Abstract.
Routine incident malaria case data have become a pillar of malaria surveillance in sub-Saharan Africa. These data provide granular, timely information to track malaria burden. However, incidence data are sensitive to changes in care seeking rates, rates of testing of suspect cases, and reporting completeness. Based on a set of assumptions, we derived a simple algebraic formula to convert crude incidence rates to a corrected estimation of incidence, adjusting for biases in variable and suboptimal rates of care seeking, testing of suspect cases, and reporting completeness. We applied the correction to routine incidence data from Guinea and Mozambique, and aggregate data for sub-Saharan African countries from the World Malaria Report. We calculated continent-wide needs for malaria tests and treatments, assuming universal testing but current care seeking rates. Countries in southern and eastern Africa reporting recent increases in malaria incidence generally had lower overall corrected incidence than countries in Central and West Africa. Under current care seeking rates, the unmet need for malaria tests was estimated to be 160 million (M) (interquartile range [IQR]: 139–188) and for malaria treatments to be 37 M (IQR: 29–51). Maps of corrected incidence were more consistent with maps of community survey prevalence than was crude incidence in Guinea and Mozambique. Crude malaria incidence rates need to be interpreted in the context of suboptimal testing and care seeking rates, which vary over space and time. Adjusting for these factors can provide insight into the spatiotemporal trends of malaria burden.
INTRODUCTION
Since 2000, with the increasing availability of funds and new tools, many sub-Saharan African countries have dramatically scaled up malaria control interventions, resulting in high coverage with vector control tools such as insecticide-treated nets, increasing access to high-quality diagnosis at point of care with rapid diagnostic tests (RDTs), and highly effective treatment with artemisinin-based combination therapy (ACT).1 As a result, parasite prevalence (as measured by large-scale, cross-sectional, community-based surveys) has fallen in many countries, in some cases to levels so low that continuing to follow trends using parasite prevalence is challenging. There is increasing interest in using alternate metrics for measuring intervention effectiveness and assessing malaria burden, particularly by tracking the incidence of confirmed malaria infection, reported by health facilities through routine data collection systems.
Accurately measuring malaria incidence is not straightforward. It requires adequate health facility (and/or community health worker) infrastructure to enable access to the healthcare sector, conditions that foster care seeking behavior, adequate supplies and training to ensure testing of patients with febrile illness, documentation of patients with positive tests, and accurate reporting into a functional database. Currently, most countries annually report the aggregate number of incident cases detected through routine systems to the World Health Organization (WHO). However, with the exception of a few low-incidence countries where routine data are used, WHO uses a mathematical model to estimate the incidence for most sub-Saharan African countries.1 The WHO approach models incidence based on prevalence data from household surveys, metadata such as climate data, and administrative data on intervention coverage. Like other methods using statistical approaches such as time series analysis, these methods are difficult to reproduce, are dependent on the availability of additional metadata, require advanced statistical skills, and are in general not readily suited for routine use by endemic country programs.
National Malaria Control Programs (NMCPs) increasingly track and report the number of confirmed malaria cases, typically calculating the incidence of confirmed cases per 1,000 population by dividing the number of reported confirmed cases by the population of each catchment area and multiplying by 1,000. With the increase in malaria donor support and subsequent scale-up of malaria control interventions, there is often great pressure to demonstrate a decrease in incidence. However, incidence of confirmed cases is an indicator that needs to be interpreted with care because of several sources of potential bias. It is highly sensitive to a number of factors, including suboptimal care seeking, testing practices, and completeness of reporting, which are not uniform in space and time, but all of which tend to decrease reported incidence. Completeness of data, both in terms of proportion of structures reporting and in terms of inclusion of all cases diagnosed, plays a major role in the number of cases reported. As the number of health facilities and community health workers reporting increases, the number of confirmed cases reported is likely to increase, and as efforts to improve reporting quality ensure more complete reporting of patients seen at individual facilities, this is also likely to increase numbers of cases reported. As ministries of health make efforts to improve access to and quality of care, and care seeking increases, many malaria cases that would have gone undetected are now diagnosed, treated, and counted. Finally, as RDTs are increasingly accepted and available and testing patients with febrile illness at point of care is increasingly normalized, the number of patients with suspected malaria who receive testing and a confirmed diagnosis also increases. Many NMCPs that previously reported decreases in incidence are now seeing reported increases.1 Many of these are also making great efforts to improve health services, including data quality and completeness of reporting, access to and quality of care, and increasing testing of suspected malaria cases, complicating efforts to determine whether the observed increases in incidence are real.
A more accurate and less biased measure of incidence is of crucial importance to NMCPs and their partners. It would allow for characterization of short- and long-term trends in malaria transmission, which in turn would permit programs to track the impact of interventions, and to identify areas that require more focused interventions. In addition, a more accurate measure of the number of incident malaria cases is critical for decisions around procurement of malaria commodities, particularly in the context of striving to reach universal testing and treatment.
As routine health facility data improve in quality and availability in endemic countries, there arises the potential to capitalize on those data to provide a robust estimator of malaria incidence based on routine data. Here, we introduce a method for adjusting routinely reported malaria incidence to account for biases in healthcare seeking and testing rates. We apply the method to routine data from Guinea and Mozambique as case studies in the potential utility of the indicator. Finally, we apply the method to aggregate country data on incident cases from the WHO World Malaria Report to obtain a more accurate estimate for the number of incident malaria cases seen by health systems and the subsequent needs for RDTs and ACTs.
