documentation and the accuracy of administrative data coding to improve passive surveillance of transient ischaemic attacks. BMJ Open 2017;7:e015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nissen F, Morales DR, Mullerova H, Smeeth L, Douglas IJ, Quint JK.. Validation of asthma recording in the clinical practice research datalink (CPRD). BMJ Open 2017;7:e017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jurek AM, Maldonado G, Greenland S, Church TR.. Exposure-measurement error is frequently ignored when interpreting epidemiologic study results. Eur J Epidemiol 2007;21:871–76. [DOI] [PubMed] [Google Scholar]
- 20. Brakenhoff TB, Mitroiu M, Keogh RH, Moons KGM, Groenwold RHH, van Smeden M.. Measurement error is often neglected in medical literature: a systematic review. J Clin Epidemiol 2018;98:89–97. [DOI] [PubMed] [Google Scholar]
- 21. Shaw PA, Deffner V, Keogh RH. et al. Epidemiologic analyses with error-prone exposures: review of current practice and recommendations. Ann Epidemiol 2018;28:821–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sorahan T, Gilthorpe MS.. Non-differential misclassification of exposure always leads to an underestimate of risk: an incorrect conclusion. Occup Environ Med 1994;51:839–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brenner H, Loomis D.. Varied forms of bias due to nondifferential error in measuring exposure. Epidemiology 1994;5:510–17. [PubMed] [Google Scholar]
- 24. Jurek AM, Greenland S, Maldonado G.. Brief report: How far from non-differential does exposure or disease misclassification have to be to bias measures of association away from the null. Int J Epidemiol 2008;37:382–85. [DOI] [PubMed] [Google Scholar]
- 25. Hutcheon JA, Chiolero A, Hanley JA.. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 26. Loken E, Gelman A.. Measurement error and the replication crisis. Science 2017;355:584–85. [DOI] [PubMed] [Google Scholar]
- 27. Carroll RJ. Measurement error in epidemiologic studies In: Armitage P and Colton T (eds). Encyclopedia of Biostatistics. Chichester, UK: Wiley, 2005. [Google Scholar]
- 28. Rosenbaum PR, Rubin DB.. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 29. Rothman KJ, Greenland S, Lash T.. Modern Epidemiology. 3rd edn Philadelphia: Lippincott Williams & Wilkins (Wolters Kluwer Health), 2008. [Google Scholar]
- 30. Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM.. Measurement Error in Nonlinear Models: A Modern Perspective. Boca Raton, FL: Chapman and Hall/CRC, 2006. [Google Scholar]
- 31. Hand DJ. Statistics and the theory of measurement. J R Stat Soc Ser A 1996;159:445–92. [Google Scholar]
- 32. Kristensen P. Bias from nondifferential but dependent misclassification of exposure and outcome. Epidemiology 1992;3:210–15. [DOI] [PubMed] [Google Scholar]
- 33. Hernan MA, Cole SR.. Invited commentary: causal diagrams and measurement bias. Am J Epidemiol 2009;170:959–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brooks DR, Getz KD, Brennan AT, Pollack AZ, Fox MP.. The impact of joint misclassification of exposures and outcomes on the results of epidemiologic research. Curr Epidemiol Rep 2018;5:166–74. [Google Scholar]
- 35. Copeland KT, Checkoway H, Mcmichael AJ, Holbrook RH.. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol 1977;105:488–95. [DOI] [PubMed] [Google Scholar]
- 36. Greenland S, Gustafson P.. Accounting for independent nondifferential misclassification does not increase certainty that an observed association is in the correct direction. Am J Epidemiol 2006;164:63–68. [DOI] [PubMed] [Google Scholar]
- 37. McKeown-Eyssen GE, Tibshirani R.. Implications of measurement error in exposure for the sample sizes of case-control studies. Am J Epidemiol 1994;139:415–21. [DOI] [PubMed] [Google Scholar]
- 38. Devine OJ, Smith JM.. Estimating sample size for epidemiologic studies: the impact of ignoring exposure measurement uncertainty. Stat Med 1998;17:1375–89. [DOI] [PubMed] [Google Scholar]
- 39. Spearman C. The proof and measurement of association between two things. Am J Psychol 1904;15:72–101. [PubMed] [Google Scholar]
- 40. Bross I. Misclassification in 2 x 2 tables. Biometrics 1954;10:478–86. [Google Scholar]
- 41. Liu K. Measurement error and its impact on partial correlation and multiple linear regression analyses. Am J Epidemiol 1988;127:864–74. [DOI] [PubMed] [Google Scholar]
- 42. Hausman J. Mismeasured variables in econometric analysis: problems from the right and problems from the left. J Econ Perspect 2001;15:57–67. [Google Scholar]
- 43. Jurek AM, Greenland S, Maldonado G, Church TR.. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int J Epidemiol 2005;34:680–87. [DOI] [PubMed] [Google Scholar]
