Abstract.
Human liver fluke infection caused by Opisthorchis viverrini increases the risk of cholangiocarcinoma (CCA) reported along the Mekong basin including Thailand, Lao People’s Democratic Republic (PDR), Cambodia, and Vietnam. The highest incidence of CCA has been reported in northeastern Thailand where liver fluke infection is prevalent. This study aimed to investigate the prevalence of O. viverrini infection in a northeastern-descendent community in rural Sa Kaeo Province, eastern Thailand, using stool examination and molecular technique. The Kato–Katz method was performed to determine eggs per gram (EPG) for infection intensity. Phosphate-buffered saline–ethyl acetate concentration was used to prepare specimens for polymerase chain reaction (PCR) and restriction fragment length polymorphism of the internal transcribed spacer 2 (ITS2) region of the ribosomal RNA. From 1,245 specimens, 105 (8.4%) samples were identified as Opisthorchis-like eggs from stool examination, and all positive specimens indicated light infection (< 1,000 EPG). From positive Opisthorchis-like egg samples, 55.2% (58/105) were identified as O. viverrini eggs from ITS2-PCR assay for which low infection intensity might result in a negative PCR result (44.8%). Using multiple logistic regression analysis, males were at 3.1 times higher risk of acquiring O. viverrini infection than females. From phylogenetic analysis, in eastern Thailand, nucleotide sequences of O. viverrini were grouped as a monoclade as those isolated from Greater Mekong, Vietnam, Myanmar, and west Siberia. The results revealed that the surveyed community is a low-grade endemic area of O. viverrini infection. Thus, data from this study can be used to improve health-promoting programs and activities to control the infection and its subsequent CCA.
INTRODUCTION
Opisthorchis viverrini, a human liver fluke, is a major public health problem in Thailand and in Southeast Asian countries where people consume the infective metacercariae in raw or undercooked cyprinoid fish.1 In Thailand, O. viverrini infection in humans has been reported for almost 100 years,2 and about eight million people are infected with O. viverrini.3 The prevalence of O. viverrini infection is high in north and northeastern Thailand where people frequently eat a raw or undercooked dish called “koi pla.”4 The disease is caused by mechanical, chemical, and immunological irritation, and severity of the disease depends on the intensity and duration of the infection. Organs involved in the disease occurrence are the bile ducts, gallbladder, and liver.5 Chronic infection of O. viverrini is associated with cholangiocarcinoma (CCA),6 and the world’s highest incidence of CCA has been reported in the northeastern area of Thailand.7 Prevention and control of liver fluke infection has been a difficult task in Thailand because of habits of local people eating uncooked freshwater fish in endemic areas.
Microscopic examination is a gold standard to diagnose O. viverrini eggs in stool specimens.8 However, eggs could not be differentiated with those of other human liver flukes (Clonorchis sinensis and Opisthorchis felineus) and small intestinal flukes (Heterophyes heterophyes, Heterophyes pumilio, Heterophyes taichui, and Heterophyes yokogawai).6 Thus, molecular techniques have been used to improve the specificity of the diagnostic test to determine the true prevalence of O. viverrini infection in the study population. Polymerase chain reaction (PCR)–based assays have been developed that could discriminate O. viverrini eggs from those of C. sinensis and small intestinal flukes using the internal transcribed spacer 1 (ITS1) and ITS2 regions of the ribosomal RNA (rRNA) gene.9
In 2009, the prevalence of O. viverrini infection was reported in north (19.3%), northeast (15.7%), central (3.8%), and southern (0%) Thailand.4 Using stool examination, from 2002 to 2009, prevalence of Opisthorchis-like eggs in studies conducted in a rural community of Sanamchaikaet district, Chachoengsao Province, central Thailand, was 17.4–21.3%. Because of eating habits of local people who originally migrated from the northeast to this rural community, the prevalence of O. viverrini infection was much higher than the average prevalence reported in central Thailand.10 Phra Phloeng village, a rural community in Sa Kaeo Province, eastern Thailand, is a nearby province next to Chachoengsao Province where a true prevalence study of O. viverrini infection in stool specimens has never been conducted using a molecular technique. Moreover, in eastern areas, the highest incidence of liver cancer and CCA has also been reported in Sa Kaeo Province11 where eating habits of the local people are the same as those living in the northeast. The study of prevalence using the ITS2-PCR assay and associated risk factors of O. viverrini infection in this study would be very helpful and support the control programs of liver fluke infection in this area. Information from this study can be used to improve health-promoting programs and activities to control the infection and progression of the disease.