METHODS
We developed a simple algebraic method to calculate a corrected incidence that could be used by NMCPs and their partners, without a background in modeling or statistical software, to interpret routinely reported surveillance data, adjusting for testing practices, care seeking behavior, and reporting completeness. The overall objective was to develop a robust estimator of incidence that would allow comparison of incidence of symptomatic malaria infection between districts or regions and evaluation of trends over time.
Derivation of estimator.
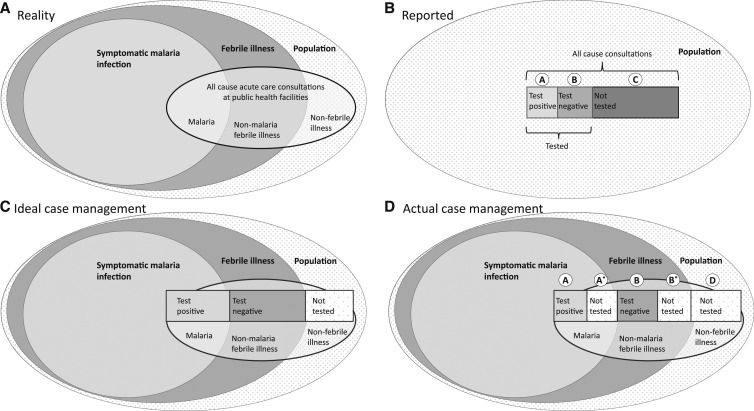
We developed a framework to estimate community incidence of symptomatic (febrile) malaria infection, based on four routinely reported data elements: population, number of all-cause acute care consultations, number of patients tested for malaria, and number of patients with confirmed malaria infection. In a given community over the course of a year, a certain number of individuals will develop febrile illnesses, and a certain proportion of these febrile illnesses will be associated with symptomatic malaria infection (Figure 1A). This approach is designed to estimate the approximate number of these febrile malaria episodes. By contrast, the health surveillance system typically reports population of the catchment area, all-cause acute care consultations, patients tested, and patients who test positive (Figure 1B). If case management was ideal (all people seeking care with fever and malaria infection received a diagnostic test for malaria), it would be relatively straightforward to estimate the community incidence of febrile malaria infection by adjusting for care seeking (Figure 1C).
Figure 1.
Schematic of process of inferring true malaria burden from routine data, showing the true universe of malaria cases (A), the subset that are reported through data information systems (B), what would be reported under ideal case management practices (C), and what is reported under actual case management practices (D).
Unfortunately, data from both health facility–based and community surveys have demonstrated that a substantial proportion of those seeking care for febrile illness do not receive a diagnostic test. Of patients with febrile illness, some will have malaria infection and some will not. Among those with malaria infection, some will be tested (and most likely test positive) and others will not be tested. Among febrile patients without malaria infection, some will be tested (and most will test negative) and some will not (Figure 1D). For the purposes of this analysis, we assume that those without febrile illness are not tested.
From the routine surveillance data, the number of patients who were tested and received a positive test result (A), the number of patients who were tested and received a negative test result (B), and the number of patients not tested (C) are readily reported or calculated and are available. Among those not tested are those with febrile illness and malaria infection (A*), those with febrile illness and without malaria infection (B*), and those without febrile illness (D). Whereas A, B, and C are known, A*, B*, and D are unknown. These unknown variables can be imputed following two assumptions related to testing practices (Box 1, Assumptions 1 and 2).
Box 1.
Description of model variables, parameters, and assumptions
| Variable/parameter | Definition | Source |
|---|---|---|
| A | Patients who tested positive | Routine data |
| A* | Patients with febrile illness and malaria infection, but not tested | Estimated using Equation (2) |
| B | Patients who tested negative | Routine data |
| B* | Patients with febrile illness and no malaria infection, but not tested | Estimated using Equation (1) |
| C | Patients not tested | Routine data |
| D | Patients without febrile illness | Estimated using Equation (3) |
| α | Ratio of test positivity rate among patients with febrile illness not tested to those tested | Estimated from health facility surveys with systematic fever screening and re-testing |
| β | Proportion of fever among nonmalaria consults | Estimated from health facility surveys with systematic fever screening and re-testing |
| γ | Mean number of nonmalaria fevers per person per year | Estimated from population-based longitudinal cohorts |
| λ | Malaria-attributable fraction among test-positive patients | Estimated by comparing antigen concentration in febrile and afebrile patients |
Box 2.
Assumptions
| Assumption 1 | The proportion of nonmalaria consults that are for febrile illness (β) is constant |
| Assumption 2 | The ratio of the test positivity rate among febrile individuals not tested for malaria is proportional to the test positivity rate among febrile individuals tested for malaria (at a constant ratio α) |
| Assumption 3 | There is a constant mean number of nonmalaria fevers per person per year (γ) |
| Assumption 4 | The test positivity rate in those with febrile malaria infection who do not seek care is proportional to the test positivity rate in those who seek care |
To translate the results reported by the surveillance system to reflect community incidence (and thereby account for those not reflected in the data, whether through lack of care seeking or lack of reporting, i.e., not being counted and reported), two further assumptions are needed: the incidence of nonmalaria fevers in the population and how the test positivity rate among those who seek care for fever compares with the test positivity rate among those who do not seek care or are otherwise not reflected in the data (Box 1, Assumptions 3 and 4).
