Highlights
-
•
A clear relationship exists between fly abundance and transmission of pathogens predisposing to intramammary infections in dairy cattle.
-
•
The use of deltamethrin reduced fly abundance and the possibility of transmitting pathogenic bacteria in the mammary gland.
-
•
Fly repellency aids the reduction of the use of antibiotic treatment of mastitis and therefore may minimize the antibiotic resistance (One Health).
-
•
Fly control should be included in mastitis management strategy.
Keywords: Deltamethrin, Fly repellency, Flies, Intramammary infections, Bacteria
Abstract
The aim of this study was to assess the possible effect of the fly repellent deltamethrin on the full microbiological profile of the intramammary infections as well as on the somatic cell count in one Greek dairy cattle farm under intensive management, during peak fly season. Fifty five multiparous cows, stabled in the same farm, were randomly divided in three groups; cows of Group A were dressed on their back with deltamethrin, cows of Group B remained untreated within the same pen and cows of Group C remained untreated in a separate pen. Clinical records of the herd showed a history of clinical and subclinical mastitis (especially during spring and early summer) and fly infestation. Deltamethrin reduced fly population, landing on the cows of Group A, facilitating a significant decrease of S. aureus, coagulase negative staplylococci, E. coli intramammary infections and somatic cell count, throughout the study period. Consequently, there is a relationship between fly abundance and transmission of bacteria resulting in intramammary infections and mastitis in dairy cows. The use of the pyrethroid deltamethrin reduced fly abundance and therefore, the possibility of transmitting pathogenic bacteria in the mammary gland.
1. Introduction
Intramammary infections, i.e. clinical and subclinical mastitis, remain a severe problem of dairy cattle farms, worldwide [1]. Mastitis can, directly, affect the mammary gland of the cow, leading to significant reduction of the quantity and quality of the milk and therefore, to reduced value of the production [2]. Despite decades of implementation of control strategies, mastitis continues to be one of the most significant and economically challenging problems of dairy cows [2]. According to estimations of the National Mastitis Council (USA), mastitis costs more than 2 billion $, to dairy farmers annually [3].
Mastitis is difficult to be controlled due to a variety of microorganisms that infect the udder and provoke the disease [4]. The most common microorganisms, causing mastitis, can be divided into 2 groups: a. contagious bacteria (e.g. Staphylococcus aureus, Mycoplasma spp. and Streptococcus agalactiae) and b. environmental bacteria (e.g. Streptococcus uberis, Streptococcus dysgalactiae subsp. dysgalactiae, coliforms including Escherichia coli and Klebsiella spp.) [3]. Due to difficulties in controlling intramammary infections, udder health programs are increasingly focused on the prevention of mastitis [2].
To understand, control or prevent mastitis in dairy cows, it is essential to determine the probable risk factors causing intramammary infections [5]. Factors, such as the milking process, the teat hygiene and the animals’ habitat, have been proved to enhance the probability of clinical or subclinical mastitis [[6], [7], [8], [9]]. Among the agents arising from the animals’ habitat, fly abundance and its absence of control consist a triggering factor, contributing to the infection of the mammary gland of the dairy cows [9].
During spring and early summer, flies maximize their numbers leading to severe welfare and productivity problems [10,11]. The relationship between the number of the flies and the prevalence of the intramammary infections of cows has been well established by many surveys [5,[12], [13], [14]]. Flies consist important vectors of infectious pathogens predisposing to mastitis through immediate teat contact or through teat injury [5,7,9,15]. As a result, farms where some kind of fly control was applied, there have been less mastitis cases in comparison to farms without fly control [12].
Given the impact of intramammary infections in dairy cows on the milk quantity and quality during the upcoming summer (reduced quantity as well as low fat and protein concentration) [17], it would seem helpful to reduce the excessive number of flies, and their role to transmit pathogens (e.g. S. aureus, E. coli) that are associated with this disease [16]. Little information is available on the relationship between high fly numbers of mixed genera and the variety of bacteria transmitted in the mammary gland causing mastitis in dairy cattle farms under intensive management, throughout the Mediterranean region (temperate climatic conditions).
Therefore, the aim of this study was to assess the possible effect of the fly repellent deltamethrin on the establishment rate of common bacteria (contagious, i.e. S. aureus, Mycoplasma spp. and others and environmental, i.e. coagulase negative staphylococci, E. coli and others) causing intramammary infections in a dairy cattle farm of Greece under intensive management, during peak fly season.
2. Materials and methods
2.1. Herd history
The study was conducted in one herd that was consisted of 150 dairy Holstein cows in lactation reared under intensive management in Larissa (Thessaly, Greece). The animals consumed total mixed ration (TMR) according to the nutrient requirements of their productive stage. The average milk production of the herd was approximately 27 liters per cow. The structure of the buildings of the farm permitted the animals to have adequate rest area as well as to express normal feeding and drinking behavior. The routine vaccination program included a single vaccination one month before parturition of the heifers (approximately 24 months old) and the cows (at each gestation) against rotavirus, coronavirus and Escherichia coli K99 (Rotavec Corona®, MSD).
Moreover, the herd had a history of subclinical and clinical mastitis (especially during spring and early summer) combined with fly infestation. Subclinical and clinical mastitis are defined as intramammary infections. Subclinical mastitis is characterized by the detection of more than 200.000 somatic cells/ml of each milk sample [44], while the clinical one includes a more severe picture of either the milk (i.e. flakes, clots and watery or other unusual appearance) or the udder (i.e. swellings, redness) [36], in at least one quarter of the mammary gland. None of the participated cows were treated with antimicrobials during the previous 30 days.
2.2. Experimental design
The study was conducted between May and June 2016. The experimental group consisted of only fifty five multiparous cows, because all animals had to be similar, as much as possible, in order to generate comparable results. They were between 3 to 5 years old and in lactation period 1–4 months post partum. These animals were fed with the same diet and premix, they had similar milk production and were living under the same hygiene conditions. These animals were randomly divided in 3 groups; cows of Group A (n = 25) were individually dressed once on their back with deltamethrin (Butox® 7.5 pour-on, MSD) post morning milking (Day 0). The application of Butox® 7.5 pour-on (MSD) was performed only once, because the registration instructions of this product state that it can be applied once at minimum every 4 weeks. At the same day (Day 0), placebo treatment was also applied once on the cows of Group B (n = 20, control group). All animals (n = 45) of groups A and B remained in the same pen allowing contact with each other. Additionally, another group (Group C), consisted of 10 cows, was left untreated and kept in a separate pen, a long distance away from the animals of Group A and B, in order to avoid any accidental drug transfer.
2.3. Milking routine and technical information of the milking machine
According to the milking routine of the farm, all milkers were wearing disposable plastic gloves, which were discarded after the end of the milking process of each group of cows. The teats of the cows were cleaned with warm water and dried with a paper towel individually. The foremilking (i.e. the removal of the first 2–3 lines of milk) was performed in every teat before the milking units were attached on the teats. Immediately after the end of the milking process, the vaccum supply to the clusters of the milking machine was turned off, to remove the milk units. Finally, the teats were disinfected with a solution of 1% iodine, in order to prevent the transmittance of bacteria into the teat canal.
