Abstract.
Schistosomiasis is an acute and chronic parasitic disease caused by blood flukes of the genus Schistosoma. More than 220 million people worldwide were estimated to have active schistosomiasis in 2017, 90% of whom live on the African continent, but only 102 million were reported to have received treatment. Africa is also disproportionately burdened by HIV, with an estimated 26 million people living with HIV in 2017. Given these overlapping epidemics, we conducted a systematic review to ascertain the contribution of schistosomes to HIV acquisition risk, the contribution of HIV to schistosome acquisition, the impact of HIV on schistosomiasis-related morbidity, the impact of schistosomes on HIV disease progression and immune response, the impact of HIV on the efficacy of praziquantel treatment, and the impact of HIV on egg shedding. We reviewed studies of people living in sub-Saharan Africa coinfected with HIV and Schistosoma spp. between January 1996 and July 2018. We found that 1) infection with Schistosoma haematobium increases the risk of HIV acquisition, 2) there is currently a lack of data on whether HIV infection increases the risk of Schistosoma acquisition, 3a) HIV coinfection was not an accelerating factor for adverse Schistosoma outcomes, 3b) schistosomiasis may be an important contributor to immune activation in HIV coinfected people, 4) praziquantel use in coinfected people may improve immune reconstitution on antiretroviral therapy for HIV, and 5) there is evidence that HIV infection reduces egg excretion in individuals infected with Schistosoma mansoni.
INTRODUCTION
Rationale.
In 2017, more than 36 million people were living with HIV worldwide and 1.8 million more were newly infected.1 Sub-Saharan Africa is disproportionally burdened by the HIV epidemic, with 25.7 million people living with HIV (70% of total cases). Of the 1.8 million new HIV infections, approximately 1.2 million (68%) occurred in sub-Saharan Africa.1 Sub-Saharan Africa is also home to more than 90% of the world’s cases of schistosomiasis,2 an acute and chronic parasitic disease caused by blood flukes (trematode worms) of the genus Schistosoma. Estimates show that more than 220 million people required treatment for schistosomiasis, of which 102 million were reported to have received treatment.2
This review focuses on the interactions of HIV infection and two species of schistosomes: Schistosoma haematobium and Schistosoma mansoni. Schistosoma haematobium adult worms live mainly in the venous plexus surrounding the bladder and genital tissue, depositing eggs, infecting the urinary tract, and causing urogenital schistosomiasis. Schistosoma mansoni causes schistosomiasis of the intestine and portal veins. Eggs of S. mansoni become embedded in the vessels of the liver, large intestine, and rectum, but rarely cause genital lesions.3 Accumulation of eggs in the splanchnic veins can lead to periportal fibrosis (PPF), which contributes to portal hypertension and esophageal varices. The geographic overlap of schistosomiasis and HIV epidemics in sub-Saharan Africa has led to a substantial burden of coinfection in the region, leading to potential consequences in host immune response and health outcomes related to both the viral and parasitic pathogens.
Objectives.
We reviewed published studies from sub-Saharan Africa on people coinfected, or at risk of coinfection, with HIV and Schistosoma spp. (S. haematobium or S. mansoni). We conducted a systematic review to ascertain 1) the effects of schistosomes on the risk of HIV acquisition; 2) the effects of HIV on the risk of schistosome acquisition; 3) the effects of comorbidity, broken down into a) the impact of HIV on schistosome morbidity and b) the impact of schistosomes on HIV disease progression and immune response; 4) the effects of HIV on the efficacy of praziquantel treatment and the impact of schistosomes on antiretroviral therapy with regard to immune reconstitution; and 5) the effect of HIV on egg shedding.
METHODS
Protocol and registration.
We did not register a protocol for this review.
Eligibility criteria.
We reviewed studies that assessed people living in sub-Saharan Africa coinfected with HIV and Schistosoma spp. (S. haematobium or S. mansoni) between 1996 and July 2018 using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.4
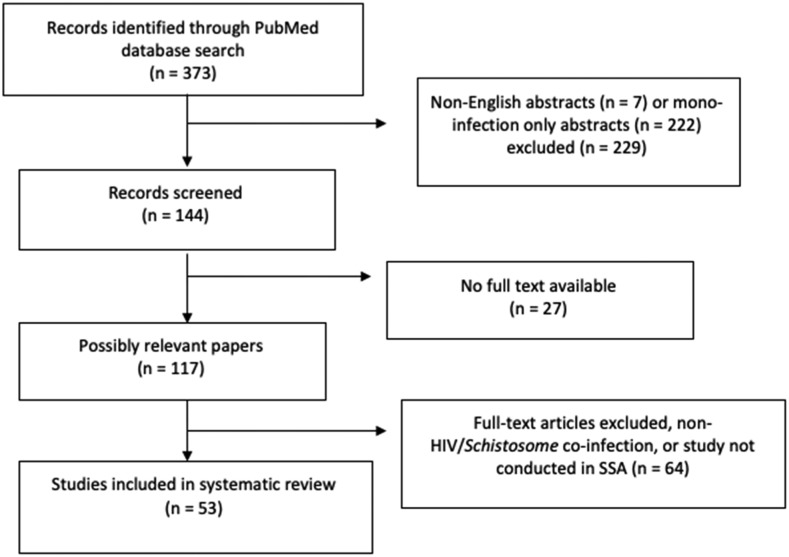
Published articles were included if they contained basic immunology research; case–control, cross-sectional, and prospective clinical studies; mathematical models; short reports; or epidemiological analyses that addressed the contribution of schistosomiasis to the risk of HIV/AIDS acquisition and vice versa; the immune response to either the viral or parasitic condition; and morbidity/mortality. Studies that did not involve human subjects, were not about HIV/Schistosoma coinfection, or were not published in English were excluded from this review. Studies that reported on schistosome infections in non-African countries, or those in African immigrants to non-African countries, or visitors to the African continent who became ill on returning to their home country were also excluded. The selection process and criteria are shown in Figure 1.