To solve for the unknowns and understand how to translate this incidence to community febrile malaria infection incidence, we need to make a number of assumptions, as detailed in Box 1. Although the values for these assumptions have been drawn from the literature, and are described in the following paragraphs, if country-specific values for these assumptions become available, it would be appropriate for a country to use those values in its calculations.
For parameterizing assumption 1, we relied on high-quality health facility surveys in which all patients are screened for febrile illness and those with febrile illness are tested for malaria (N. Bayoh et al., unpublished data).2–14 β in this scenario is calculated as follows: (number of patients who test negative)/(number of patients who test negative + number of patients with nonfebrile illness). Although the number of published health facility surveys in which this was carried out is limited, a literature search found that β was approximately 0.73 (n = 14, range: 0.56–0.94, interquartile range [IQR]: 0.65–0.84) for children aged < 5 years and 0.57 (n = 10, range: 0.42–0.75, IQR: 0.49–0.61) for patients aged ≥ 5 years2 (Table 1; Supplemental Figure S1). Alternately, if such a health facility survey exists for a given country, β could be calculated and used for corrected incidence for that country.
Table 1.
Estimates for parameters for correction of routine health facility data to account for under-testing, derived from empirical distributions of parameters from health facility surveys with systematic fever screening and retesting
| Median (Interquartile range) | |||
|---|---|---|---|
| Age < 5 years | Age ≥ 5 years | All ages | |
| Proportion of fever among nonmalaria consults (β) | 0.73 (0.65–0.84) | 0.57 (0.49–0.61) | 0.59 (0.54–0.66) |
| Ratio of malaria test positivity in untested vs. tested febrile patients (α) | – | – | 0.48 (0.42–0.55) |
For assumption 2, data to calculate α, the ratio of the test positivity rate among patients whom healthcare workers do not test to the test positivity rate among those whom healthcare workers test, are available from the health facility surveys in which exit interviews are conducted, test results among those tested by the healthcare provider are recorded, and tests are offered to patients identified as having a febrile illness but not tested. Supplemental Table S1 provides values of α calculated from health facility surveys in three countries,13–15 although country-specific values could be used if there has been a recent health facility survey in which malaria testing is offered to all patients with febrile illness at an exit interview. In most settings, healthcare providers do not test all patients with febrile illness but factor in clinical judgment to decide whom to test. If clinical judgment was perfect (no patients with malaria infection untested), α would be zero. The higher the α, the higher the test positivity rate among patients with febrile illness not tested compared to the test positivity rate among patients with febrile illness tested. If α = 100%, the test positivity rate among patients with febrile illness not tested would be equal to the test positivity rate among patients with febrile illness tested.
Few published data are available to estimate γ, the estimated annual incidence per person of nonmalaria fevers per year (assumption 3). In Dielmo and Ndiop, Senegal, a population-based longitudinal cohort was followed weekly with fever screening and malaria testing since 1990, allowing reporting of annual incidence of malarial and nonmalarial fevers.16 Of note, the investigators defined malarial fevers as those with parasite density above the estimated pyrogenic threshold, which was calculated annually; thus, some fevers with low-density malaria infection were categorized as nonmalarial fever. Whereas the annual incidence of symptomatic malaria infection per person decreased from 2.76 in 1990 to 0.05 in 2012 among children, and from 0.39 in 1990 to 0.05 in 2012 among adults, nonmalaria fever incidence was relatively more stable. Although nonmalaria fever incidence per person fluctuated (up to 3.67 among children and up to 2.83 among adults) with other infectious disease trends, it remained consistently above 2.0 for children and 1.0 for adults. To ensure that periods of high incidence of nonmalarial febrile illness do not artificially elevate the estimates, we used γ = 2 for children younger than 5 years and γ = 1 for those aged 5 years and older.
To parameterize assumption 4, we used the results of a meta-analysis of Malaria Indicator Surveys and Demographic and Health Surveys from 2003 to 2015 to estimate the test positivity rate among patients who do not seek care compared with the test positivity rate among patients who seek care.17 During these household surveys, caregivers of children younger than 6–59 months were asked whether the child had fever in the last 2 weeks and whether care was sought with a trained provider. These children were also all tested for malaria as part of the survey. For each survey, RDT positivity among children for whom care was not sought was plotted against RDT positivity among children for whom care was sought. The ratio of the test positivity rate among children with reported febrile illness for whom care was not sought to the test positivity among children with reported febrile illness for whom care was sought was equal to or slightly greater than 1. Data were not available for those aged 5 years and older. For the purposes of this analysis, we assumed that the test positivity rate of individuals with febrile illness who are not represented in the reported surveillance data (whether through not seeking care or through data incompleteness) is equal to the test positivity rate of those represented in the surveillance data.
The first two equations (Equations (1) and (2)) were solved to provide estimates for B*, A*, and D:
| (1) |
| (2) |
| (3) |
To adjust for febrile illnesses with malaria infection that are not captured in the surveillance data, we scale by the incidence per person of nonmalarial fever (as this is assumed to be independent of malaria transmission intensity).
