Abstract
In mammals, more than 2000 genes are specifically or abundantly expressed in testis, but gene knockout studies revealed several are not individually essential for male fertility. Tesmin (Metallothionein-like 5; Mtl5) was originally reported as a testis-specific transcript that encodes a member of the cysteine-rich motif containing metallothionein family. Later studies showed that Tesmin has two splicing variants and both are specifically expressed in male and female germ cells. Herein, we clarified that the long (Tesmin-L) and short (Tesmin-S) transcript forms start expressing from spermatogonia and the spermatocyte stage, respectively, in testis. Furthermore, while Tesmin-deficient female mice are fertile, male mice are infertile due to arrested spermatogenesis at the pachytene stage. We were able to rescue the infertility with a Tesmin-L transgene, where we concluded that TESMIN-L is critical for meiotic completion in spermatogenesis and indispensable for male fertility.
Keywords: spermatogenesis, meiotic arrest, male infertility, knockout
TESMIN protein, a member of metallothionein family, is essential for mouse spermatogenesis, especially in the meiotic stage.
Introduction
Spermatogenesis is a complicated process involving mitotically dividing spermatogonial cells, spermatocytes dividing meiotically, and spermatid maturation into spermatozoa. While spermatogonial stem cells reside at the basement of the seminiferous tubules and continuously produce progeny cells to maintain the stem cell pool, some of the progeny cells start differentiation into primary spermatocyte (4C). The primary spermatocytes undergo meiosis I to form secondary spermatocytes (2C) and meiosis II to form spermatids (1C). The spermatids elongate their cilia and drastically change their morphology to form mature spermatozoa, which are released into the lumen of seminiferous tubules. Each of these events is supported by stage- and cell-specific gene expression.
In mammals, gene-expression analysis estimates that more than 2300 genes are expressed predominantly in the male germ line [1], and these testis-enriched genes are believed to play critical roles for male fertility. While knockout (KO) mouse studies have revealed essential genes for male fertility, recent CRISPR/Cas9-mediated KO studies suggested that many of the testis-enriched genes are not individually essential [2]. One can posit that paralogous genes may complement the testis-specific gene functions. However, it should be noted that many genes shown essential for male fertility are simultaneously expressed with their paralogues during spermatogenesis (e.g., Ccna2/Ccna1 [3], Hspa1a/Hspa2 [4]). Thus, the KO approach remains suitable to determine whether the target gene has an essential role in vivo.
Metallothioneins (MTs) belong to a group of low-molecular-weight cysteine-rich proteins, which bind to heavy metal ions via their cysteine-rich (CXC) motif [5, 6]. MTs fall into four subgroups (MT1-4), Mt1 and Mt2 are ubiquitously expressed in mice, Mt3 is expressed mostly in the brain, and Mt4 was detected in stratified squamous epithelial cells. All are located on a single chromosome (chromosome 8 in mice and chromosome 11 in human) [7]. Additionally, Tesmin was identified as a fifth member, which is strongly expressed in testis [8]. It was discovered that Tesmin encodes two variants with the longer variant having an additional 180 amino acids at the N-terminus [9]. We refer to the long form as TESMIN-L and the short form as TESMIN-S, respectively. Both TESMIN-variant proteins contain the CXC-domain like cysteine-rich domains and a hinge region as described in previous reports [9, 10]. Since TESMIN-L had been reported to change its localization in response to metal stress in vivo and in vitro [9, 11], TESMIN proteins are assumed to chelate metal ions like other MTs. However, TESMIN-L and TESMIN-S have some differences compared with other members of the MT family. For example, TESMIN proteins are relatively larger than other family members: TESMIN-L consists of 475 amino acids and TESMIN-S consists of 295 amino acids, while MT1–4 consists of 61, 61, 68, and 62 amino acids, respectively. Moreover, Tesmin is located on chromosome 19 in mice, while the other Mt1–4 genes cluster on chromosome 8.
TESMIN-L has also been reported to translocate from the cytoplasm to the nucleus before meiotic division even without metal ion stress [9, 11]. Furthermore, as described in a previous study, meiosis-stage-specific expression of Tesmin suggests a germ-cell-specific function independent of the metal ion stress response [12]. Despite these findings, it remains unclear whether TESMIN proteins play critical roles in meiosis and which isoform (TESMIN-L and TESMIN-S) works in testis. In the present study, we generated Tesmin KO mice and demonstrated its essential role in the completion of meiosis in male germ cells.
Materials and methods
Animals
All animal experiments were conducted in accordance with the guidelines of “Animal experiment rules” established by the Research Institute for Microbial Diseases, Osaka University, and were approved by the Animal Care and Use Committee of the Research Institute for Microbial Diseases, Osaka University. B6D2F1 and ICR mice were purchased from CLEA (Tokyo, Japan) and SLC (Shizuoka, Japan).