MATERIALS AND METHODS
Ethics.
The research proposal was reviewed and approved by the Ethics Committee of the Royal Thai Army Medical Department (IRBRTA 31/2559) and the Ethics Committee of Mahidol University (MU-CIRB 2018/098.0205). Informed consent was obtained from the enrolled participants or the parents of the enrolled participants aged younger than 18 years. The study area was Thung Phra Phloeng subdistrict, a rural community of Sa Kaeo Province, 200 km east of Bangkok, Thailand. The community comprises 13 villages, of which 1,279 participants were recruited in this study in March 2017.
Stool and quantitative examination.
A single stool sample was collected from each subject. The fecal specimens were examined using the following three methods: simple wet smear, Kato–Katz technique, and phosphate-buffered saline–ethyl acetate concentration (PBS-ECT) methods. A fecal sample was considered positive for O. viverrini–like eggs when one or more eggs were observed in any of the three methods.
Intensity of infection was measured by counting numbers of O. viverrini–like eggs under a microscope and calculated into egg per gram (EPG) of feces using the Kato–Katz technique. The intensity of Opisthorchis-like egg was classified into light infection (< 1,000 EPG), moderate infection (1,000–10,000 EPG), and heavy infection (> 10,000 EPG).12
DNA preparation and extraction of O. viverrini–like eggs.
A concentration technique based on PBS-ECT was used to prepare fecal samples before DNA extraction. Phosphate-buffered saline replaced formalin because of the benefits of further PCR amplifications. Approximately 2 g of feces were added with 10 mL PBS and filtered through gauze to dissolve stool specimens. And 3 mL of ethyl acetate was added, followed by shaking continuously until a complete suspension of stools could be observed. The homogenizing stool mixtures were centrifuged at 2,000 g for 10 minutes. The expected four layers were present after centrifugation, such as, layers of ethyl acetate, wastes, PBS, and parasite eggs or other protozoa, in order. The three upper layers were removed, leaving the sediment at the retaining bottom layer. The sediment was washed three times continuously with PBS at 3,000 g for 10 minutes. At final PBS washing, the sediment was examined for O. viverrini–like eggs under a light microscope. The positive samples for O. viverrini–like eggs in PBS were kept at −20°C.
Each positive sample from Opisthorchis-like eggs collected by PBS-ECT was centrifuged at 3,000 g for 10 minutes to precipitate the pellets and used for DNA extraction. And 180 µL ATL tissue lysis buffer was added to the pellets and mixed continuously using a pestle homogenizer until the pellets were thoroughly homogenized. The suspension was subjected for five cycles of freezing in liquid nitrogen and thawing at 98–100°C.10 Subsequently, the DNA was extracted using DNeasy Blood and Tissue Kits (Qiagen, Hilden, Germany) according to the manufacturer’s protocol with occasional vortexing using a thermomixer every 5 minutes for 3 hours during incubation at 56°C. The DNA was eluted with 100 μL elution buffer.
Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) for discriminating O. viverrini–like eggs.
RTFluke primers designed for the ITS2 region of rRNA of opisthorchiid and heterophylid flukes were used to amplify Opisthorchis-like eggs’ DNA.13 Polymerase chain reaction amplifications were performed under a final volume of 50 μL, consisting of a DNA template, 1× buffer PCR, 2 mM of MgCl2, 200 μM dNTP, 12.5 pmole of each primer (RTFlukeFa; 5′–CTTGAACGCACATTGCGGCC and RTFlukeRa; 5′–CACGTTTGAGCCGAGGTCAG), and 1 unit of Taq polymerase (5 U/μL) (Promega, Madison, WI). The PCR profile was one cycle of initial amplification; 15-minute denaturation at 94°C, 1-minute annealing at 60°C, and 1-minute extension at 72°C, followed by 35 cycles of amplification; 30-second denaturation at 94°C, 30-second annealing at 60°C, and 30-second extension at 72°C, and one cycle of 7-minute extension at 72°C and held at 12°C to complete the amplification. The positive control was DNA extraction of O. viverrini eggs confirmed by DNA sequencing, whereas the negative control was double-distilled water. The expected sizes of O. viverrini, C. sinensis, and H. taichui amplicons were 375, 381, and 526 bp, respectively.13 The 375 bp and 381 bp of PCR products were subjected to PCR-RFLP to discriminate eggs of O. viverrini and C. sinensis using a restriction enzyme that digested the PCR amplicons with 2 units of FauI (New England Biolabs, Ipswich, MA) in a total volume of 20 μL at 55°C overnight.10
Agarose gel electrophoresis and DNA sequencing.
