Abstract
Rationale: Acute kidney injury (AKI), a common complication of sepsis, is associated with substantial morbidity and mortality and lacks definitive disease-modifying therapy. Early, reliable identification of at-risk patients is important for targeted implementation of renal protective measures. The updated Pediatric Sepsis Biomarker Risk Model (PERSEVERE-II) is a validated, multibiomarker prognostic enrichment strategy to estimate baseline mortality risk in pediatric septic shock.
Objectives: To assess the association between PERSEVERE-II mortality probability and the development of severe, sepsis-associated AKI on Day 3 (D3 SA-AKI) in pediatric septic shock.
Methods: We performed secondary analysis of a prospective observational study of children with septic shock in whom the PERSEVERE biomarkers were measured to assign a PERSEVERE-II baseline mortality risk.
Measurements and Main Results: Among 379 patients, 65 (17%) developed severe D3 SA-AKI. The proportion of patients developing severe D3 SA-AKI increased directly with increasing PERSEVERE-II risk category, and increasing PERSEVERE-II mortality probability was independently associated with increased odds of severe D3 SA-AKI after adjustment for age and illness severity (odds ratio, 1.4; 95% confidence interval, 1.2–1.7; P < 0.001). Similar associations were found between increasing PERSEVERE-II mortality probability and the need for renal replacement therapy. Lower PERSEVERE-II mortality probability was independently associated with increased odds of renal recovery among patients with early AKI. A newly derived model incorporating the PERSEVERE biomarkers and Day 1 AKI status predicted severe D3 SA-AKI with an area under the received operating characteristic curve of 0.95 (95% confidence interval, 0.92–0.98).
Conclusions: Among children with septic shock, the PERSEVERE biomarkers predict severe D3 SA-AKI and identify patients with early AKI who are likely to recover.
Keywords: sepsis, septic shock, acute kidney injury, precision medicine
At a Glance Commentary
Scientific Knowledge on the Subject
Acute kidney injury is a common complication of pediatric septic shock that is known to be associated with poor outcomes; however, no effective disease-modifying therapies have been identified. Consequently, implementation of renal-protective strategies and supportive care remain the mainstays of therapy, but we currently lack adequate tools to identify patients most likely to benefit from these interventions.
What This Study Adds to the Field
We developed a clinically useful tool for the prediction of pediatric sepsis–associated acute kidney injury using the Pediatric Sepsis Biomarker Risk Model (PERSEVERE) biomarkers and clinical data collected in the first 24 hours of admission. Pending prospective validation, this tool could be utilized to identify both high- and low-risk patients to personalize care and inform future clinical trial enrollment.
Septic shock is a common and consequential diagnosis in critically ill adults and children that is associated with significant morbidity, mortality, and substantial cost to the healthcare system (1–3). Similarly, acute kidney injury (AKI) affects up to half of patients admitted to the ICU, increasing the risk of poor outcomes in those afflicted (4–6). Given the substantial independent consequences of these conditions, it is not surprising that the coincidence of septic shock and AKI is associated with even worse outcomes than either condition alone, with mortality rates as high as 70% cited in some studies (7–11). Sepsis-associated AKI (SA-AKI) is a common phenomenon that affects up to half of all critically ill patients admitted with a diagnosis of septic shock (7–11).
Compounding the significant consequences of SA-AKI is the lack of available disease-modifying therapies once present. As a result, the mainstay of therapy remains supportive care aimed at renal protection, which is likely more effective if coupled with early detection of at-risk patients (7). Although epidemiologic data are available to suggest certain patient characteristics that may predispose to SA-AKI (12–14), more advanced attempts to identify those at risk have not resulted in a reliable prediction strategy thus far (15–22). Consequently, SA-AKI remains difficult to accurately predict in a time frame that is clinically useful, resulting in late application of renal-protective measures that have uncertain benefit.
Part of the difficulty in both predicting SA-AKI and developing successful treatment strategies is the complex pathophysiology of the disease itself. Sepsis is known to be a heterogeneous disease process (23, 24); therefore, it is reasonable to suspect that SA-AKI is similarly heterogeneous (7, 25, 26). As such, it is likely that the development of precision medicine strategies for SA-AKI will rely on an understanding of the underlying sepsis molecular signature of a given patient.
The Pediatric Sepsis Biomarker Risk Model (PERSEVERE) is a validated multibiomarker stratification tool for estimating the baseline risk of mortality among children with septic shock (27, 28). PERSEVERE-II is an updated version of the model that incorporates admission platelet count as an additional predictor variable and that was recently shown to outperform PERSEVERE in a prospectively enrolled cohort (27).