According to manufacturer guidelines, the milking machine (Westfalia, Germany) was a “low line” milking machine inspected by authorized technical personnel every six months. Its vacuum pump was set at 41 kilopascals, the pulsation rate at 60 cycles per minute and the pulsation ratio was 60/40. Moreover, the liners of the milking machine were new as they had been changed just 2 months before the start of the trial. The cleaning process of the milking machine included the use of alkaline and acid detergents (Desintec®, Germany) at a ratio 2/1, according to manufacturer instructions. Finally, the temperature of the circulating water at the second phase of the cleaning process was 75–78 °C.
2.4. Fly monitoring
Numbers of flies landing on the animals of all groups (Group A, B, C) were recorded by direct observation of the animals. This procedure has been, previously, reported by many fly studies and is considered accurate, when the same observers are used. Thus, inter-observer inconsistencies are avoided [[18], [19], [20]]. The fly population was enumerated on half of the animal’s body from the neck to the start of the tail and from the spinous processes of the vertebrae to the belly and the lower legs [18]. Each cow of all groups (n = 55) was observed by the same blinded person from a distance of about 3–4 meters away and for 2 min. The enumeration of the fly burden, in order to access the long term repellency, was carried out at Day 0, 10, 20 and 30, at about 11:00 pm. On Day 0 an additional enumeration was done, 6 h post treatment, i.e. at about 5:00 am, in order to access the short term repellency of deltamethrin.
2.5. Meteorological data
Meteorological data for Larissa (Thessaly, Greece) were obtained from the Hellenic National Meteorological Service (Athens, Greece). The mean air temperature and mean relative humidity of the location of the farm per day of sampling were accessed to determine the possible effect of weather conditions on fly population of the farm.
2.6. Fly trapping and identification
Ten fly traps (with sticky surface) were set in locations within the pens at the animals level, but not accessible by them, at equal distances on Day 0 and 30, in order to capture and identify fly species present in the farm. These traps (after use) were put individually into plastic bags and were transported to the Laboratory of Parasitology and Parasitic Diseases of the Veterinary School of the Aristotle University of Thessaloniki, where flies were separately transferred into tightly closed containers (20 ml capacity) with a mixture of 70% ethanol and 30% glycerol [21]. Then, these specimens were identified according to morphological keys of Wall and Shearer [22] and Couri et al. [23] and their different species were enumerated.
2.7. Milk sampling
Milk samples (containing milk from all 4 quarters of each cow) were taken individually from the total (n = 55) of the cows, starting from Day 0 and every 10 days for 3 times (Day 10, 20 and 30), after the single application of Butox® pour-on in Group A. Cows were walked calmly to the milking parlor, where they were individually restrained, while milk samples were taken. Teat ends were sanitized using a cotton ball soaked in 90% isopropyl alcohol. After sample collection, teats were disinfected with a solution of 1% iodine, in order to prevent the transmittance of bacteria into the teat canal.
2.8. Somatic cell count (SCC)
The measurement of the somatic cells in each milk sample was performed with Fossomatic (Foss Company).
2.9. Microbiological profile of the milk samples
Aseptically collected milk samples (as described above) were placed in isothermal box, under cooled conditions (approx. 4 °C), and were transported to the “Vet-Analyseis” Veterinary Microbiology Laboratory, in Larissa (Thessaly, Greece), for bacteriological culture. Briefly, 50 μl of undiluted milk sample were placed on sheep blood agar (Oxoid Company) and incubated aerobically at 37 ± 1 °C for 22 ± 2 h. Samples yielding 3 or more different bacterial species on sheep blood agar were considered to be contaminated In addition, 50 μl of each undiluted milk sample as well as one tenfold dilution of each milk sample were cultured on mannitol salt agar (Oxoid Company) and on McConkey agar (Biolife Company). These plates were incubated aerobically at 37 ± 1 °C for 48 ± 2 h and for 24 ± 2 h, respectively. After the end of the incubation time, bacteria were identified according to colony morphology, gram-staining, catalase reaction and other biochemical reactions (i.e. DNAse activity, coagulase test, heamolysis pattern, lactose, catalase, indole reaction and esculin hydrolysis), as appropriate. More precisely, for Gram-positive cocci, which were cultivated in mannitol salt agar, catalase-tests were used to differentiate between catalase-positive staphylococci and catalase-negative cocci. DNAse activity, colony morphology, coagulase tube test and hemolysis pattern were used to discriminate S. aureus from CNS. Oxidase negative E. coli, which were cultivated in McConkey agar, were distinguished from other coliforms due to the lactose, catalase and indole positive activity on 44 °C. Finally, catalase as well as esculin negative streptococci, which were cultivated in sheep blood agar, were identified according to the hemolytic reaction. Colonies, on mannitol salt and McConkey agars, were counted (CFU/ml) in order to estimate the bacterial population of staphylococci (S. aureus and CNS) and E. coli [24].
The presence or absence of other environmental bacteria, such as Enterobacter spp., Citrobacter spp., Pasteurella spp., Proteus spp., and fungi was estimated according to conventional methods [25]. All the oxidase negative, lactose positive and lactose negative enterobacteriaceae colonies were subcultured from McConkey agar to the Nutrient agar (Oxoid Company) for further investigation. This process (subculture to the Nutrient agar) was repeated for the Gram negative, oxidase positive bacteria of the sheep blood agar. GN-A (convenient 12 substrates identity system for the most commonly isolated Enterobacteriaceae) and GN-B (24 substrates identity system for the complete range of Enterobacteriaceae and oxidase positive non fastidious Gram negative bacilli) tests (Microgen Company) were used in order to identify the above bacteria.
The milk samples were checked for the presence of Mycoplasma spp. using Eatons agar and broth (prepared in the laboratory). Briefly 0.1 ml of each milk sample was streaked on Eatons agar [26] and incubated for 3–7 days at 37 °C in a humidified incubator supplied with 5% CO2. Meanwhile 0.5 mL of each milk sample was incubated in 10 mL Eatons broth [26] at 37 °C aerobically. Broth-to-broth passages and subcultures to agar plates were made twice at 48 to 72-h intervals. Isolates were purified three times on solid medium, after which a single colony was selected, transferred to Eatons broth and incubated aerobically for 2–3 days. DNA extraction from mycoplasma broth cultures was carried out using the QIAmp Tissue kit (QIAGEN, Gmbh, Germany) according to the manufacturer. The DNA samples were analyzed by multiplex polymerase chain reaction (PCR) using two sets of primers, specific for Mycoplasma bovis and the M. mycoides cluster species, respectively.