The PCR products were detected under 2% agarose gel in 1× tris/borate/EDTA buffer. The SYBR® Safe DNA Gel Stain (Invitrogen, Grand Island, NY) was used to detect DNA by dissolving 10 mL of agarose gel with 1× SYBR Safe DNA Gel Stain (Invitrogen). Ten microliters of the PCR product was added and mixed with loading dye, then loaded on 2% agarose gel. A 100-bp DNA ladder (Vivantis Technologies, Selangor Darul Ehsan, Malaysia) was used as a marker to estimate the expected sizes of PCR products. The PCR products were run at 100 volts for 45 minutes at room temperature. Finally, PCR products were detected by ultraviolet (UV) visualization using a Molecular Imager® Gel Doc™ XR + imaging system (Bio-Rad, Hercules, CA). The expected PCR products were purified using a QIAquick Gel Extraction Kit (Qiagen) according to the manufacturer’s protocol and then sent to Bioneer Sequencing Service for nucleotide sequencing.
Phylogenetic trees of the ITS2 of O. viverrini and other trematodes.
The ITS2 sequences were aligned with the reference ITS2 genes of different trematode species retrieved from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) using MUSCLE (MEGA7 program, Molecular Evolutionary Genetics Analysis, version 7.0) to generate a phylogenetic relationship among parasitic trematode families, including Opisthorchiidae, Heterophyidae, Fasciolidae, and Schistosomatidae. The blood fluke, Schistosoma japonicum, was chosen as an outgroup. RAxML analysis was used to construct the ITS2 tree based on RAxML, version 7.4.2, with a GTR matrix (GTR + Γ model)14 using RaxmlGUI, version 1.15 The clade stability of the tree topology was evaluated using 1,000 replicates of RAxML bootstrap values.
The reference sequences were O. viverrini isolates from west Siberia, Russia (KC427196 and KC427197), Greater Mekong, Lao PDR (HQ328550, HQ328548, and HQ328549), central Vietnam (KT726408 and KT726409), central Myanmar (MG797539 and MG797538), and northeastern Thailand (AY584735); Opisthorchis felineus (DQ513403 and DQ513404); Opisthorchis sinensis (KJ137226 and JQ048598); Haplorchis taichui (HM004157, KP165440, and KC999100); Fasciola gigantica (KR676570, KT899309, KR676568, and KT899310); and Fasciola hepatica (MG569980 and MH048706). Schistosoma japonicum (FJ852568 and FJ852572) was chosen for the outgroup.
Data collection and analysis.
Study identification number (ID) was generated for each subject and used to label specimen containers and questionnaires concealed each subject’s identity. Data for age and gender were obtained from the local health-promoting hospital’s database after matching with study ID under subjects’ consent. Additional risk factors of Opisthorchis-like egg infections were collected using self-administered standardized questionnaires covering demographic data, alcoholic consumption, and eating habits such as consuming traditional uncooked fish dishes.
Prevalence and risk factors of Opisthorchis-like egg infections were analyzed using STATA/SE, version 9.2 (StataCorp LP, College Station, TX). Prevalence was reported with percentage, and risks were reported with odds ratio (OR), P-value, and 95% CI. Univariate logistic regression analysis was initially performed to determine relationships between study covariate and infection status (yes/no), and covariates with P-value < 0.2 were incorporated in a multivariate model using multiple logistic regression analysis.
RESULTS
From 1,279 enrolled participants, 1,245 specimens were returned for stool examination with 97.3% response rate; each specimen ID of 1,245 was later matched to database for age and gender, of which 701 (56.3%) of the study participants were male (female = 544), 497 (39.9%) in the age-group 40–59 years. The mean (±SD) age of participants was 43.11 (±22.9) years. Returned questionnaires totaled 417 (32.6%). In all, 84.9% of subjects received formal education. The population or households mostly comprised farmers working in paddy fields.
Prevalence and risk factors of Opisthorchis-like infection.