Given the knowledge gaps related to SA-AKI pathophysiology and prediction, we examined the association between PERSEVERE-II mortality probability and the development of severe SA-AKI at Day 3 of septic shock (D3 SA-AKI), a clinically relevant time point in the evolution of AKI (29). Furthermore, we sought to develop a model for estimating the risk of severe D3 SA-AKI, based on the premise that a reliable model would have clinical utility for targeted implementation of renal protective measures. Some results of this study have been presented previously in the form of abstracts (30, 31).
Methods
Study Design and Patient Selection
We conducted a secondary analysis of a prospective observational cohort study of children admitted with septic shock to 14 pediatric ICUs (PICUs) in the United States from January 2015 to December 2018 (27). The study protocol was approved by the institutional review board at each participating institution and was previously described in detail (28, 32). Briefly, patients between the ages of 1 week and 18 years were enrolled after obtaining informed consent from parents or legal guardians. The inclusion criteria were based on pediatric-specific consensus criteria (33); there were no exclusion criteria other than the inability to obtain informed consent. Clinical and laboratory data were collected daily while in the PICU. Mortality was tracked for 28 days after enrollment. PICU-free days were calculated by subtracting the number of days in the PICU from a maximum of 28 days. Patients with a PICU length of stay greater than 28 days or those who died by 28 days were assigned a value of 0 PICU-free days.
Patients from the original study (n = 461) were included in the analyses if they had serum creatinine (SCr) data available from both admission (Day 1) and Day 3 of septic shock (n = 328). We also included patients who had been discharged from the PICU alive before Day 3 and without evidence of AKI (n = 51); these patients were presumed not to have AKI on Day 3. We excluded patients without any SCr data, those still admitted to the PICU on Day 3 of septic shock but with missing Day 3 SCr values, those discharged from the PICU alive before Day 3 with evidence of AKI, and those with preexisting kidney disease (n = 82).
Primary Outcome
The primary outcome of interest was the development of severe D3 SA-AKI. Severe SA-AKI was defined as Kidney Disease Improving Global Outcomes (KDIGO) stage 2 AKI or higher, which is at least a twofold increase in SCr from baseline (25). Day 3 was selected based on the definition of persistent AKI provided by the 16th Consensus Conference of the Acute Dialysis Quality Initiative, which is kidney injury sustained beyond 48 hours, well documented to be associated with poor outcomes (29).
Baseline SCr was estimated for each patient using calculated body surface area (in square meters) and an estimated glomerular filtration rate of 120 mL/min per 1.73 m2, as validated in the literature (6, 34). When patient height data were unavailable (n = 20), the age-based Pottel method was used; this method was recently validated as a reliable height-independent method for estimating baseline SCr in children (35).
Secondary Outcomes
Secondary outcomes included the incidence of renal replacement therapy (RRT) use and of renal recovery by Day 3 of septic shock. Renal recovery was defined as any improvement of AKI stage by Day 3 among patients with early AKI, which was defined as KDIGO stage 1 or higher on Day 1 or 2 of septic shock.
PERSEVERE Biomarkers and Baseline Mortality Risk Assignment
The PERSEVERE biomarkers—C-C chemokine ligand 3, GZMB (granzyme B), HSPA1B (heat shock protein 70 kD 1B), IL-8, and matrix metallopeptidase 8—were measured from serum samples obtained within 24 hours of a septic shock diagnosis, as described previously (27, 28). Using the biomarker data and admission platelet counts, each patient was classified according to the previously published and validated PERSEVERE-II decision tree, which was derived using classification and regression tree methodology (36). Using prespecified biomarker concentration and platelet count decision rules, the PERSEVERE-II decision tree assigns patients to terminal nodes, each of which provides a prespecified baseline mortality probability ranging from 0.000 to 0.571. The PERSEVERE-II–derived mortality probabilities were used for the logistic regression and modeling procedures described next. In other analyses, patients were grouped into one of three previously defined PERSEVERE-II–based categorical mortality risk strata: low risk (mortality probability, 0.000–0.019), intermediate risk (mortality probability, 0.167–0.189), and high risk (mortality probability, 0.300–0.571) (27).
Statistical Analysis and Modeling
Data were initially described using medians, interquartile ranges, frequencies, and percentages. Comparisons between groups were performed using Wilcoxon rank sum, χ2, or Fisher exact test, as appropriate. Multivariable logistic regression analyses were performed to assess the ability of PERSEVERE-II mortality probability to predict the development of severe D3 SA-AKI, the need for RRT use, and Day 3 renal recovery, after correction for severity of illness (Pediatric Risk of Mortality III score [PRISM III score], which incorporates 17 physiologic variables to predict PICU mortality risk) and patient age, which are potential confounders of interest. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using Sigmaplot 14.0 (Systat Software).