2.10. Statistical analysis
Both parametric and non-parametric methods were applied for the statistical evaluation of the data. The assumptions of normality and homogeneity of variances for the continuous variables were tested using the Shapiro-Wilk and Levene’s test, respectively. The differences of the fly population, landing on the cows of Group A, B and C were evaluated using the non-parametric Kruskal-Wallis test, while differences between median values of specific groups were evaluated using the non-parametric Wilcoxon rank sum test (Mann-Whitney U test). The differences in the proportions of each genera of the identified fly species (categorical variables) at Day 0 and Day 30 were estimated through the application of chi-square test. Areas under the curve (AUC) for Day 0–10, Day 10–20 and Day 20–30 were calculated to represent the effect of the fly repellency using deltamethrin on the microbiological profile of the collected milk samples due to S. aureus, CNS and E. coli intramammary infections as well as on the SCC in Group A, B and C. The differences of 0–10, 10–20, 20–30 AUC as well as the total AUC were evaluated using the parametric one-way ANOVA method, while differences between AUC of specific groups were evaluated using the Bonferroni’s correction. A P value ≤ 0.05 was considered statistically significant. All analyses were conducted with the statistical software program SPSS (version 22.0).
3. Results
3.1. Deltamethrin repellency (short and long term)
Deltamethrin application reduced the fly burden within the same day (Day 0, 6 h post treatment) by 88% in Group A, while the fly number landing on the animals of Group B (untreated control group) remained stable. The differences of the mean (±sd) number of fly population pre/post treatment at Day 0 are presented in Table 1 .
Table 1.
Mean (±sd) number of fly population landing on the cows of all groups (Group A, B and C) throughout the study period.
| Groups |
||||
|---|---|---|---|---|
| Day | A | B | C | |
| 0 | (pre) | 145a (±32) | 173a (±24) | 167a (±28) |
| (post) | 17.4a (±4.1) | 178b (±39.8) | NA | |
| 10 | 12.4a (±4.3) | 206.5b (±48.2) | 202.4b (±23.4) | |
| 20 | 10.5a (±2.7) | 214.6b (±35.6) | 205.2b (±32.9) | |
| 30 | 7.3a (±2.5) | 7.8a (±3) | 234b (±35.5) | |
a,bDifferent uppercase letters in each row indicate statistical differences of the mean (±sd) number of fly population per day of sampling among the groups (P ≤ 0.05).
In order to calculate the long term deltamethrin repellency, the enumeration of the fly number landing on the animals of both Groups A and B was repeated for extra 3 times (Day 10, 20, 30). Significant (P ≤ 0.05) differences of the mean number of flies between deltamethrin treated cows (Group A) and untreated ones (Group B) were recorded at both Days 10 and 20, while no significant (P > 0.05) differences were recorded at Day 30. More precisely, at Day 10 fly number increased in untreated control group (Group B), but tended to decline on deltamethrin treated animal (Group A). At Day 20, the mean (±sd) number of flies in Group B reached 214.6 (±35.6), while the corresponding one of Group A was 10.5 (±2.7). Finally, at Day 30 the mean (±sd) number of flies minimized in both Groups A and B, reaching 7.3 (±2.5) and 7.8 (±3), respectively. The differences of the mean (±sd) number of fly population at Day 10, 20 and 30 are presented in Table 1.
The enumeration of the fly burden landing on the cows of Group C was carried out at the same days (Day 0, 10, 20, 30), as described above. More precisely, the mean (±sd) number of flies per day of sampling was 167 (±28), 202.4 (±23.4), 205.2 (±32.9) and 234 (±35.5), respectively. There were no significant (P > 0.05) differences of the mean number of flies between Group B and C at Day 0, 10 and 20. At Day 30, significant (P ≤ 0.05) differences of the mean number of flies between Group B and C were recorded. The differences of the mean (±sd) number of fly population in both Group B and C throughout the study are presented in Table 1.
3.2. Meteorological data
Climatic conditions for the location of the farm (Larissa, Thessaly, Greece) were typical for the season of the year (May and June 2016) according to the Hellenic National Meteorological Service. More precisely, at Day 0, 10, 20 and 30 the mean (±sd) air temperature was 31.6 (±3.2)°C, 24.8 (±2.6)°C, 26.9 (±4.4)°C and 26.7 (±5.1)°C, respectively and the mean (±sd) relative humidity was 38.8 (±12.3)%, 39.4 (±10.6)%, 45.5 (±9.6)% and 36.3 (±13.4)%, respectively.
3.3. Fly identification
The most prevalent fly species caught on the traps were Musca domestica at a percentage of 73.6%, followed by Haematobia spp., Stomoxys calcitrans, Simulium spp. and other minor species at 11.3%, 6.3%, 3.8% and 5%, respectively. There were no significant (P > 0.05) differences in the proportions of each fly genera identified in all groups of cows, throughout the trial.
3.4. Major pathogens
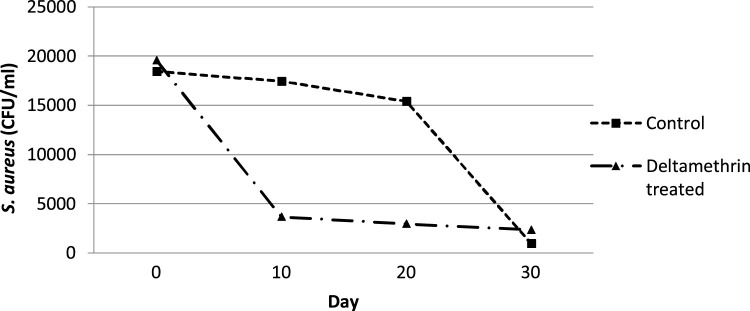
Microbiological analysis of milk samples, taken on Day 0, demonstrated S. aureus intramammary infections. According to the mean curves of S. aureus infection (Fig. 1 ), the isolation rate of S. aureus (CFU/ml) in deltamethrin treated cows (Group A) was decreased from Day 0 to 30, reaching at low levels (2375.71 ± 987.03 CFU/ml). The mean number of S. aureus colonies, cultured from the milk samples of the untreated control cows (Group B), remained stable from Day 0 to 20, while at Day 30 reached similar low levels with Group A (Fig. 1). Significant (P ≤ 0.05) differences of the mean (±sd) total AUC, regarding S. aureus intramammary infections, were recorded between deltamethrin treated group (Group A) and untreated control group (Group B), favoring the first one (Table 2 ). In other words, fly repellency reduced faster S. aureus intramammary infections in Group A than in Group B. The differences of mean (±sd) S. aureus AUC among the different days of sampling are presented in Table 2.
Fig. 1.
Mean curves showing the effect of the fly repellent on S. aureus intramammary infections (CFU/ml) between deltamethrin treated group (Group A) and untreated control group of cows (Group B).
Table 2.
