Abstract.
There are an estimated 19.4 million sepsis cases every year, many of them in low-income countries. The newly adopted definition of sepsis uses Sequential Organ Failure Assessment Score (SOFA), a score which is not feasible in many low-resource settings. A simpler quick-SOFA (qSOFA) based solely on vital signs score has been devised for identification of suspected sepsis. This study aimed to determine in-hospital prevalence and outcomes of sepsis, as defined as suspected infection and a qSOFA score of 2 or more, in two hospitals in Malawi. The secondary aim was to evaluate qSOFA as a predictor of mortality. A cross-sectional study of adult in-patients in two hospitals in Malawi was conducted using prospectively collected single-day point-prevalence data and in-hospital follow-up. Of 1,135 participants, 81 (7.1%) had sepsis. Septic patients had a higher hospital mortality rate (17.5%) than non-septic infected patients (9.0%, p = 0.027, odds ratio 2.1 [1.1–4.3]), although the difference was not statistically significant after adjustment for baseline characteristics. For in-hospital mortality among patients with suspected infection, qSOFA ≥ 2 had a sensitivity of 31.8%, specificity of 82.1%, a positive predictive value of 17.5%, and a negative predictive value of 91.0%. In conclusion, sepsis is common and is associated with a high risk of death in admitted patients in hospitals in Malawi. In low-resource settings, qSOFA score that uses commonly available vital signs data may be a tool that could be used for identifying patients at risk—both for those with and without a suspected infection.
INTRODUCTION
Globally, there are an estimated 19.4 million sepsis cases and 5.3 million sepsis deaths every year.1,2 Approximately 30% of patients in intensive care units (ICUs) have sepsis.3 The data comes almost exclusively from high-income countries—only 1.4% of the patients were from Africa—and the prevalence of sepsis may be even higher in low-income countries.1,3
The recent “Third International Consensus” adopted a new definition of sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection.”4 The consensus proposed the Sequential (sepsis-related) Organ Failure Assessment Score (SOFA) criteria for the identification of sepsis in ICUs. For SOFA, each organ system (respiration, cardiovascular, coagulation, liver, central nervous, and renal) is assigned a number from 0 to 4 depending on its level of dysfunction. The numbers are added up to generate a SOFA score: the higher the score, the more severe the illness. The derivative studies from the USA found that an increased SOFA score of ≥ 2 was associated with a mortality rate of greater than 10%.4 A simpler tool called quick-SOFA (qSOFA) was also devised to identify elevated risk of death among patients with suspected infection in emergency departments and general wards, but in low-income countries, it may be appropriate to use qSOFA to define sepsis among patients with suspected infection.5 quick-SOFA assigns one point for each of a respiratory rate of 22/minute or greater, altered mentation, or a systolic blood pressure of 100 mmHg or less. A score of ≥ 2 plus a clinical suspicion of infection suggests sepsis. quick-SOFA was found to have a greater predictive validity for mortality outside the ICU than the SOFA score in a U.S. population.4,6
In Malawi, a recent single-center study found that, at admission, 29% of medical patients had sepsis as defined by a suspected infection and qSOFA ≥ 2.7 When planning hospital services, the in-hospital prevalence of sepsis is an equally important measure as admission prevalence as it provides information about the burden of sepsis within the hospital. The in-hospital prevalence of sepsis in Malawi and other low-income countries and the outcomes of these patients have not been investigated. The primary aim of this study was to determine the in-hospital point-prevalence and outcomes of sepsis in two hospitals in Malawi using the definition of suspected infection and a qSOFA score of ≥ 2. The secondary aim was to evaluate the predictive value of a qSOFA score of ≥ 2 for mortality among hospitalized adults and among those with suspected infection.
METHODOLOGY
A cross-sectional study of adult in-patients in two hospitals in Malawi using prospectively collected, single-day point-prevalence data and in-hospital follow-up.
Study setting.
The study took place in Queen Elizabeth Central Hospital (QECH) and Chiradzulu District Hospital (CDH). The Queen Elizabeth Central Hospital is a national referral center in Blantyre with 1,000 beds, an immediate catchment population of one million, and the availability of many specialist services and physicians. The Chiradzulu District Hospital is a district hospital with a catchment population of 230,000, 300 beds, and, at the time of the study, one physician. These two hospitals were chosen to provide settings of different resources; personnel and epidemiology CDH does not have an ICU, whereas QECH has a four-bedded adult unit capable of providing continual monitoring, mechanical ventilation, and vasopressor infusions.
