Abstract
Rationale: Admissions to ICUs are common during terminal hospitalizations, but little is known about how ICU care affects the end-of-life experience for patients dying in hospitals and their families.
Objectives: We measured the association between ICU care during terminal hospitalization and family ratings of end-of-life care for patients who died in 106 Veterans Affairs hospitals from 2010 to 2016.
Methods: Patients were divided into four categories: no-ICU care, ICU-only care, mixed care (died outside ICU), and mixed care (died in ICU). Multivariable linear probability models were adjusted for patient and hospital characteristics. Patients receiving mixed care were also analyzed based on percentage of time in ICU.
Measurements and Main Results: Of 57,550 decedents, 28,062 (48.8%) had a survey completed by a family member or close contact. In adjusted models, ICU-only care was associated with more frequent optimal ratings than no-ICU care, including overall excellent care (56.6% vs. 48.1%; P < 0.001), care consistent with preferences (78.7% vs. 72.4%; P < 0.001), and having pain controlled (51.3% vs. 46.7%; P < 0.001). Among patients with mixed care, increasing ICU time was associated with higher ratings on these same measures (all P < 0.001 for comparisons of those spending >75% time in ICU vs. ≤25% time).
Conclusions: Among hospital decedents, ICU care was associated with higher family ratings of quality of end-of-life care than ward care. Reducing ICU use among hospital decedents may not improve end-of-life quality, and efforts to understand how ICU care improves end-of-life quality could help provide better care outside ICUs.
Keywords: palliative care, health services research, terminal care
At a Glance Commentary
Scientific Knowledge on the Subject
Dying in the ICU is commonly viewed as an unfavorable end-of-life outcome. However, among patients who die in the hospital, little is known about how ICU use may affect ratings of end-of-life care.
What This Study Adds to the Field
Compared with ward-based care, ICU care is associated with higher family ratings of overall care, pain management, clinician–family communication, emotional support, and spiritual support.
Treatment in an ICU remains common near the end of life (1–4), with nearly 30% of Medicare beneficiaries receiving ICU care in their final 30 days (3). Reducing such late ICU use has been an important research and policy objective because doing so is postulated to reduce costs and improve the quality of the end-of-life experience for patients and their families (5–8). For example, ICU care has been associated with low family ratings of end-of-life care for cancer patients, possibly because it increases the likelihood of experiencing aggressive care (9). For these and other reasons, multiple studies have focused on reducing ICU utilization for patients at high risk of death (8, 10, 11), and ICU utilization near the end of life has been used as a measure of low-quality care and high end-of-life treatment intensity (12–15).
A contrasting view is that, for some patients, dying in an ICU may represent goal-concordant care (16, 17). Furthermore, ICUs might actually increase care quality because they offer more favorable staffing ratios and other features that may improve symptom management and other facets of care (18). However, although dying in a hospital has been associated with lower quality of care than dying at home or in nonacute institutional settings (9, 19, 20), only limited evidence, predominantly among patients with cancer, is available regarding the effect of ICU care specifically (9, 20, 21). Thus, to guide clinicians who influence ICU triage, seriously ill patients and their caregivers who may be asked to consider whether they would want ICU care, and investigators and quality measure developers, we sought to quantify relationships between ICU care and family ratings of end-of-life care among decedents in Veterans Affairs (VA) acute care hospitals.
Methods
Data Sources
Three data sources were used for this study. The first was the Bereaved Family Survey (BFS), which, 2 to 6 weeks after a patient’s death, is distributed by telephone or mail to a family member or close contact of every veteran who dies in a VA acute care hospital or other VA institutional setting, such as inpatient VA hospices and nursing homes (22–25). The BFS asks the patient’s next of kin to evaluate the quality of care in the last month of life and is used for operational purposes to monitor end-of-life care in VA facilities. The BFS overall care measure has been endorsed as an end-of-life quality measure by the National Quality Forum (26). The second source was data on patient and hospitalization characteristics extracted from the VA Corporate Data Warehouse. Finally, two care process measures (do-not-resuscitate status and chaplain visits) were abstracted through chart review for patients who died from 2010 to 2013.
Institutional review board approval was obtained from the Corporal Michael J. Crescenz Philadelphia VA Medical Center.