Figure 1.
Study selection regarding HIV/Schistosoma spp. coinfection.
Information sources.
We performed a PubMed search via NCBI (https://www.ncbi.nlm.nih.gov/pubmed/) for studies on HIV and Schistosoma spp. infection.
Search.
The search was limited to studies of humans reported in English. The terms that were used as MeSH terms or direct keywords for the search were (“schistosomiasis”[MeSH Terms] odds ratios [OR] “schistosomiasis”[All Fields]) AND (“HIV”[MeSH Terms] OR “hiv”[All Fields]). We did not include the specific search terms “AIDS,” “S. haematobium,” or “S. mansoni.” All titles with abstracts and full text in English were reviewed.
Study selection.
Three hundred seventy-three articles were retrieved from the PubMed search of published articles, of which 229 were excluded because they were not in English or the subject pertained to either HIV or schistosome mono-infection only, 27 had no available full text, and 64 had available full text but did not address HIV and schistosome coinfection or the studies were not conducted in sub-Saharan Africa. Fifty-three studies of HIV/Schistosoma coinfection were identified and included in the analysis: case–control (7), case study (1), cross-sectional (20), longitudinal (22), and mathematical model (3) (Figure 1).
Data collection process.
One reviewer extracted data from the PubMed search for tabulation and review. The studies were very heterogenous, and no formal analysis was performed to assess publication bias, but all limitations related to possible bias are listed in the “Limitations” section of this review. We did not retrieve individual patient data.
Data items.
Based on article presentation, “cases” are defined as HIV-positive (+) and Schistosoma-positive (+) individuals, that is, H+/S+ coinfected participants. “Controls” are defined as mono-infected participants—those infected with either HIV or schistosomes but not both, that is, HIV negative (−)/Schistosoma positive (+) (H−/S+), or HIV positive (+)/Schistosoma negative (−) (H+/S−). Data extracted from the study articles (as available) included country; year; gender; age; HIV infection status; use of antiretroviral therapy; use of the anti-helminthic drug praziquantel; measurements of circulating cathodic antigen (CCA) or circulating anodic antigen (CAA) in urine to identify active infection with adult worms and as a proxy for worm burden; Kato–Katz assay results; measurements of CC chemokine receptor type 5, also known as CCR5 or CD195, and CXC chemokine receptor type 4 (CXCR4), also known as fusin or CD184, proteins on the surface of white blood cells that serve as the main receptor and entry point for HIV; host immune response and granuloma formation; CD4+ T cells, soluble CD8+ T cells, assessments of CD4+ Th1 and Th2 cellular responses; measurements of plasma- or serum-soluble interleukin (IL)-4, IL-6, and IL-10; measurements of HIV-1 RNA or plasma viremia quantification (quantifiable ≥ 20 copies/mL; detectable but not quantifiable < 20 copies/mL; or not detectable); and hepatic imaging for the presence of PPF. Mortality was recorded when appropriate.
Summary measures.
We included previously calculated and reported risk ratios and ORs and their 95% CIs, and available ORs for HIV acquisition in the context of current schistosomiasis infection were averaged to create a composite OR. All reported P-values of < 0.05 were considered statistically significant.
RESULTS
Fifty-three reports from 1996 to July 2018 were identified from Ethiopia (2), Kenya (9), Madagascar (1), Nigeria (1), South Africa (3), Tanzania (9), Uganda (9), Zambia (2), Zimbabwe (8), and incorporating data from multiple countries in sub-Saharan Africa (9). Three studies compared schistosomiasis with soil-transmitted helminths (STHs): Ascaris lumbricoides, hookworm, Trichuris trichiura, Trichostrongylus, and Strongyloides stercoralis.5–7 Two studies included children and adolescents only8,9; four reviewed schistosome eggs and viral shedding in the semen and included males only.10–13 Five articles assessed female genital schistosomiasis (FGS) and included women only,14–18 whereas all other articles included male and female adults defined as 18 years and older.
Effects of schistosomiasis on HIV acquisition.
Table 1 describes eleven studies that were published from 2003 to 2017, including studies from Kenya (1), Madagascar (1), South Africa (1), Tanzania (3), Uganda (1), Zimbabwe (3), and broadly sub-Saharan Africa (1). One study, which involved men only, suggested that male urogenital schistosomiasis might constitute a risk factor for HIV transmission, as a result of egg-induced inflammation in the semen-producing pelvic organs.11 A 2006 study examined the association between FGS and HIV in rural Zimbabwe and included women only19; and a 2014 study from South Africa defined “cases” as women with FGS and “controls” as FGS-negative (FGS−) women18 to determine HIV risk. The remaining eight studies included both male and female adults. The average number of participants from the eleven studies was 627, and the average number of cases (H+/S+) was 110. All the articles concerned S. mansoni infection, or S. haematobium, or both. Schistosoma haematobium appeared to have greater risk of HIV transmission as these eggs embed in the venous plexus and mucosa of the genital tract (average OR = 4.0).
