Abstract.
Zika virus (ZIKV) has recently been confirmed as endemic in Indonesia, but no congenital anomalies (CA) related to ZIKV infection have been reported. We performed molecular and serological testing for ZIKV and other flaviviruses on cord serum and urine samples collected in October 2016 to April 2017 during a prospective, cross-sectional study of neonates in Jakarta, Indonesia. Of a total of 429 neonates, 53 had CA, including 14 with microcephaly. These 53, and 113 neonate controls without evidence of CA, were tested by ZIKV-specific real-time reverse transcription polymerase chain reaction (RT-PCR), pan-flavivirus RT-PCR, anti-ZIKV and anti-DENV IgM ELISA, and plaque reduction neutralization test. There was no evidence of ZIKV infection among neonates in either the CA or non-CA cohorts, except in three cases with low titers of anti-ZIKV neutralizing antibodies. Further routine evaluation throughout Indonesia of pregnant women and their newborns for exposure to ZIKV should be a high priority for determining risk.
Zika virus (ZIKV), an arthropod-borne member of the Flaviviridae, has been implicated as the cause of severe neurological abnormalities in a proportion of fetuses and newborns of infected women in the Western Hemisphere.1 Zika virus infection typically produces a mild, self-limiting febrile illness in adults, which can be overlooked. Neonatal microcephaly has been the syndrome predominantly linked to maternal ZIKV infection, especially during the first trimester, but there are reports of other developmental defects, including intrauterine growth restriction, brain anomalies, ocular defects, and fetal demise.2 From May 2015 to January 2018, the WHO recorded 3,720 confirmed congenital Zika syndrome (CZS) cases presenting with microcephaly and other neurodevelopmental defects in 27 countries in the Americas.3
Although there is evidence that ZIKV has circulated in Southeast Asia long before the epidemic in the Western Hemisphere,4,5 there have been few reports linking CZS to maternal ZIKV infection in the region, notably two cases of microcephaly from Thailand and one from Vietnam.6,7 Zika virus is endemic in Indonesia.8 The aim of this study was to determine whether congenital anomalies (CA) in neonates born in Jakarta, Indonesia, might be associated with prenatal ZIKV infection.
We performed molecular and serological testing for ZIKV and dengue virus (DENV) on samples collected during October 2016 to April 2017 at the Integrated Service Unit of the Perinatology and Neonatal Intensive Care Unit, Department of Paediatrics, Dr. Cipto Mangunkusumo National Central Hospital, Jakarta, Indonesia. The specimens were collected as part of a study designed to determine the prevalence of symptomatic and asymptomatic congenital cytomegalovirus infection.9 Additional testing of the anonymized specimens was approved by the Ethics Committee of the Faculty of Medicine, University of Indonesia (no. 925/UN2.F1/ETIK/2016). Neonates consecutively born in the hospital were selected for enrollment into the study if the parents provided consent. Umbilical cord blood and infant urine were collected; maternal and newborn blood were not tested. Serum was obtained from cord blood samples collected using a 3-mL syringe after delivery of the placenta. Urine samples were collected with a pediatric urine collector bag (males) and with sterile cotton wool balls (females). The newborns were screened by the attending pediatrician for CA, which included structural birth defects