Study Cohort
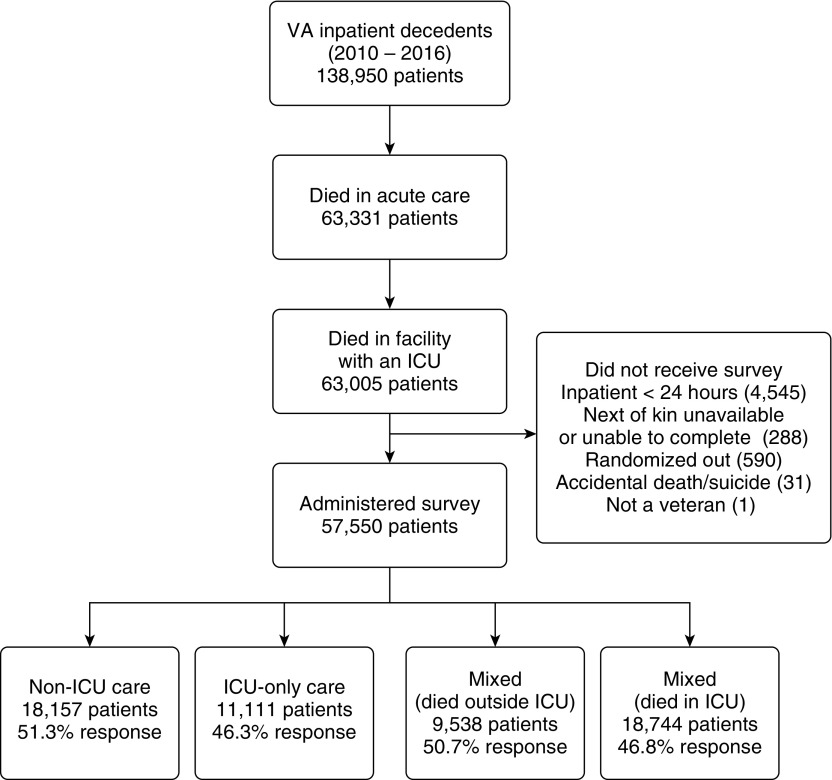
The study included veterans who died in VA acute care hospitals with ICUs from January 1, 2010, to December 31, 2016. Thirty patients were excluded because of missing data on the location of death, and three were eliminated because they were misattributed as dying in the ICU of a facility without an ICU. Of the remaining 63,005 decedents, 5,455 were not sent the BFS for reasons described in Figure 1, including mainly ineligibility due to hospitalization <24 hours. Thus, 57,550 decedents in all VA facilities with ICUs were eligible for inclusion. Of those, 28,062 patients had a close contact complete the BFS, and these patients were included in BFS analyses.
Figure 1.
Study population and sample. Shown is the total number of VA inpatient decedents across all levels of care (January 1, 2010–December 31, 2016), the number who were administered the Bereaved Family Survey, and the response rate by category. VA = Veterans Affairs.
Outcome Measures
The BFS asks respondents to rate several aspects of the patient’s care in the last month of life on a 4-point or 5-point Likert scale. The primary outcome in this study was the global rating of care, dichotomized as “excellent” versus “very good” to “poor.” Secondary outcomes included eight measures of individual aspects of care, including pain control, patient and family communication, emotional support, and spiritual support (see Table E1 in the online supplement). This approach followed prior work with the BFS (20–22, 27, 28). Outcomes and categorizations were all selected before analysis.
Exposure Variables
During terminal hospitalizations, decedents received care in one or more settings of care. ICU and non-ICU acute locations were identified based on the patient’s bed unit and specialty of primary service. Patients were classified as being in the ICU at a given time if the bed unit, specialty of care, or both indicated ICU or intensive care. Non-ICU days were primarily those in medical and surgical ward units but also included inpatient observation, telemetry units, and step-down units. Patients were grouped into four exposure categories based on the type of care they received during terminal hospitalization: no-ICU care, ICU-only care, mixed care (died outside ICU), and mixed care (died in ICU). We excluded patients who died in other institutional settings, such as an inpatient hospice or a nursing home.
Statistical Analysis and Survey Weights
In primary analyses, patients with ICU-only care were compared with patients receiving no-ICU care. Patients receiving mixed care who died in an ICU versus a ward were compared with these other categories in secondary analyses. Pearson’s χ2 was used for categorical variables and one-way ANOVA for normally distributed continuous variables. Given the large sample sizes, we also calculated standardized differences. We then fit separate multivariable linear regression models for the primary outcome and eight secondary outcomes, treating ICU category as the primary exposure and adjusting for patient characteristics, including age, sex, race, relationship of next of kin, inpatient or outpatient palliative care consultation in the last 90 days of life, the length of terminal hospitalization, the number of care transitions (i.e., change in specialty of primary service and/or physical location), and Elixhauser comorbidities (29). We also included fixed effects for year and facility to mitigate confounding and account for within-facility correlation of outcomes. Linear probability models are an appropriate alternative to logistic models with binary outcomes in most circumstances, provided the outcomes of interest are not rare events (30). To assess if model choice influenced results, we fit logistic models for all outcomes in the no-ICU to ICU-only comparison. Because no adjusted probabilities changed by >10% and all results remained statistically significant, we present results from the linear models. We deliberately excluded from our models essential distinguishing characteristics of ward or ICU settings of care, such as differences in nursing staff ratios, which could mediate causal relationships between the care setting and our outcomes of interest.