We used classification and regression tree methodology to derive a new model estimating the probability of severe D3 SA-AKI (Salford Predictive Modeler v8.0; Salford Systems). Predictor variables included Day 1 KDIGO AKI stage, PERSEVERE-II mortality probability, and the PERSEVERE biomarkers. We pruned terminal nodes having <5% of the patients in the root node and terminal nodes that did not improve classification, based on the class probability method. Weighting of cases and costs for misclassification was not used. Performance of the derived model is reported using diagnostic test statistics and was tested using a 10-fold cross-validation procedure. Model performance was further tested using a historical cohort of children with septic shock previously reported in a study evaluating the association between hyperchloremia and severe D3 SA-AKI (37). The historical cohort was enrolled between 2002 and 2015. Among the 691 participants reported in that previous study, 461 had PERSEVERE-II data, and among those were 102 (21%) with D3 SA-AKI. Net reclassification improvement (NRI) was used to estimate the incremental predictive ability of the newly derived model, relative to knowing the Day 1 AKI status alone (38). The NRI was computed using the R package Hmisc (39).
Results
Baseline Characteristics
The study cohort consisted of 379 patients, of whom 65 (17%) had severe D3 SA-AKI. Table 1 provides the clinical and demographic characteristics of the cohort according to the presence of severe D3 SA-AKI. Compared with those without severe D3 SA-AKI, patients with severe D3 SA-AKI had higher PRISM III scores, higher PERSEVERE-II mortality probabilities, higher 28-day mortality, greater PICU length of stay, and fewer PICU-free days. No other differences were noted.
Table 1.
Clinical and Demographic Variables according to the Presence of Severe AKI on Day 3 of Septic Shock
| Variable | No Severe D3 SA-AKI | Severe D3 SA-AKI | P Value |
|---|---|---|---|
| n (%) | 314 (83) | 65 (17) | — |
| Age, yr* | 6.5 (2.5–12.4) | 5.1 (1.3–13.5) | 0.32 |
| Sex, M, n (%) | 162 (52) | 33 (51) | 0.99 |
| PRISM III* | 10 (6–14) | 14 (10–20.5) | <0.001 |
| PERSEVERE II mortality risk* | 0.007 (0.007–0.167) | 0.189 (0.019–0.33) | <0.001 |
| Organism, n (%) | |||
| Gram positive | 68 (22) | 13 (20) | 0.90 |
| Gram negative | 77 (25) | 19 (29) | 0.52 |
| Viral | 23 (7) | 6 (9) | 0.79 |
| Fungal | 6 (2) | 4 (6) | 0.13 |
| None | 140 (45) | 23 (35) | 0.22 |
| Mortality, n (%) | 18 (6) | 24 (37) | <0.001 |
| PICU LOS, d* | 6 (3–12) | 10 (7–21) | <0.001 |
| PICU-free days* | 22 (14–25) | 5 (0–19) | <0.001 |
Definition of abbreviations: D3 SA-AKI = sepsis-associated acute kidney injury on Day 3; LOS = length of stay; PERSEVERE-II = updated Pediatric Sepsis Biomarker Risk Model; PICU = pediatric ICU; PRISM III = Pediatric Risk of Mortality score.
Continuous variables are reported as median (interquartile range).
Primary Analysis
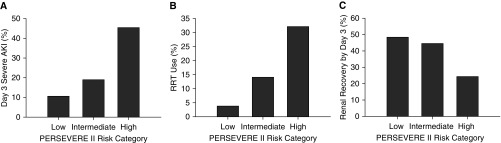
The primary analysis focused on the association between PERSEVERE-II mortality probability and severe D3 SA-AKI. The proportion of patients who developed severe D3 SA-AKI increased directly with PERSEVERE-II risk assignment category (Figure 1A and Table E1 in the online supplement). To further assess the association between PERSEVERE-II mortality probability and the development of severe D3 SA-AKI, we conducted multivariable logistic regression (Table 2). After adjusting for PRISM III and age, the PERSEVERE-II mortality probability was independently associated with increased odds of severe D3 SA-AKI.
Figure 1.
The incidence of severe sepsis-associated acute kidney injury (AKI) on Day 3, renal replacement therapy (RRT) use, and renal recovery from early AKI according to the updated Pediatric Sepsis Biomarker Risk Model (PERSEVERE-II) risk category. The cohort was grouped into one of three PERSEVERE-II risk categories: low risk (mortality risk, 0.000–0.019), intermediate risk (mortality risk, 0.167–0.189), and high risk (mortality risk, 0.300–0.571). (A) Proportion of patients with severe sepsis-associated AKI on Day 3 (P < 0.001, χ2, 2 degrees of freedom [df ]). (B) Proportion of patients requiring RRT (P < 0.001, χ2, 2 df ). (C) Proportion of patients with renal recovery by Day 3 of septic shock (P = 0.039, χ2, 2 df ).
Table 2.