From stool examination, prevalence of Opisthorchis-like infection was 8.4%. In addition, prevalence of the other top three intestinal parasites was 6.1% for Blastocystis sp., 1.6% for Strongyloides stercoralis, and 1.1% for hookworm; others are shown in Table 1. Prevalence in each age-group was 1.7% (< 20 years), 4.8% (20–39 years), 8.7% (40–59 years), and 15.4% (> 59 years), respectively. The prevalence in each age-group differed significantly (P < 0.01). As shown in Table 2, of 105 positive Opisthorchis-like egg samples, EPG of feces examined are as follows: 1–99 EPG (100 samples), 100–199 EPG (three samples), and 200–499 EPG (two samples), for which all positive samples were considered light intensity.
Table 1.
Intestinal parasitic infections in 1,245 stool specimens returning from 1,279 enrolled participants in Thung Phra Phloeng subdistrict, Sa Kaeo Province, eastern Thailand
| Intestinal parasitic infection | No. positive | % Positive |
|---|---|---|
| Opisthorchis-like eggs | 105 | 8.4 |
| Blastocystis sp. | 76 | 6.1 |
| Strongyloides stercoralis | 20 | 1.6 |
| Hookworms | 14 | 1.1 |
| Giardia intestinalis | 9 | 0.7 |
| Trichomonas hominis | 8 | 0.6 |
| Enterobius vermicularis | 5 | 0.4 |
| Taenia spp. | 4 | 0.3 |
| Trichuris trichiura | 3 | 0.2 |
| Entamoeba coli | 2 | 0.2 |
| Large intestinal flukes | 2 | 0.2 |
| Others (parasitic amoeba and fluke) | 3 | 0.2 |
Three examination methods including simple wet smear, Kato–Katz, and phosphate-buffered saline–ethyl acetate concentration techniques were used to examine intestinal parasitic infections.
Table 2.
Detection of Opisthorchis-like eggs in stool samples using the internal transcribed spacer 2 PCR assay according to the number of EPG of feces
| Number of EPG | Number of positive Opisthorchis viverrini–like eggs in stool samples by Kato–Katz | Number positive by PCR (%) |
|---|---|---|
| 1–99 | 100 | 54 (54.0) |
| 100–199 | 3 | 2 (66.7) |
| 200–499 | 2 | 2 (100) |
| 105 | 58 |
EPG = eggs per gram; PCR = polymerase chain reaction.
From Table 3, univariate analysis revealed that the age-group 40–59 years (OR = 5.6, 95% CI = 2.2–14.4) and age-group more than 59 years (OR = 10.8, 95% CI = 4.2–27.5) significantly acquired the infection compared with age-group < 20 years. Using multiple logistic regression analysis, men were 3.1 (95% CI = 1.1–8.4) times at higher risk of acquiring O. viverrini infection than women after adjusting for age, gender, and alcohol and koi pla consumption.
Table 3.
Univariate and multivariate analyses for Opisthorchis viverrini–like egg infection among villagers in Phra Phloeng village, Sa Kaeo Province, eastern Thailand
| Factor | Prevalence of infection (%) | Crude OR (95% CI) | P-value | AOR (95% CI) | P-value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 7.1 | 1 | – | 1 | – |
| Female | 9.7 | 1.4 (0.9–2.1) | 0.10 | 3.1 (1.1–8.4) | 0.03 |
| Age (years) | |||||
| < 20 | 1.7 | 1 | – | 1 | – |
| 20–39 | 4.8 | 3.0 (0.9–9.9) | 0.08 | 0.6 (0.03–11.6) | 0.75 |
| 40–59 | 8.7 | 5.6 (2.2–14.4) | < 0.001 | 1.1 (0.1–10.1) | 0.91 |
| > 59 | 15.4 | 10.8 (4.2–27.5) | < 0.001 | 1.5 (0.2–11.3) | 0.70 |
| Alcohol | |||||
| No | 10.4 | 1 | – | 1 | – |
| Yes | 14.3 | 1.5 (0.8–2.7) | 0.19 | 1.0 (0.4–2.7) | 0.99 |
| Fish menus | |||||
| Chopped raw fish salad (koi pla) | |||||
| No | 5.5 | 1 | – | 1 | – |
| Yes | 9.6 | 1.8 (0.7–4.4) | 0.19 | 1.3 (0.5–3.4) | 0.59 |
| Briefly fermented fish (pla som) | |||||
| No | 7.3 | 1 | – | – | – |
| Yes | 7.3 | 1.0 (0.4–2.4) | 0.99 | – | – |
AOR = adjusted odds ratio; OR = odds ratio. Crude OR and AOR were analyzed using univariate and multivariate analyses, respectively.