To adjust for incident parasitemia/antigenemia in febrile patients, the number of febrile illness of nonmalaria etiology is a sum of patients without malaria infection and a certain proportion of febrile test-positive patients ([1−λ] [A + A*]), where λ is the malaria-attributable fraction among test-positive patients. We modeled λ to be 1 when the test positivity rate was 0, decreasing linearly to a minimum of 0.75 (range: 0.5–0.95) when the test positivity rate reached 1.18
| (4) |
| Number of febrile illnesses of nonmalaria etiology calculated from surveillance data | B + B* + (1−λ) (A + A*) |
| Incidence per person of nonmalarial febrile illness who sought care and were reported in surveillance data | (B + B* + [1−λ] [A + A*])/population |
| Adjustment factor, based on incidence of nonmalarial fever |
The number of episodes of febrile malaria illnesses among patients seeking care imputed from the surveillance data is A + A*. Assuming the test positivity rate among those reflected in the data is similar to those not reflected in the data (assumption 4), the incidence of febrile illness with malaria infection at the community level can be estimated by multiplying the incidence of malaria per person as calculated from the surveillance data by the correction factor:
| (5) |
Incidence per 1,000 can be calculated by multiplying by 1,000.
For all results presented, we report malaria incidence counting all malaria fevers with malaria infection, regardless of the attribution of fever etiology.
Case study 1: Estimating RDT and ACT needs for sub-Saharan Africa.
We abstracted the following data elements from routine health information data submitted by malaria-endemic sub-Saharan African countries to the 2016 World Malaria Report: population at risk, total number of all-cause consultations, number of patients tested for malaria, and confirmed malaria cases. We then applied Equations (1)–(3) to estimate the expected number of fever cases and true malaria cases seeking care. To estimate the full community-level burden, we used Equation 5 to calculate the expected number of fever and true incident malaria fevers, assuming universal access and healthcare seeking.
We calculated the median and IQR for the α and β parameters from our literature search for studies for which the parameters could be estimated (Table 1). Because the World Malaria Report data are not stratified by age, we used the all-ages estimates. We used a conservative rate of 1.5 nonmalaria fevers per year (range: 1–2).
We compared the relative ranking of malaria-endemic sub-Saharan African countries when considering the reported (crude) malaria incidence and the corrected malaria incidence. We calculated the expected number of malaria tests and treatments needed to attain universal testing of fever cases and treatment of all confirmed cases under two scenarios: 1) current rates of care seeking (using total patient encounters reported by the countries, Equations (1)–(3) and 2) universal access and complete care seeking, representing the scenario in which all community cases of fever are tested and treated if positive for malaria (additionally applying Equation (5)). We estimated the needs using the median values of α and β. We calculated lower and upper bounds on the estimates by applying the first and third quartiles of the empirical distributions of these parameters (Table 1). By subtracting the number of tested and confirmed cases that were reported from the estimated needs for tests and treatments, we estimated country-level gaps for malaria tests and treatments.
Case study 2: Analyzing spatiotemporal trends in Guinea and Mozambique.
We exported data from the Guinea malaria-specific Routine Malaria Information System and the Mozambique integrated Health Management Information System on the number of all-cause consults, patients tested for malaria, and confirmed malaria cases. The time period included in the analysis was 2012–2017 for Guinea and 2017–2018 for Mozambique. Data from Mozambique were stratified for children aged < 5 years and children and adults aged ≥ 5 years, whereas data from Guinea were aggregated across all ages.
We applied Equations (1)–(5) to estimate the corrected annualized malaria incidence by month by district/province. We used the median for α and β for all ages for Guinea (Table 1) but used country-specific estimates for α (0.443 in < 5 years, 0.603 in ≥ 5 years) and β (0.783 in < 5 years, 0.544 in ≥ 5 years) for Mozambique because of the availability of a recent health facility survey with systematic fever screening and retesting of patients during exit interviews.13
Because the parameters and assumptions are independent of seasonality, assuming relatively stable rates of nonmalarial fever over the course of a year, the results were not adjusted for high versus low transmission period.
We plotted the crude and corrected incidence for the most recent year by district/province for each country. For illustrative examples, we plotted the monthly crude and corrected incidence over time for Labé Region in Guinea and Zambézia Province in Mozambique. Both represent zones that in recent years have received intensive malaria case management interventions, including supervision and training of healthcare workers and expansions of the community healthcare worker program.
RESULTS
Case study 1.