Multivariable Logistic Regression Testing for an Association between PERSEVERE-II Mortality Probability and Septic Shock–associated Acute Kidney Injury Outcomes
| Outcome | Variable | n | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|---|
| Severe D3 SA-AKI | PERSEVERE-II* | 379 | 1.4 | 1.2–1.7 | <0.001 |
| PRISM III | 1.1 | 1.0–1.1 | <0.001 | ||
| Age (yr) | 1.0 | 0.9–1.0 | 0.972 | ||
| RRT use | PERSEVERE-II* | 379 | 1.6 | 1.3–2.0 | <0.001 |
| PRISM III | 1.1 | 1.0–1.1 | 0.003 | ||
| Age (yr) | 1.0 | 1.0–1.1 | 0.581 | ||
| Day 3 renal recovery | PERSEVERE-II* | 131 | 1.3† | 1.0–1.6 | 0.047 |
| PRISM III | 1.0 | 0.9–1.1 | 0.745 | ||
| Age (yr) | 1.0 | 0.9–1.1 | 0.962 |
Definition of abbreviations: CI = confidence interval; D3 SA-AKI = sepsis-associated acute kidney injury on Day 3; PERSEVERE-II = updated Pediatric Sepsis Biomarker Risk Model; PRISM III = Pediatric Risk of Mortality score; RRT = renal replacement therapy.
The raw PERSEVERE-II mortality probability was transformed by a factor of 10 for the logistic regression analyses.
Lower PERSEVERE-II mortality probability associated with increased odds of renal recovery.
Secondary Analyses
We assessed the requirement for RRT and incidence of renal recovery as secondary outcomes. Among the 379 patients in the cohort, 38 (10%) required RRT. Similar to severe D3 SA-AKI, the incidence of RRT use increased directly with increasing PERSEVERE-II mortality risk category (Figure 1B and Table E1). After adjusting for age and PRISM III, the PERSEVERE-II mortality probability was independently associated with increased odds of needing RRT (Table 2).
In total, 131 patients (35%) had early AKI. Among these patients, the proportion who had renal recovery by Day 3 decreased directly with PERSEVERE-II risk assignment category (Figure 1C and Table E1). After adjusting for PRISM III and age, a lower PERSEVERE-II mortality probability was independently associated with increased odds of renal recovery by Day 3 of septic shock (Table 2).
Estimating the Risk of Severe D3 SA-AKI
The data suggest that PERSEVERE-II, or the PERSEVERE biomarkers, could have clinical utility for identifying children at risk for severe D3 SA-AKI and those who have evidence of AKI in the first 48 hours but will recover by Day 3 of septic shock. The PERSEVERE-II mortality probability had an area under the receiver operating characteristic curve (AUROC) of 0.73 (95% confidence interval [CI], 0.66–0.79; P < 0.001) for discriminating between patients with and without severe D3 SA-AKI. This AUROC reflects a sensitivity of 62% (95% CI, 49–73%) and a specificity of 69% (95% CI, 63–74).
Although the AUROC of PERSEVERE-II for predicting severe D3 SA-AKI is statistically significant, the corresponding sensitivity and specificity are not sufficiently robust to inform clinical decision making. Accordingly, we derived a new model, using the PERSEVERE biomarkers and AKI status at admission, to more reliably estimate the risk of severe D3 SA-AKI. The severity of AKI on Day 1 of septic shock by itself had reasonable performance for discriminating between patients with and without severe D3 SA-AKI (AUROC, 0.87; 95% CI, 0.82–0.93). Accordingly, we conducted our modeling procedures with the prespecified goal of significantly improving on this performance.
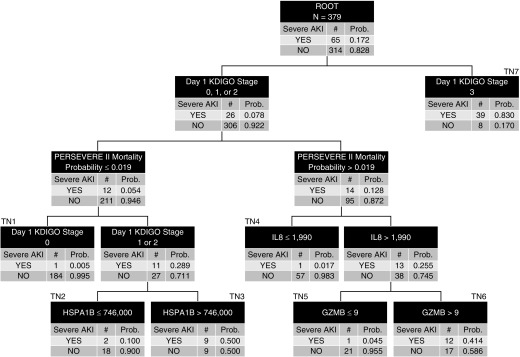
Figure 2 shows the classification and regression tree–derived model. Day 1 AKI status informed the first-level decision rule. Patients with KDIGO stage 3 AKI on Day 1 had a 0.83 probability of having severe D3 SA-AKI (terminal node 7, right side of the decision tree). The remaining patients, those with KDIGO stage 0, 1, or 2 on Day 1, occupy the left side of the decision tree. Among these patients, the PERSEVERE-II mortality probability, HSPA1B, GZMB, and IL-8 added to the predictive capacity for severe D3 SA-AKI. The AUROC of the decision tree for discriminating between patients with and without severe D3 SA-AKI was 0.95 (95% CI, 0.92–0.98). Table 3 shows the other diagnostic test characteristics of the decision tree for estimating the risk of severe D3 SA-AKI, compared with Day 1 KDIGO status alone. Among the 5 false-negative participants in the model, only 1 required RRT and all survived to 28 days.