Table 1.
Risk of HIV acquisition in current schistosomiasis infection
| Author and year of publication | Country [reference number] | Helminth species | Study design and participants | Major findings |
|---|---|---|---|---|
| Risk of HIV infection | ||||
| Downs et al., 2012 | Tanzania [14] | S. mansoni | Cross-sectional (n = 345), cases = 185 | HIV prevalence was higher among women with more intense schistosome infections (P = 0.005), and the median schistosome intensity was higher in HIV-infected than HIV-uninfected women (400 vs. 15 per g circulating anodic antigen/mL, P = 0.01). |
| Kjetland et al., 2006 | Zimbabwe [15] | S. haematobium | Cross-sectional (n = 479), cases = 134 | S. haematobium infection of the genital mucosa was significantly associated with HIV seropositivity (adjusted odds ratios, 2.9; 95% CI: 1.11–7.5; P = 0.030). |
| Downs et al., 2017 | Tanzania [29] | Schistosoma (species not specified) | Case–control (n = 338), cases = 73 | Women with schistosome infections had higher odds of HIV-1 acquisition than those without (adjusted odds ratios = 2.8 [1.2–6.6], P = 0.019). |
| Ssetaala et al., 2015 | Uganda [30] | S. mansoni | Case–control (n = 200), cases = 50 | Not having taken praziquantel in the last 2 years was associated with HIV acquisition. |
| Ndhlovu et al., 2007 | Zimbabwe [31] | S. haematobium | Cross-sectional (n = 544) | Women with urinary schistosomiasis had a higher HIV prevalence (33.3%) than women without urinary schistosomiasis (HIV prevalence of 25.6%, P = 0.053). |
| Immunological mechanisms | ||||
| Midzi et al., 2017 | Zimbabwe [10] | S. haematobium | Observational (n = 2,825) | Individuals with S. haematobium were found with higher cell surface densities of CCR5 and CXCR4 receptors on CD4+ T cells and monocytes in peripheral blood, which made them more susceptible to HIV infection. |
| Leutscher et al., 2005 | Madagascar [11] | S. haematobium | Cross-sectional (n = 240) | S. haematobium egg excretion in semen was associated with the presence of neutrophil, lymphocyte, and eosinophil leukocytes that contribute to the transmission of sexually transmitted infections (Chlamydia trachomatis, P < 0.05). |
| Kleppa et al., 2014 | South Africa [18] | S. haematobium | Case–control (n = 39), cases = 14 | CD4+ T cells expressing CCR5 were more frequent in FGS–positives than FGS–negatives (median 4.7% (IQR 1.7–8.1) vs. 1.5% (IQR 0.6–3.4), respectively, P = 0.018), with blood samples showing that the level of CCR5 expression on CD4+ cells decreased significantly after treatment (median 3.4% (IQR 1.8–7.2) vs. 0.5% (IQR 0.4–1.8) respectively, P = 0.036). |
| Secor et al., 2003 | Kenya [19] | S. mansoni | Cross-sectional (n = 42) | Patients with active schistosomiasis displayed higher cell surface densities of chemokine receptors CCR5 and CXCR4 than did cured schistosomiasis patients. |
| Mathematical models | ||||
| Mbah et al., 2014 | Sub-Saharan Africa [32] | S. haematobium | Mathematical model | Twenty years of intervention, treatment with praziquantel annually, would reduce HIV incidence by 15%. |
| Secor et al., 2012 | Tanzania [33] | S. haematobium | Mathematical model | Schistosoma haematobium plays a greater role for increased susceptibility and transmission of HIV because of its association with urogenital disease. |
FGS = female genital schistosomiasis; S. haematobium = Schistosoma haematobium; S. mansoni = Schistosoma mansoni.
Key findings from five studies provide strong evidence that there is increased risk of HIV acquisition in people infected with schistosomes (Table 1). The mechanism of this increased risk may include increased density of CCR5 and CXCR4 receptors on CD4 cells,10,18,19 and S. haematobium egg excretion in semen is associated with the transmission of sexually transmitted infections.11 In addition, it can cause genital lesions in women from eggs embedding in the cervical mucosa, and it may increase the risk for sexually transmitted infections, including HIV.2
Effects of HIV on Schistosoma spp. acquisition.
We found three studies that investigated the risk of Schistosoma spp. acquisition as a direct result of HIV infection. In one study, male gender was associated with 2.6-fold greater odds of S. mansoni infection,20 but there was no evidence of an effect of HIV infection on the risk of acquiring schistosomiasis as assessed by Kato–Katz or urine CCA. The same study did not find an association between S. mansoni infection intensity and HIV infection status as assessed by adjusted odds ratio (aOR) of Kato–Katz (aOR = 1.04; 95% CI: 0.74–1.47, P = 0.81) or urine CCA (aOR = 1.53; 95% CI: 0.78–3.00, P = 0.19).20 In the second study, elevated percentages of circulating eosinophils were associated with resistance to reinfection by S. mansoni in HIV-negative people. However, among those with HIV, low CD4+ T-cell counts were associated with a less intense eosinophilia and an increased risk of S. mansoni reinfection.18,21
Effects of comorbidity.