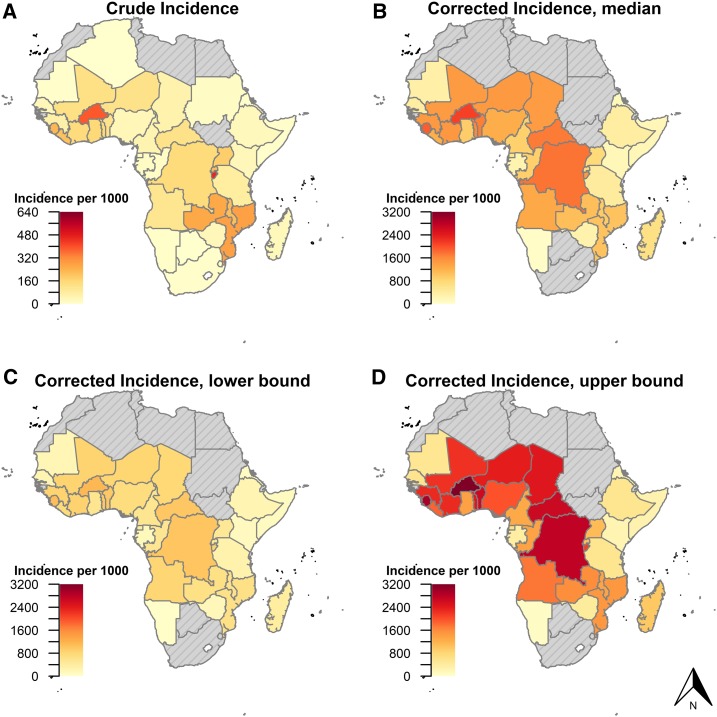
The median country-level corrected incidence for sub-Saharan Africa was 901 cases/1,000 population, ranging from 16/1,000 (Comoros) to 2,107/1,000 (Burkina Faso) (Figure 2). This was several fold higher than the reported crude incidence, which ranged from 2/1,000 (Comoros) to 462/1,000 (Burundi), with a median of 79/1,000.
Figure 2.
Crude (A) and corrected (B) malaria incidence rates, as reported and estimated from routine health information data submitted by countries to the World Malaria Report, 2016. (C and D) show the lower and upper bounds of the corrected estimates. Note the difference in scales between crude and corrected maps.
Whereas the crude incidence was generally highest in countries along the southern and western branches of the Rift Valley (Mozambique, Malawi, Zambia, Burundi, and Rwanda), with a second cluster of high incidence in West Africa, the corrected incidence map showed different patterns. The corrected incidence estimates showed very high incidence in central Africa, as well as throughout West Africa. The Rift Valley countries that ranked highly in the crude reported incidence generally fell in the corrected incidence rankings (Supplemental Figure S2), and these countries typically had a lower ratio of corrected to crude incidence, suggesting a larger proportion of true cases being detected and reported (Supplemental Figure S3).
We estimated that of the 471 million (M) reported outpatient consults, 329 M (70%) were for patients with fever, representing the true need for malaria tests under current rates of care seeking (Table 2, Supplemental Figure S4). Countries reported testing only 170 M patients, thus representing a gap of 160 M (IQR: 139–188) malaria tests across sub-Saharan Africa. We estimated that 126 M (IQR: 117–139) of these tests were for febrile patients with malaria (Table 2, Supplemental Figure S5). Against a total of 89 M reported cases, this left a 37 M (IQR: 29–51) gap in malaria treatments under current care seeking rates. When considering the true, community-level incidence of fever and malaria cases, we estimated a total need for 2.283 billion (IQR: 1.4–3.1) malaria tests and 871 M (IQR: 515–1,339) malaria treatments per year across the continent.
Table 2.
Estimates for malaria tests and treatments under universal testing of fever policy after correction for under-testing and incomplete reporting as estimated from routine health information data submitted by sub-Saharan African countries to the World Malaria Report, 2016
| Current care seeking rates | Universal access scenario | ||||
|---|---|---|---|---|---|
| Reported (M) | True need (M), median (IQR) | Gap (M), median (IQR) | True need (M), median (IQR) | Gap (M), median (IQR) | |
| All-cause consults | 471 | – | – | – | – |
| Malaria tests | 170 | 329 (308–358) | 160 (139–188) | 2,173 (1,383–3,075) | 2,002 (1,212–2,905) |
| Malaria treatments | 89 | 126 (117–139) | 37 (29–51) | 871 (515–1,339) | 782 (426–1,251) |
IQR = interquartile range; M = million.
Case study 2.
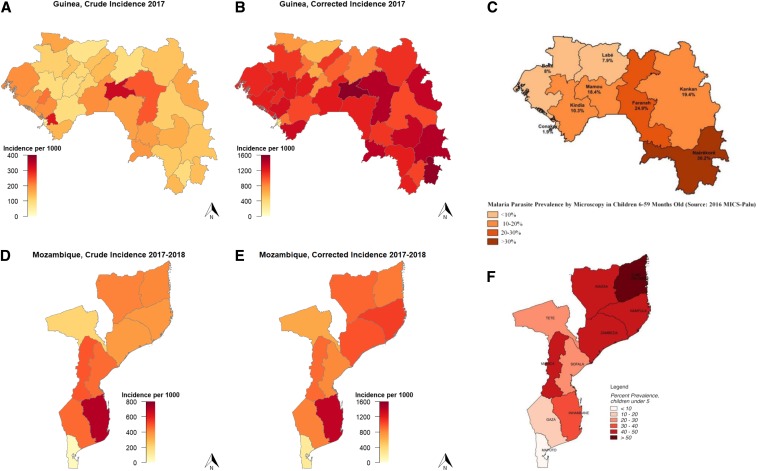
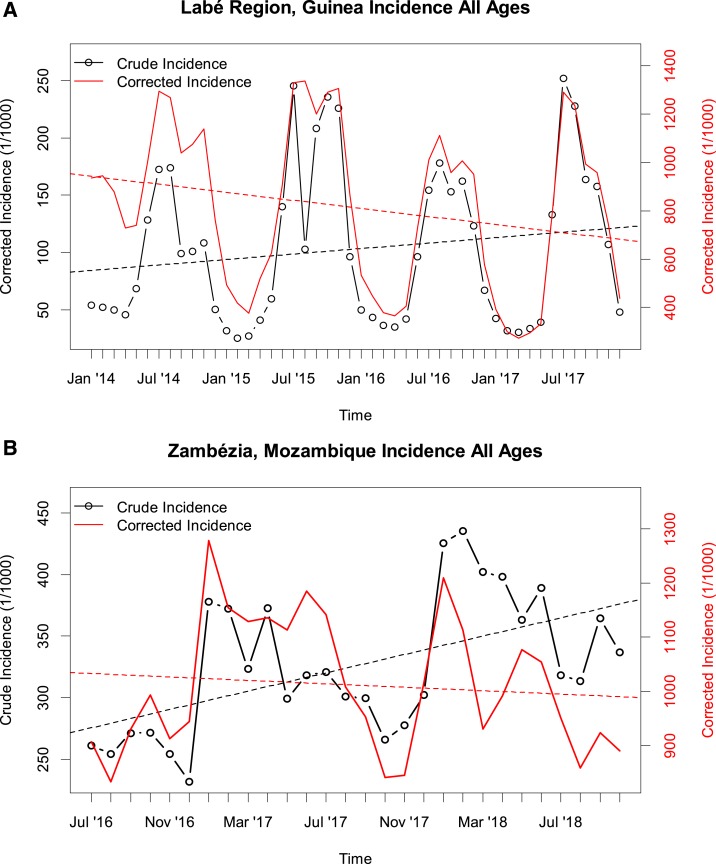
In Guinea, the health district-level crude incidence showed a different pattern than the corrected incidence (Figure 3A and B). The crude incidence was generally lower in the holoendemic southeast of the country, which is highly forested and has the highest prevalence as measured during the most recent household survey19 (Figure 3C), compared with the Sahelian north of the country, where prevalence is substantially lower. By contrast, the corrected incidence showed a pattern more consistent with the prevalence data, with the lowest incidence in the north of the country. In Guinea’s Labé region, the crude incidence was reported to be consistently increasing since 2014, at a rate of 11% per year (Figure 4A). After correction, the incidence showed an overall annual decrease of −7.1% per year.
Figure 3.
Crude (A and D) and corrected (B and E) malaria incidence rates, as reported and estimated from routine health information data from Guinea and Mozambique. (C and F) show the parasite prevalence in children younger than 5 years for the most recent household survey in each country.19,20 Note the difference in axis scales between the crude and corrected incidence and difference in aggregation level for Guinea, where incidence data are by health district and prevalence data are by region.