Figure 2.
The derived decision tree for estimating the risk of severe sepsis-associated acute kidney injury on Day 3 (D3 SA-AKI). All patients (n = 379) are included in the root node at the top of the tree, with the corresponding number of those with and without D3 SA-AKI and the respective rates. Patients were subsequently allocated to daughter nodes using decision rules, as indicated in the top row of each node. All biomarker data are shown as pg/ml. Each daughter node provides the number of patients with and without D3 SA-AKI and the respective rates. Subsequent daughter nodes were generated, ending in terminal nodes (TNs). The TNs are used to assign the risk of D3 SA-AKI to a patient classified to a given TN, which is used for construction of the area under the receiver operating characteristic curve. For calculation of the diagnostic test characteristics, the D3 SA-AKI risk is dichotomized into those who are predicted to not have D3 SA-AKI and those who are predicted to have D3 SA-AKI. Patients allocated to TN1, TN2, TN4, and TN5 (D3 SA-AKI risk, 0.005–0.100) were classified as predicted to not have D3 SA-AKI. Patients allocated to TN3, TN6, and TN7 (D3 SA-AKI risk, 0.414–0.830) were predicted to have D3 SA-AKI. GZMB = granzyme B; HSPA1B = heat shock protein 70 kD 1B; KDIGO = Kidney Disease Improving Global Outcomes; PERSEVERE-II = updated Pediatric Sepsis Biomarker Risk Model; Prob. = probability.
Table 3.
Diagnostic Test Characteristics of the Newly Derived CART Model for Estimating the Risk of Severe Acute Kidney Injury on Day 3 of Septic Shock
| Variable | CART-derived Model |
Day 1 KDIGO Status Model |
||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| AUC | 0.95* | 0.92–0.98 | 0.87 | 0.82–0.93 |
| True positive, n | 60 | — | 55 | — |
| True negative, n | 280 | — | 254 | — |
| False positive, n | 34 | — | 60 | — |
| False negative, n | 5 | — | 10 | — |
| Sensitivity, % | 92 | 82–97 | 85 | 73–92 |
| Specificity, % | 89 | 85–92 | 81 | 76–85 |
| Positive predictive value, % | 64 | 53–73 | 48 | 38–57 |
| Negative predictive value, % | 98 | 96–99 | 96 | 93–98 |
| Positive likelihood ratio | 8.5 | 6.2–11.8 | 4.4 | 3.4–5.7 |
| Negative likelihood ratio | 0.09 | 0.04–0.20 | 0.2 | 0.1–0.3 |
Definition of abbreviations: AUC = area under the curve; CART = classification and regression tree; CI = confidence interval; KDIGO = Kidney Disease Improving Global Outcomes.
Ten-fold cross-validation, AUC = 0.88.
The AUROC of the derived model was superior to that of Day 1 AKI status alone (P = 0.001). The NRI of the model, relative to Day 1 AKI status alone, was 1.0 (95% CI, 0.8–1.2; P < 0.001). This NRI reflected an NRI for events of 0.3 (95% CI, 0.1–0.4; P < 0.001) and for nonevents of 0.7 (95% CI, 0.6–0.8; P < 0.001).
On 10-fold cross-validation, the decision tree had a summary AUROC of 0.88. When tested in the historical test cohort (n = 461), the decision tree had an AUROC of 0.83 (95% CI, 0.79–0.88; P < 0.001).
Discussion
In this secondary analysis of a large prospective study of critically ill children with septic shock, we found that the PERSEVERE-II baseline mortality probability was associated with development of severe D3 SA-AKI. The proportion of patients developing severe D3 SA-AKI increased directly with increasing PERSEVERE-II mortality risk category. In addition, the PERSEVERE-II mortality probability was independently associated with severe D3 SA-AKI after accounting for the potential confounders of age and illness severity. We also evaluated two related SA-AKI end points: the need for RRT and renal recovery among those with early AKI. The PERSEVERE-II mortality probability was also independently associated with these two clinically relevant outcomes.
Although the association between PERSEVERE-II and severe D3 SA-AKI was robust, it did not provide sufficient discrimination between those with and without severe D3 SA-AKI to have clinical utility. Accordingly, we derived a new model to more reliably estimate the risk of severe D3 SA-AKI, incorporating the severity of AKI on Day 1 of septic shock, the PERSEVERE-II mortality probability, and a subset of the PERSEVERE biomarkers. The model generates positive and negative likelihood ratios that meet thresholds for clinical utility (40, 41) and performed well in a 10-fold cross-validation procedure and when tested in a separate historical cohort.