Molecular study.
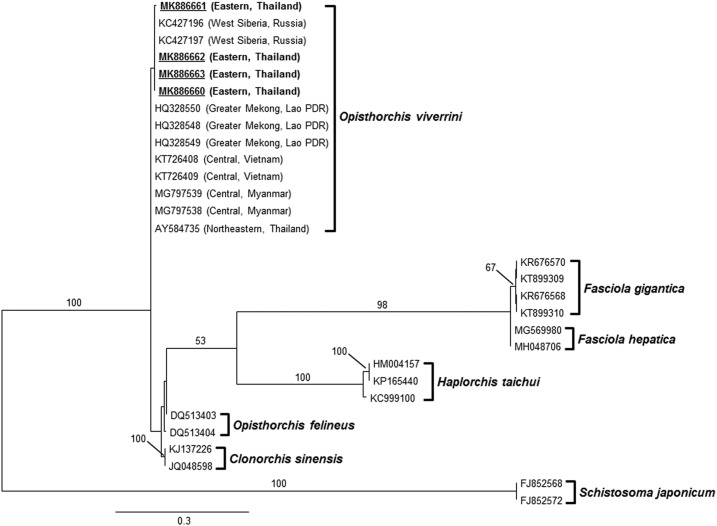
From the molecular study, PCR-RFLP of the ITS2 region was used to discriminate eggs of O. viverrini, C. sinensis, and H. taichui, revealing that 58/105 (55.24%) positive samples of Opisthorchis-like eggs were O. viverrini eggs. There were no specific amplicons of 526 bp for H. taichui found in the study. The sensitivity of ITS2-PCR was 54% and 66.7% when EPG in feces was less than 100 and 200, respectively, whereas EPG in feces ≥ 200 showed 100% sensitivity (Table 2). Phylogenetic relationships among parasitic trematode families were generated based on 377 bp of the ITS2 region using RAxML (Figure 1). Topology of the ITS2 tree demonstrated all species groups in four families were supported with bootstrap value higher than 98%, and our samples were clustered within the O. viverrini group (bootstrap value = 100%). Regarding strong support of the bootstrap value at each branch, the cluster of each species in four families was less likely to exchange between branches representing the strictly conserved topology. No difference was found between O. viverrini in this study and others. Opisthorchis viverrini in this study collected from eastern Thailand was monophyletic, with others collected from different regions and countries including northeastern Thailand and Southeast Asian countries, such as Lao PDR, Myanmar, and Vietnam, as well as Russia.
Figure 1.
The randomized axelerated maximum likelihood tree of Opisthorchis viverrini and other trematodes based on 377 nucleotide sequences of ITS2 gene. Schistosoma japonicum was selected as an outgroup. Bootstrap values higher than 50% are labeled over branches (1,000 replicates). Isolates in this report are underlined and shown in boldface, accession number: MK886660-MK886663. The collecting site of O. viverrini is in parenthesis. Scale bar indicates nucleotide substitutions per site.