RT-PCR
Mouse cDNA was prepared from various tissues of adult ICR, and testes from 0 to 35 days after birth. RT-PCR was performed using 10 ng of cDNA with the following forward and reverse primers: 5′-CCAGCAGGGCTAGGGATAGA-3′ and 5′-CATGGGGCTGGAGTCTTTCTTCC-3′ for Tesmin-S, 5′-ATGGAGGACGCGCTGCTCGG-3′ and 5′-CATGGGGCTGGAGTCTTTCTTCC-3′ for Tesmin-L, 5′-AAGTGTGACGTTGACATCCG-3′ and 5′-GATCCACATCTGCTGGAAGG-3′ for the Actb gene. The amplification conditions were 1 min at 94 °C, followed by 40 cycles (for Tesmin-S) or 35 cycles (for Tesmin-L, Actb) of 94 °C for 30 s, 65 °C for 30 s, and 72 °C for 30 s, with a final 1-min extension at 72 °C.
Plasmid construction
The cDNA encoding the mouse Flag-Tesmin-S and Flag-Tesmin-L variants were amplified by PCR using wild-type (WT) testis cDNA as a template. The PCR primer sets used were 5′-AAGAATTCGCCGCCATGGACTACAAAGACGATGACGACAAGGGCGGCGTGATTTGTCAGCTGAAAGG-3′ and 5′-AAGTCGACCTACTCAATTTTCAGCCCCTTGGACTTG-3′ for Flag-Tesmin-S, and 5′-AAGAATTCGCCGCCATGGACTACAAAGACGATGACGACAAGGGCGGCGAGGACGCGCTGCTCGG-3′ and 5′-AAGTCGACCTACTCAATTTTCAGCCCCTTGGACTTG-3′ for Flag-Tesmin-L. The EcoRI and SalI sites included in the PCR primers were used to introduce the amplified cDNA into a pCAG ubiquitous expression vector carrying chicken Actb promoter with a cytomegalovirus enhancer [13, 14]. We named them pCAG-Flag-Tesmin-S and pCAG-Flag-Tesmin-L, respectively.
Antibodies
Rabbit anti-mouse TESMIN-L polyclonal antiserum was produced by immunization with mouse TESMIN-L polypeptide (AYLGATEPGEPLLRALS) (Sigma-Aldrich, MO, USA, IB 1:200). Anti-FLAG antibody (M2, #F1804) from Sigma-Aldrich, anti-CALNEXIN antibody (#sc-11397, IB 1:1000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA), anti-SYCP polyclonal antibody (#ab15092) from Abcam (Cambridge, UK), anti-phospho-histone H2A.X (Ser139) antibody (#05-636) from Merck Millipore (Darmstadt, Germany) were used. Secondary polyclonal antibodies were used as follows: rabbit anti-mouse HRP 1:1000 (Dako, Hamburg, Germany), goat anti-rabbit HRP 1:2000 (Dako).
Cell culture and transfection
Cos7 cells were maintained with Dulbeccos modified Eagles medium, 100 U/ml of penicillin, 0.1 mg/ml of streptomycin sulfate, and 10% fetal bovine serum under 5% CO2 at 37 °C. For transfection, the cells were transfected at approximately 70% confluence with 1 μg of pCAG-Flag-Tesmin-S or pCAG-Flag-Tesmin-L using Lipofectamine LTX & PLUS technology (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Immunofluorescence staining of Cos7
Transfected Cos7 cells were seeded onto coverslips in 6-well plates. After 2 days, cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min. After washes with PBS, cells were permeabilized with 0.2% Triton X-100 for 7 min and then blocked with 10% goat serum in PBS. Then, coverslips were incubated with anti-FLAG (1:200) antibody for overnight at 4 °C. After incubation with Alexa Fluor 488 conjugated secondary antibody (1:200) (Thermo Fisher Scientific, MA, USA) at room temperature for 1 h, cells were counterstained with Hoechst33342 (Life Technologies) and mounted on slides.
Guide RNA design and cloning
Tesmin-specific guide sequences (sgRNAs) were designed and inserted into the pX330 plasmid (#42230, Addgene, Cambridge, MA, USA). Validation of DNA cleavage activity of these plasmids was performed as described previously [15, 16].
Generation of Tesmin KO mice and transgenic mice
B6D2F1 superovulated females were mated with B6D2F1 males and then fertilized eggs were collected. Circular pX330 plasmids were injected into one of the pronuclei at 5 ng/μl. Surviving zygotes were cultured in potassium simplex optimization medium (KSOM) medium for 1 day. Developing two-cell embryos were transferred into the oviducts of pseudo-pregnant foster mice. KO mice were genotyped by PCR with the primers 5′-CTCAGAGGTAGGAAATTGAG-3′ and 5′-CTCAAGCGCTCATTGACCTG-3′. The Tesmin-L transgenic mouse line was established by pronuclear injection of the CAG-Tesmin-L transgene. The cDNA encoding the mouse Tesmin-L gene was amplified by PCR with the primers 5′-AAGAATTCGCCGCCATGGAGGACGCGCTGCTCGG-3′ and 5′-AAGTCGACCTACTCAATTTTCAGCCCCTTGGACTTG-3′ using WT testis cDNA as a template. The EcoRI and SalI sites included in the PCR primers were used to introduce the amplified Tesmin-L cDNA into a pCAG expression vector described above. The transgene fragment (3.7 kb) was digested with ClaI, PacI, and ScaI and purified by gel electrophoresis. Transgenic mice were genotyped by PCR with primers 5′-GCCTTCTTCTTTTTCCTACAGC-3′ and 5′-AGCAGGTCCAGAGGGAAAGGCAGC-3′. Frozen spermatozoa from Tesmin disrupted males (B6D2-Mtl5<em1Osb>, RBRC#09987, CARD#2542) and Tesmin-L transgenic males (B6D2-Mtl5<em1Osb> Tg(CAG-Mtl5)1Osb, RBRC#10131, CARD#2604) will be available through RIKEN BRC (http://en.brc.riken.jp/index.shtml) and CARD R-BASE (http://cardb.cc.kumamoto-u.ac.jp/transgenic/).