Beyond estimating the risk of severe D3 SA-AKI, the model also provides insight regarding the natural history of severe SA-AKI in the pediatric septic shock population. The model shows that the presence of stage 3 AKI on Day 1 is the strongest predictor of severe D3 SA-AKI; among the 47 participants having stage 3 AKI on Day 1, 39 (83%) developed severe D3 SA-AKI, and 29 (74%) of those required RRT. This finding is perhaps not surprising from a clinical standpoint, but to our knowledge, it has not been reported previously among children with septic shock. Among the remaining subjects, those with AKI stages 0–2 on Day 1, the model was able to reliably estimate the risk of severe D3 SA-AKI. An added benefit of this estimate is the ability to identify participants with early AKI who will recover renal function; combined, terminal nodes 2, 4, and 5 identified 47 participants (12% of the whole cohort) with stage 1 or 2 AKI on Day 1 who had a low risk of severe D3 SA-AKI. These 47 participants demonstrated renal recovery by Day 3 of septic shock, as defined in the current study. We note that among the 4 false-negative participants allocated to terminal nodes 2, 4, and 5, only 1 required RRT.
Collectively, these findings suggest that the development of severe D3 SA-AKI, and renal recovery, can be predicted early in the course of septic shock through the utilization of the PERSEVERE biomarkers and clinical data collected in the first 24 hours. The importance of early recognition and proactive intervention in patients at risk for severe SA-AKI is highlighted by its association with poor outcomes and the lack of available disease-modifying therapies once present (7–10, 14, 42–44). Recognition of these consequences has resulted in several prior attempts to develop reliable enrichment strategies for SA-AKI prediction. These include the utilization of known kidney-injury biomarkers (15–18), attempts at novel candidate biomarker discovery (19), and the use of easily accessible clinical parameters (20–22). However, few attempts thus far have provided effective strategies for SA-AKI prediction with diagnostic test characteristics having clinical utility. Our prediction model meets these thresholds for clinical utility, offering a potentially clinically feasible strategy for early identification of high-risk patients who may benefit from proactive intervention.
Although early recognition of SA-AKI is considered a mainstay of therapy, no interventional studies specific to sepsis have examined the impact of early preventive care in those at risk. Our prediction model could be utilized to identify high-risk patients who would be most likely to benefit from these interventions. For example, the implementation of standardized care bundles promoting renal protection in high-risk patients has been shown to reduce AKI severity and associated morbidity in adult postsurgical patients (45, 46). Although the pathophysiology of SA-AKI is likely more complex than that of postoperative patients, a similar approach to early strategies for renal protection in those at risk for severe, persistent SA-AKI warrants consideration. In addition, the identification of patients at highest risk of requiring RRT is potentially useful given the ongoing debate surrounding the optimal timing of RRT initiation in critically ill patients, including those with sepsis, with some data suggesting that early RRT initiation improves outcomes (47–53). Finally, the identification of patients likely to have renal recovery by Day 3 of septic shock—a subset of patients about whom little data exist—is also useful because it identifies a low-risk cohort that may not require significant intervention and could inform SA-AKI clinical trial enrollment in the future. Our data provide a potential strategy to identify all 3 of these subsets of patients early and accurately, allowing for a more personalized approach to their care.
The underlying biology of SA-AKI is not well understood. Although previously thought to be secondary to renal hypoperfusion, a growing body of evidence suggests a more complex process involving the interplay between the hemodynamic consequences of sepsis and the systemic inflammatory response to infection (7, 26, 54). In this context, our findings raise the possibility that the PERSEVERE biomarkers are not simply associated with SA-AKI but are directly involved in the biological pathway leading to SA-AKI. Further study is required to formally test this assertion.
This study has several strengths. First, this large cohort reflects multiple centers across the United States, enhancing generalizability to critically ill children with septic shock as a whole. Second, this study examines the presence of severe, persistent AKI at Day 3 of septic shock, an end point that has been demonstrated to be associated with poor outcomes in critically ill patients (29). Finally, we examined the concept of renal recovery by Day 3 of septic shock, an entity with the potential to impact clinical care and inform clinical trial design but that is poorly studied to date.
Our work also has important limitations. This work is a secondary analysis of an observational study, and although our hypotheses were generated a priori, these data were not originally collected with the intent of studying SA-AKI, raising the possibility of unintended bias. Similarly, the lack of standardized criteria for the initiation of RRT across institutions and providers could also result in bias. Baseline SCr data were not available; therefore, we relied on estimated values for all patients. Furthermore, accurate urine output data were not available for all patients in our cohort, forcing us to rely on SCr-based definitions and raising the possibility that rates of SA-AKI were underestimated. Finally, given the lack of a prospective independent cohort to test the prediction model, we relied on a cross-validation procedure and testing in a historical cohort.