Table 2 summarizes 1) the impact of HIV on schistosomiasis-related morbidity in the coinfected and 2) the impact of schistosomes on HIV morbidity in the coinfected population. Seven articles report progression to PPF, liver damage, anemia, and further immunosuppression as a consequence of HIV/Schistosoma coinfection. All studies included male and female adults with concurrent HIV and S. mansoni infection (cases); the average number of participants was 449, and the average number of cases was 97.
Table 2.
Adverse effects of schistosomiasis on morbidity and in HIV-infected adults
| Author and year of publication | Country [reference number] | Helminth species | Study design and participants | Major findings |
|---|---|---|---|---|
| Impact of HIV on schistosomiasis infection | ||||
| Mazigo et al., 2015 | Tanzania [22] | S. mansoni | Cross-sectional (n = 1,671), cases = 129 | There was no significant difference in the prevalence of PPF between coinfected individuals (n = 129) and those who were not infected with HIV-1 (n = 118). |
| Ocama et al., 2017 | Uganda [23] | S. mansoni | Cross-sectional (n = 299), cases = 206 | Among HIV+ people, 206 (69%) of all participants had periportal fibrosis; of those, 183 (68%) were positive for S. mansoni. |
| Of the 93 HIV + participants without PPF, 85 (32%) were positive for S. mansoni. | ||||
| Impact of schistosomiasis on HIV infection | ||||
| Mulu et al., 2013 | Ethiopia [5] | A. lumbricoides, hookworm, T. trichiura, S. mansoni, S. stercoralis | Longitudinal (n = 220), cases = 87 | Helminth-infected individuals had a higher level of CD8+ T cells at baseline (P < 0.001), which were significantly reduced (P < 0.01) at 12 weeks after anti-helminthic treatment. Helminths were also found to be associated with increased HIV RNA but were reduced after successful treatment. |
| Brown et al., 2004 | Uganda [6] | A. lumbricoides, hookworm, T. trichiura, S. mansoni, S. stercoralis | Case–control (n = 663), cases = 172 | Helminth infection was not associated with higher viral load, lower CD4+ cell count, or faster decrease in CD4+ cell count preceding anti-helminthic therapy. |
| Obuku et al., 2016 | Uganda [24] | S. mansoni | Observational (n = 34), cases = 18 | Plasma viral load and CD4 count were similar between HIV+ SM+ and HIV+ SM− individuals. The frequency of HIV-specific IFN-γ+IL-2-TNF-α- CD8 T cells and IFN-γ+IL-2-TNF-α+ CD4 T cells was significantly higher in HIV/S. mansoni coinfected individuals than in HIV mono-infected individuals. |
| Colombe et al., 2018 | Tanzania [34] | Schistosoma (species not specified) | Longitudinal (n = 172), cases = 43 | There was an 82% reduction in the risk of reaching CD4 counts of < 350 and/or death in HIV/Schistosoma coinfected compared with HIV mono-infected patients, suggesting that schistosomes may confer a protective effect. |
| Mwinzi et al., 2004 | Kenya [35] | S. mansoni | Case–control (n = 81), cases = 23 | Those with HIV+/Sm+ coinfection and their HIV−/Sm+ mono-infected counterparts with fibrosis both demonstrated reduced CD4+ T-cell counts. |
PPF = periportal fibrosis; S. haematobium = Schistosoma haematobium; S. mansoni = Schistosoma mansoni.
Impact of HIV on schistosomiasis-related morbidity.
Key findings suggest that there is little evidence that HIV increased rates of progression to portal vein fibrosis. The risk of morbidity remains high as schistosome infection in childhood increases the risk for infection in adulthood.8 Male gender, age 21–30 years, and more than 10 years of continuous residence in an endemic area are all factors that increase the risk of developing PPF in HIV/Schistosoma coinfected individuals, just as they are in those who are schistosome mono-infected,22 suggesting that HIV has no significant effect. Coinfection was associated with the development of PPF; however, coinfection was not an accelerating factor for adverse outcomes such as anemia, hepatotoxicity, PPF, increase in the portal vein diameter, varices, or reinfection.23
Impact of schistosomiasis on HIV morbidity.
Key findings suggest that coinfection may be protective against death and HIV disease progression. One study shows that data on CD4 counts in coinfection are inconsistent,5 as are data suggesting reduced levels of inflammatory markers following treatment. In two studies, inflammatory biomarkers were significantly elevated in the coinfected compared with HIV mono-infected people.24 Soluble tumor necrosis factor-RII and IL-8 were positively associated with schistosome intensity as measured by CAA, regardless of HIV status.25 Schistosomes may be an important contributor to immune activation as evidenced by sustained decreases in IL-10 in H+/S+ individuals after antiretroviral therapy initiation25; however, it is not clear what the clinical consequences of this are.
Impact of HIV on praziquantel therapy and the impact of schistosomes on antiretroviral therapy: Immune reconstitution.