Our approach enabled us to obtain adjusted proportions of patients in each exposure category for whom the next-of-kin reported “excellent” (for the primary outcome) or “always” for the measure of interest. The one exception was pain control, for which we calculated adjusted proportions of patients with pain controlled (no pain or not uncomfortable from pain). We report 95% confidence intervals for each estimate and the P value for the two-way comparison of interest. A P value of 0.05 was considered statistically significant.
We also analyzed patients receiving mixed care based on the percentage of hospitalization time spent in the ICU, measured in quartiles (≤25%, 26–50%, 51–75%, and >75%). We fit multivariable linear probability models with percentage of ICU time by quartile as the exposure variable, adjusting for patient and hospitalization characteristics and fixed effects for year and facility. We then created adjusted proportions of favorable outcomes by ICU time and reported 95% confidence intervals. We further reported P values for differences between the highest and lowest quartile.
Models were weighted for survey nonresponse as in past BFS studies (20, 22, 27, 28). Nonresponse weights were created by fitting multivariable logistic regression models predicting survey response as a function of decedent characteristics. Estimates were used to create inverse probability weights (31). Among survey respondents, item nonresponse was <4% for all outcomes except pain control, for which it was 18.8%, primarily due to respondents being unsure about the decedent’s pain level (16.4%).
Sensitivity Analyses
We performed five sensitivity analyses. First, because of large differences in the number of care transitions between no-ICU and ICU-only care, we compared outcomes among decedents who had no care transitions during their terminal hospitalization. Second, to assess for the possibility that results might be influenced by lower complexity facilities with low-intensity ICUs, we analyzed outcomes by category for patients in high-complexity facilities alone, which correspond to VA complexity level 1 facilities with high patient risk, high teaching and/or research, and generally the highest intensity ICUs. Third, because palliative care consultation in the last 90 days of life could be considered a mediator rather than confounder, we refit models without controlling for such consultations. Fourth, because patients with serious illness may have different end-of-life experiences from those with unexpected acute illness, we reran the primary model among a population of patients with serious illness, which included those with cancer, congestive heart failure, chronic obstructive pulmonary disease, dementia, and end-stage renal disease. Patients were classified using the primary admission diagnosis code, based on a modified version of a classification employed in prior work with the BFS (28). Finally, we excluded patients who had surgery within 1 calendar day of the date of admission, thereby mitigating any potential confounding by differences in the postoperative composition of no-ICU and ICU-only patients.
All analyses were performed with Stata version 15.1 (StataCorp).
Results
Decedent Characteristics
Of the 57,550 hospital decedents, 18,157 (31.5%) received no-ICU care, 11,111 (19.3%) received ICU-only care, 9,538 (16.6%) received mixed care and died outside the ICU, and 18,744 (32.6%) received mixed care and died in the ICU (Table 1). Of patients who died outside the ICU, 25,982 (93.8%) died on a general medical or surgical ward. Most standardized differences between ICU-only and no-ICU patients were small. Only age, care transitions, facility complexity, and palliative care consultation yielded standardized differences >0.20. ICU-only decedents compared with no-ICU decedents were younger (mean age, 70.1 yr vs. 75.5 yr; standardized difference, 0.46), more likely to have no care transitions (89.7% vs. 63.6%; standardized difference, 0.65), more likely to be admitted to a high-complexity facility (89.5% vs. 81.3%; standardized difference, 0.23), and less likely to have a palliative care consultation (31.4% vs. 44.2%; standardized difference, 0.27).
Table 1.