Study population.
All patients older than 18 years who were being treated as in-patients in any of the wards in the two hospitals on the study days (2 days in QECH: Saturday, January 21, 2017 and Tuesday, May 8, 2018; three times in CDH: Friday, November 24, 2017; Wednesday, February 7, 2018; and Tuesday, July 3, 2018 in CDH) were included. The exclusion criteria were 1) women in active labor or 2) admitted solely to wait for their delivery, 3) patients with a primary psychiatric diagnosis, 4) patients in the final stage of dying (those identified by the ward clinician or nurse-in-charge as terminal patients who were expected to die within minutes or hours), and 5) refusal to consent.
Data collection.
Data on patient demographics, diagnoses, and prescribed treatments were collected from the patients’ files and clinical data on the patients’ vital signs and ongoing therapies were collected by direct clinical observation at the time of inclusion. Blood pressures were measured using Omron M2 digital blood pressure machines, and oxygen saturations and heart rates were measured using Lifebox pulse oximeters. Consciousness was measured using both Glasgow coma score and Alert, V responding to verbal stimuli, P- Responding to painful stimuli, U- Unresponsive (AVPU). Altered mental status was defined as a GCS of equal to or less than 14 or an AVPU score of P, V, or U. Data were collected by qualified nurses and senior nursing students following training that was conducted the day before to ensure a standardized methodology.
Sepsis was defined as a qSOFA score ≥ 2 plus suspected infection, where suspected infection was any documented current infectious disease in the patient’s medical records. A positive HIV status alone was not defined as a suspected infection. Although qSOFA was used in the derivative studies from the United States for suspecting sepsis rather than a definitive diagnosis,6,8 the SOFA score is not possible to calculate in Malawian hospitals and so a pragmatic decision was made to use qSOFA as the illness severity criterion for the diagnosis of sepsis. An alternative definition of suspected infection as “the current prescription of an antimicrobial” was used in the secondary analysis.
The primary mortality outcome was in-hospital mortality (censored at 30 days). The secondary outcome was 48-hour mortality. The 48-hour outcomes were collected by direct observation of each participant on the ward, and from the ward clerk if the patient had been discharged or died. The ward clerks kept records of every participant, and the data were reviewed daily by the researchers to identify those who died and those who were discharged (alive). Outcomes were censored at 30 days, so patients still in the hospital at 30 days were recorded as alive.
Ethical clearance for the study was granted by the University of Malawi College of Medicine Research and Ethics Committee (COMREC P.08/16/2007).
Statistical analysis.
Each patient’s qSOFA score was calculated from the vital signs. The prevalence of patients with suspected infection and with sepsis and their 48-hour and 30-day in-hospital mortalities were calculated. The patients were cared for by specialty care teams which were grouped into five specialties: medicine, surgery, obstetrics and gynecology, ophthalmology, and other. If the hospital mortality at 30 days was missing, the participant was included in the prevalence analyses, but excluded from outcomes analyses.
The risk of death was analyzed primarily using univariable logistic regression. Multivariable logistic regression was also conducted with the covariates decided a priori because of a plausible biological effect: age, gender, HIV status, and surgery in this admission. The model’s goodness-of-fit was evaluated using the Hosmer–Lemeshow test. We tested for collinearity by quantifying variance inflation factors. For the performance of the binary variable of qSOFA ≥ 2 for predicting death, sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs), and likelihood ratios were calculated. Area under the receiver operating characteristic curves (AUROC) were calculated. Missing data for vital signs included in qSOFA (systolic blood pressure, respiratory rate, and conscious level) were imputed a score of 1, as we adjudged the most common reason for missing data to be the recognition of a patient in a critical state leading to unobtainable measurements or necessitating immediate action by the data collector. Sensitivity analyses were carried out with an imputed score of 0. Missing data for covariates were excluded. Stata (Release 15, StataCorp, College Station, TX) was used for the analyses. P-values less than 0.05 were considered statistically significant.