Mean (±sd) areas under the curve (AUC) of the deltamethrin treated group (Group A) compared to the untreated control group of cows (Group B) regarding changes of S. aureus and CNS intramammary infections (CFU/ml) throughout the study period.
|
Staphylococcus aureus |
Coagulase negative staphylococci |
|||
|---|---|---|---|---|
| Day | AUC Group A | AUC Group B | AUC Group A | AUC Group B |
| 0-10 | 116475.00a (±18673.23) | 179645.85b (±21523.13) | 29928.80a (±7569.78) | 37892.85b (±6586.03) |
| 10-20 | 33458.30a (±5123.19) | 164633.25b (±24332.00) | 18830.80a (±12398.65) | 52250.00b (±19436.34) |
| 20-30 | 26861.85a (±4351.45) | 82360.75b (±13221.21) | 10040.85a (±3985.81) | 29232.15b (±11337.00) |
| Total | 176795.15a (±33423.27) | 426639.85b (±82332.32) | 58800.45a (±28569.84) | 119375.00b (±38593.73) |
a,bDifferent uppercase letters in each row indicate statistical differences of the mean (±sd) AUC between Group A and B throughout the study period (P ≤ 0.05).
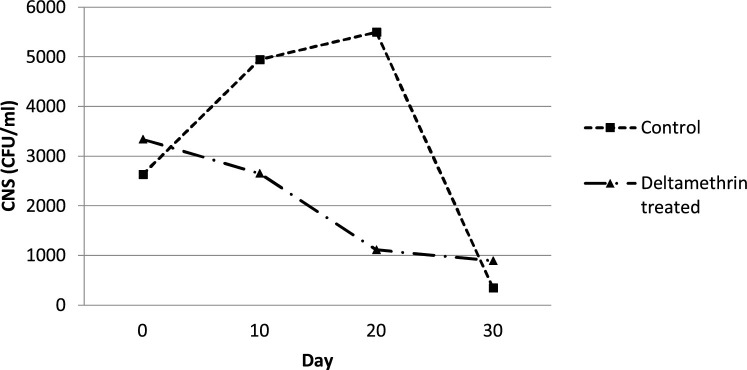
The mean number of CNS colonies (CFU/ml), enumerated in milk samples of deltamethrin treated animals (Group A), followed a stable reduction rate throughout the study (Fig. 2 ). On the contrary, the isolation rate of CNS (CFU/ml) increased according to the bacteriological cultivation of the milk samples of the untreated control animals (Group B) from Day 0 to 20, following a markedly reduction of the mean number of their colonies (CFU/ml) until Day 30 (Fig. 2). According to one-way ANOVA method, the mean (±sd) total AUC regarding CNS intramammary infections revealed that the cows dressed on their back with the fly repellent, deltamethrin reduced significantly (P ≤ 0.05) the shedding rate of CNS in milk samples compared to the untreated one (Table 2), leading to improvement of the intramammary infections status. The differences of mean (±sd) CNS AUC among the different days of sampling are presented in Table 2.
Fig. 2.
Mean curves showing the effect of the fly repellent on CNS intramammary infections (CFU/ml) between deltamethrin treated group (Group A) and untreated control group of cows (Group B).
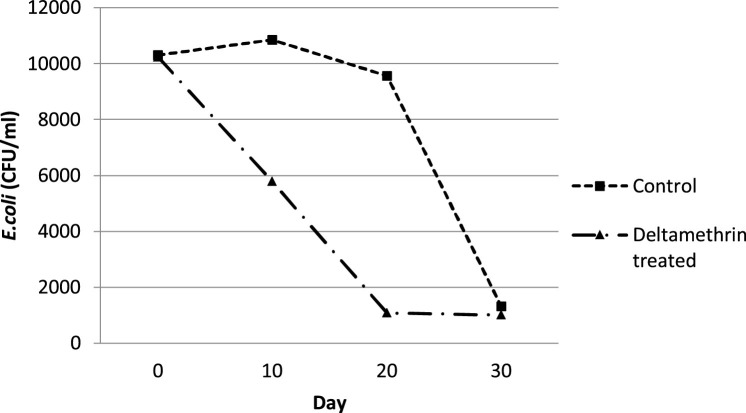
According to the mean curves of E. coli infection (Fig. 3 ), the isolation rate of E. coli (CFU/ml) reduced significantly in deltamethrin treated cows (Group A) from Day 0 to 30, reaching at low levels (mean ± sd, 1017.27 ± 562.23 CFU/ml). The mean (±sd) number of E. coli colonies (CFU/ml), cultured from the milk samples of the untreated control cows (Group B), increased slightly from Day 0 to 10, following a markedly reduction of their mean number (CFU/ml) from Day 10 to 30 (Fig. 3). Significant (P ≤ 0.05) differences of the mean (±sd) total AUC, regarding E. coli intramammary infections, were recorded between deltamethrin treated group (Group A) and untreated control group (Group B). In other words, fly repellency reduced faster E. coli intramammary infections in Group A than in Group B (Table 3 ). The differences of the mean (±sd) E. coli AUC among the different days of sampling are presented in Table 3.
Fig. 3.
Mean curves showing the effect of the fly repellent on E. coli intramammary infections (CFU/ml) between deltamethrin treated group (Group A) and untreated control group of cows (Group B).
Table 3.
Mean (±sd) areas under the curve (AUC) of the deltamethrin treated group (Group A) compared to the untreated control group of cows (Group B) regarding changes of E. coli intramammary infections (CFU/ml) and SCC (*103 cells/ml) throughout the study period.
|
Escherichia coli |
Somatic cell count |
|||
|---|---|---|---|---|
| Day | AUC Group A | AUC Group B | AUC Group A | AUC Group B |
| 0-10 | 80092.55a (±53245.67) | 105867.40b (±43478.06) | 8570.00a (±1843.00) | 13154.00b (±3296.87) |
| 10-20 | 34367.55a (±11234.32) | 102086.10b (±32749.39) | 6217.55a (±2649.76) | 16052.50b (±5612.21) |
| 20-30 | 10530.10a (±2375.38) | 54400.00b (±19472.81) | 5402.30a (±1004.57) | 13468.85b (±3481.49) |
| Total | 124990.20a (±47821.58) | 262353.50b (±84286.01) | 20189.85a (±5623.69) | 42675.35b (±18932.39) |
a,bDifferent uppercase letters in each row indicate statistical differences of the mean (±sd) AUC between Group A and B throughout the study period (P ≤ 0.05).
The mean (±sd) number of S. aureus colonies (CFU/ml), enumerated in milk samples of the untreated control group C, remained stable throughout the study i.e. 17532 (±2516.53) CFU/ml, even though a slight increase of their mean number (CFU/ml) was recorded at Day 30. Significant (P ≤ 0.05) differences of the mean (±sd) AUC from Day 20 to 30, regarding S. aureus intramammary infections, were recorded between Group B and C. More precisely, the mean (±sd) 20–30 AUC for Group B and C was 82360.75 (±13221.21) and 163453.32 (±29562.79) respectively.
At the same frame, CNS and E. coli colonies (CFU/ml) counted in milk samples of Group C remained stable from the start (Day 0) to the end (Day 30) of the study, with a mean (±sd) number at 2956.73 (±675.32) CFU/ml and 10234.42 (±2375.62) CFU/ml, respectively. It must be mentioned that the mean (±sd) AUC from Day 20 to 30 regarding CNS and E. coli intramammary infections presented significant (P ≤ 0.05) differences between Group B and C, with the second one of Group C to be higher than of Group B.