Table 3 describes the nine articles that discuss the effects of combined anti-schistosomal and antiretroviral treatment on the improvement of immunological parameters of coinfection. The articles reviewed were from Ethiopia (1), Kenya (1), Tanzania (1), Uganda (2), Zimbabwe (2), and broadly sub-Saharan Africa (2) and included adult male and female participants. The average number of participants was 650, and the average number of cases was 125. Three articles included HIV/S. mansoni and STH-positive individuals,5–7 and one mathematical model that examined the co-dynamics of schistosomiasis and HIV/AIDS.
Table 3.
Efficacy of praziquantel and/or antiretroviral therapy on immune reconstitution
| Author and year of publication | Country [reference number] | Helminth species | Study design and participants | Major findings |
|---|---|---|---|---|
| Praziquantel treatment | ||||
| Mulu et al., 2013 | Ethiopia [5] | A. lumbricoides, hookworm, T. trichiura, S. mansoni, S. stercoralis | Longitudinal (n = 220), cases = 87 | At baseline, helminth-infected individuals had a higher level of CD8+ T cells (P < 0.001) and significantly lower baseline viral load (5.01 log10 vs. 3.41 log10, P < 0.001). Viral load remained reduced posttreatment. |
| Brown et al., 2004 | Uganda [6] | A. lumbricoides, hookworm, T. trichiura, S. mansoni, S. stercoralis | Case–control (n = 663), cases = 172 | Helminth infection was not associated with higher viral load, lower CD4+ cell count, or faster decrease in CD4+ cell count preceding anti-helminthic therapy. |
| Walson et al., 2008 | Sub-Saharan Africa [7] | A. lumbricoides, hookworm, T. trichiura, Trichostrongylus, Schistosoma spp., S. stercoralis | Longitudinal | Coinfected individuals receiving treatment for schistosomiasis had a significantly lower change in plasma HIV-1 RNA over 3 months (−0.001 log10 copies/mL) than those receiving no treatment (+0.21 log10 copies/mL) (P = 0.03). |
| Midzi et al., 2017 | Zimbabwe [10] | S. haematobium | Observational (n = 2,825) | Following treatment with praziquantel, there was a decline in HIV-1 RNA viral load in men with urogenital schistosomiasis. |
| Secor et al., 2003 | Kenya [19] | S. mansoni | Cross-sectional (n = 42) | The presence of HIV-1 immune cells in the seminal vesicles and the prostate declines after praziquantel treatment with diminished viral shedding in the semen as a result. |
| Mushayabasa and Bhunu 2011 | Sub-Saharan Africa [26] | Schistosoma (species not specified) | Mathematical model | Schistosomiasis may increase the prevalence of HIV/AIDS in the community, but schistosomiasis treatment can reduce the burden of schistosomiasis and HIV/AIDS coinfection in areas of extreme poverty. |
| Kallestrup et al., 2005 | Zimbabwe [36] | Schistosoma (species not specified) | Case–control (n = 287), cases = 130 | Those with early treatment with praziquantel had a significantly lower increase in plasma HIV-1 RNA load than did those who received delayed treatment (n = 66) (P < 0.05). |
| Brown et al., 2005 | Uganda [37] | S. mansoni | Longitudinal (n = 163) | Treatment of S. mansoni infection in adults coinfected with HIV-1 results in a transient increase in viral replication. |
| Antiretroviral treatment | ||||
| Efraim et al., 2013 | Tanzania [38] | Schistosoma (species not specified) | Cross-sectional (n = 351), cases = 112 | Schistosome infection was strongly associated with immunological failure (odds ratio 4.6 [95% CI: 1.9–11.2], P = 0.0009) in coinfected people, with significantly lower CD4+ count increases on antiretroviral therapy than in schistosome-uninfected patients. |
S. haematobium = Schistosoma haematobium; S. mansoni = Schistosoma mansoni.
These studies do not permit any generalization, suggesting treatment with praziquantel either increases viral load or decreases viral load, provides no change, or has a positive impact on HIV viral suppression.26
Efficacy of praziquantel in coinfected persons: Egg excretion.
Table 4 summarizes studies on praziquantel treatment for schistosomiasis among persons with HIV as measured by egg excretion. We reviewed 10 articles from four countries. One study from Kenya included men only,12 and all other studies included adult males and females with concurrent HIV/Schistosoma infection (cases); the average number of participants was 722, and the average number of cases was 287. One Kenyan study spanned from 1997 to 2009 and included 569 men with 823 episodes of schistosomiasis. The overall mean egg reduction was 83% and ranged from 62% to 91% annually, and the overall mean number of praziquantel doses needed to achieve cure was 1.8, ranging from 1.4 to 3.1 annually.12
Table 4.