involving the brain, spinal cord, heart, and gastrointestinal tract. Head ultrasound was conducted in some cases at the discretion of the attending neonatologist. The occipitofrontal or head circumference (HC) was taken around the widest possible circumference of the head. Microcephaly was defined as HC less than the third percentile, and macrocephaly as HC greater than the 97th percentile based on the gender, age, and gestational age at birth by the Fenton growth chart (for preterm babies) and the WHO chart (for term babies).10,11
Cord serum and urine samples were tested by ZIKV-specific real-time RT-PCR using QuantiTect Probe RT-PCR Kit (Qiagen, Valencia, CA) with the analytical sensitivity of 100 copies of ZIKV RNA.12 Pan-flavivirus RT-PCR, designed to detect all flaviviruses including ZIKV, DENV, and Japanese encephalitis virus (JEV), was performed on cord serum samples. The analytical sensitivity of the assay was 0.4 PFU/mL for DENV-1, DENV-2, DENV-4, and JEV; 4 PFU/mL for ZIKV; and 400 PFU/mL for DENV-3.13 The RT-PCR assays were performed using OneStep RT-PCR Kit (Qiagen). Commercial serological assays were used to detect anti-ZIKV IgM (ZIKV Detect IgM Capture ELISA, InBios, Seattle, WA) and anti-DENV IgM (Dengue IgM Capture DxSelect, Focus Diagnostics, Cypress, CA) in cord serum. The InBios anti-ZIKV IgM ELISA sensitivity is 100% compared with the recommended U.S. CDC diagnostic assays,14 whereas the Focus anti-DENV IgM ELISA kit sensitivity is 98.6% compared with the reference standard ELISAs used by U.S. CDC and the Armed Forces Research Institute of Medical Science, Bangkok, Thailand.15
Plaque reduction neutralization tests (PRNT) of cord serum samples for ZIKV were performed in a two-tier approach as described previously.8 Briefly, all serum samples were screened for the presence of anti-ZIKV neutralizing antibodies (NAb) at 1:10 dilution against ZIKV strain JMB-185. Cord serum samples that suppressed the formation of plaque-forming units by ≥ 90% (PRNT90) in BHK-21 cell culture were considered to be potentially positive for ZIKV NAb and were further tested at dilutions of 1:10, 20, 40, 80, 160, and 320 against ZIKV alongside all four DENV serotypes (combo ZIKV-DENV PRNT90).8 The DENV strains used were DENV-1 strain Hawaii, DENV-2 strain NGC, DENV-3 strain H87, and DENV-4 strain H241. Specimens were classified as true ZIKV if positive for ZIKV NAb without any detectable levels of DENV NAb or if ZIKV NAb titers were ≥ 4-fold higher than that of DENV NAb.
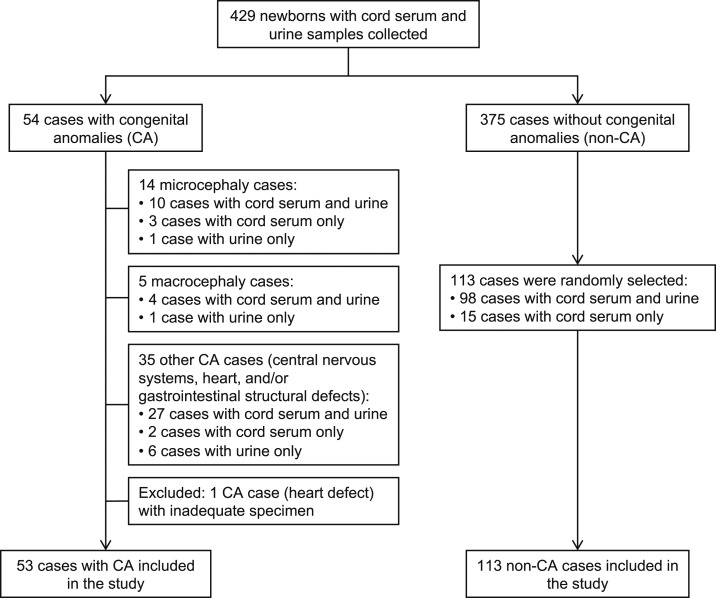
Of a total of 429 neonates enrolled, 54 (13%) were determined to have CA, 53 of whom were represented by at least one specimen (Figure 1). Fourteen neonates were diagnosed with microcephaly, five with macrocephaly, and 35 others with one or more CAs including heart, central nervous system, gastrointestinal, genitourinary, eye, hearing, musculoskeletal, and/or maxillofacial defects (Table 1). The proportion of CA cases in our study was higher than that in the national data16 because Dr. Cipto Mangunkusumo National Central Hospital is a referral hospital for the management of pregnant women at high risk or those with complications. From 375 non-CA cases, 113 were randomly selected as case controls by Microsoft Excel–generated random lists. Controls were not selected to match CA cases either by gender, gestational age, or any clinical history of the mother. Ninety-seven percent (161/166) of all the studied cases were from the greater Jakarta region in western Java.