Conclusions
The PERSEVERE-II mortality probability is independently associated with severe D3 SA-AKI, the need for RRT use, and renal recovery from early AKI. A newly derived model has robust diagnostic test characteristics for both estimating the risk of severe D3 SA-AKI and identifying patients who will likely recover from early AKI. The model also provides insight into the natural history of SA-AKI among children with septic shock. Pending prospective independent validation, this model has the potential not only to identify patients who are most likely to benefit from early preemptive measures to mitigate the risk of SA-AKI but also to reduce exposure to more aggressive interventions such as RRT in low-risk patients and to inform enrollment procedures for clinical trials in the future.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Kelli Harmon and Patrick Lahni (Cincinnati Children’s Research Foundation) for technical assistance in the conduct of these studies, and Christopher Lindsell and Kimberly Hart (Vanderbilt University) for conducting the net reclassification improvement analysis.
Footnotes
Supported by National Institute of General Medical Sciences, R35GM126943 (H.R.W.).
Author Contributions: Study concept and design: N.L.S., E.K.S., and H.R.W. Acquisition of data: N.Z.C., S.L.W., J.C.F., M.T.B., P.N.J., A.S., R.L., J.N., G.L.A., N.J.T., J.R.G., T.B., M.Q., B.H., and H.R.W. Analysis and interpretation of data: N.L.S. and H.R.W. Drafting of the manuscript: N.L.S. and H.R.W. Critical revision of the manuscript for important intellectual content: N.L.S., E.K.S., N.Z.C., S.L.W., J.C.F., M.T.B., P.N.J., A.S., R.L., J.N., G.L.A., N.J.T., J.R.G., T.B., M.Q., B.H., and H.R.W. Statistical analysis: N.L.S. and H.R.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201911-2187OC on January 9, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191:1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 5.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Neonatal Kidney Collaborative (NKC) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–194. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35:2–11. doi: 10.1016/j.semnephrol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald JC, Basu RK, Akcan-Arikan A, Izquierdo LM, Piñeres Olave BE, Hassinger AB, et al. Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network. Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med. 2016;44:2241–2250. doi: 10.1097/CCM.0000000000002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 13.Bagshaw SM, George C, Bellomo R ANZICS Database Management Committee. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald JC, Ross ME, Thomas NJ, Weiss SL, Balamuth F, Anderson AH. Risk factors and inpatient outcomes associated with acute kidney injury at pediatric severe sepsis presentation. Pediatr Nephrol. 2018;33:1781–1790. doi: 10.1007/s00467-018-3981-8. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Kim H-J, Ahn H-S, Song JY, Um T-H, Cho C-R, et al. Is plasma neutrophil gelatinase-associated lipocalin a predictive biomarker for acute kidney injury in sepsis patients? A systematic review and meta-analysis. J Crit Care. 2016;33:213–223. doi: 10.1016/j.jcrc.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Tu Y, Wang H, Sun R, Ni Y, Ma L, Xv F, et al. Urinary netrin-1 and KIM-1 as early biomarkers for septic acute kidney injury. Ren Fail. 2014;36:1559–1563. doi: 10.3109/0886022X.2014.949764. [DOI] [PubMed] [Google Scholar]
- 18.Honore PM, Nguyen HB, Gong M, Chawla LS, Bagshaw SM, Artigas A, et al. Sapphire and Topaz Investigators. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med. 2016;44:1851–1860. doi: 10.1097/CCM.0000000000001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu RK, Standage SW, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, et al. Identification of candidate serum biomarkers for severe septic shock-associated kidney injury via microarray. Crit Care. 2011;15:R273. doi: 10.1186/cc10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song J, Wu W, He Y, Lin S, Zhu D, Zhong M. Value of the combination of renal resistance index and central venous pressure in the early prediction of sepsis-induced acute kidney injury. J Crit Care. 2018;45:204–208. doi: 10.1016/j.jcrc.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Lee C-W, Kou H-W, Chou H-S, Chou H-H, Huang S-F, Chang C-H, et al. A combination of SOFA score and biomarkers gives a better prediction of septic AKI and in-hospital mortality in critically ill surgical patients: a pilot study. World J Emerg Surg. 2018;13:41. doi: 10.1186/s13017-018-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Bai Y, Wang X, Yang J, Fu P, Cai D, et al. A simple risk score for prediction of sepsis associated-acute kidney injury in critically ill patients. J Nephrol. 2019;32:947–956. doi: 10.1007/s40620-019-00625-y. [DOI] [PubMed] [Google Scholar]
- 23.Atreya MR, Wong HR. Precision medicine in pediatric sepsis. Curr Opin Pediatr. 2019;31:322–327. doi: 10.1097/MOP.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol. 2020;16:20–31. doi: 10.1038/s41581-019-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 26.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong HR, Caldwell JT, Cvijanovich NZ, Weiss SL, Fitzgerald JC, Bigham MT, et al. Prospective clinical testing and experimental validation of the pediatric sepsis biomarker risk model. Sci Transl Med. 2019;11:eaax9000. doi: 10.1126/scitranslmed.aax9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong HR, Salisbury S, Xiao Q, Cvijanovich NZ, Hall M, Allen GL, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16:R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 30.Stanski NL, Stenson EK, Wong HR. PERSEVERE-II predicts acute kidney injury and renal recovery in children with septic shock [abstract]. Presented at the 25th International Conference on Advances in Critical Care Nephrology AKI and CRRT, February 24–27, 2020, San Diego, CA.