Figure 4.
Longitudinal trends in crude (black) and corrected (red) malaria incidence in Labé Region, Guinea, (A) and Zambézia Province, Mozambique (B). Note the difference in axis scales between the crude and corrected incidence. This figure appears in color at www.ajtmh.org.
In Mozambique, the crude incidence was highest in the southern provinces (excluding Maputo) (Figure 3D). By contrast, the corrected incidence was highest in the north, with the exception of Inhambane Province (Figure 3D), more closely matching data from the most recent household survey20 (Figure 3E).
In Zambézia Province located in central Mozambique, the crude and corrected incidence showed different trends over the last 2 years. Whereas the crude incidence increased at an annual rate of 16% over that time period, the corrected incidence showed a −1.8% decrease (Figure 4B). The pattern held separately for children < 5 years and older children and adults (Supplemental Figure S6).
DISCUSSION
Our analysis of routinely reported health data show large and variable discrepancies between crude (reported) incidence and our corrected incidence measure, which corresponds more closely with other metrics of malaria burden (e.g., prevalence). These discrepancies reinforce the notion that crude incidence data should be interpreted in the context of several key biases. Our method for addressing two important biases—uneven rates of access/care seeking and under-testing of fever cases—can provide NMCPs with a simple method for capitalizing on routine health system data for making timely, granular, evidence-based decisions, while minimizing the biases inherent in routine data.
This method comes at a time when the malaria community is moving away from periodic surveys toward real-time analysis of routine data. As the prevalence of malaria decreases, rendering parasite prevalence less useful as a metric of malaria burden, the incidence of malaria has been increasingly used to understand trends in burden, monitor effectiveness of interventions, and target interventions. During the last decade, as ministries of health have moved to increase access to health care and malaria control programs have scaled up the use of RDTs at point of care, reported incidence has been increasing, often in zones in which malaria control activities have most intensified, leading to consternation among malaria control programs.
Although there have been many model-based approaches to understanding incidence of malaria, the approach reported here relies on an accessible, simple algebraic formula applied to routine health information system data, based on several simple assumptions to account for differences in testing and in care seeking or data reporting. This approach allows NMCP managers without a background in modeling or training to easily calculate a corrected incidence that accounts for difference in data completeness, care seeking, and testing, and allows them to better understand trends in malaria incidence.
The estimates presented here for corrected incidence define any febrile patient with a positive malaria test as a malaria case. A certain proportion of these will have a coinfection with another infectious agent, and for some of these, malaria might not be the etiologic agent of the current febrile illness. Accounting for the malaria attributable fraction would likely result in estimates of malaria-attributable fever incidence more in line with the prevalence-to-incidence model results typically reported by WHO. Nevertheless, programmatically, inferring etiology of a fever is of secondary importance, and the more important indicator to follow for programs is the corrected incidence of malaria-positive fevers. Because all malaria-positive fevers should be treated to clear parasitemia, for the purposes of commodity quantification, programs should rely on the incidence of malaria-positive fevers. Moreover, because population-level inference of fever etiology is inherently noisy and imprecise, incidence of malaria-positive fever cases is a more appropriate indicator for programs to track.