Characteristics of Veterans Affairs Patients Who Died during Acute Hospitalization
| All Decedents (N = 57,550) | No-ICU Care (n = 18,157) | ICU-Only Care (n = 11,111) | P Value | Standardized Difference | |
|---|---|---|---|---|---|
| Age, mean (SD), yr | 72.5 (11.8) | 75.5 (12.1) | 70.1 (11.4) | <0.001 | 0.46 |
| Sex, M | 56,226 (97.7) | 17,764 (97.8) | 10,819 (97.4) | 0.010 | 0.03 |
| Race | |||||
| African American | 11,017 (19.1) | 3,182 (17.5) | 2,256 (20.3) | <0.001 | 0.07 |
| White | 41,770 (72.6) | 13,547 (74.6) | 7,902 (71.1) | 0.08 | |
| Asian and other | 919 (1.6) | 248 (1.4) | 216 (1.9) | 0.04 | |
| Unknown | 3,844 (6.7) | 1,180 (6.5) | 737 (6.6) | 0.00 | |
| Next of kin | |||||
| Spouse | 22,897 (39.8) | 7,032 (38.7) | 4,669 (42.0) | <0.001 | 0.07 |
| Parent | 2,227 (3.9) | 572 (3.2) | 518 (4.7) | 0.08 | |
| Child | 18,466 (32.1) | 6,315 (34.8) | 3,226 (29.0) | 0.12 | |
| Sibling | 7,714 (13.4) | 2,187 (12.0) | 1,570 (14.1) | 0.06 | |
| Other | 6,246 (10.9) | 2,051 (11.3) | 1,128 (10.2) | 0.04 | |
| Elixhauser index, mean (SD) | 10.6 (9.8) | 11.9 (10.1) | 10.0 (9.8) | <0.001 | 0.20 |
| High-complexity facility* | 49,798 (86.5) | 14,758 (81.3) | 9,940 (89.5) | <0.001 | 0.23 |
| Length of terminal hospitalization | |||||
| Under 3 d | 13,061 (22.7) | 5,343 (29.4) | 4,313 (38.8) | <0.001 | 0.20 |
| 3–6.9 d | 14,861 (25.8) | 5,766 (31.8) | 2,915 (26.2) | 0.12 | |
| 7–13.9 d | 14,6061 (24.4) | 4,308 (23.7) | 2,057 (18.5) | 0.13 | |
| 14 d or longer | 15,567 (27.0) | 2,740 (15.1) | 1,826 (16.4) | 0.04 | |
| Number of care transitions† | |||||
| None | 21,508 (37.4) | 11,539 (63.6) | 9,969 (89.7) | <0.001 | 0.65 |
| One | 17,091 (29.7) | 4,468 (24.6) | 998 (9.0) | 0.43 | |
| Two | 9,447 (16.4) | 1,445 (8.0) | 116 (1.0) | 0.34 | |
| Three or more | 9,504 (16.5) | 705 (3.9) | 28 (0.3) | 0.25 | |
| Palliative care consultation in last 90 d of life | 21,881 (38.0) | 8,017 (44.2) | 3,486 (31.4) | <0.001 | 0.27 |
| Do-not-resuscitate order‡ | 22,459 (84.3) | 7,316 (87.5) | 4,228 (82.0) | <0.001 | 0.15 |
| Chaplain visit with patient/family§ | 23,429 (66.3) | 6,837 (60.9) | 4,396 (65.0) | <0.001 | 0.09 |
All values are shown as n (%) unless otherwise noted.
Low complexity includes Veterans Affairs facility levels 2 and 3. High complexity includes levels 1a to 1c.
Changes in physical location or specialty of primary care team. Transitions for ICU patients include transfers from one ICU setting to another (e.g., from a medical ICU to a surgical ICU or vice versa).
Data from 2010 to 2012 only.
Data from 2010 to 2013 only.
BFS
The BFS was completed by 28,062 close contacts of eligible patients (48.8%). Response rates differed slightly but statistically significantly (P < 0.001) by category, with the highest response rate in the no-ICU category (51.3%) and the lowest in the ICU-only category (46.3%). Standardized differences between patients with BFS respondents and patients without respondents were small. Only spouse and child respondent proportions had a standardized difference >0.20. There were also differences in Elixhauser score, facility complexity, and length of terminal hospitalization. Similar differences between respondents and nonrespondents were observed for no-ICU patients and ICU-only patients (see Table E3).
In adjusted models, respondents of decedents receiving ICU-only care compared with those of decedents receiving no-ICU care more commonly reported overall excellent care (56.6% vs. 48.1%; P < 0.001), and more commonly responded favorably across all eight secondary outcomes (Table 2). For example, respondents of decedents receiving ICU-only care more commonly reported care always consistent with patient and family preferences (78.7% vs. 72.4%; P < 0.001) and pain controlled (51.3% vs. 46.7%; P < 0.001). In unadjusted analyses, all BFS measures were more commonly rated as optimal for patients receiving ICU-only care, with differences in all measures being smaller than in adjusted analyses (see Tables E4 and E5).
Table 2.
Adjusted Measures of Quality of End-of-Life Care Based on a Bereaved Family Survey
| No-ICU Care (n = 9,314)* | ICU-Only Care (n = 5,141) | P Value | |
|---|---|---|---|
| Overall rating of care was excellent | 48.1 (47.0–49.2) | 56.6 (55.1–58.1) | <0.001 |
| Pain was controlled | 46.7 (45.6–47.9) | 51.3 (49.6–53.0) | <0.001 |
| Staff always provided care that patient and family wanted | 72.4 (71.4–73.5) | 78.7 (77.4–80.1) | <0.001 |
| Staff always treated patient with kindness and respect | 77.1 (76.1–78.0) | 82.4 (81.1–83.6) | <0.001 |
| Staff always took time to listen | 66.8 (65.7–67.8) | 73.8 (72.4–75.3) | <0.001 |
| Staff always provided emotional support before death | 54.9 (53.8–56.0) | 62.1 (60.5–64.6) | <0.001 |
| Staff always provided emotional support after death | 61.3 (60.2–62.4) | 68.0 (66.5–69.5) | <0.001 |
| Staff always kept patient and family informed | 62.5 (61.4–63.6) | 70.6 (69.2–72.1) | <0.001 |
| Staff always provided spiritual support | 55.2 (54.1–56.3) | 61.1 (59.6–62.7) | <0.001 |
Percentage and 95% confidence intervals are shown. Values were adjusted for age, sex, race, next of kin, Elixhauser comorbidities, length of admission, number of care transitions, palliative care consultation last 90 days of life, fixed year, and facility effects, and they were weighted for nonresponse.