DISCUSSION
Opisthorchis viverrini infection remains a public health problem and a major risk factor for CCA16,17 in the Greater Mekong subregions, especially in north and northeast Thailand.18 In 2009, the first survey of intestinal parasitic infections in Chachoengsao Province, central Thailand, revealed that the incidence rate of O. viverrini infection was 21.6/100 person-years,19 which was relatively high compared with other provinces in the central area. As a result, prevention and control programs have been implemented in that study area. However, in 2013, the incidence rate was still as high as 21.4/100 person-years, which did not differ when compared with a related study reported in 2009.20 Thus, changing the habit of consuming uncooked fish is considered a difficult task to implement among local people. In this study, the area was Thung Phra Phloeng subdistrict, Sa Kaeo Province, an adjacent area to Chachoengsao Province. Sa Kaeo Province has been placed in the top list of having the highest incidence of liver cancer and CCA in eastern Thailand. Patients with liver cancer increased 56% from 1999 to 2003.11 The elderly are a high-risk group of acquiring O. viverrini infection in Sa Kaeo Province, and they also migrated from northeast Thailand where eating habits of uncooked freshwater fish have not changed. In 2004, using microscopic examination, the prevalence of O. viverrini infection totaled 47.9% in Sa Kaeo Province21 for which the true prevalence of O. viverrini has not yet been confirmed by molecular technique. Using microscopic examination, this study showed a lower prevalence of 8.4% Opisthorchis-like egg infection among villagers residing in Thung Phra Phloeng subdistrict, Sa Kaeo Province. The prevalence was lower than that found in Chachoengsao Province, central Thailand, which was recently reported as 16.8% in 2017.10
Discrimination of human liver fluke eggs and those of small intestinal flukes was introduced using PCR assays of the ITS2 region.13 A related study showed that the ITS2-PCR assay gave a sensitivity of 71.0%, with detection limits as low as 0.6–3 pg of Opisthorchis-like eggs.13 In this study, the ITS2-PCR-RFLP assay was used to differentiate O. viverrini eggs from those of C. sinensis, a human liver fluke reported in 2009,13 and H. taichui, a common small intestinal fluke coinfection with O. viverrini.22 The O. viverrini infection in Phra Phloeng community should be classified as very light infection considering that 93% (54/58) was lower than 100 EPG (Table 2) because the criterion for light infection was < 1,000 EPG. Thus, a modified DNA extraction method for light intensity of O. viverrini–like eggs (EPG) was developed in this study to increase the ability of breaking down the thick shell of eggs and improve DNA quality, in that additional proteinase K and 3-hour extension during the incubation period at 56°C were applied in between the standard protocol of DNA extraction. Using positive samples by the PBS-ECT method, the ITS2-PCR assay proved to be useful; however, successful PCR amplification was 55.2% (58/105). Interestingly, the reduction in successful detection was found to correlate with the intensity of eggs, where 100% was observed in 200–499 EPG and 46% missing detection was found in 1–99 EPG. Because of the very low intensity of eggs, insufficient DNA concentration could be obtained using the modified DNA extraction method, of which 44.8% (47/105) samples could not be amplified using the ITS2-PCR assay. Another reason could be specific primers used in PCR targeting the ITS2 region might be nonspecific to the ITS2 region of other small intestinal fluke eggs. Unfortunately, those of 47 negative ITS2-PCR samples were not further molecularly identified using other target genes, of which an existence of other small intestinal flukes could not be proved in the study area. Non-Opisthorchis eggs were likely to be C. sinensis and H. taichui because of the finding of mixed infections in closely adjacent district to this study area: Sanamchaikaet district, Chachoengsao Province.10,13 The intensity of Opisthorchis-like egg infection was estimated as the number of EPG of individual feces for which 100% of low intensity of Opisthorchis-like egg infection (EPG < 1,000) was observed among all age-groups. This could be the result of a control program using praziquantel treatment annually provided by local public health officers. The treatment aimed to reduce the intensity and severity of the infection, which constitutes one key factor to reduce progression of O. viverrini infection to CCA.
The main risk factor associated with O. viverrini infection in Thailand was reported and confirmed in several studies, that is, uncooked fish consumption, mainly koi pla (chopped fish salad).1,4,20,23 Other associated risks include male gender,24 age-group ≥ 60 years, and alcohol consumption. The higher prevalence of Opisthorchis-like egg infection among the elderly could have been related to their unchanged habits of uncooked fish consumption. However, our study could not show any significant difference between those who consumed uncooked fish dishes and those who did not. The highest prevalence of O. viverrini infection (15.3%) was still observed among individuals aged > 59 years and decreased prevalence in younger age-groups, which was similar to related studies conducted in other endemic areas of Thailand.20 In this study, using multivariate analysis, males were at 3.1 times higher risk of acquiring O. viverrini infection than females after adjusting for gender, age, and koi pla consumption. A related study reported that males were at 10 times higher risk than females to acquire O. viverrini infection25 because of their social behavior of consuming raw fish dishes.26,27 However, nonresponse questionnaires were over 70%, which might have resulted in bias when interpreting risk factors regarding consumption behaviors.
Presently, effective prevention and control programs of O. viverrini infection have focused on all school-aged groups toward health education together with providing collective consistent treatment to all infected individuals. Moreover, annual screenings of O. viverrini infection in the endemic areas have been conducted for primary prevention programs of liver fluke infection. A decline in the prevalence and incidence of O. viverrini infection as well as CCA could be reached under the effective strategies of these control programs.