- 44. Greenland S. The effect of misclassification in the presence of covariates. Am J Epidemiol 1980;112:564–69. [DOI] [PubMed] [Google Scholar]
- 45. VanderWeele TJ, Hernan MA.. Results on differential and dependent measurement error of the exposure and the outcome using signed directed acyclic graphs. Am J Epidemiol 2012;175:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buzas JS, Stefanski LA, Tosteson TD.. Measurement error In: Ahrens W and Pigeot I (eds). Handbook of Epidemiology. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. [Google Scholar]
- 47. Brakenhoff TB, van Smeden M, Visseren FLJ, Groenwold R.. Random measurement error: why worry? An example of cardiovascular risk factors. PLoS One 2018;13:e0192298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dosemeci M, Wacholder S, Lubin JH.. Does nondifferential miclassification of exposure always bias a true effect toward the null value? Am J Epidemiol 1990;132:746–48. [DOI] [PubMed] [Google Scholar]
- 49. Brenner H. Bias due to non-differential misclassification of polytomous confounders. J Clin Epidemiol 1993;46:57–63. [DOI] [PubMed] [Google Scholar]
- 50. Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 1998;55:651–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muff S, Keller LF.. Reverse attenuation in interaction terms due to covariate measurement error. Biom J 2015;57:1068–83. [DOI] [PubMed] [Google Scholar]
- 52. Jaccard J, Wan CK.. Measurement error in the analysis of interaction effects between continuous predictors using multiple regression: multiple indicator and structural equation approaches. Psychol Bull 1995;117:348–57. [Google Scholar]
- 53. Le Cessie S, Debeij J, Rosendaal FR, Cannegieter SC, Vandenbroucke JP.. Quantification of bias in direct effects estimates due to different types of measurement error in the mediator. Epidemiology 2012;23:551–60. [DOI] [PubMed] [Google Scholar]
- 54. VanderWeele TJ, Valeri L, Ogburn EL.. The role of measurement error and misclassification in mediation analysis. Epidemiology 2012;23:561–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Drews CD, Greeland S.. The impact of differential recall on the results of case-control studies. Int J Epidemiol 1990;19:1107–12. [DOI] [PubMed] [Google Scholar]
- 56. White E. Design and interpretation of studies of differential exposure measurement error. Am J Epidemiol 2003;157:380–87. [DOI] [PubMed] [Google Scholar]
- 57. Flegal KM, Keyl PM, Nieto FJ.. Differential misclassification arising from nondifferential errors in exposure measurement. Am J Epidemiol 1991;134:1233–46. [DOI] [PubMed] [Google Scholar]
- 58. Blas Achic BG, Wang T, Su Y, Kipnis V, Dodd K, Carroll RJ.. Categorizing a continuous predictor subject to measurement error. Electron J Stat 2018;12:4032–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wacholder S, Dosemeci M, Lubin JH.. Blind assignment of exposure does not always prevent differential misclassification. Am J Epidemiol 1991;134:433–37. [DOI] [PubMed] [Google Scholar]
- 60. Carroll RJ, Spiegelman CH, Lan KKG, Bailey KT, Abbott RD.. On errors-in-variables for binary regression models. Biometrika 1984;71:19–25. [Google Scholar]
- 61. Stefanski LA. Unbiased estimation of a nonlinear function a normal mean with application to measurement-error models. Commun Stat Theory Methods 1989;18:4335–58. [Google Scholar]
- 62. Fuller WA. Measurement Error Models. New York: Wiley, 1987. [Google Scholar]
- 63. Cook JR, Stefanski LA.. Simulation-extrapolation estimation in parametric measurement error models. J Am Stat Assoc 1994;89:1314–28. [Google Scholar]
- 64. Carroll RJ, Stefanski LA.. Approximate quasi-likelihood estimation in models with surrogate predictors. J Am Stat Assoc 1990;85:652–63. [Google Scholar]
- 65. Hui SL, Walter SD.. Estimating the error rates of diagnostic tests. Biometrics 1980;36:167–71. [PubMed] [Google Scholar]
- 66. Sánchez BN, Budtz-Jørgensen E, Ryan LM, Hu H.. Structural equation models. J Am Stat Assoc 2005;100:1443–55. [Google Scholar]
- 67. Cole SR, Chu H, Greenland S.. Multiple-imputation for measurement-error correction. Int J Epidemiol 2006;35:1074–81. [DOI] [PubMed] [Google Scholar]
- 68. Gravel CA, Platt RW.. Weighted estimation for confounded binary outcomes subject to misclassification. Stat Med 2018;37:425–36. [DOI] [PubMed] [Google Scholar]
- 69. Gustafson P. Measurement Error and Misclassification in Statistics and Epidemiology: Impacts and Bayesian Adjustments. Boca Raton, FL: Chapman and Hall (CRC Press), 2003. [Google Scholar]
- 70. Buonaccorsi JP. Measurement Error. Boca Raton, FL: Chapman and Hall/CRC, 2010. [Google Scholar]