RESULTS
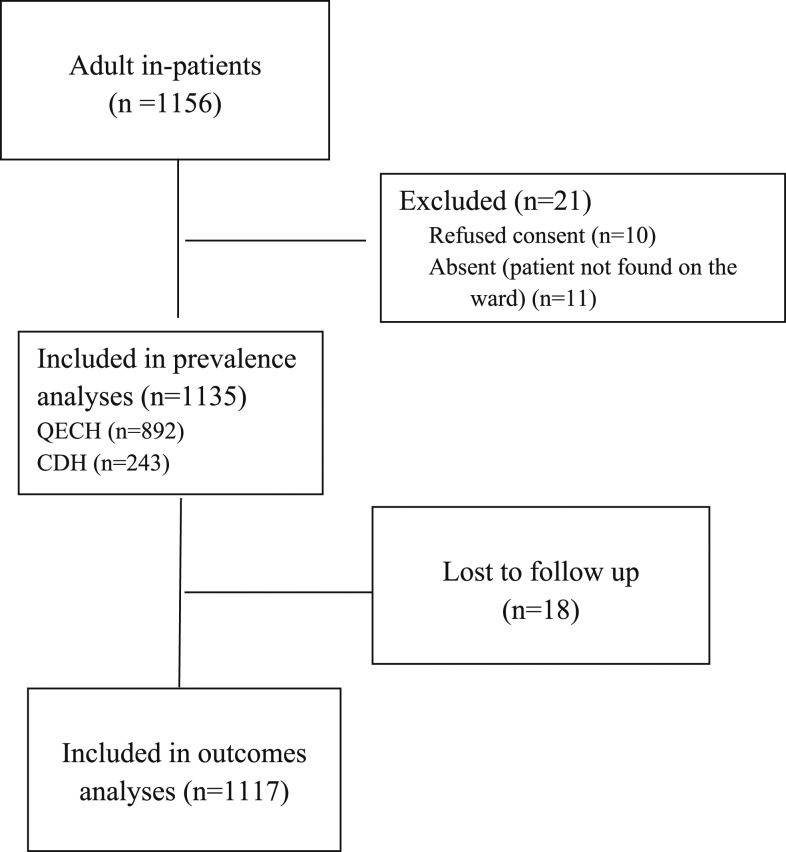
All the 1,135 adult in-patients (excluding waiting mothers) in the hospitals on the days of data collection were included in the prevalence analyses, and 1,117 patients with known outcomes were included in the outcome analyses (Figure 1.) Eight hundred ninety-two patients were in QECH and 243 in CDH. Fifty-nine percent were female, the patients’ mean age was 39 years, and they were in the hospital for a median of 5 days before inclusion and 4 days after inclusion (Table 1). There were 20 (0.7%) missing vital sign data points that required imputation.
Figure 1.
Consolidated Standards for Trials (CONSORT) diagram. CDH = Chiradzulu District Hospital; QECH = Queen Elizabeth Central Hospital.
Table 1.
Participant characteristics
| Variable | All patients | All patients without suspected infection | Patients with suspected infection* | Patients with suspected infection and qSOFA < 2 | Patients with sepsis† |
|---|---|---|---|---|---|
| All patients, n/N (%) | 1,135 (100) | 716/1,135 (63.1) | 419/1,135 (37.0) | 338/1,135 (34.1) | 81/1,135 (7.1) |
| Female (%) | 673 (59.3) | 450 (62.9) | 223 (53.2) | 179 (53.0) | 44 (54.3) |
| Age (years), mean (range) | 39 (18–98) | 38 (18–96) | 40 (18–98) | 40 (18–98) | 41 (19–80) |
| HIV-positive/HIV status known (%) | 367/846 (43.4) | 149/487 (30.6) | 218/359 (60.7) | 163/287 (56.8) | 55/72 (76.4) |
| Had surgery in hospital (%) | 265 (23.4) | 227 (31.7) | 38 (9.1) | 35 (10.4) | 3 (3.7) |
| Specialty | |||||
| Medicine (%) | 473 (41.7) | 164 (23.0) | 309 (73.8) | 237 (70.1) | 72 (88.9) |
| Surgery (%) | 319 (28.1) | 260 (36.3) | 59 (14.1) | 53 (15.4) | 7 (8.6) |
| Obstetrics and Gynecology (%) | 292 (25.7) | 249 (34.8) | 43 (10.3) | 42 (12.4) | 1 (1.2) |
| Ophthalmology (%) | 20 (1.8) | 15 (2.1) | 5 (1.2) | 5 (1.5) | 1 (1.2) |
| Other (%) | 31 (2.7%) | 28 (4.0%) | 3 (0.7%) | 3 (0.6%) | 0 |
| Length of hospital stay (days), median (ICQ) | |||||
| Before data collection | 5 (2–12) | 5 (2–12) | 6 (2–14) | 5 (2–14) | 7 (3–15) |
| After data collection‡ | 4 (2–10) | 4 (2–10) | 5 (2–10) | 5 (2–9) | 7 (3–11) |
| Total length of stay | 11 (5–22) | 11 (5–23) | 12 (6–21) | 12 (6–20) | 12 (8–26) |
IQR = interquatile range. All data n (%), unless otherwise stated.