- 31.Stanski NL, Stenson EK, Wong HR. PERSEVERE-II predicts acute kidney injury and renal recovery in children with septic shock [abstract]. Presented at the 49th Critical Care Congress, February 16–19, 2020, Orlando, FL.
- 32.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, et al. Genomics of Pediatric SIRS/Septic Shock Investigators. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein B, Giroir B, Randolph A International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 34.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy J-P, Johnson C, Towne B, Menke F, Kiger S, Young W, et al. Use of height-independent baseline creatinine imputation method with renal angina index. Pediatr Nephrol. 2019;34:1777–1784. doi: 10.1007/s00467-019-04294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, et al. Pediatric sepsis biomarker risk model-II: redefining the pediatric sepsis biomarker risk model with septic shock phenotype. Crit Care Med. 2016;44:2010–2017. doi: 10.1097/CCM.0000000000001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenson EK, Cvijanovich NZ, Allen GL, Thomas NJ, Bigham MT, Weiss SL, et al. Hyperchloremia is associated with acute kidney injury in pediatric patients with septic shock. Intensive Care Med. 2018;44:2004–2005. doi: 10.1007/s00134-018-5368-5. [DOI] [PubMed] [Google Scholar]
- 38.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderbilt biostatistics wiki. Hmisc Package. Nashville, TN: Vanderbilt University [revised 2018 Sep; accessed 2019 Oct 4]. Available from: http://biostat.mc.vanderbilt.edu/wiki/Main/Hmisc.
- 40.Gallagher EJ. Clinical utility of likelihood ratios. Ann Emerg Med. 1998;31:391–397. doi: 10.1016/s0196-0644(98)70352-x. [DOI] [PubMed] [Google Scholar]
- 41.Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet. 2005;365:1500–1505. doi: 10.1016/S0140-6736(05)66422-7. [DOI] [PubMed] [Google Scholar]
- 42.Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13:176–181. doi: 10.1016/j.ijid.2008.05.1231. [DOI] [PubMed] [Google Scholar]
- 43.Lima RSA, Marques CN, Silva Júnior GB, Barbosa AS, Barbosa ES, Mota RMS, et al. Comparison between early and delayed acute kidney injury secondary to infectious disease in the intensive care unit. Int Urol Nephrol. 2008;40:731–739. doi: 10.1007/s11255-008-9352-9. [DOI] [PubMed] [Google Scholar]
- 44.Oppert M, Engel C, Brunkhorst F-M, Bogatsch H, Reinhart K, Frei U, et al. German Competence Network Sepsis (Sepnet) Acute renal failure in patients with severe sepsis and septic shock: a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23:904–909. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 45.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018;267:1013–1020. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 47.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 48.Yang X-M, Tu G-W, Zheng J-L, Shen B, Ma G-G, Hao G-W, et al. A comparison of early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. BMC Nephrol. 2017;18:264. doi: 10.1186/s12882-017-0667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Li H, Zhang D. Timing of continuous renal replacement therapy in patients with septic AKI: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16800. doi: 10.1097/MD.0000000000016800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Fernández X, Sabater-Riera J, Sileanu FE, Vázquez-Reverón J, Ballús-Noguera J, Cárdenas-Campos P, et al. Clinical variables associated with poor outcome from sepsis-associated acute kidney injury and the relationship with timing of initiation of renal replacement therapy. J Crit Care. 2017;40:154–160. doi: 10.1016/j.jcrc.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Gulla KM, Sachdev A, Gupta D, Gupta N, Anand K, Pruthi PK. Continuous renal replacement therapy in children with severe sepsis and multiorgan dysfunction: a pilot study on timing of initiation. Indian J Crit Care Med. 2015;19:613–617. doi: 10.4103/0972-5229.167044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh HJ, Kim MH, Ahn JY, Ku NS, Park JT, Han SH, et al. Can early initiation of continuous renal replacement therapy improve patient survival with septic acute kidney injury when enrolled in early goal-directed therapy? J Crit Care. 2016;35:51–56. doi: 10.1016/j.jcrc.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 53.Gaudry S, Quenot J-P, Hertig A, Barbar SD, Hajage D, Ricard J-D, et al. Timing of renal replacement therapy for severe acute kidney injury in critically ill patients. Am J Respir Crit Care Med. 2019;199:1066–1075. doi: 10.1164/rccm.201810-1906CP. [DOI] [PubMed] [Google Scholar]
- 54.Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14:217–230. doi: 10.1038/nrneph.2017.184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.