Assessment of the fertility of TESMIN deficient mice
Sexually mature male mice of genotypes Tesmin +/em1 and KO were caged with B6D2F1 female mice (at least 2 months old) for 3 months, and the number of pups in each cage was counted within a week of birth.
Immunoblotting
Protein extracts from whole testis were subjected to 10% polyacrylamide gel electrophoresis and blotted onto a polyvinylidene fluoride membrane. Membranes were blocked in 10% skim milk powder in tris-buffered saline with Tween20 and probed with primary (overnight at 4 °C) and secondary (1 h at RT) antibodies in blocking solution. Chemiluminescent signals were detected using ImageQuant LAS 4000 mini (GE Healthcare, Munich, Germany) after incubation of the membrane with ECL prime western blotting detection reagent (GE Healthcare).
Histological analysis
Testes were fixed in 4% paraformaldehyde in PBS and embedded in Technovit 8100 resin (Heraeus-Kulzer, Wehrheim, Germany) according to the manufacturer’s instructions. Sections were cut at 5 μm and stained with periodic acid-Schiff (PAS) and then counterstained with Mayers hematoxylin solution (Wako, Japan).
Immunohistochemistry
The spreading procedure of testicular cells was carried out as described [17]. In brief, hypotonic treated cells were spread and fixed on slides with 1% paraformaldehyde and 0.1% Triton X-100. Spread samples were blocked with 10% goat serum in PBS and then incubated with anti-SYCP3 (1:500) and anti-phospho-histone H2A.X (1:500) antibodies overnight at 4 °C in blocking solution. After incubation with Alexa Fluor 488 or Alexa Fluor 546-conjugated secondary antibody (1:250) at room temperature for 1 h, samples are counterstained with Hoechst33342, and mounted with Immu-Mount (Thermo Fisher Scientific).
Results
Expression of Tesmin isoforms
Tesmin was originally identified as a gene encoding a testis-specific 30-kDa protein in mice [8] but later shown to have another splicing variant [9]. Because Tesmin-L and Tesmin-S transcripts utilize different starting exons but share common exons 2–9, we designed specific primers and performed RT-PCR with various adult tissues (Figure 1a). While the Tesmin-S signal was weak but specifically observed in testis, the Tesmin-L signal was strongly detected in testis with weak signals in spleen, liver, and kidney (Figure 1b). As shown in Figure 1b, we further detected two bands of Tesmin-S by RT-PCR. Sequencing analysis revealed that the upper band includes an additional 69 bp outside the coding region (Figure S1a). When we assessed the expression pattern of Tesmin variants during spermatogenesis (Figure 1c), Tesmin-L appears in a 6-day-old testis, which contains only spermatogonia and somatic cells, whereas Tesmin-S appears in a 18-day-old testis in which spermatocytes finish meiosis and haploid spermatids appear.
Figure 1.
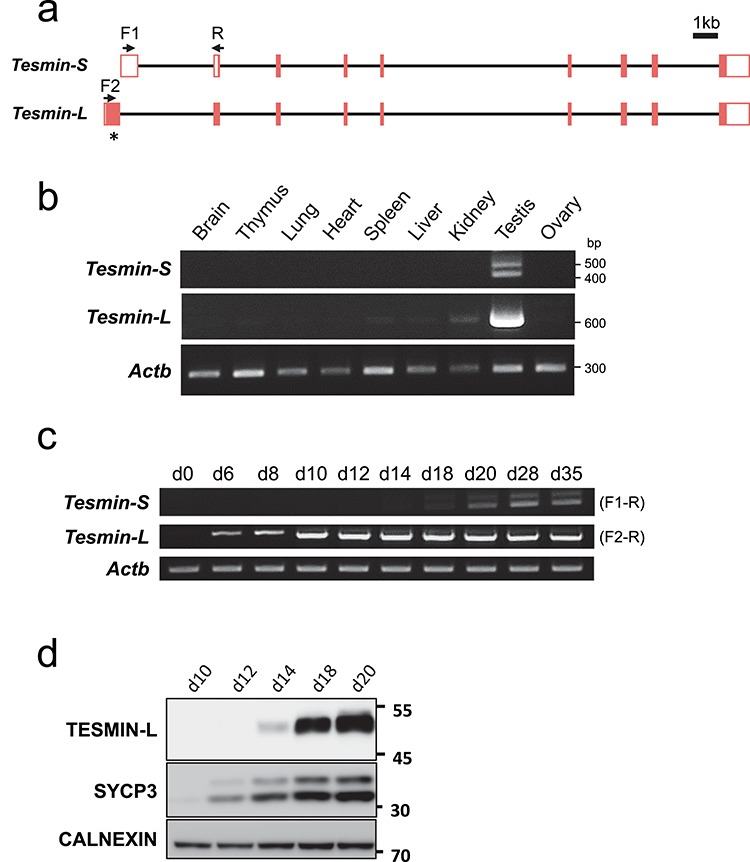
Expression profile of Tesmin isoforms. (a) Schematic representation of two splicing variants of mouse Tesmin. Filled box: protein-coding sequence, white box: untranslated sequences, arrows: primer binding sites. * shows the region that the antibody was raised against. (b) RT-PCR of Tesmin using cDNAs obtained from various tissues. Actb as control. (c) Expression of each variant analyzed with RT-PCR using testicular cDNA obtained from 0-, 6-, 8-, 10-, 12-, 14-, 18-, 20-, 28-, and 35-day-old mice. Actb as control. Primer sets used for each Tesmin-S and Tesmin-L are indicated at right, whose annealing sites are shown in Figure 1a. (d) TESMIN-L expression in spermatogenesis detected by western blotting. SYCP3 (a meiosis marker) and CALNEXIN (ubiquitously expressed marker) were used as controls.