Effects of HIV on schistosome egg output
| Author and year of publication | Country [reference number] | Helminth species | Study design and participants | Major findings |
|---|---|---|---|---|
| Egg shedding posttreatment with praziquantel | ||||
| Sanya et al., 2015 | Uganda [20] | S. mansoni | Cross-sectional (n = 2,316), cases = 1,412 | Median S. mansoni eggs per gram (EPG) for HIV-positive participants (median = 102, IQR 36–321) was lower than that for the HIV-negatives (median = 180, IQR 60–660). |
| Mwinzi et al., 2004 | Kenya [35] | S. mansoni | Case–control (n = 81), cases = 23 | Schistosoma mansoni egg count per gram was lower in the HIV-positive participants (P = 0.005). |
| Kallestrup et al., 2005 | Zimbabwe [36] | Schistosoma (species not specified) | Case–control (n = 287), cases = 130 | Those who received treatment with praziquantel (n = 64) had no significant difference in urine and fecal egg count between early and late treatment groups. |
| Secor et al., 2004 | Kenya [39] | Schistosoma (species not specified) | Longitudinal (n = 172), cases = 43 | Fecal egg and circulating antigen levels were reduced by praziquantel; however, there was no effect on viral load. The anti-schistosome antibody responses critical for praziquantel efficacy may have developed before depletion of CD4+ T cell help that may be necessary for antibody production. |
| Karanja et al., 1998 | Kenya [40] | S. mansoni | Case–control (n = 47), cases = 15 | Praziquantel was useful for treating schistosomiasis in people with HIV-1 coinfection and lowered CD4 lymphocyte percentages. Those who were excreting eggs 4 weeks after initial drug treatment still had an average 93% decrease in their S. mansoni eggs per gram, regardless of HIV-1 serostatus. |
| Egg shedding post antiretroviral treatment | ||||
| Muok et al., 2013 | Kenya [27] | S. mansoni | Longitudinal (n = 90) | Kato–Katz–negative individuals at enrollment subsequently started excreting S. mansoni eggs once antiretroviral therapy begun, accompanied by a significant increase in CD4+ T lymphocytes (P = 0.004). |
| Host immunity post combination treatment | ||||
| Black et al., 2009 | Kenya [12] | S. mansoni | Longitudinal (n = 178) | Among HIV-infected individuals, there was a 26.5% cure failure after single-dose praziquantel treatment, although there was no comparison group. |
| Kallestrup et al., 2006 | Zimbabwe [28] | S. haematobium | Longitudinal (n = 1,545), cases = 287 | HIV-positive individuals experience significantly less clearance of schistosomiasis (cure rate, 31%) than HIV-negative individuals (cure rate, 52%). |
| Fontanet et al., 2000 | Ethiopia [41] | S. mansoni | Cross-sectional (n = 1,239) | Schistosoma mansoni egg output was significantly lower in the HIV-positives than in the HIV-negatives (Mann–Whitney test; P = 0.03; ratio of geometric means = 0.74) and remained so after controlling for potential confounders. |
| Karanja et al., 1997 | Uganda [42] | Schistosoma (species not specified) | Cross-sectional (n = 351), cases = 97 | Patients who were infected with S. mansoni and were seropositive for HIV had similar levels of circulating cathodic antigen but excreted fewer eggs (643 ± 622 EPG; n = 16) than individuals who were not seropositive for HIV infection (1,891 ± 1,779 EPG; n = 37) (P = 0.009). |
S. haematobium = Schistosoma haematobium; S. mansoni = Schistosoma mansoni.
The key findings of this study are that these articles provide strong and consistent evidence that HIV reduces egg excretion and clear evidence that antiretroviral therapy increases egg output (P = 0.004).27 HIV probably reduces praziquantel efficacy,12,27,28 but few studies have looked at this, and more work is required.
DISCUSSION
This systematic review examined 53 articles from sub-Saharan Africa focusing on HIV/Schistosoma coinfected individuals. Based on the studies we reviewed, we found the following:
1. There is strong evidence for increased risk of HIV acquisition in people infected with schistosomes probably because the density of CCR5 and CXCR4 receptors on CD4 cells increases together with an increase in egg-induced mucosal damage.10,19
2. Few studies have examined the risk of schistosome acquisition in people with versus without HIV; these studies show no evidence of an effect of HIV infection on the risk of acquiring schistosomes as assessed by Kato–Katz or urine CCA.32
3a. There is little evidence that HIV increased rates of progression to portal vein fibrosis; coinfection was not an accelerating factor for adverse outcomes, such as anemia, hepatotoxicity, PPF, increase in portal vein diameter, varices, or reinfection23; and 3b) schistosomiasis may be an important contributor to immune activation as evidenced by sustained decreases in IL-10 in H+/S+ individuals after antiretroviral therapy initiation25; however, it is not clear what the clinical consequences of this are. There are also inconsistent data about CD4 counts.
4. There were inconclusive data regarding praziquantel, suggesting that treatment increases viral load, decreases viral load, or shows no change, and schistosomiasis treatment has a positive impact on HIV viral suppression.26
5. There is strong and consistent evidence that HIV reduces schistosome egg excretion, antiretroviral therapy increases egg output, and praziquantel is effective during antiretroviral therapy. A key question is the timing of praziquantel: whether early treatment with praziquantel can counteract the increased egg excretion that occurs during antiretroviral therapy initiation.
Limitations of reviewed articles.
Based on the articles that met our criteria, there were insufficient data available to determine the potential benefits of helminth eradication, more specifically Schistosoma, in HIV-1 coinfected adults, and insufficient data to support a reduction of HIV-1 viral load, which could be very important for disease progression and viral transmission. Our search overlooked the role of CD4 cells in HIV infections, as they are key contributors to the inflammation pathway and to the development of fibrosis in schistosomiasis. At the same time, CD4-mediated granuloma formation is important for walling off eggs and further egg excretion, which plays an important role in immune activation and schistosome-related morbidity in coinfected individuals. The impact of deworming on markers of HIV-1 progression should be addressed in pilot studies that treat every HIV-positive person with praziquantel while evaluating species-specific effects, with a sufficient duration of follow-up to document potential differences in clinical outcomes and CD4+ decline.29 Other limitations observed were small sample sizes and the predominance of women versus men.23 Choice of cross-sectional study design was also a limiting factor, as these studies could not provide an accurate time line for how long patients were on antiretroviral therapy or how long they had been infected or reinfected with schistosomes. Also, choice of diagnostic test should be considered as the CCA test can reliably detect S. mansoni, whereas CAA testing detects both without the ability to determine species. Similarly, Kato–Katz is only useful for detecting S. mansoni; urine filtration is used to detect S. haematobium eggs, but both can miss low-intensity infections as egg burden fluctuates daily. Finally, no article reported on pediatric coinfected participants without also including adults, as most schistosome acquisition occurs in childhood.