Figure 1.
Study flowchart of patient enrollment and sample collection.
Table 1.
Characteristics and laboratory assay results from neonates with and without congenital anomalies (CA)
| CA (n = 53) | Non-CA (n = 113) | |
|---|---|---|
| Neonates characteristics | ||
| Gender | ||
| Male | 22 (42%) | 54 (48%) |
| Female | 31 (58%) | 59 (52%) |
| Gestational age (weeks) | ||
| Term (≥ 37) | 22 (42%) | 53 (47%) |
| Premature (≤ 36) | 31 (58%) | 60 (53%) |
| Birthweight (grams) | ||
| ≥ 2,500 | 19 (36%) | 53 (47%) |
| 1,500−2,499 | 25 (47%) | 44 (39%) |
| < 1,500 | 9 (17%) | 16 (14%) |
| Mode of delivery | ||
| Spontaneous delivery | 15 (28%) | 34 (30%) |
| Cesarean section | 38 (72%) | 79 (70%) |
| Congenital anomalies (CA) | ||
| Neonates with more than one birth defect | 13 (25%) | – |
| Microcephaly | 14 (26%) | – |
| Macrocephaly | 5 (9%) | – |
| CNS defect | 10 (19%) | – |
| Ventriculomegaly | 6 (11%) | – |
| Hydrocephalus | 1 (2%) | – |
| Multiple periventricular cysts | 1 (2%) | – |
| Hypoxic ischemic encephalopathy | 1 (2%) | – |
| Agenesis of the corpus callosum | 1 (2%) | – |
| Heart defect | 25 (47%) | – |
| Gastrointestinal defect | 7 (13%) | – |
| Genitourinary defect | 4 (8%) | – |
| Eye defect | 2 (4%) | – |
| Hearing defect | 2 (4%) | – |
| Musculoskeletal defect | 1 (2%) | – |
| Maxillofacial defect | 1 (2%) | – |
| Results of laboratory assays | ||
| ZIKV real-time RT-PCR | ||
| Cord serum | 0/45 (0%) | 0/113 (0%) |
| Urine | 0/49 (0%) | 0/98 (0%) |
| Pan-flavivirus RT-PCR | ||
| Cord serum | 0/45 (0%) | 0/113 (0%) |
| Anti-ZIKV IgM ELISA | 0/45 (0%) | 0/113 (0%) |
| Anti-DENV IgM ELISA | 1/46 (2%) | 1/112 (1%) |
| ZIKV-DENV plaque reduction neutralization test90* | 1/46 (2%) | 2/110 (2%) |
CA = Congenital anomalies; CNS = central nervous system; DENV = dengue virus; ZIKV = Zika virus. Data are n (%) or number of positive/tested samples (%).
* Result indicates samples that were true ZIKV positive (presence of anti-ZIKV NAb without any detectable levels of anti-DENV NAb or ≥ 4-fold higher anti-ZIKV NAb titers than that of anti-DENV NAb).
All of the specimens from CA and non-CA groups were negative for ZIKV by both real-time RT-PCR and anti-ZIKV IgM ELISA. No other flavivirus RNA was detected in cord sera by pan-flavivirus RT-PCR (Table 1). Because fetal IgM response can be low, all cord sera were also screened by PRNT90 for anti-ZIKV NAb; a high ZIKV IgG titer could indicate transplacental transfer from a recently infected mother. The PRNTs were marginally positive for one CA (anti-ZIKV NAb titer: 20) and two non-CA specimens (anti-ZIKV NAb titers: 10 and 40) (Table 2). No anti-DENV IgM was detected in these three cases, but one other cord serum specimen from a CA and one from a non-CA did test positive for anti-DENV IgM; DENV PRNT90 were not done (Table 2)
Table 2.