Next, to confirm the presence of TESMIN protein, we attempted to produce anti-TESMIN antibodies. One TESMIN-L specific antibody worked and detected a band near 50 kD, which is the expected size of TESMIN-L. Western blot analysis also detects TESMIN-L expression in a 14-day-old testis when differentiating germ cells enter the early pachytene stage of meiosis (Figure 1d). Since our anti-TESMIN-L antibody did not work for immunofluorescence in testis, we stained FLAG-tagged TESMIN-L and TESMIN-S transiently transfected into the Cos7 cell line with anti-FLAG antibody to know the localization of each variant (Figure S1b). Interestingly, TESMIN-L localized to the cytoplasm but TESMIN-S localized in close proximity to the nucleus, suggesting different roles between TESMIN-L and TESMIN-S in cells.
CRISPR/Cas9-mediated generation of Tesmin KO mice
To analyze the physiological function of TESMIN proteins in vivo, we generated Tesmin KO mice using CRISPR/Cas9-mediated genome-editing in mouse zygotes. To disrupt both Tesmin-L and Tesmin-S genes, we designed a single guide RNA (sgRNA) against the common third exon (Figure 2a). We microinjected the sgRNA/Cas9 expressing plasmid into 105 zygotes and obtained eight newborn pups. By PCR amplification of the target region and subsequent sequence analysis, we confirmed three pups carrying mutations. While two of the three mice had indel mutations (+/i3 and +/i5d3), the third had a 316 bp-deletion (hereinafter, referred to as “em1” mutation) that includes the entire exon 3 (Figure 2b). Because exon 3 consists of 121 nucleotides, the lack of exon 3 results in a frameshift mutation in both Tesmin-L and Tesmin-S transcripts. We, therefore, bred this em1 mutant mouse line (B6D2-Mtl5<em1Osb>) to obtain Tesmin KO mice. Tesmin KO mice were born at the expected Mendelian ratio (+/+: 27, +/em1: 62, em1/em1: 29; n = 118), and no overt abnormality was observed in both females and males throughout their life. We confirmed the disappearance of TESMIN-L protein in the Tesmin KO mouse by western blotting (Figure 2c). Since an antibody against TESMIN-S was not available, we performed RT-PCR with Tesmin-S specific primers and confirmed the frameshift mutation (Figure 2d). These results indicate that both the TESMIN-L and TESMIN-S proteins are deleted in the Tesmin KO mice.
Figure 2.
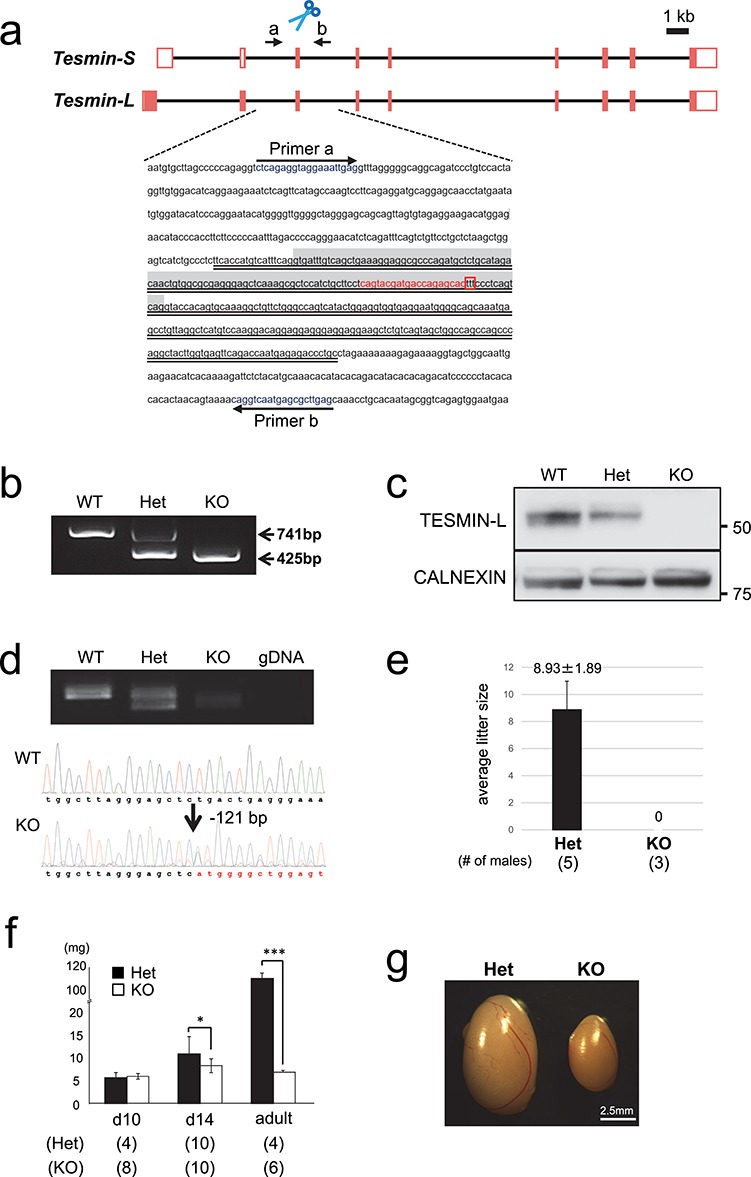
TESMIN deficiency leads to male infertility with spermatogenesis defects. (a) Targeting scheme of the Tesmin KO. Tesmin consists of nine exons. Exon 3, which is shared with both splicing variants, was targeted to generate the Tesmin KO mice. Sequence highlighted in gray indicates exon 3. Red characters and red box indicate the target of sgRNA and PAM sequence, respectively. Deleted region is underlined with a double line. Arrows indicate primers. (b) Genotyping by PCR. The 316-bp sequence was deleted in em1-mutant mice. (c) Tesmin-L expression in testis detected by western blotting. CALNEXIN (ubiquitously expressed marker) was used as a control. (d) Expression of Tesmin analyzed with RT-PCR using testicular cDNA obtained from Tesmin +/+, +/em1 and KO mice (top panel). Although a faint band was observed in Tesmin KO testis, the sequence of PCR products showed that Tesmin KO mouse had a 121-bp deletion that causes a frameshift (bottom panel). Red characters indicate the sequence after deletion. (e) Number of pups that were obtained by crossing Tesmin +/em1 (n = 5) and KO (n = 3) male mice with WT female partners. (f) Comparison of the testicular weight among Tesmin +/em1 and 10- and 14-day-old KO mice and adults. (g) Comparison of the gross morphology of the testis from Tesmin +/em1 and KO male adult mice (21 weeks old).