One patient was excluded because of missing covariate data. Number for specific outcomes will differ based on item nonresponse.
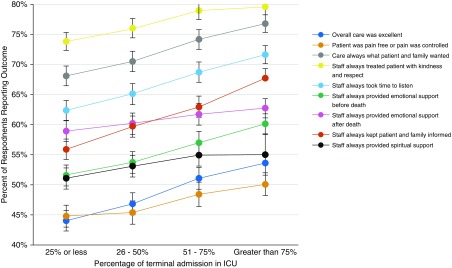
For patients receiving mixed care, increased time in the ICU was associated with higher adjusted family ratings of care (Figure 2). In comparison with decedents with ≤ 25% ICU care, decedents with >75% ICU care more commonly reported excellent overall care (53.6% for >75% time vs. 44.0% for ≤ 25% time; P < 0.001), care consistent with preferences (76.7% for >75% vs. 68.1% for ≤ 25%; P < 0.001), pain controlled (50.1% for >75% vs. 44.8% for ≤ 25%; P < 0.001), and all other outcomes. Both categories of mixed care had lower ratings than ICU-only care for most measures, including overall care. Mixed care with death outside the ICU had a higher proportion report overall excellent care than did for mixed care with death in ICU. Other comparisons between the two categories of mixed care were not significant (see Table E6).
Figure 2.
Adjusted Bereaved Family Survey responses in relation to percentage of time in ICU for patients with mixed care. Adjusted proportion of respondents reporting the outcome of interest is shown in relation to the percentage of time the decedent received ICU care. Error bars indicate 95% confidence intervals.
Sensitivity Analyses
Sensitivity analyses revealed no changes in the significance or direction of effects. First, in models comparing care by category for decedents with no care transitions, ICU-only care had statistically superior proportions of favorable ratings on all measures compared with no-ICU care (see Table E7). Second, models limited to complex facilities yielded similar results (see Table E8). Third, omitting palliative care consultation as a covariate did not change the magnitude, direction, or significance of comparisons (see Table E9). Fourth, outcomes were similar when limiting analyses to patients with chronic, serious illness, except that the difference in spiritual care was rendered nonsignificant in this subgroup (see Tables E10 and E11). Finally, excluding postoperative patients did not affect the main results (see Table E12), and comparisons restricted to postoperative patients generally produced similar results, albeit with small sample sizes (see Table E13).
Discussion
In a multiyear national cohort of VA acute care decedents, ICU care during terminal hospitalization was associated with higher family ratings of end-of-life care quality. ICU-only care was associated with higher global ratings of care, as well as higher ratings of emotional support, communication, and pain control. For patients with mixed care, an increasing percentage of time in ICU care was associated with higher ratings across all measures. Confidence in these results is enhanced by their consistency across different methods of classifying the exposure variable and multiple sensitivity analyses, and by the observation that the magnitudes of the associations were uniformly stronger following multivariable adjustment.
There are several reasons why the quality of end-of-life care may be viewed more favorably, on average, among family members of patients cared for in ICUs. First, clinical staff ratios are higher in ICUs than other acute care settings. Past work has suggested that higher nursing ratios may improve outcomes in hospitals and nursing homes (32–34). Second, ICU staff may have more experience with end-of-life care given their patient population. ICUs have been the focus of significant research and guidelines on end-of-life care (35–37). Greater experience may translate into improved communication and symptom management. Third, family members of patients receiving ICU care may take solace in knowing that all reasonable efforts to extend life were attempted. These structural characteristics of the setting of care were deliberately not included in our models because they may lie on the causal pathway with the outcomes of interest. These factors may outweigh other characteristics of ICUs that could lead to worse patient and family experiences, such as the burdens and intensity of interventions commonly provided in ICUs. However, more work is needed to understand the mediators of high-quality end-of-life care for hospitalized patients because we also found that surrogates of patients receiving mixed care more commonly reported excellent overall care when death occurred outside the ICU. Apart from the setting of death, other factors, such as unexpected changes in clinical course prompting ward-to-ICU transfers, may influence the end-of-life experience.