CONCLUSION
This systematic review examined the relationships between schistosomiasis and HIV in coinfected individuals; the key take-home points are as follows: 1) there is evidence that infection with schistosomes increases the risk of HIV transmission/acquisition; 2) there are insufficient data on whether HIV infection increases the risk of Schistosoma acquisition; 3) coinfection may influence the levels of inflammatory biomarkers; 4) there is inconsistent evidence on the effect coinfection has on CD4+ T-cell levels and host immune response; and 5) coinfection impedes schistosome egg excretion, which can rebound during initiation of antiretroviral therapy.
Although increased risk of HIV acquisition seems to be species dependent, on the whole, schistosomes may contribute to the spread and progression of HIV/AIDS in areas where both diseases are prevalent. Critical gaps in research include further exploration of schistosomes as an important contributor to immune activation, as evidence demonstrated sustained decreases in IL-10 in H+/S+ individuals after antiretroviral therapy initiation compared with H-/S+ individuals25 as it could pertain to immune protection. Future studies should also compare HIV+ and HIV-uninfected children to find influences of HIV on schistosome acquisition, reinfection, and resistance.
Finally, studies should consider the implications of both male and female genital schistosomiasis in HIV prevention programs to better understand the underlining immunologic contributions of coinfection as protection.
REFERENCES
- 1.World Health Organization , 2017. HIV/AIDS Fact Sheet, HIV/AIDS. Available at: https://www.afro.who.int/health-topics/hivaids. Accessed August 12, 2018. [Google Scholar]
- 2.World Health Organization , 2018. Fact Sheet Schistosomiasis. Available at: http://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed August 12, 2018. [Google Scholar]
- 3.Inobaya MT, Olveda RM, Chau TN, Olveda DU, Ross AG, 2014. Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med 2014: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li XX, Zhou XN, 2013. Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasites Vectors 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulu A, Maier M, Liebert UG, 2013. Deworming of intestinal helminths reduces HIV-1 subtype C viremia in chronically co-infected individuals. Int J Infect Dis 17: e897–e901. [DOI] [PubMed] [Google Scholar]
- 6.Brown M, Kizza M, Watera C, Quigley MA, Rowland S, Hughes P, Whitworth JA, Elliott AM, 2004. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J Infect Dis 190: 1869–1879. [DOI] [PubMed] [Google Scholar]
- 7.Walson JL, John-Stewart G, 2008. Treatment of helminth co-infection in HIV-1 infected individuals in resource-limited settings. Cochrane Database Syst Rev CD006419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D, 2003. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 86: 125–139. [DOI] [PubMed] [Google Scholar]
- 9.Olusegun AF, Ehis OC, Richard O, 2011. Proportion of urinary schistosomiasis among HIV-infected subjects in Benin city, Nigeria. Oman Med J 26: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Midzi N, Mduluza T, Mudenge B, Foldager L, Leutscher PD, 2017. Decrease in seminal HIV-1 RNA load after praziquantel treatment of urogenital schistosomiasis coinfection in HIV-positive men—an observational study. Open Forum Infectious Diseases, Vol. 4, Oxford University Press, ofx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leutscher PDC, Pedersen M, Raharisolo C, Jensen JS, Hoffmann S, Lisse I, Ostrowski SR, Reimert CM, Mauclere P, Ullum H, 2005. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium-infected individuals. J Infect Dis 191: 1639–1647. [DOI] [PubMed] [Google Scholar]
- 12.Black CL, Steinauer ML, Mwinzi PN, Evan Secor W, Karanja DM, Colley DG, 2009. Impact of intense, longitudinal retreatment with praziquantel on cure rates of Schistosomiasis mansoni in a cohort of occupationally exposed adults in western Kenya. Trop Med Int Health 14: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Silva S, Walsh J, Brown M, 2006. Symptomatic Schistosoma mansoni infection as an immune restoration phenomenon in a patient receiving antiretroviral therapy. Clin Infect Dis 42: 303–304. [DOI] [PubMed] [Google Scholar]
- 14.Downs JA, et al. 2012. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg 87: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, Mason PR, Sandvik L, Friis H, Gundersen SG, 2006. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20: 593–600. [DOI] [PubMed] [Google Scholar]
- 16.Secor WE, 2006. Interactions between schistosomiasis and infection with HIV‐1. Parasite Immunol 28: 597–603. [DOI] [PubMed] [Google Scholar]
- 17.Mutengo MM, Mudenda V, Mwansa JC, Kaonga K, Sianongo S, Wamulume HI, Shinondo CJ, 2009. Presence of Schistosomiasis in genital biopsies from patients at the university teaching hospital in Lusaka, Zambia. Med J Zambia 36: 114–118. [Google Scholar]