- 71. Yi GY. Statistical Analysis with Measurement Error or Misclassification. New York: Springer, 2017. [Google Scholar]
- 72. Keogh RH, White IR.. A toolkit for measurement error correction, with a focus on nutritional epidemiology. Stat Med 2014;33:2137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tian L, Durazo-Arvizu RA, Myers G, Brooks S, Sarafin K, Sempos CT.. The estimation of calibration equations for variables with heteroscedastic measurement errors. Stat Med 2014;33:4420–36. [DOI] [PubMed] [Google Scholar]
- 74. Edwards JK, Cole SR, Westreich D. et al. Multiple imputation to account for measurement error in marginal structural models. Epidemiology 2015;26:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR.. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the i2 statistic. Int J Epidemiol 2016;45:1961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dahm CC, Keogh RH, Spencer EA. et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst 2010;102:614–26. [DOI] [PubMed] [Google Scholar]
- 77. Schumacher SG, Van Smeden M, Dendukuri N. et al. Diagnostic test accuracy in childhood pulmonary tuberculosis: a Bayesian latent class analysis. Am J Epidemiol 2016;184:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ahrens K, Lash TL, Louik C, Mitchell AA, Werler MM.. Correcting for exposure misclassification using survival analysis with a time-varying exposure. Ann Epidemiol 2012;22:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lash TL, Fox MP, Fink AK.. Applying Quantitative Bias Analysis to Epidemiologic Data. New York: Springer-Verlag, 2009. [Google Scholar]
- 80. Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S.. Good practices for quantitative bias analysis. Int J Epidemiol 2014;43:1969–85. [DOI] [PubMed] [Google Scholar]
- 81. Tromp M, Ravelli AC, Bonsel GJ, Hasman A, Reitsma JB.. Results from simulated data sets: probabilistic record linkage outperforms deterministic record linkage. J Clin Epidemiol 2011;64:565–72. [DOI] [PubMed] [Google Scholar]
- 82. Harron K, Wade A, Gilbert R, Muller-Pebody B, Goldstein H.. Evaluating bias due to data linkage error in electronic healthcare records. BMC Med Res Methodol 2014;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pierce BL, VanderWeele TJ.. The effect of non-differential measurement error on bias, precision and power in Mendelian randomization studies. Int J Epidemiol 2014;43:1383–93. [DOI] [PubMed] [Google Scholar]
- 84. Barendse W. The effect of measurement error of phenotypes on genome wide association studies. BMC Genomics 2011;12:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gryparis A, Paciorek CJ, Zeka A, Schwartz J, Coull BA.. Measurement error caused by spatial misalignment in environmental epidemiology. Biostatistics 2009;10:258–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sanderson E, Macdonald-Wallis C, Davey Smith G.. Negative control exposure studies in the presence of measurement error: implications for attempted effect estimate calibration. Int J Epidemiol 2018;47:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fosgate G. Non-differential measurement error does not always bias diagnostic likelihood ratios towards the null. Emerg Themes Epidemiol 2006;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. de Groot JAH, Bossuyt PMM, Reitsma JB. et al. Verification problems in diagnostic accuracy studies: consequences and solutions. BMJ 2011;343:d4770. [DOI] [PubMed] [Google Scholar]
- 89. Joseph L, Gyorkos TW, Coupal L.. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol 1995;141:263–73. [DOI] [PubMed] [Google Scholar]
- 90. Pajouheshnia R, van Smeden M, Peelen LM, Groenwold R.. How variation in predictor measurement affects the discriminative ability and transportability of a prediction model. J Clin Epidemiol 2019;105:136–41. [DOI] [PubMed] [Google Scholar]
- 91. Luijken K, Groenwold RHH, Van Calster B, Steyerberg EW, van Smeden M.. Impact of predictor measurement heterogeneity across settings on the performance of prediction models: a measurement error perspective. Stat Med 2019;38:3444–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nab L, Groenwold RHH, Welsing PM, van Smeden M.. Measurement error in continuous endpoints in randomised trials: problems and solutions. Stat Med 2019;38:5182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis 2008;66:150–54. [PubMed] [Google Scholar]
- 94. Hernan MA, Robins JM.. Causal Inference. Boca Raton, FL: Chapman & Hall/CRC, 2020. (forthcoming). [Google Scholar]
- 95. Agniel D, Kohane IS, Weber GM.. Biases in electronic health record data due to processes within the healthcare system: retrospective observational study. BMJ 2018; k1479. [DOI] [PMC free article] [PubMed] [Google Scholar]