There are inherent limitations and uncertainties tied to any approach attempting to estimate community incidence from routine data. First, this approach assumes that the key data elements—number of all-cause acute care consultations, number of patients tested for malaria, and number of patients with confirmed malaria infection—are reported in an unbiased manner. Any systematic misclassification of these, for example, systematic underreporting of negative RDT results or reporting of negative RDTs as positive, will impact the validity of the model. Moreover, the model was parameterized based on data available from a limited number of studies and from a limited number of countries. As such, the precision, accuracy, and generalizability of the parameter estimates used here may be limited. More health facility surveys with rigorous fever screening from different settings will add to the evidence base and will improve the precision of the methodology, as well as allow a proper assessment of its uncertainties. Therefore, the incidence estimates presented here should be interpreted in the context of the uncertainties of the key parameters, and the results of relative comparisons (across time and space) are likely more robust than the absolute estimates. Validation of the approach, for example, with incidence directly calculated from demographic and health surveillance cohort data and testing parameters calculated from health facility surveys, is recommended to calibrate the model assumptions.
Strengths of the approach outlined here include its simplicity and transparency, as well as its flexibility in parameters being tailored to specific countries using available country-specific data. The second step of the adjustment (translating health facility incidence to community incidence) is agnostic as to the reason for under-reporting, whether it is through low access to health care or incomplete recording and reporting. As a result, longitudinal data with temporal differences in reporting and testing can still be analyzed for trends in incidence. Nevertheless, the validity of our approach is tied to the validity of the assumptions. Although there is substantial evidence to suggest a high background rate of fever among outpatients (assumption 1) and a similar rate of test positivity regardless of care seeking, at least for children aged < 5 years (assumption 4), more research is needed to expand the evidence base around expected rates of positivity among untested fever patients (assumption 2) and the annual rate of nonmalaria fevers in the community (assumption 3).
Our results underscore the importance of accounting for biases in crude incidence. Faced with limited resources, NMCPs often have to choose high-priority areas for certain interventions. This is particularly relevant in the context of indoor residual spraying, where NMCPs often have the resources to spray only a small percentage of the country’s structures and choosing the appropriate areas to have the maximum impact on transmission is an important programmatic decision. Here, our case studies from Guinea and Mozambique show that administrative areas that report low crude incidence and ostensibly are not good candidates for targeted interventions might in actuality have higher incidence than areas that have higher reported incidence because of higher access to care and higher testing rates. Risk stratification in these countries changes substantially when considering the corrected incidence rather than the crude incidence, with the corrected incidence figures more closely matching prevalence patterns from household surveys.
In addition to facilitating more accurate interpretation of incidence data, this approach (particularly the first three equations, using current levels of care seeking) may help improve the accuracy of RDT and ACT needs quantification. When RDT procurement is based on the historical use, if less than 50% of patients with febrile illness have historically benefitted from an RDT, needs will be underestimated, and care providers will be unable to test all patients with febrile illness. Procuring RDTs and ACTs based on the assumption of universal testing of patients seeking care for febrile illness will necessitate a substantial increase in ACT and RDT procurement across the continent. Current gaps in RDT and ACT needs, even under current care seeking rates, are substantial, with estimates in the 37 M ACT and 160 M RDT range.
When applied to national-level data reported to the WHO, even using conservative assumptions, corrected incidence was often an order of magnitude or more higher than reported incidence. However, this was not uniform. Countries in which malaria control programs have rapidly scaled-up access to malaria case management or countries nearing elimination had corrected malaria incidence closer to reported incidence. Similarly, countries that have shown ostensible increases in malaria incidence in recent years (particularly along the western and southern branches of the Rift Valley), triggering worries of true increases in malaria transmission, generally also had a smaller ratio of corrected incidence to crude incidence, suggesting that the trends are likely due at least in part to increasing healthcare access and better testing. Our estimates of the community burden of malaria suggest that Central and West Africa represent the highest burden areas of the continent. Similarly, when applied to longitudinal data from Mozambique and Guinea, regions that had increases in crude incidence in the context of scale-up of case management interventions had decreases in corrected incidence.
There is strong evidence of heterogeneity in terms of how well countries across the continent are capturing incident malaria cases, reflected in the large variance in the correction factor (Supplemental Figure S3). Although all countries are reporting fewer cases than estimated through our model, the magnitude of the variation in this under-detection and under-reporting points to substantial differences in the performance of malaria control programs related to care seeking, testing, and reporting completeness. In general, more mature malaria control programs would be expected to detect and report a larger proportion of their true malaria burden, and the observed variability across the continent likely reflects different stages of effectiveness of the malaria control programs.
CONCLUSION
Reporting of and decision-making based on malaria incidence is becoming increasingly important in this era of malaria control. Applying a simple algebraic formula, using several simple assumptions, to routinely reported incidence data accounts for inherent biases and renders it more useful for intervention targeting and monitoring progress, as well as estimating needs for RDTs and ACTs. Moreover, this simple tool can be used by individuals without a background in modeling or access to sophisticated software. Although not an exact measure, these corrections allow a closer understanding of the real magnitude and distribution of malaria burden and their trends over time.