* Any infectious disease.
† Suspected infection and a qSOFA score ≥ 2.
‡ Data censored at 30 days.
Among the participants, 419/1,135 (37.0%) had suspected infection and the prevalence of sepsis was 81/1,135 (7.1%). Among those with suspected infection, 81/419 (19.3%) had sepsis—72 were medical patients, seven were surgical, one was in obstetrics and gynecology, and one was in ophthalmology.
In-hospital mortality for the septic patients was 17.5%, whereas non-septic infected patients had a mortality of 9.0% (P = 0.027) (Table 2). Forty-eight–hour mortality for the septic patients was 3.8%, whereas non-septic infected patients had a 48-hour mortality of 1.5%. In univariable analysis, septic patients had increased odds of in-hospital mortality compared with non-septic infected patients (unadjusted odds ratio [OR] = 2.1, 95% CI: 1.1–4.3). When adjusting for the covariates, the association became nonsignificant: adjusted OR (aOR = 1.7, 95% CI: 0.8–3.5) (Table 2). The other covariates in the model had the following associations with mortality: age (OR per year = 1.0, 95% CI: 0.99–1.05, P 0.054), male gender (OR = 0.5, 95% CI: 0.2–0.99, P 0.047), HIV positive (OR = 4.5, 95% CI: 1.7–11.7, P 0.002). No deaths occurred among patients who had surgery in this admission. Collinearity between the independent variables was not high (all variance inflation factors were < 10). The Hosmer–Lemeshow test gave a P-value of 0.574, suggesting an adequate fit of the model to the data. Sensitivity analyses using qSOFA scores of 0 for missing variables did not substantially change any results.
Table 2.
Mortality by suspected infection and sepsis
| 48-hour mortality | In-hospital mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Died, n/N (%) | Unadjusted OR (95% CI) | P-value | Died, n/N (%) | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI)* | P-value |
| All patients | 23/1,134 (2.0%) | – | – | 84/1,117 (7.5%) | – | – | – | – |
| Patients with suspected infection† | 8/419 (2.0%) | – | – | 44/413 (10.7%) | – | – | – | – |
| Patients without sepsis but with suspected infection‡ | 5/338 (1.5%) | Reference | Reference | 30/333 (9.0%) | Reference | Reference | Reference | Reference |
| Patients with sepsis§ | 3/81 (3.7%) | 2.5 (0.6–11.0) | 0.204 | 14/80 (17.5%) | 2.1 (1.1–4.3) | 0.030 | 1.7 (0.8–3.5) | 0.115 |
OR = odds ratio.
* Adjusted for age, gender, HIV status, and surgery while in hospital.
† Any infectious disease.
‡ Suspected infection and a qSOFA score < 2.
§ Suspected infection and a qSOFA score ≥ 2.
When the alternative definition of suspected infection was used—any prescription of an antimicrobial—the prevalence of suspected infection and sepsis was 632/1,135 (55.7%) and 107/1,135 (9.4%), respectively. In-hospital mortality for the septic patients using the alternative definition was 18.9%, whereas non-septic infected patients had a mortality of 6.7% (OR 3.2, 95% CI: 1.8–5.8; aOR 2.0, 95% CI: 1.0–4.0).
quick-SOFA ≥ 2 as a predictor of in-hospital mortality among infected patients had a sensitivity of 31.8% (95% CI: 18.6–47.6), a specificity of 82.1% (95% CI: 77.8–85.9), a PPV of 17.5% (95% CI: 9.9–27.6), a NPV of 91.0% (95% CI: 87.4–93.8), a positive likelihood ratio of 1.78 (95% CI: 1.10–2.89), and a negative likelihood ratio of 0.83 (95% CI: 0.67–1.02). The AUROC for qSOFA among infected patients was 0.71 (95% CI: 0.66–0.77) (Supplemental Figure 1).