Fertility of Tesmin KO male mice
To evaluate the effect of TESMIN deficiency on fecundity, Tesmin KO female (n = 10) and male (n = 3) mice were mated with WT males or females for 3 months, respectively. Whereas the Tesmin KO female mice were fertile (litter size of +/em1: 7.9 ± 2.0, number of litters n = 15, litter size of em1/em1: 7.1 ± 2.7, number of litters n = 15), the Tesmin KO male mice were infertile despite showing normal mating behavior (litter size of 0, Figure 2e). To define the causes of male infertility, we measured testicular weights in Tesmin +/em1 and KO mice. Although the testicular weight of Tesmin KO mice was comparable with that of Tesmin +/em1 10-day-old mice, a significant decrease appeared in testis weight in Tesmin 14-day-old KO mice and remained smaller throughout their life (Figure 2f and g). These results indicated that the lack of TESMIN impairs testis function.
Meiotic defect in Tesmin KO male mice
To define the cause of the smaller testis in Tesmin KO mice, we observed testicular sections stained with hematoxylin and PAS, which detect nuclei and carbohydrate chains, respectively (Figure 3a). There were no significant differences between Tesmin KO and +/em1 testis cross sections in 10- and 14-day-old testes, respectively (Figure 3a). Sections from 5-week-old Tesmin KO testis showed apparent abnormalities compared with that of Tesmin +/em1 mice. Instead of three layers seen in control sections, only two layers of germ cells were observed at the periphery at 5 weeks in KO seminiferous tubules with a few cells beyond the meiotic stage (Figures 3a and S2). These results indicated that spermatogenesis was predominately arrested at an early stage before the appearance of haploid spermatogenic cells. Due to an apparent defect in meiosis in the KO, we focused on the meiotic events and performed immunofluorescence staining of synaptonemal complex protein 3 (SYCP3) and γ-H2AX, a histone variant (Figure 3b). During meiotic prophase I, SYCP3 protein begins to assemble along each sister-chromatid pair at leptonema to form the axial elements, which appears as short filaments dispersed in the nucleus. Then, during zygotene, these filaments begin to associate in pairs to form thicker filaments, called the lateral elements of synaptonemal complex [18, 19]. Meanwhile, γ-H2AX, which is a marker of the response to DNA double-strand breaks, localizes throughout the entire nucleus during leptonema and early zygonema when programmed double-strand breaks are being generated by the Spo11 endonuclease [19]. Thereafter, it accumulates in the chromatin of the XY body from late zygotene until diplotene [18]. We prepared meiotic nuclear spreads from heterozygous and KO males and quantified zygotene, pachytene, and diplotene spermatocytes based on SYCP3 and γ-H2AX staining patterns according to published reports [20, 21]. In heterozygous, the major population (~80%) of selected spermatocytes showed a γ-H2AX foci in the XY body, which is the typical feature of late-pachytene to diplotene spermatocytes (Figure 3b and c). This distribution of spermatocytes among different meiotic stages was consistent with that of WT mice in a previous report [22]. However, in the majority of Tesmin KO spermatocytes, the staining pattern of γ-H2AX was not restricted to the XY body in fully synapsed chromosomes, which is found in zygotene and early-pachytene spermatocytes. These data suggest that meiosis was arrested before late-pachytene stage in the absence of Tesmin.