All milk samples tested were negative for the presence of Mycoplasma spp. and Trueperella pyogenes.
3.5. Less major pathogens
Other pathogens of less significance, occasionally related to intramammary infections of dairy cattle farms, include species of the genera Enterobacter, Citrobacter, Proteus and Pasteurella. In our study, the first three microorganisms were mostly identified in milk samples of all groups at the start of the experimental period (Day 0), falling to zero numbers at Day 30. More precisely, 7 and 10% of the milk samples were positive for the presence of Enterobacter spp in Groups A and B, respectively, at Day 0. Regarding Citrobacter spp. and Proteus spp., 2 and 2.5% of the milk samples were positive for the presence of these microorganisms, respectively (Day 0), while Pasteurella spp. have been identified only in 2% of the milk samples in both cow groups at Day 10.
3.6. Somatic cell count (SCC)
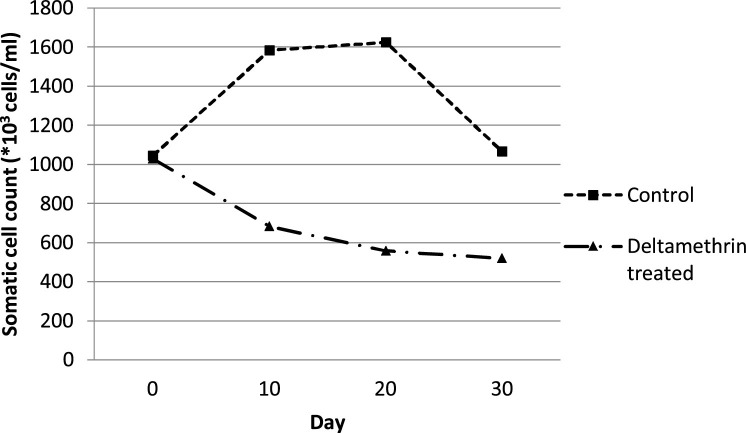
According to the mean curves of SCC, the mean (±sd) number of SCC in deltamethrin treated cows (Group A) followed a stable reduction rate from Day 0 to 30, i.e. from 1029 (±867.11)*103 cells/ml at Day 0 to 546.82 (±123.23)*103 cells/ml at Day 30 (Fig. 4 ). In the untreated control group of cows, housed together with the treated ones (Group B), SCC increased from Day 0 to 20, leading to severe intramammary infections (>1.600*103 cells/ml). However, at Day 30, a reduction of the mean number of SCC in Group B had been also recorded, following the reduction of S. aureus, CNS and E. coli infection of the mammary gland due to fly repellency. Significant (P ≤ 0.05) differences of the mean (±sd) total SCC AUC (Table 3) were recorded between deltamethrin treated group of animals (Group A) and untreated control one (Group B). The application of deltamethrin on the animals of Group A decreased SCC in milk samples, due to reduced shedding rate of S. aureus, CNS and E. coli in the same milk samples (Table 3). The differences of the mean (±sd) SCC AUC among the different days of sampling are presented in Table 3.
Fig. 4.
Mean curves showing the effect of the fly repellent on Somatic cell count (*103 cells/ml) between deltamethrin treated group (Group A) and untreated control group of cows (Group B).
In our study, 8 cows from deltamethrin treated group (Group A) at the start had SCC less than 200.000 cells/ml and this remained throughout the study period. Therefore, there is a strong evidence that uninfected cattle are less likely to become infected when carrying reduced fly burdens.
The mean (±sd) number of SCC, enumerated in milk samples of the untreated control group C, presented a constantly increase rate from Day 0 to 30 reaching at 1611.71 (±556.21)*103 cells/ml. Significant (P ≤ 0.05) differences of the mean (±sd) SCC AUC from Day 20 to 30 were recorded between Group B and C. More precisely, the mean (±sd) 20–30 SCC AUC for Group B and C was 13468.85 (±3481.49)*103 cells/ml and 16453.32 (±2478.93)*103 cells/ml, respectively.
4. Discussion
The aim of this study was to assess the possible effect of the fly repellency, using deltamethrin, on the microbiological profile (S. aureus, CNS and E. coli) of the intramammary infections in one Greek dairy cattle farm under intensive management, during peak fly season. SCC was recorded to support the microbiological findings.
In countries with temperate climatic conditions, such as Greece, flies express a temperature-moisture dependent appearance which coincides with spring and early summer [27]. This time of the year, flies reach at their peak numbers causing not only welfare but also productivity problems [10,11]. According to meteorological data from the Hellenic National Meteorological Service (Athens, Greece), the combination of mean temperature and mean relative humidity for the location of the farm (Larissa, Thessaly, Greece) were typical for the season of the year (May and June 2016), favoring increased fly activity [10].
In our study, Musca domestica and Stomoxys calcitrans were the two most commonly identified fly species with a percentage of 76.6 and 12.3%, respectively. Up to date, there was no information regarding the fly species that are found in Greek cattle farms and therefore no comparisons can be made. Another study, under tropical climatic conditions of Baghdad (Iraq), conducted by Hassan and Alkafagi [28], revealed the presence of 97.8% Musca domestica and 2.2% Stomoxys calcitrans. During a study at Beni-Suef (Egypt) of Mohammed et al. [29], it was concluded that the most predominant species were the biting midges, followed by Musca domestica (18.31%) and Stomoxys calcitrans (7.74%).
Flies act as vectors for several pathogens [29,30], such as S. aureus [5], CNS [31] and E. coli [32]. These bacteria, which are carried out on proboscis and hairs of the flies’ body and feet [33], are transmitted to the udder and teats of the cows through direct contact leading to both clinical and subclinical intramammary infections [34].
S. aureus is one of the most commonly found etiological causes of contagious mastitis in dairy cattle farms [5,8]. S. aureus is a natural habitat of the external skin of the mammary gland (teat and udder) [14] and once entry is achieved, it infects the mammary gland of the cow (especially the milk secreting tissues) causing mastitis [35]. In our study, the cows dressed on their back with deltamethrin (Group A) presented a lower shedding rate of S. aureus than the untreated ones (Group B). This is due to the fact that the fly repellent, deltamethrin reduced the number of flies on the animals of Group A, facilitating a reduced transmission of S. aureus and therefore, reduced intramammary infections. Anderson et al. [5] found that 13.6% of positive milk samples for S. aureus (in 3 dairy cattle farms of North Carolina) to be 8 times likely to had been transmitted by horn flies.