A noteworthy secondary finding was the high rate of uncontrolled pain. Across ICU and non-ICU care, respondents reported high ratings of emotional support, spiritual support, and preference-consistent care. Yet these same respondents indicated that approximately half of all patients were uncomfortable due to pain. This finding is consistent with prior work suggesting high unmet needs for symptom management near the end of life (28, 38).
Our study extends prior work relating site of death to the quality of end-of-life care by exploiting a unique source of population-level data from the VA that combines family ratings of care with granular data on hospitalizations. In-hospital deaths have been associated with lower quality than deaths in hospice or nursing facilities (9, 20). However, hospital-based deaths remain common in the United States (39, 40) and even more common in peer nations (1). Yet few studies have examined the association between ICU care and the quality of end-of-life care among acute-care decedents, particularly with rich family-reported data on patient-centered outcomes. One prior study of VA nursing homes using the BFS separated ICU decedents from those dying in non-ICU acute care, but this study did not directly compare ICU and non-ICU and looked only at the final location of care (20). A second study reported separate ICU and hospital bereavement scores, but only for cancer decedents followed at seven outpatient locations and again without linking to characteristics of terminal hospitalization (9).
Understanding the association between ICU care and the end-of-life experience for hospital decedents has important implications for patients. Ideally, we could eliminate nonbeneficial hospitalizations near the end-of-life, whether or not they include ICU use. However, predictions of the timing of death are imperfect (41–43), and not every end-of-life hospitalization or ICU stay is preventable (44). Seriously ill patients weighing the advantages and disadvantages of an ICU admission benefit from knowing how ICU care might affect the end-of-life experience for themselves and their families. Thus, while we continue to identify ways to reduce acute care deaths, we should simultaneously seek to understand what elements of ICU-based care improve the end-of-life experience and then extrapolate those lessons to hospital wards.
A key strength of this study is the use of a family rating instrument endorsed by the National Quality Forum to assess end-of-life care (26). Although such ratings are subject to recall bias, other measures commonly used to assess end-of-life care quality, such as rates of palliative care consultation, documentation of advance care planning, and other process measures, may not capture the patient and family experience.
Our study also has limitations. First, findings may not generalize beyond the VA system. The inclusion of more than 100 facilities nationally helps mitigate this concern. Moreover, the VA, as the largest integrated health system in the United States, offers lessons on care processes and outcomes for end-of-life care in other health systems. Although the VA has invested in improving end-of-life care, it has done so across multiple settings of care, not just in ICUs (45). However, further understanding how ICU-based care may improve quality for hospitalized patients near the end of life will require nationally representative data on family perceptions of care for hospital decedents outside the VA setting. Second, bereavement scores may imperfectly reflect actual differences in pain control, communication, and other facets of care. However, biases due to outcome misclassification are likely nondifferential across exposure groups, and, if so, the direction of bias would be toward the null. Third, we cannot rule out the possibility that unmeasured patient-level, family-level, or facility-level factors confound these results, although use of facility-fixed effects substantially reduces the possibility of facility-level confounding. The finding that ICU-only included a smaller proportion of seriously ill patients than did no-ICU, for example, may suggest that ICU differentially included those patients for whom intensive care was preference-concordant. However, the aforementioned robustness of results across multiple exposure definitions and sensitivity analyses, and the larger observed differences in care quality after adjustment for potential confounders, all reduce the likelihood of bias.
Finally, slightly under half of patients had surrogates who completed the BFS survey, and patients represented by respondents versus nonrespondents differed along multiple characteristics. Although we adjusted for nonresponse, we cannot exclude the possibility, for example, that more satisfied next of kin were more likely to respond to the survey than less satisfied individuals. Still, such bias would tend to affect absolute estimates rather than relative estimates between exposure groups. We would not expect differential bias across exposure groups and indeed found that observed differences between respondents and nonrespondents were similar for no-ICU and ICU-only patients.
Conclusions
Given the costs and burdens associated with ICU care, and evidence that many patients and families prefer death at home, our data do not suggest that ICU use should be promoted near the end of life. Our results only apply to ICU use conditional on in-hospital death. Rather, our results suggest that ICU use specifically, as opposed to in-hospital death more generally, may not be an appropriate measure of poor-quality end-of-life care or a failure of care provision for patients dying in the hospital. Perhaps more importantly, these findings suggest that instead of focusing on decreasing ICU deaths, efforts should be made to improve end-of-life care in hospital wards and other care settings, perhaps using insights into what aspects of ICU care lend value to this experience.
Supplementary Material
Footnotes
Supported by NIH grant K24 HL143289 (S.D.H.).