Regarding the highly conserved ITS2 region, this gene region is unsuitable for predicting genetic relationships among O. viverrini. Divergence and genetic diversity of each isolate could not be solved and found. The use of variable gene regions such as cox1 and nad1 could be more advantageous to discriminate the genetic diversity to understand the distribution and transmission of O. viverrini in eastern Thailand. Interestingly, Buathong et al.10 found that the populations of O. viverrini obtained from three villages in central Thailand were monophyletic and agreed with the populations of O. viverrini along the Mekong River including Thailand, Lao PDR, and Cambodia based on nad1 sequences.28 Thus, the genetic structure of O. viverrini in Southeast Asia is homogeneous and tends to be monophyletic probably because of population migration within the region. Although nad1 was described as a powerful molecular maker for studying genetic relationships of O. viverrini,28 no significant differences of genetic diversity were observed within and among O. viverrini populations.10
In conclusion, the prevalence of Opisthorchis-like egg infection was 8.4%, of which 55.2% O. viverrini eggs were identified using the ITS2-PCR assay. Using EPG of feces, low-intensity infection (EPG < 1,000) was observed among all infected individuals. Primary prevention should be implemented to reduce incidence of infection in low-prevalence groups, such as age-group < 20, and to prevent them from acquiring the infection by consuming uncooked fish. In higher risk groups, men and older age-groups, infected cases should be treated as secondary prevention, and long-term plans should be suggested to implement prevention programs and reduce reinfection.
Acknowledgments:
We gratefully thank the local health volunteers and all participants from Thung Phra Phloeng subdistrict for their contributions.
REFERENCES
- 1.Suwannahitatorn P, Webster J, Riley S, Mungthin M, Donnelly CA, 2019. Uncooked fish consumption among those at risk of Opisthorchis viverrini infection in central Thailand. PLoS One 14: e0211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaewpitoon N, et al. 2015. Review and current status of Opisthorchis viverrini infection at the community level in Thailand. Asian Pac J Cancer Prev 16: 6825–6830. [DOI] [PubMed] [Google Scholar]
- 3.Sayasone S, et al. 2017. Efficacy and safety of praziquantel against light infections of Opisthorchis viverrini: a randomized parallel single-blind dose-ranging trial. Clin Infect Dis 64: 451–458. [DOI] [PubMed] [Google Scholar]
- 4.Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, 2008. Opisthorchiasis in Thailand: review and current status. World J Gastroenterol 14: 2297–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A, 2012. The tumorigenic liver fluke Opisthorchis viverrini-multiple pathways to cancer. Trends Parasitol 28: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ, 2011. Opisthorchiasis and opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop 120 (Suppl 1): S158–S168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamsa-ard S, Kamsa-ard S, Luvira V, Suwanrungruang K, Vatanasapt P, Wiangnon S, 2018. Risk factors for cholangiocarcinoma in Thailand: a systematic review and meta-analysis. Asian Pac J Cancer Prev 19: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogoch II, Sayasone S, Vonghachack Y, Meister I, Utzinger J, Odermatt P, Andrews JR, Keiser J, 2016. Diagnosis of Opisthorchis viverrini infection with handheld microscopy in Lao People’s Democratic Republic. Am J Trop Med Hyg 94: 158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato M, Thaenkham U, Dekumyoy P, Waikagul J, 2009. Discrimination of O. viverrini, C. sinensis, H. pumilio and H. taichui using nuclear DNA-based PCR targeting ribosomal DNA ITS regions. Acta Trop 109: 81–83. [DOI] [PubMed] [Google Scholar]