Many researchers have identified S. aureus as a major cause of heifer mastitis in dairy cattle farms. It is worth noted that heifers’ infections represents almost one third of the new cases of S. aureus mastitis [9]. This is due to the fact that S. aureus can be transmitted from the infected mammary gland of the cows to unbred and pregnant heifers, especially with vectors such as flies [7,13,36]. The percentages of infection with S. aureus, among heifers, range from 0.6 to 8% [31]. Ryman et al. [14] reported a much higher (59.5%) prevalence of S. aureus intramammary infections in heifers than this reported by Fox et al. [31]. According to Ryman et al. [14], this result was attributed to the role that horn flies play in the spread of S. aureus infections. Another study, conducted by Oliver et al. [9], agreed with the above findings supporting that cows in lactation period were a source of S. aureus infection; thus leading to increased cases of heifer mastitis (spread of the disease) in the absence of fly control programs in the farm.
CNS have been considered as less important pathogens, associated with either clinical or subclinical intramammary infections [34]. Due to the fact that they, commonly, colonize the teat skin, teat end and teat canal of the cow, it is difficult to estimate if these are the cause of mastitis or simply teat end contaminants [13]. Considering that there is little information available regarding the epidemiology and transmission routes of CNS [37], these bacteria have been associated with a moderate increase in milk SCC while, in rare cases, have been involved in clinical mastitis [38]. In our study, the total AUC regarding CNS intramammary infections revealed a lower shedding rate of these bacteria in cows of Group A than in cows of Group B. Mohammed et al. [29] reported 18.2% and 9.1% infection of Musca domestica and Stomoxys calcitrans, respectively, with CNS. In heifers, these bacteria are considered the primary cause of intramammary infections, associated with subclinical mastitis near calving [34]. Ryman et al. [14] found 64.3% prevalence of CNS intramammary infections among heifers.
Considering that other major causes of mastitis (e.g. S. aureus) were identified in milk samples, CNS might have not been the main pathogens causing mastitis, but the result of teat contamination or poor hygiene. In cases were CNS is the cause of mastitis, then the cultural isolation should produce pure growth of CNS [38]. Unfortunately, CNS isolated from the milk samples examined in our study, were not identified at species or strain level.
Several authors reported an increasing rate of E. coli mastitis, despite the fact that worldwide the cases of bovine mastitis have been reduced [39,43]. E. coli is the second (after Streptococcus uberis) most prevalent environmental bacteria causing intramammary infections. It is present in large numbers in feces and therefore, it can infect primarily dairy cows under the intensive management system, when conditions are wet and humid or the hygiene is poor [38]. According to Nevala et al. [40], in Finland, the mastitis cases caused by E. coli were fewer than 20%, while Shpigel et al. [41] and Shpigel et al. [42] reported a higher percentage (60%) of E. coli mastitis in Israel. One interesting point is that E. coli does not adhere to the teat and udder endothelium, leading, in most cases, to self recovery [38].
The association between the fly burden and intrammamary infections of the cows through transmission of several pathogens (e.g. E. coli) has been established by many surveys [43,[47], [48], [49]]. Flies, including M. domestica, H. irritans and S. calcitrans, play an active role in spreading of E. coli strains among cows, leading to mastitis [5,43]. This association reinforces the significance of controlling fly population in dairy cattle farms in order to prevent the transmission of these pathogens. In our study, fly repellency using deltamethrin reduced the fly burden of the treated cows (Group A) leading to quick reduction of E. coli excretion in milk samples.
Interestingly, the untreated cows of Group B also presented low numbers of S. aureus, CNS and E. coli colonies in milk samples from Day 20 to 30. According to pharmacokinetics of deltamethrin, cattle may excrete active metabolites of this drug in feces and urine [44]. For this reason, the breeding success of flies in dairy cattle farms under intensive management is decreased, leading to a significant reduction of the fly population of the farm [16]. Another possible explanation is that the co-existence of both cow groups allowed the cows to lick each other and therefore, to ingest deltamethrin [45,50].
Summer mastitis (a special form of mastitis) has a different aetiology and epidemiology compared to other forms of mastitis. Trueperella pyogenes is the most frequent isolated among other bacteria and is responsible for severe necrosis and destruction of the infected quarter of the mammary gland of the cow [12]. The major mean of transmission of this type of mastitis is thought to be Hydrotaea irritans, a blood sucking fly [12,51]. In cattle farms, high numbers of Hydrotaea irritans have been detected on the ventral abdomen and udder, and since the microorganisms involved in summer mastitis have been isolated from these flies, there is a strong evidence that these flies can transmit the disease. One survey, conducted in Sweden from 2002 to 2003, revealed the presence of Trueperella pyogenes in 64 out of 987 (6.1%) udder quarters milk samples [52], while this percentage was lower in New York State, reaching 1.0% for multiparous cows [53]. In our study, Trueperella pyogenes and Hydrotaea irritans were not isolated.
Intramammary infections usually fluctuate between subclinical and clinical state [46]. Cows with subclinical mastitis have higher possibility to develop clinical mastitis than are uninfected cows [46]. SCC is the number of white blood cells and epithelial cells, present in milk, as distinguished from invading bacterial cells [38]. Individual somatic cell count consists the best indicator to discriminate mastitis category, as SCC over 200.000 indicates subclinical mastitis [46]. In our study, the high numbers of SCC indicated severe intramammary infections of the cows. The application of deltamethrin reduced fly infestation, leading to lower shedding rate of the responsible bacteria for intramammary infections (e.g. S. aureus, CNS and E. coli) in milk samples. This reduced shedding resulted in lower number of SCCs in the same milk samples.
In order to confirm that there were no significant changes of the fly population in the farm due to reasons other than the use of deltamethrin, i.e. environmental factors, enumeration of the number of flies as well as full microbiological control of the milk samples of Group C cows’ were performed. At Day 30, the comparison of fly burden, in Group B and C, enhanced the possible explanations regarding the reduction of the mean fly population in untreated control group of cows (Group B) from Day 20 to 30. In addition, the mean numbers of S. aureus, CNS and E. coli intramammary infections and SCC in milk samples of Group C remained in high levels at the end of the study, something that was confirmed by the significant differences of the 20–30 AUC of these parameters. Therefore, the reduction of the mean fly burden as well as of the shedding rate of S. aureus, CNS, E. coli and SCC were largely attributed to the application of the fly repellent, deltamethrin.
Consequently, there is a clear relationship between fly abundance and transmission of pathogens predisposing to intramammary infections in the dairy cattle farm where the study was conducted. The use of the pyrethroid, deltamethrin, reduced fly abundance and therefore, the possibility of transmitting pathogenic bacteria in the mammary gland. Finally, this finding aids the reduction of the use of antibiotic treatment of mastitis and therefore minimizes the antibiotic resistance, a very important issue within the «One Health» aspect.