Figure 3.
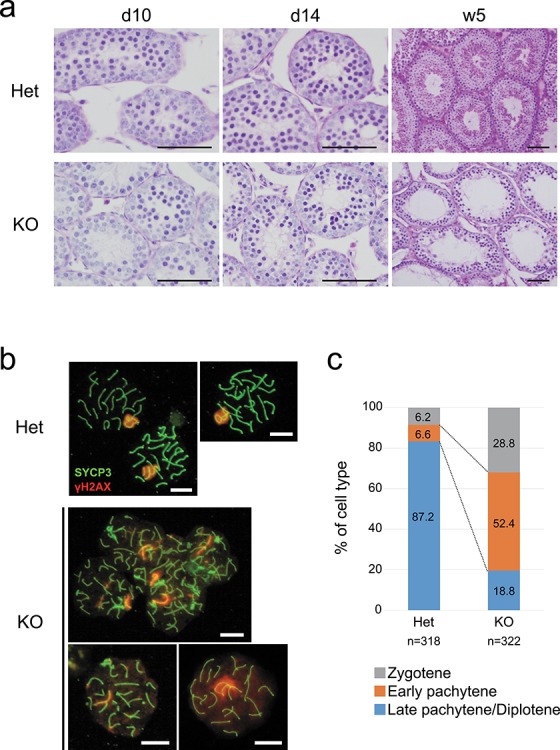
Spermatogenesis arrest occurred at meiosis. (a) PAS–hematoxylin staining of the testis from Tesmin +/em1 and 10-, 14-day-old, and 5-week-old KO mice . Scale bars: 50 μm. (b) Immunostaining of meiotic spreads from Tesmin +/em1 and KO spermatocytes for the synaptonemal complex component SYCP3 (green) to identify chromosome axes, and γH2AX (red) as a marker for DNA damage. Three spermatocytes from heterozygous are in late-pachytene stage. Top and bottom left spermatocytes of KO are categorized as early pachytene stage, and bottom right is categorized as late pachytene stage. Scale bars: 10 μm. (c) Percentage of spermatocytes from Tesmin +/em1 and KO male mice that are categorized as late zygotene, early pachytene and late pachytene by the feature of SYCP3 and γH2AX staining. Three mice (over 5 weeks old) are used for each genotype and more than 50 nuclei were observed from one mouse. The numbers in bar graph indicate the ratio (%) of each category. P-values of each categories are; zygotene: 0.0373, early pachytene: 0.0033, and late pachytene/diplotene: 0.0002, respectively. Welch t test was used for statistical analysis.
Transgenic rescue of Tesmin KO mice
To exclude the possibility that the infertility phenotype was caused by an off-target effect from CRISPR/Cas9 cleavage, or an aberrant genetic modification near the Tesmin locus, we performed a rescue experiment by generating transgenic mouse lines expressing Tesmin-L driven by the CAG promoter on a Tesmin KO background (Figure 4a). CAG promoter is a strong ubiquitous promoter frequently used to drive high levels of gene expression [13]. From pronuclear injection of 88 zygotes, we generated five founder lines. Western blotting analysis confirmed the expression of the transgene in these mice (Figure 4b). In Tesmin KO mice carrying the Tesmin-L transgene, the testicular weight (99.3 ± 9.8 mg) and spermatogenesis were comparable with the control Tesmin +/em1 mice (Figure 4c and d). In addition, the infertile phenotype was rescued (litter size of 7.8 ± 2.5, number of litters n = 21). These data that indicate infertility in Tesmin KO mice are due to the absence of TESMIN protein.
Figure 4.
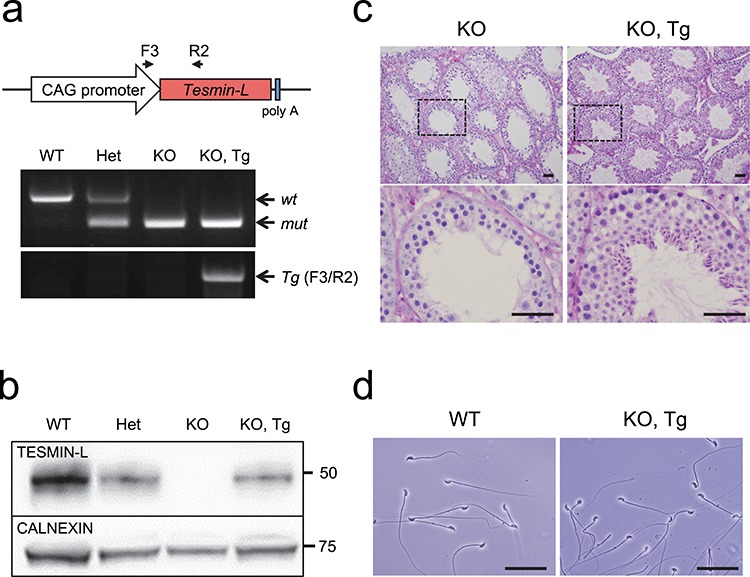
The sterile phenotype of Tesmin KO male mice was rescued by a Tesmin-L transgene. (a) Upper: schematic representation of the Tesmin-L transgene. Black arrows: primer annealing sites. Lower: PCR genotyping to check the Tesmin mutation and transgene. The Tesmin mutation was checked with primer set a/b described in Figure 2a and the Tesmin-L transgene was checked with primer set F3/R2. (b) TESMIN-L expression in Tesmin +/+, +/em1, KO and KO mice carrying the Tesmin-L transgene was detected by western blotting. CALNEXIN was used as a control. (c) PAS–hematoxylin staining of the testis from Tesmin KO and KO mice expressing the Tesmin-L transgene. Scale bars: 50 μm. (d) Cauda epididymal spermatozoa from Tesmin +/+ and KO mice. Scale bars: 50 μm.