Author Contributions: Conception, design, and drafting of the manuscript for important intellectual content: J.A.R. and S.D.H. Acquisition of the data for the work: J.A.R. and M.E. Analysis and interpretation: all authors. Critical revision for important intellectual content: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201907-1423OC on January 15, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bekelman JE, Halpern SD, Blankart CR, Bynum JP, Cohen J, Fowler R, et al. International Consortium for End-of-Life Research (ICELR) Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315:272–283. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 2.Gozalo P, Teno JM, Mitchell SL, Skinner J, Bynum J, Tyler D, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212–1221. doi: 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teno JM, Gozalo P, Trivedi AN, Bunker J, Lima J, Ogarek J, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320:264–271. doi: 10.1001/jama.2018.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, et al. Robert Wood Johnson Foundation ICU End-Of-Life Peer Group. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 5.Luce JM, Rubenfeld GD. Can health care costs be reduced by limiting intensive care at the end of life? Am J Respir Crit Care Med. 2002;165:750–754. doi: 10.1164/ajrccm.165.6.2109045. [DOI] [PubMed] [Google Scholar]
- 6.Curtis JR, Engelberg RA, Bensink ME, Ramsey SD. End-of-life care in the intensive care unit: can we simultaneously increase quality and reduce costs? Am J Respir Crit Care Med. 2012;186:587–592. doi: 10.1164/rccm.201206-1020CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandelwal N, Curtis JR. Economic implications of end-of-life care in the ICU. Curr Opin Crit Care. 2014;20:656–661. doi: 10.1097/MCC.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano AM, Gade KE, Nielsen G, Havard R, Harrison JH, Jr, Barclay J, et al. Early palliative care reduces end-of-life intensive care unit (ICU) use but not ICU course in patients with advanced cancer. Oncologist. 2017;22:318–323. doi: 10.1634/theoncologist.2016-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28:4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med. 2015;43:1102–1111. doi: 10.1097/CCM.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11:180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61:209–214. doi: 10.1111/jgs.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Barnato AE, Farrell MH, Chang C-CH, Lave JR, Roberts MS, Angus DC. Development and validation of hospital “end-of-life” treatment intensity measures. Med Care. 2009;47:1098–1105. doi: 10.1097/MLR.0b013e3181993191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CEP, Kamal AH, Kluger M, Coke P, Kelley MJ. National trends in end-of-life care for veterans with advanced cancer in the Veterans Health Administration: 2009 to 2016. J Oncol Pract. 2019;15:e568–e575. doi: 10.1200/JOP.18.00559. [DOI] [PubMed] [Google Scholar]
- 16.Halpern SD. Goal-concordant care: searching for the holy grail. N Engl J Med. 2019;381:1603–1606. doi: 10.1056/NEJMp1908153. [DOI] [PubMed] [Google Scholar]
- 17.Hua M, Wunsch H. Placing value on end-of-life care-is it time for a new taxonomy? JAMA Netw Open. 2019;2:e1914466. doi: 10.1001/jamanetworkopen.2019.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White DB, Ernecoff N, Billings JA, Arnold R. Is dying in an ICU a sign of poor quality end-of-life care? Am J Crit Care. 2013;22:263–266. doi: 10.4037/ajcc2013604. [DOI] [PubMed] [Google Scholar]
- 19.Higgins PC, Garrido MM, Prigerson HG. Factors predicting bereaved caregiver perception of quality of care in the final week of life: implications for health care providers. J Palliat Med. 2015;18:849–857. doi: 10.1089/jpm.2015.29001.hp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ersek M, Thorpe J, Kim H, Thomasson A, Smith D. Exploring end-of-life care in Veterans Affairs community living centers. J Am Geriatr Soc. 2015;63:644–650. doi: 10.1111/jgs.13348. [DOI] [PubMed] [Google Scholar]
- 21.Ersek M, Miller SC, Wagner TH, Thorpe JM, Smith D, Levy CR, et al. Association between aggressive care and bereaved families’ evaluation of end-of-life care for veterans with non-small cell lung cancer who died in Veterans Affairs facilities. Cancer. 2017;123:3186–3194. doi: 10.1002/cncr.30700. [DOI] [PubMed] [Google Scholar]
- 22.Ersek M, Smith D, Cannuscio C, Richardson DM, Moore D. A nationwide study comparing end-of-life care for men and women veterans. J Palliat Med. 2013;16:734–740. doi: 10.1089/jpm.2012.0537. [DOI] [PubMed] [Google Scholar]
- 23.Casarett D, Pickard A, Bailey FA, Ritchie CS, Furman CD, Rosenfeld K, et al. A nationwide VA palliative care quality measure: the family assessment of treatment at the end of life. J Palliat Med. 2008;11:68–75. doi: 10.1089/jpm.2007.0104. [DOI] [PubMed] [Google Scholar]
- 24.Casarett D, Shreve S, Luhrs C, Lorenz K, Smith D, De Sousa M, et al. Measuring families’ perceptions of care across a health care system: preliminary experience with the Family Assessment of Treatment at End of Life Short Form (FATE-S) J Pain Symptom Manage. 2010;40:801–809. doi: 10.1016/j.jpainsymman.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Thorpe JM, Smith D, Kuzla N, Scott L, Ersek M. Does mode of survey administration matter? Using measurement invariance to validate the mail and telephone versions of the Bereaved Family Survey. J Pain Symptom Manage. 2016;51:546–556. doi: 10.1016/j.jpainsymman.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 26.National Quality Forum. Endorsed Measures for Person and Family-Centered Care, Measure 1623 [accessed 2020 Feb 29]. Available from: http://www.qualityforum.org/Projects/n-r/Person_and_Family_Centered_Care/Final_Report_-_Phase_1.aspx.