Cases with positive anti-ZIKV neutralizing antibodies and anti-DENV IgM in cord serum
| Case no. | Neonates group | Anti-ZIKV IgM ELISA | Anti-DENV IgM ELISA | ZIKV-DENV PRNT90* | ||||
|---|---|---|---|---|---|---|---|---|
| ZIKV | DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
| 1 | CA | Negative | Negative | 20 | < 10 | < 10 | < 10 | < 10 |
| 2 | Non-CA | Negative | Negative | 10 | < 10 | < 10 | < 10 | < 10 |
| 3 | Non-CA | Negative | Negative | 40 | < 10 | < 10 | 10 | 10 |
| 4 | CA | Negative | Positive | < 10 | ND | ND | ND | ND |
| 5 | Non-CA | Negative | Positive | < 10 | ND | ND | ND | ND |
CA = Congenital anomalies; DENV = dengue virus; ND = not determined; PRNT = Plaque reduction neutralization test; ZIKV = Zika virus.
* Value indicates PRNT endpoint at which the highest serum dilution inhibited formation of plaque-forming units by ≥ 90%.
Serological evidence for ZIKV transmission on the islands of Java and Lombok was reported in the 1980s,17,18 but the first molecular confirmation, from Sumatra, was in 2016.19 In specimens collected during 2014 from asymptomatic children throughout western and central Indonesia, the overall ZIKV seroprevalence in 1- to 4-year-olds was 9.1%; the seroprevalence in metropolitan Jakarta, from which most our study subjects came, was greater than 10%.8 None of the cord blood or urine tested in our study, including from infants with microcephaly, indicated recent congenital ZIKV infection; the low level of anti-ZIKV antibody detected by PRNT in three positive cord blood specimens suggests transplacental transfer of maternal antibody rather than fetal infections.20–22 A limitation of this study was that no maternal blood was available for testing.
The apparent rarity of microcephaly associated with ZIKV in Asia has led some to speculate that a mutation in the Asian lineage, which caused the American epidemic, might underlie the observed teratogenicity; such a mutation has yet to be identified.23 A case of congenital ZIKV infection with microcephaly in Thailand was caused by an Asian lineage virus nearly identical to that sequenced from Indonesia.24 Although ZIKV is endemic in Indonesia and anti-ZIKV NAb were detected in children living in greater Jakarta in 2014, we do not know the ZIKV incidence in the area during 2016−2017. Alternately, many of our study subjects, predominately women aged 20–40 years, might have been naturally immunized by mosquito-borne ZIKV infection before reaching the childbearing age.
Our study suggests no evidence of ZIKV infection among infants born at this referral hospital in Jakarta during the study period, including among infants with microcephaly and other CA well recognized as complications of ZIKV infection. Birth defect surveillance was initiated by the Indonesian Ministry of Health in September 2014 in 28 hospitals across 18 provinces, but reports on the infectious etiology of birth defects have so far been limited.25 To explore the role of ZIKV infection in causing birth defects confounding etiologies, such as toxoplasmosis, rubella, herpes simplex virus, measles, mumps, and syphilis, must also be accurately diagnosed. More extensive seroprevalence surveys, as well as routine testing of pregnant women and their babies, must be performed to determine the frequency of ZIKV teratogenicity in Indonesia and other endemic countries.
Acknowledgments:
We would like to thank the patients and physicians involved in this study, and Dr. Susan L. Hills (U.S. Centers for Disease Control and Prevention, Fort Collins, CO) for the critical comments and suggestions to improve and clarify this article.