To develop more robust and country-specific values for the assumptions, health facility surveys for malaria should incorporate screening of all patients for fever and offer tests to those who did not receive testing from health facility providers. As malaria burden decreases and children aged < 5 years are no longer at highest risk for parasite carriage, community-based surveys should also assess care seeking and parasite carriage among those aged 5 years and older. Finally, longitudinal, all-cause fever surveillance would help translate incidence measured at the health facility level to the community. Although the values used for these assumptions need to be confirmed and refined, the conservative estimates used here demonstrate that community-level incidence of malaria infection with fever is an order of magnitude higher than what is reported through health facilities. However, many countries are making much progress in improving access to diagnosis and treatment, and increases in reported incidence must be interpreted in the light of increased care seeking, testing, and reporting.
Supplemental Annex
Acknowledgments:
R. Z., J. P., and M. M. P. were supported by the U.S. President’s Malaria Initiative.
Note: Supplemental table and figures appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2018. World Malaria Report 2018. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Plucinski MM, Guilavogui T, Camara A, Ndiop M, Cisse M, Painter J, Thwing J, 2018. How far are we from reaching universal malaria testing of all fever cases? Am J Trop Med Hyg 99: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe AK, Onikpo F, Lama M, Cokou F, Deming MS, 2001. Management of childhood illness at health facilities in Benin: problems and their causes. Am J Public Health 91: 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber M, Mulholland EK, Jaffar S, Troedsson H, Gove S, Greenwood BM, 1997. Evaluation of an algorithm for the integrated management of childhood illness in an area with seasonal malaria in the Gambia. Bull World Health Organ 75 (Suppl 1): 25–32. [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe AK, Ponce de León GF, Mihigo J, Santelli ACF, Miller NP, Van-Dúnem P, 2009. Quality of malaria case management at outpatient health facilities in Angola. Malar J 8: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plucinski MM, et al. 2017. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar J 16: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Namuyinga RJ, et al. 2017. Health worker adherence to malaria treatment guidelines at outpatient health facilities in southern Malawi following implementation of universal access to diagnostic testing. Malar J 16: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhardt LC, Chinkhumba J, Wolkon A, Luka M, Luhanga M, Sande J, Oyugi J, Ali D, Mathanga D, Skarbinski J, 2014. Quality of malaria case management in Malawi: results from a nationally representative health facility survey. PLoS One 9: e89050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MalariaCare Madagascar , 2015. Health Facility Assessment Report. White Paper. Antananarivo, Madagascar.
- 10.Johansson EW, Nsona H, Carvajal-Aguirre L, Amouzou A, Hildenwall H, 2017. Determinants of Integrated Management of Childhood Illness (IMCI) non–severe pneumonia classification and care in Malawi health facilities: analysis of a national facility census. J Glob Health 7: 020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redd SC, Luby S, Hightower A, Kazembe P, Nwanyanwu O, Ziba C, Chitsulo L, Franco C, Olivar M, Wirima J, 1996. Clinical algorithm for treatment of Plasmodium falciparum malaria in children. Lancet 347: 223–227. [DOI] [PubMed] [Google Scholar]
- 12.Nsimba SE, Massele AY, Eriksen J, Gustafsson LL, Tomson G, Warsame M, 2002. Case management of malaria in under‐fives at primary health care facilities in a Tanzanian district. Trop Med Int Health 7: 201–209. [DOI] [PubMed] [Google Scholar]
- 13.Candrinho B, et al. 2019. Quality of malaria services offered in public health facilities in three provinces of Mozambique: a cross-sectional study. Malar J 18: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davlantes E, et al. 2019. Quality of malaria case management and reporting at public health facilities in six health districts in Guinea, 2018. Am J Trop Med Hyg 101: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thwing J, et al. 2017. Assessment of the utility of a symptom-based algorithm for identifying febrile patients for malaria diagnostic testing in Senegal. Malar J 16: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trape J-F, et al. 2014. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis 14: 476–488. [DOI] [PubMed] [Google Scholar]
- 17.Bennett A, et al. 2017. Population coverage of artemisinin-based combination treatment in children younger than 5 years with fever and Plasmodium falciparum infection in Africa, 2003–2015: a modelling study using data from national surveys. Lancet Glob Health 5: e418–e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plucinski MM, Candrinho B, Dimene M, Smith T, Thwing J, Colborn J, Rogier E, Zulliger R, 2019. Estimation of malaria-attributable fever in malaria-test-positive febrile outpatients in three provinces of Mozambique, 2018. Am J Trop Med Hyg doi:10.4269/ajtmh.19-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institut National de la Statistique (INS), Programme National de Lutte contre le Paludisme (PNLP), ICF , 2017. Enquête de Prévalence Parasitaire du Paludisme et de l’anémie en Guinée 2016. Rockville, MD: INS, PNLP et ICF. [Google Scholar]
- 20.Instituto Nacional de Saúde (INS), Instituto Nacional de Estatística (INE), ICF , 2019. Malaria Indicator Survey, Mozambique 2018. Rockville, MD: INS, INE, and ICF. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.