5. Conclusions
This study, conducted in one dairy cattle farm of Greece, showed evidence that fly abundance plays a significant role in the development of intramammary infections among dairy cows, enhancing the role of flies as possible vectors for pathogens provoking intramammary infections. After the administration of the fly repellent, deltamethrin, a decrease of pathogens and SCC was observed, after the reduction of the fly population, suggesting that fly control should be included in a mastitis management strategy. Finally, more research should be done in a larger scale study in order to investigate this relationship.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Oliver S.P., Calvinho L.F. Influence of inflammation on mammary gland metabolism and milk composition. J. Anim. Sci. 1995;73(Suppl. 2):18–33. [Google Scholar]
- 2.Ruegg P.L. New perspectives in udder health management. Vet. Clin. Food. Anim. 2012;28:149–163. doi: 10.1016/j.cvfa.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.National Mastitis Council (NMC) fifth ed. National Mastitis Council; Verona: 2011. Current Concepts of Bovine Mastitis. [Google Scholar]
- 4.Oliver S.P., Murinda S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. Food Anim. 2012;28:165–185. doi: 10.1016/j.cvfa.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Anderson K.L., Lyman R., Moury K., Ray D., Watson D.W., Correa M.T. Molecular epidemiology of Staphylococcus aureus mastitis in dairy heifers. J. Dairy Sci. 2012;95:4921–4930. doi: 10.3168/jds.2011-4913. [DOI] [PubMed] [Google Scholar]
- 6.Wegener H.C., Nielsen P., Rosdahl V.T. Staphylococcus aureus mastitis in heifers. An epidemiological study by conventional and molecular typing methods, Dansk. Veterinartidsskrift. 1993;76:457–461. [Google Scholar]
- 7.Owens W.E., Oliver S.P., Gillespie B.E., Ray C.H., Nickerson S.C. Role of horn flies (Haematobia irritans) in Staphylococcus aureus-induced mastitis in dairy heifers. Am. J. Vet. Res. 1998;59:1122–1124. [PubMed] [Google Scholar]
- 8.Fox L.K., Bayles K.W., Bohach G.A. Staphylococcus aureus mastitis. In: Honeyman A.L., Friedman H., Bendinelli M., editors. Staphylococcus aureus Infection and Disease. Springer; New York: 2001. pp. 271–294. [Google Scholar]
- 9.Oliver S.P., Gillespie B.E., Headrick S.J., Lewis M.J., Dowlen H.H. Prevalence, risk factors, and strategies for controlling mastitis in heifers during the periparturient period. Int. J. Appl. Res. Vet. Med. 2005;3:150–162. [Google Scholar]
- 10.Taylor D.B., Moon R.D., Mark D.R. Economic impact of stable flies (Diptera: Muscidae) on dairy and beef cattle production. J. Med. Entomol. 2012;49:198–209. doi: 10.1603/me10050. [DOI] [PubMed] [Google Scholar]
- 11.Arsenopoulos K., Triantafillou E., Papadopoulos E. Reduced stress and fatigue indicators (cortisol and creatinine kinase) in dairy cattle due to fly repellency using deltamethrin (Butox®, MSD), Bulg. J. Vet. Med. 2017;20(Suppl. 1):123–129. [Google Scholar]
- 12.Nickerson S.C., Owens W.E., Boddie R.L. Mastitis in dairy heifers: initial studies on prevalence and control. J. Dairy Sci. 1995;78:1607–1618. doi: 10.3168/jds.S0022-0302(95)76785-6. [DOI] [PubMed] [Google Scholar]
- 13.Piepers S., Peeters K., Opsomer G., Barkema H.W., Frankena K., De Vliegher S. Pathogen-specific risk factors at the herd, heifer and quarter level for intramammary infections in early lactating dairy heifers. Prev. Vet. Med. 2011;99:91–101. doi: 10.1016/j.prevetmed.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Ryman V.E., Nickerson S.C., Hurley D.J., Berghaus R.D., Kautz F.M. Influence of horn flies (Haematobia irritans) on teat skin condition, intramammary infection, and serum anti-S. aureus antibody titres in holstein heifers. Res. Vet. Sci. 2013;95:343–346. doi: 10.1016/j.rvsc.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Foster M., Klimpel S., Mehlhorn H., Sievert K., Messler S., Pfeffer K. Pilot study on synathotropic flies as vectors of pathogenic microorganisms. Parasitol. Res. 2007;101:243–246. doi: 10.1007/s00436-007-0522-y. [DOI] [PubMed] [Google Scholar]
- 16.Scott D.W. Skin diseases. In: Divers T., Peck S., editors. Rebhun’s Diseases of Dairy Cattle. Saunders Elsevier; Missouri: 2008. pp. 295–326. [Google Scholar]
- 17.Garry F. Miscellaneous infectious diseases. In: Divers T., Peck S., editors. Rebhun’s Diseases of Dairy Cattle. Saunders Elsevier; Missouri: 2008. pp. 606–639. [Google Scholar]
- 18.Castro E., Gil A., Solari M.A., Farias N.A. Validation of a subjective counting method for a horn flies (Haematobia irritans irritans) (Diptera: Muscidae) population in a cattle herd. Vet. Parasitol. 2005;133:363–367. doi: 10.1016/j.vetpar.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Mullens B.A., Soto D., Gerry A.C. Estimating field densities of Haematobia irritans(Diptera: Muscidae) using direct visual field counts versus photographic assessments. J. Med. Entomol. 2016;53:703–706. doi: 10.1093/jme/tjv246. [DOI] [PubMed] [Google Scholar]
- 20.Mullens B.A., Watson D.W., Gerry A.C., Sandelin B.A., Soto D., Rawls D., Denning S., Guisewite L., Cammack J. Field trials of fatty acids and geraniol applied to cattle for suppression of horn flies,Haematobia irritans (Diptera: Muscidae), with observations on fly defensive behaviors. Vet. Parasitol. 2017;245:14–28. doi: 10.1016/j.vetpar.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Hannan A.M. Prevalence of dipterous flies with veterinary importance in selected sheep farms and slaughter houses in Jazan, Saudia Arabia, Egypt. Acad. J. Biol. Sci. 2010;3:63–73. [Google Scholar]
- 22.Wall R., Shearer D. second ed. Blackwell Science Ltd.; USA: 2001. Veterinary Ectoparasites: Biology, Pathology and Control. [Google Scholar]
- 23.Couri M.S., Pont A.C., Penny N.D. Muscidae (Diptera) from Madagascar: identification keys, descriptions of new species and new records. Proc. Calif. Acad. Sci. 2006;57:799–923. [Google Scholar]
- 24.National Mastitis Council (NMC) third ed. National Mastitis Council; Madison: 1999. Laboratory Handbook on Bovine Mastitis. [Google Scholar]
- 25.Quin P.J., Carter M.E., Arkey B.K., Carter G.R. first edition. Mosby Scientific Publications; Wolfe: 1994. Clinical Veterinary Microbiology. [Google Scholar]