When used for all patients, not just those with suspected infection, 12.5% of patients had qSOFA ≥ 2% and 22.0% of patients with qSOFA ≥ 2 died (cf. 5.4% with qSOFA < 2, OR 5.0 [3.0–8.0], aOR [adjusting for the other covariates age, gender, HIV status, and surgery in this admission] 3.4 [2.0–6.0]). As a predictor of mortality for all inpatients, qSOFA ≥ 2 had a sensitivity of 37.0% (95% CI: 26.6–48.1), specificity of 89.4% (95% CI: 87.3–91.2), PPV 22.0% (95% CI: 15.5–29.7), NPV 94.6% (95% CI: 93.0–95.9), a positive likelihood ratio of 3.5 (95% CI: 2.4–4.8), and a negative likelihood ratio of 0.7 (95% CI: 0.6–0.8). The AUROC for qSOFA for all patients was 0.74 (95% CI: 0.69–0.79) (Supplemental Figure 2).
DISCUSSION
We have found a prevalence of sepsis among in-patients in two hospitals in Malawi of 9.3%. Septic patients had a hospital mortality rate of 17.5%, which was higher than non-septic infected patients (9.0%) and higher than for the whole patient population (7.5%), although the difference was not statistically significant after adjustment for baseline characteristics.
Our results confirm other authors’ findings: the large burden of disease due to sepsis.1,9–12 Our study is the first, to our knowledge, to measure the in-hospital prevalence in a mixed population in a low-income country. Admission prevalence has been found to be 21% among emergency department cases in Haiti13 and 29% among medical patients at admission in Lilongwe, Malawi.7 Older studies from the United States and the West Indies found a sepsis prevalence of 0.4% and 1.3%, respectively, among emergency department patients, but these studies were from higher resourced settings and used sepsis-related diagnosis codes.14,15 Other authors have looked at population incidence—a systematic review found a population incidence of 288 hospital-treated sepsis cases per 100,000 person-years,1 although almost all the data came from high-income countries.
Mortality rates for sepsis in high-income countries are estimated to be around 26%.1 Mortality rates in low-income countries may be expected to be higher because of resource limitations, but research is limited.16,17 Some studies from low- and middle-income countries have found mortality rates of 22–55%.11,18 In Malawi in 2008–2009, mortality for patients using a previous definition of sepsis that used the systemic inflammatory response (SIRS) criteria was 22%9 and in Haiti in 2014 was 24%13—similar to the findings in our study.
Our finding of an increased risk of mortality among infected patients if qSOFA ≥ 2 supports the findings of the original Sepsis 3 studies, and a subsequent retrospective secondary analysis from low- and middle-income countries.5,6 However, as a predictor of mortality for infected patients, qSOFA ≥ 2 did not perform very well in our population. A sensitivity of 31.6% and a PPV of 17.5% indicate that qSOFA ≥ 2 cannot be used for identifying the patients who will die in their hospital stay. quick-SOFA has somewhat better discrimination for mortality among all hospital inpatients, with a higher positive likelihood ratio (3.5 versus 1.78 for patients with suspected infection). A study from Norway also found a poor performance of qSOFA at predicting mortality.19 In Gabon, a sensitivity of 87%, specificity of 75%, and an AUROC of 0.83 among infected emergency patients20 suggested a better performance of qSOFA ≥ 2, but we would still be concerned that such a performance may lack clinical utility as so many patients would be misclassified—a concern shared with other authors conducting prognostic acute medical research in Africa.21
As well as qSOFA ≥ 2, the Sepsis 3 Consensus requires a “suspected infection” to suspect sepsis.4 The Consensus explicitly refrains from defining suspected infection, leaving it open to interpretation, which is notoriously difficult22 and does not require that the infection is the known cause of the deranged physiology. Our primary definition of suspected infection was any documented infectious disease. This may have misclassified those patients whose diagnosis was not confirmed. When we used an alternative definition of a current prescription of antimicrobial, we found markedly different results for the prevalence of suspected infection and sepsis but not for mortality risk. This alternative definition risks misclassifying patients who were receiving antibiotics as a prophylaxis. To avoid the problem of deciding when infection was suspected, we looked at qSOFA as a tool for all patients and found a similar performance. A study from an emergency department in the United States also looked at all patients and found that 7.7% of those with documented vital signs measurements had qSOFA ≥ 2 and qSOFA scores were associated with mortality.23 It may be that qSOFA should be seen as a compound score of physiological abnormality and is as useful as a marker of critical illness, as it is a marker of sepsis in particular. This potentially increases the utility of qSOFA (or other measures of physiological abnormality), particularly in settings of low-resources where a lack of diagnostic facilities hampers the diagnosis of an infectious cause of a patient’s critical illness. Indeed, we would argue that the main clinical purpose of identifying sepsis in a critically ill patient is to decide whether to treat with antibiotics or other anti-infectious agents. Given that the signs of infection can be so nonspecific and diagnosing infectious etiologies can be difficult, we believe that such agents should be considered whenever a critically ill patient is identified and should be promptly stopped if and when an infection is ruled out or the patient improves. It may be beneficial to focus further research efforts on predictive tools for all patients with critical illness rather than just sepsis.