REFERENCES
- 1.Yi-Pin Lee C, Ng LFP, 2018. Zika virus: from an obscurity to a priority. Microbes Infect 20: 635–645. [DOI] [PubMed] [Google Scholar]
- 2.Del Campo M, et al. 2017. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A 173: 841–857. [DOI] [PubMed] [Google Scholar]
- 3.PAHO/WHO , 2018. Zika Cases and Congenital Syndrome Associated with Zika Virus Reported by Countries and Territories in the Americas, 2015–2018 Cumulative Cases. Available at: https://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=1170&gid=43297&lang=en. Accessed August 4, 2018. [Google Scholar]
- 4.Wikan N, Smith DR, 2016. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis 16: e119–e126. [DOI] [PubMed] [Google Scholar]
- 5.Ruchusatsawat K, Wongjaroen P, Posanacharoen A, Rodriguez-Barraquer I, Sangkitporn S, Cummings DAT, Salje H, 2019. Long-term circulation of Zika virus in Thailand: an observational study. Lancet Infect Dis 3099: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization , 2016. Situation Report: Zika Virus, Microcephaly and Guillain-Barré Syndrome 6 October 2016, 1–6. Available at: http://apps.who.int/iris/bitstream/10665/250244/1/zikasitrep29Sep16-eng.pdf?ua=1. Accessed April 3, 2018. [Google Scholar]
- 7.Moi ML, et al. 2017. Zika virus infection and microcephaly in Vietnam. Lancet Infect Dis 17: 805–806. [DOI] [PubMed] [Google Scholar]
- 8.Sasmono RT, et al. 2018. Zika virus seropositivity in 1–4-year-old children, Indonesia, 2014. Emerg Infect Dis 24: 1740–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putri ND, et al. 2019. Birth prevalence and characteristics of congenital cytomegalovirus infection in an urban birth cohort, Jakarta, Indonesia. Int J Infect Dis 86: 31–39. [DOI] [PubMed] [Google Scholar]
- 10.Fenton TR, 2003. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO , 2014. Head Circumference-For-Age. WHO; Available at: http://www.who.int/childgrowth/standards/hc_for_age/en/. Accessed November 17, 2018. [Google Scholar]
- 12.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR, 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawuntu AH, et al. 2018. Detection of central nervous system viral infections in adults in Manado, North Sulawesi, Indonesia. PLoS One 13: e0207440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safronetz D, et al. 2017. Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis 23: 1577–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunsperger EA, et al. 2009. Evaluation of commercially available anti-dengue virus immunoglobulin M tests. Emerg Infect Dis 15: 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christianson A, Howson CP, Modell B, 2006. Global Report on Birth defects. The Hidden Toll of Dying and Disabled Children. Available at: https://www.marchofdimes.org/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-full-report.pdf. [Google Scholar]
- 17.Olson J, Ksiazek T, Suhandiman T, 1981. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 75: 389–393. [DOI] [PubMed] [Google Scholar]
- 18.Olson J, Ksiazek T, Gubler D, Lubis S, Simanjuntak G, Lee V, Nalim S, Juslis K, See R, 1983. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol 77: 131–137. [DOI] [PubMed] [Google Scholar]
- 19.Yudhaputri FA, et al. 2017. Genomic characterization of Zika virus isolated from Indonesia. Virology 510: 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordeiro MT, et al. 2016. Results of a zika virus (ZIKV) immunoglobulin M-specific diagnostic assay are highly correlated with detection of neutralizing anti-ZIKV antibodies in neonates with congenital disease. J Infect Dis 214: 1897–1904. [DOI] [PubMed] [Google Scholar]
- 21.Moreira-Soto A, et al. 2017. Evidence for congenital Zika virus infection from neutralizing antibody titers in maternal sera, northeastern Brazil. J Infect Dis 216: 1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castanha PMS, et al. 2018. Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: a microcephaly case-control study in an area of high dengue endemicity in Brazil. PLoS Negl Trop Dis 13: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun SR, Wassenaar TM, Wanchai V, Patumcharoenpol P, Nookaew I, Ussery DW, 2017. Suggested mechanisms for Zika virus causing microcephaly: what do the genomes tell us? BMC Bioinformatics 18 (Suppl 14): 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wongsurawat T, et al. 2018. Case of microcephaly after congenital infection with Asian lineage Zika virus, Thailand. Emerg Infect Dis 24: 1758–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kementerian Kesehatan RI, 2018. Kelainan Bawaan. Available at: http://www.depkes.go.id/download.php?file=download/pusdatin/infodatin/infodatin kelainan bawaan.pdf. Accessed June 18, 2019. [Google Scholar]