- 27.Kutney-Lee A, Smith D, Thorpe J, Del Rosario C, Ibrahim S, Ersek M. Race/ethnicity and end-of-life care among veterans. Med Care. 2017;55:342–351. doi: 10.1097/MLR.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 28.Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176:1095–1102. doi: 10.1001/jamainternmed.2016.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 30.Hellevik O. Linear versus logistic regression when the dependent variable is a dichotomy. Qual Quant. 2009;43:59–74. [Google Scholar]
- 31.Smith D, Kuzla N, Thorpe J, Scott L, Ersek M. Exploring nonresponse bias in the Department of Veterans Affairs’ Bereaved Family Survey. J Palliat Med. 2015;18:858–864. doi: 10.1089/jpm.2015.0050. [DOI] [PubMed] [Google Scholar]
- 32.Castle NG, Engberg J. Further examination of the influence of caregiver staffing levels on nursing home quality. Gerontologist. 2008;48:464–476. doi: 10.1093/geront/48.4.464. [DOI] [PubMed] [Google Scholar]
- 33.Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288:1987–1993. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- 34.Lang TA, Hodge M, Olson V, Romano PS, Kravitz RL. Nurse-patient ratios: a systematic review on the effects of nurse staffing on patient, nurse employee, and hospital outcomes. J Nurs Adm. 2004;34:326–337. doi: 10.1097/00005110-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 35.White DB, Angus DC, Shields A-M, Buddadhumaruk P, Pidro C, Paner C, et al. PARTNER Investigators. A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378:2365–2375. doi: 10.1056/NEJMoa1802637. [DOI] [PubMed] [Google Scholar]
- 36.Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med. 2005;171:844–849. doi: 10.1164/rccm.200409-1267OC. [DOI] [PubMed] [Google Scholar]
- 37.Sprung CL, Truog RD, Curtis JR, Joynt GM, Baras M, Michalsen A, et al. Seeking worldwide professional consensus on the principles of end-of-life care for the critically ill: the consensus for Worldwide End-of-Life Practice for Patients in Intensive Care Units (WELPICUS) study. Am J Respir Crit Care Med. 2014;190:855–866. doi: 10.1164/rccm.201403-0593CC. [DOI] [PubMed] [Google Scholar]
- 38.Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Is care for the dying improving in the United States? J Palliat Med. 2015;18:662–666. doi: 10.1089/jpm.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howell DA, Wang HI, Roman E, Smith AG, Patmore R, Johnson MJ, et al. Preferred and actual place of death in haematological malignancy. BMJ Support Palliat Care. 2017;7:150–157. doi: 10.1136/bmjspcare-2014-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer S, Min S-J, Cervantes L, Kutner J. Where do you want to spend your last days of life? Low concordance between preferred and actual site of death among hospitalized adults. J Hosp Med. 2013;8:178–183. doi: 10.1002/jhm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Einav L, Finkelstein A, Mullainathan S, Obermeyer Z. Predictive modeling of U.S. health care spending in late life. Science. 2018;360:1462–1465. doi: 10.1126/science.aar5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detsky ME, Harhay MO, Bayard DF, Delman AM, Buehler AE, Kent SA, et al. Discriminative accuracy of physician and nurse predictions for survival and functional outcomes 6 months after an ICU admission. JAMA. 2017;317:2187–2195. doi: 10.1001/jama.2017.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients BMJ 2003327195–198.12881260 [Google Scholar]
- 44.Angus DC, Truog RD. Toward better ICU use at the end of life. JAMA. 2016;315:255–256. doi: 10.1001/jama.2015.18681. [DOI] [PubMed] [Google Scholar]
- 45.Edes T, Shreve S, Casarett D. Increasing access and quality in Department of Veterans Affairs care at the end of life: a lesson in change. J Am Geriatr Soc. 2007;55:1645–1649. doi: 10.1111/j.1532-5415.2007.01321.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.