Discussion
The study of MTs stretches back decades when they were first identified in the late 1950s [23]. MTs belong to the group of intracellular cysteine-rich, metal-binding proteins that have been found in bacteria, plants, invertebrates, and vertebrates [24]. Mammalian MTs are involved in diverse intracellular functions, with their role in detoxification of heavy metals and maintenance of essential metal ion homeostasis having been mostly investigated. However, TESMIN, which was identified as a MT-like protein (also known as Mtl5) and abundantly expressed in testes had not been studied well. In our experiments using KO mice, we demonstrated that TESMIN is an essential protein for meiotic progression in male germ cells.
Previous studies revealed two Tesmin splicing variants, named Tesmin-S and Tesmin-L in this paper [8, 9]. In this study, we confirmed that Tesmin-S and Tesmin-L encoding mRNAs that begin expression before and after meiosis, respectively and their localization in Cos7 cells were completely different. These results are consistent with the previous report, which claimed that TESMIN localizes in the cytoplasm of spermatocytes and then begins to localize on the nuclear membrane just under the acrosome vesicle of elongated spermatids [9]. It allows us to speculate that the splicing variants of Tesmin may have distinct roles in spermatogenesis. However, we demonstrated that the Tesmin-L transgene alone could rescue the meiotic defect and male fertility in Tesmin KO mice, suggesting that TESMIN-S is not essential for spermatogenesis.
A previous report showed that TESMIN-L localizes to the cytoplasm until the pachytene stage of meiosis then moves into the nucleus just before meiotic division [9, 11]. Our immunofluorescence staining of FLAG-TESMIN-L in Cos7 cells showed TESMIN-L localization in the cytoplasm, which is consistent with the first localization in testis. From immunofluorescence staining of meiotic markers SYCP3 and γ-H2AX in meiotic nuclear spreads, we found spermatogenesis arrests at the early pachytene stage in the majority of spermatocytes in Tesmin KO testis. The timing of the arrest in spermatogenesis corresponds to the change of Tesmin-L localization from the cytoplasm to nucleus, where TESMIN-L is thought to play an important role for meiosis in cytoplasm, and then moves into nucleus.
Previously, a similar phenotype, spermatogenesis arrest at the early pachytene stage with abnormal localization of γ-H2AX, has been reported in several mice lines deficient in proteins related to chromosome-structure during prophase I [25–27]. Based on these reports, TESMIN-L may be involved in such specific events in the nucleus at meiosis-I, but it could be indirect because of its cytoplasmic localization. Moreover, although a great majority of Tesmin KO spermatocytes are arrested at the early pachytene stage, a small fraction of cells complete meiosis and cease development at the metaphase/anaphase or haploid stage (Figure S2). To address these questions, more precise assessment of the arrested stages by immunofluorescence staining with other meiotic markers such as RAD51 and SYCP1 would be beneficial.
Tesmin was originally identified as an MT family-like gene because of its metal-binding, cysteine-rich domain, and early studies showed that metal ion-treated mice translocated TESMIN from the cytoplasm to the nucleus during meiosis [8, 11]. These results indicate that TESMIN responds to metal ions, like other MT genes; however, there are some differences between canonical MTs and TESMIN, as discussed previously [9]. TESMIN is ten times larger than other MT family proteins, and its cysteine composition is only 7.8% compared to 30% in other MT proteins (Figure S3). This suggests that the non-CXC regions of TESMIN might have different roles from other MTs. Furthermore, the metal-responsive element (MRE), a DNA sequence located upstream of MT family genes that are activated by the binding of the metal-responsive transcription factor (MTF-1), is not present upstream of Tesmin [9]. MTF-1 is known to be translocated from the cytoplasm into the nucleus upon heavy metal exposure or stress stimuli and bind to the MRE [28, 29]. The translocation of TESMIN in a similar manner to MTF-1 upon metal exposure suggests it may have a similar function during meiosis. Notably, with the absence of an MRE, Tesmin expression would be regulated by meiosis-specific transcription factors rather than in a metal-dependent manner.