- 26.Nicholas R., Baker S. Recovery of mycoplasma from animals. In: Miles R., Nicholas R., editors. Methods in Molecular Biology. Humana Press; Totawa: 1988. pp. 37–43. [Google Scholar]
- 27.Mehlhorn H., Al-Rasheid K.A.S., Abdel-Ghaffar F., Klimpel S., Pohle H. Life cycle and attacks of ectoparasites on ruminants during the year in Central Europe: recommendations for treatment with insecticides (e.g. Butox®) Parasitol. Res. 2010;107:425–431. doi: 10.1007/s00436-010-1957-0. [DOI] [PubMed] [Google Scholar]
- 28.Hassan H.E., Alkafagi M. Association of Escherichia coli with the prevalence of flies population. AJABS. 2013;8:217–221. [Google Scholar]
- 29.Mohammed A.N., Abdel-Latef G.K., Abdel-Azeem N.M., El-Dakhly K.M. Ecological study on antimicrobial-resistant zoonotic bacteria transmitted by flies in cattle farms. Parasitol. Res. 2016;115:3889–3896. doi: 10.1007/s00436-016-5154-7. [DOI] [PubMed] [Google Scholar]
- 30.Graczyk T.K., Knight R., Gilman R.H., Cranfield M.R. The role of non-biting flies in the epidemiology of human infectious diseases. Microbes Infect. 2001;3:231–235. doi: 10.1016/s1286-4579(01)01371-5. [DOI] [PubMed] [Google Scholar]
- 31.Fox L.K. Prevalence, incidence and risk factors of heifer mastitis. Vet. Microbiol. 2009;134:82–88. doi: 10.1016/j.vetmic.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Lane R.P., Crosskey R.W. Chapman and Hall; London: 1993. Medical Insects and Arachnids. [Google Scholar]
- 33.Durden L.A., Muller C.R. Academic Press; U.S.A: 2002. Medical and Veterinary Entomology. [Google Scholar]
- 34.De Vliegher S., Fox L.K., Piepers S., McDougall S., Barkema H.W. Invited review: mastitis in dairy heifers: nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 2012;95:1025–1040. doi: 10.3168/jds.2010-4074. [DOI] [PubMed] [Google Scholar]
- 35.Trinidad P., Nickerson S.C., Alley T.K. Prevalence of intramammary infection and teat canal colonization in unbred and primigravid dairy heifers. J. Dairy Sci. 1990;73:107–114. doi: 10.3168/jds.S0022-0302(90)78652-3. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie B.E., Owens W.E., Nickerson S.C., Oliver S.P. Deoxyribonucleic acid fingerprinting of Staphylococcus aureus from heifer mammary secretions and from horn flies. J. Dairy Sci. 1999;82:1581–1585. doi: 10.3168/jds.S0022-0302(99)75386-5. [DOI] [PubMed] [Google Scholar]
- 37.Zadoks R.N., Watts J.L. Species identification of coagulase-negative staphylococci: genotyping is superior to phenotyping. Vet. Microbiol. 2009;134:20–28. doi: 10.1016/j.vetmic.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Blowey R., Edmondson P. second edition. Cab International; Oxfordshire: 2010. Mastitis Control in Dairy Herds. [Google Scholar]
- 39.Fairbrother J.H., Dufor S., Fairbrother J.M., Francoz D., Nadeau E., Messier S. Characterization of persistent and transient Escherichia coli isolates recovered from clinical mastitis episodes in dairy cows. Vet. Microbiol. 2015;176:126–133. doi: 10.1016/j.vetmic.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Nevala M., Taponen S., Pyorala S. Bacterial etiology of clinical mastitis: data from Saari Clinic Ambulatory in 2003-2004. Finn. Vet. J. 2004;110:363–369. [Google Scholar]
- 41.Shpigel N.Y., Winkler M., Ziv G., Saran A. Clinical, bacteriological and epidemiological aspects of clinical mastitis in Israeli dairy herds. Prev. Vet. Med. 1998;35:1–9. doi: 10.1016/s0167-5877(98)00052-x. [DOI] [PubMed] [Google Scholar]
- 42.Shpigel N.Y., Elazar S., Rosenshine I. Mammary pathogenic Escherichia coli. Curr. Opin. Microbiol. 2008;11:60–65. doi: 10.1016/j.mib.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Castro B.G., Souza M.M.S., Regua-Mangia A.H., Bittencourt A.J. Genetic relationship between Escherichia coli strains isolated from dairy mastitis and from the stable fly Stomoxys calcitrans. Pesqui. Vet. Bras. 2016;36:479–484. [Google Scholar]
- 44.Heitzman R.J. Deltamethrin. In: FAO, editor. Residues of Some Veterinary Drugs in Animals and Foods. FAO/WHO; Rome: 2001. pp. 1–20. [Google Scholar]
- 45.Whalen C. Nature of the residues in milk and tissues following dermal application of 14C-benzyl-deltamethrin or 14C-gem-dimethyl-deltamethrin to lactating dairy cattle. HWI. 1995 6187-6143. [Google Scholar]
- 46.Rhoda D.A., Pantoja J.C.F. Using mastitis records and somatic cell count data. Vet. Clin. Food Anim. 2012;28:347–361. doi: 10.1016/j.cvfa.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Macovei L., Miles B., Zurek L. The potential of house flies to contaminate ready-to-eat food with antibiotic-resistant enterococci. J. Food Prot. 2008;71:435–439. doi: 10.4315/0362-028x-71.2.435. [DOI] [PubMed] [Google Scholar]
- 48.Mian L.S., Maag H., Tacal J.V. Isoloation of Salmonella from muscoid flies at commercial animal establishments in San Bernardino County, California. J. Vector Ecol. 2002;27:82–85. [PubMed] [Google Scholar]
- 49.Morsi O.P.G., Agbozele G., Ukwandu N.C.D. Some aspects of epidemiology of filth flies: Musca domestica, Musca domestica vicina, Drosophilia melanogasterand associated bacteria pathogens in Ekpoma, Nigeria. Vector Borne Zoonotic Dis. 2007;7:107–117. doi: 10.1089/vbz.2006.0539. [DOI] [PubMed] [Google Scholar]
- 50.Bousquet-Melou A., Jacquiet P., Hoste H., Clement J., Bergeaud J.P., Alvinerie M., Toutain P.L. Licking behavior induces partial anthelmintic efficacy of ivermectin pour-on formulation in untreated cattle. Int. J. Parasitol. 2011;41:563–569. doi: 10.1016/j.ijpara.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Chirico J., Jonsson P., Kjellberg S., Thomas G. Summer mastitis experimentally induced by Hydrotaea irritans exposed to bacteria. Med. Vet. Entomol. 1997;11:187–192. doi: 10.1111/j.1365-2915.1997.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 52.Ericsson Unnerstad H., Lindberg A., Persson Waller K., Ekman T., Artursson K., Nilsson-Ost M., Bengtsson B. Microbial aetiology of acute clinical mastitis and agent-specific risk factors. Vet. Microbiol. 2009;137:90–97. doi: 10.1016/j.vetmic.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Cha E., Hertl J.A., Schukken Y.H., Tauer L.W., Welcome F.L., Grohn Y.T. The effect of repeated episodes of bacteria-specific clinical mastitis on mortality and culling in Holstein dairy cows. J. Dairy Sci. 2013;96:4993–5007. doi: 10.3168/jds.2012-6232. [DOI] [PubMed] [Google Scholar]