In our study, we used qSOFA rather than SOFA as the illness severity criterion for sepsis. Sepsis 3 uses qSOFA only for suspecting sepsis, but in Malawi and other low-resource settings where the SOFA score cannot be measured, we took the decision to use qSOFA to enable feasibility. We could not identify any patient as having septic shock, nor could we compare qSOFA with SIRS because of a lack of the data necessary for the definition of septic shock or SIRS. The Sepsis-3 Consensus definition requires the use of vasopressors and serum lactate results to diagnose septic shock and these were not done in any patient in this setting. These issues highlight a limitation of Sepsis-3 for global use—either the definitions should be modified or alternative definitions should be developed that are suitable for use in low-resource settings.
A limitation of our study was that it was conducted in only two centers, one of which was a tertiary care institution, and on single defined days in each hospital. These days might not be representative of the patient population in the hospitals, either because of random variation or non-random variation such as seasonal variation or variation on different days of the week. Our results may not be generalizable to all days in the two study sites or to other hospitals or low-income countries. In this study, we were not able to include other potential risk factors in our models, such as socioeconomic status, body mass index, or smoking, and we could not confirm infection by blood culture or other diagnostic tests. An HIV prevalence rate among inpatients of 43.4% is not unusually high for hospitals in southern Africa, but results may be different in populations with different rates of HIV infection or different if HIV-infected patients were excluded. Our population was small, which resulted in wide confidence intervals for the aORs. Our 30-day hospital outcome might have misclassified some patients with a poor prognosis who were discharged home to die, which may have worsened the performance of predictions based on vital sign abnormalities.
Our study has several strengths. We included all the wards in government-run hospitals in Malawi, a low-income country: a setting from which data are sparse or non-existent.1,10 Our prospective data collection and inclusion of all adult patients, regardless of specialty, avoided the selection bias and missing data that can affect studies using retrospective or routinely collected data. Our study investigates in-hospital sepsis, a different measure than sepsis at admission to hospital. In-hospital prevalence and outcomes are useful for estimating the hospital burden due to sepsis. The mortality rates of patients who are septic on admission and those who become septic in hospital could differ. Indeed, patients without sepsis on admission but with sepsis in hospital have been found to have a higher mortality of 35% compared with 12% of those who had sepsis on admission24—our study found an in-hospital mortality rate for septic patients in hospital of 17.5%. To date, most work has been on admission sepsis, and we believe an increased focus on in-patient sepsis could be beneficial.
CONCLUSION
Sepsis is common and is associated with a high risk of death in admitted patients in hospitals in Malawi. In low-resource settings, the qSOFA score that uses commonly available vital signs data may be a tool that could be used for identifying patients at risk—both for those with and without a suspected infection—but its predictive value is low and its clinical usefulness may be limited.