- 10.Buathong S, Leelayoova S, Mungthin M, Ruang-Areerate T, Naaglor T, Suwannahitatorn P, Piyaraj P, Taamasri P, Tan-Ariya P, 2017. Molecular discrimination of Opisthorchis-like eggs from residents in a rural community of central Thailand. PLoS Negl Trop Dis 11: e0006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amon JJ, Nedsuwan S, Chantra S, Bell BP, Dowell SF, Olsen SJ, Wasley A, 2005. Trends in liver cancer, Sa Kaeo province Thailand. Asian Pac J Cancer Prev 6: 382–386. [PubMed] [Google Scholar]
- 12.Maleewong W, Intapan P, Wongwajana S, Sitthithaworn P, Pipitgool V, Wongkham C, Daenseegaew W, 1992. Prevalence and intensity of Opisthorchis viverrini in rural community near the Mekong River on the Thai-Laos border in northeast Thailand. J Med Assoc Thai 75: 231–235. [PubMed] [Google Scholar]
- 13.Traub RJ, Macaranas J, Mungthin M, Leelayoova S, Cribb T, Murrell KD, Thompson RC, 2009. A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand. PLoS Negl Trop Dis 3: e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamatakis A, 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 15.Silvestro D, Michalak I, 2012. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12: 335–337. [Google Scholar]
- 16.Jongsuksuntigul P, Imsomboon T, 2003. Opisthorchiasis control in Thailand. Acta Trop 88: 229–232. [DOI] [PubMed] [Google Scholar]
- 17.Sripa B, Pairojkul C, 2008. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 24: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews RH, Sithithaworn P, Petney TN, 2008. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol 24: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangsin R, Mungthin M, Taamasri P, Mongklon S, Aimpun P, Naaglor T, Leelayoova S, 2009. Incidence and risk factors of Opisthorchis viverrini infections in a rural community in Thailand. Am J Trop Med Hyg 81: 152–155. [PubMed] [Google Scholar]
- 20.Suwannahitatorn P, Klomjit S, Naaglor T, Taamasri P, Rangsin R, Leelayoova S, Mungthin M, 2013. A follow-up study of Opisthorchis viverrini infection after the implementation of control program in a rural community, central Thailand. Parasit Vectors 6: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nontasut P, Muennoo C, Sa-nguankiat S, Fongsri S, Vichit A, 2005. Prevalence of strongyloides in Northern Thailand and treatment with ivermectin vs albendazole. Southeast Asian J Trop Med Public Health 36: 442–444. [PubMed] [Google Scholar]
- 22.Radomyos B, Wongsaroj T, Wilairatana P, Radomyos P, Praevanich R, Meesomboon V, Jongsuksuntikul P, 1998. Opisthorchiasis and intestinal fluke infections in northern Thailand. Southeast Asian J Trop Med Public Health 29: 123–127. [PubMed] [Google Scholar]
- 23.Yoon HJ, Ki M, Eom K, Yong TS, Chai JY, Min DY, Rim HJ, Sohn WM, Insisiengmay B, Phommasack B, 2014. Risk factors for Opisthorchis viverrini and minute intestinal fluke infections in Lao PDR, 2009–2011. Am J Trop Med Hyg 91: 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaewnongiew K, Singthong S, Kutchamart S, Tangsawad S, Promthet S, Sailugkum S, Wongba N, 2014. Prevalence and risk factors for Opisthorchis viverrini infections in upper northeast Thailand. Asian Pac J Cancer Prev 15: 6609–6612. [DOI] [PubMed] [Google Scholar]
- 25.Chaiputcha K, Promthet S, Bradshaw P, 2015. Prevalence and risk factors for infection by Opisthorchis viverrini in an urban area of Mahasarakham province, northeast Thailand. Asian Pac J Cancer Prev 16: 4173–4176. [DOI] [PubMed] [Google Scholar]
- 26.Kaewpitoon SJ, Rujirakul R, Kaewpitoon N, 2012. Prevalence of Opisthorchis viverrini infection in Nakhon Ratchasima province, northeast Thailand. Asian Pac J Cancer Prev 13: 5245–5249. [DOI] [PubMed] [Google Scholar]
- 27.Kaewpitoon SJ, Rujirakul R, Ueng-Arporn N, Matrakool L, Namwichaisiriku N, Churproong S, Wongkaewpothong P, Nimkuntod P, Sripa B, Kaewpitoon N, 2012. Community-based cross-sectional study of carcinogenic human liver fluke in elderly from Surin province, Thailand. Asian Pac J Cancer Prev 13: 4285–4288. [DOI] [PubMed] [Google Scholar]
- 28.Kaewkes S, Elkins DB, Sithithaworn P, Haswell-Elkins MR, 1991. Comparative studies on the morphology of the eggs of Opisthorchis viverrini and lecithodendriid trematodes. Southeast Asian J Trop Med Public Health 22: 623–630. [PubMed] [Google Scholar]