- 18.Kleppa E, et al. 2014. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS One 9: e98593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secor WE, Shah A, Mwinzi PM, Ndenga BA, Watta CO, Karanja DM, 2003. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4+ T cells and monocytes of patients with Schistosoma mansoni infection. Infect Immun 71: 6668–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E, Webb EL, Elliott AM; LaVIISWA Study Team , 2015. Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Trop Med Int Health 20: 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, Colley DG, Secor WE, 2006. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun 74: 2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazigo HD, Dunne DW, Morona D, Lutufyo TE, Kinung’hi SM, Kaatano G, Nuwaha F, 2015. Periportal fibrosis, liver and spleen sizes among S. mansoni mono or co-infected individuals with human immunodeficiency virus-1 in fishing villages along Lake Victoria shores, North-Western, Tanzania. Parasites Vectors 8: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocama P, Opio KC, Seremba E, Ajal P, Apica BS, Aginya EO, 2017. The burden, pattern and factors that contribute to periportal fibrosis in HIV-infected patients in an S. mansoni endemic rural Uganda. Afr Health Sci 17: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obuku AE, et al. 2016. Effect of Schistosoma mansoni infection on innate and HIV-1-specific T-cell immune responses in HIV-1-infected Ugandan fisher folk. AIDS Res Hum Retroviruses 32: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erikstrup C, et al. 2008. Schistosomiasis and infection with human immunodeficiency virus 1 in rural Zimbabwe: systemic inflammation during co-infection and after treatment for schistosomiasis. Am J Trop Med Hyg 79: 331–337. [PubMed] [Google Scholar]
- 26.Mushayabasa S, Bhunu CP, 2011. Modeling schistosomiasis and HIV/AIDS co-dynamics. Comput Math Methods Med 2011: 846174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muok EM, Simiyu EW, Ochola EA, Secor WE, Karanja DM, Mwinzi PN, 2013. Association between CD4+ T-lymphocyte counts and fecal excretion of Schistosoma mansoni eggs in patients coinfected with S. mansoni and human immunodeficiency virus before and after initiation of antiretroviral therapy. Am J Trop Med Hyg 89: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Gerstoft J, Erikstrup C, Ullum H, 2006. Schistosomiasis and HIV in rural Zimbabwe: efficacy of treatment of schistosomiasis in individuals with HIV coinfection. Clin Infect Dis 42: 1781–1789. [DOI] [PubMed] [Google Scholar]
- 29.Downs JA, et al. 2017. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: a nested case-control study. PLoS Negl Trop Dis 11: e0005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ssetaala A, et al. 2015. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case–control study. Trop Med Int Health 20: 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndhlovu PD, Mduluza T, Kjetland EF, Midzi N, Nyanga L, Gundersen SG, Friis H, Gomo E, 2007. Prevalence of urinary schistosomiasis and HIV in females living in a rural community of Zimbabwe: does age matter? Trans R Soc Trop Med Hyg 101: 433–438. [DOI] [PubMed] [Google Scholar]
- 32.Mbah MLN, Gilbert JA, Galvani AP, 2014. Evaluating the potential impact of mass praziquantel administration for HIV prevention in Schistosoma haematobium high-risk communities. Epidemics 7: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secor WE, 2012. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS 7: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombe S, et al. 2018. Impact of schistosome infection on long-term HIV/AIDS outcomes. PLoS Negl Trop Dis 12: e0006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwinzi PN, Karanja DM, Kareko I, Magak PW, Orago AS, Colley DG, Secor WE, 2004. Evaluation of hepatic fibrosis in persons co-infected with Schistosoma mansoni and human immunodeficiency virus 1. Am J Trop Med Hyg 71: 783–786. [PubMed] [Google Scholar]
- 36.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, Van Dam GJ, Gerstoft J, Erikstrup C, Ullum H, 2005. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis 192: 1956–1961. [DOI] [PubMed] [Google Scholar]
- 37.Brown M, Mawa PA, Joseph S, Bukusuba J, Watera C, Whitworth JA, Dunne DW, Elliott AM, 2005. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J Infect Dis 191: 1648–1657. [DOI] [PubMed] [Google Scholar]
- 38.Efraim L, Peck RN, Kalluvya SE, Kabangila R, Mazigo HD, Mpondo B, Bang H, Todd J, Fitzgerald DW, Downs JA, 2013. Schistosomiasis and impaired response to antiretroviral therapy among HIV-infected patients in Tanzania. J Acquir Immune Defic Syndr 62: e153–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Secor WE, Karanja D, Colley DG, 2004. Interactions between schistosomiasis and human immunodeficiency virus in Western Kenya. Mem Inst Oswaldo Cruz 99: 93–95. [DOI] [PubMed] [Google Scholar]
- 40.Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, Secor WE, 2002. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360: 592–596. [DOI] [PubMed] [Google Scholar]
- 41.Fontanet AL, Woldemichael T, Sahlu T, Van Dam GJ, Messele T, Rinke de Wit T, Masho W, Yeneneh H, Coutinho RA, Van Lieshout L, 2000. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol 94: 145–155. [PubMed] [Google Scholar]
- 42.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE, 1997. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg 56: 515–521. [DOI] [PubMed] [Google Scholar]