Supplemental tables and figures
Acknowledgments:
We thank the QECH and CDH hospital administration and the staff for their assistance in conducting this research.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists , 2016. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193: 259–272. [DOI] [PubMed] [Google Scholar]
- 2.Reinhart K, Daniels R, Kissoon N, O’Brien J, Machado FR, Jimenez E, 2013. The burden of sepsis-a call to action in support of World Sepsis Day 2013. J Crit Care 28: 526–528. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, et al. ICON Investigators , 2014. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2: 380–386. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd KE, et al. Sepsis Assessment and Identification in Low Resource Settings (SAILORS) Collaboration , 2018. Association of the quick sequential (sepsis-related) organ failure assessment (qsofa) score with excess hospital mortality in adults with suspected infection in low- and middle-income countries. JAMA 319: 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour CW, et al. 2016. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huson MAM, Katete C, Chunda L, Ngoma J, Wallrauch C, Heller T, van der Poll T, Grobusch MP, 2017. Application of the qSOFA score to predict mortality in patients with suspected infection in a resource-limited setting in Malawi. Infection 45: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waitt PI, Mukaka M, Goodson P, SimuKonda FD, Waitt CJ, Feasey N, Allain TJ, Downie P, Heyderman RS, 2015. Sepsis carries a high mortality among hospitalised adults in Malawi in the era of antiretroviral therapy scale-up: a longitudinal cohort study. J Infect 70: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudd KE, Tutaryebwa LK, West TE, 2017. Presentation, management, and outcomes of sepsis in adults and children admitted to a rural Ugandan hospital: a prospective observational cohort study. PloS One 12: e0171422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado FR, Azevedo LCP, 2018. Sepsis: a threat that needs a global solution. Crit Care Med 46: 454–459. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J, et al. 2015. Sepsis: a roadmap for future research. Lancet Infect Dis 15: 581–614. [DOI] [PubMed] [Google Scholar]
- 13.Papali A, Verceles AC, Augustin ME, Colas LN, Jean-Francois CH, Patel DM, Todd NW, McCurdy MT, West TE; Haiti Resource Limited Intensive Care (Haiti-RELIC) Study Group , 2016. Sepsis in Haiti: prevalence, treatment, and outcomes in a Port-au-Prince referral hospital. J Crit Care 38: 35–40. [DOI] [PubMed] [Google Scholar]
- 14.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA Jr. 2006. National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med 48: 326–331, 31.e1–3. [DOI] [PubMed] [Google Scholar]
- 15.Edwards R, Hutson R, Johnson J, Sherwin R, Gordon-Strachan G, Frankson M, Levy P, 2013. Severe sepsis in the emergency department–an observational cohort study from the university hospital of the West Indies. West Indian Med J 62: 224–229. [PubMed] [Google Scholar]
- 16.Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, Reynolds SJ, Kenya-Mugisha N, Mayanja-Kizza H, Scheld WM; Promoting Resource-Limited Interventions for Sepsis Management in Uganda (PRISM-U) Study Group, 2009. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One 4: e7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR, 2014. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med 42: 2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aluisio AR, Garbern S, Wiskel T, Mutabazi ZA, Umuhire O, Ch’ng CC, Rudd KE, D’Arc Nyinawankusi J, Byiringiro JC, Levine AC, 2018. Mortality outcomes based on ED qSOFA score and HIV status in a developing low income country. Am J Emerg Med 36: 2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Askim Å, Moser F, Gustad LT, Stene H, Gundersen M, Åsvold BO, Dale J, Bjørnsen LP, Damås JK, Solligård E, 2017. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality–a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med 25: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huson MAM, Kalkman R, Grobusch MP, van der Poll T, 2017. Predictive value of the qSOFA score in patients with suspected infection in a resource limited setting in Gabon. Trav Med Infect Dis 15: 76–77. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler I, Price C, Sitch A, Banda P, Kellett J, Nyirenda M, Rylance J, 2013. Early warning scores generated in developed healthcare settings are not sufficient at predicting early mortality in Blantyre, Malawi: a prospective cohort study. PloS One 8: e59830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho KM, Lan NS, 2017. Combining quick sequential organ failure assessment with plasma lactate concentration is comparable to standard sequential organ failure assessment score in predicting mortality of patients with and without suspected infection. J Crit Care 38: 1–5. [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, Ng J, Thode HC, Jr., Spiegel R, Weingart S, 2017. Quick SOFA scores predict mortality in adult emergency department patients with and without suspected infection. Ann Emerg Med 69: 475–479. [DOI] [PubMed] [Google Scholar]
- 24.Rothman M, Levy M, Dellinger RP, Jones SL, Fogerty RL, Voelker KG, Gross B, Marchetti A, Beals J, 4th, 2017. Sepsis as 2 problems: identifying sepsis at admission and predicting onset in the hospital using an electronic medical record–based acuity score. J Crit Care 38: 237–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.