The CXC in TESMIN are also present in LIN54, which is an essential cell cycle regulator. The CXC domains form a larger domain within LIN54 called the CXC-hinge-CXC (CHC) domain that is conserved in many LIN54 orthologs in various species, such as plants, invertebrates, and mammals [30–32]. Previous reports showed that LIN54 binds to the promoters of mitotic genes, such as cdc2 through the CHC domain and that LIN54 depletion through RNAi arrested the cell cycle [33, 34]. In addition, LIN54 has been assumed to change its subcellular localization in a cell-cycle-dependent manner [35]. Moreover, in Drosophila, mutation of a member of the CXC-domain family, tombola (tomb), which is expressed specifically in testis, fails to express Cyclin B and shows meiotic arrest in the male germline [31]. These reports allowed us to anticipate that TESMIN might have a similar function to LIN54 and tomb such as cell-cycle regulation in the nucleus at the end of meiosis during spermatogenesis.
Here, we demonstrated that TESMIN is essential for the completion of meiosis in male germ cells. Further studies on TESMIN will elucidate the mechanism of meiosis in male germ cells and shed light on the study of male infertility. We would expect that Tesmin KO mice would provide a clue to elucidate how TESMIN relates to meiosis in future research.
Supplementary Material
Acknowledgement
The authors would like to thank Naoko Nagasawa for technical assistance.
Conflicts of interest
The authors declare no competing financial interests.
Author Contributions
AO, AI, and MI designed the research; AO, AI, YF and SO performed the research; AO, AI, YF, JMC, and MI analyzed the data, and AO, JMC, and MI wrote the paper.
References
- [1]. Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA 2003; 100:12201–12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Miyata H, Castaneda JM, Fujihara Y, Yu Z, Archambeault DR, Isotani A, Kiyozumi D, Kriseman ML, Mashiko D, Matsumura T, Matzuk RM, Mori M et al. Genome engineering uncovers 54 evolutionarily conserved and testis-enriched genes that are not required for male fertility in mice. Proc Natl Acad Sci USA 2016; 113:7704–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet 1998; 20:377–380. [DOI] [PubMed] [Google Scholar]
- [4]. Dix DJ, Allen JW, Collins BW, Poorman-Allen P, Mori C, Blizard DR, Brown PR, Goulding EH, Strong BD, Eddy EM. HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development 1997; 124:4595–4603. [DOI] [PubMed] [Google Scholar]
- [5]. Coyle P, Philcox JC, Carey LC, Cellular RAM. Molecular life sciences metallothionein: the multipurpose protein. Cell Mol Life Sci 2002; 59:627–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Henkel G, Metallothioneins KB. Zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features—structural aspects and biological implications. Chem Rev 2004; 104:801–824. [DOI] [PubMed] [Google Scholar]
- [7]. Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R. The role of metallothionein in oxidative stress. Int J Mol Sci 2013; 14:6044–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Sugihara T, Wadhwa R, Kaul SC, Mitsui Y. A novel testis-specific metallothionein-like protein, tesmin, is an early marker of male germ cell differentiation. Genomics 1999; 57:130–136. [DOI] [PubMed] [Google Scholar]
- [9]. Sutou S, Miwa K, Matsuura T, Kawasaki Y, Ohinata Y, Mitsui Y. Native tesmin is a 60-kilodalton protein that undergoes dynamic changes in its localization during spermatogenesis in mice. Biol Reprod 2003; 68:1861–1869. [DOI] [PubMed] [Google Scholar]
- [10]. Hauser BA, He JQ, Park SO, Gasser CS. TSO1 is a novel protein that modulates cytokinesis and cell expansion in arabidopsis. Development 2000; 127:2219–2226. [DOI] [PubMed] [Google Scholar]
- [11]. Matsuura T, Kawasaki Y, Miwa K, Sutou S, Ohinata Y, Yoshida F, Mitsui Y. Germ cell-specific nucleocytoplasmic shuttling protein, tesmin, responsive to heavy metal stress in mouse testes. J Inorg Biochem 2002; 88:183–191. [DOI] [PubMed] [Google Scholar]
- [12]. Olesen C, Moller M, Byskov AG. Tesmin transcription is regulated differently during male and female meiosis. Mol Reprod Dev 2004; 67:116–126. [DOI] [PubMed] [Google Scholar]
- [13]. Hitoshi N, Ken-ichi Y, Jun-ichi M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991; 108:193–199. [DOI] [PubMed] [Google Scholar]
- [14]. Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. Green mice’ as a source of ubiquitous green cells. FEBS Lett 1997; 407:313–319. [DOI] [PubMed] [Google Scholar]
- [15]. Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep 2013; 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Fujihara Y, Ikawa M. CRISPR/Cas9-based genome editing in mice by single plasmid injection. Methods Enzymol 2014; 546:319–336. [DOI] [PubMed] [Google Scholar]
- [17]. Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol 2001; 229:468–479. [DOI] [PubMed] [Google Scholar]
- [18]. De La Fuente R, Parra MT, Viera A, Calvente A, Gómez R, Suja JÁ, Rufas JS, Page J. Meiotic pairing and segregation of achiasmate sex chromosomes in eutherian mammals: the role of SYCP3 protein. PLoS Genet 2007; 3:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Mahadevaiah SK, Turner JMA, Baudat F, Rogakou EP, De Boer P, Blanco-Rodríguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 2001; 27:271–276. [DOI] [PubMed] [Google Scholar]
- [20]. Gaysinskaya V, Soh IY, van der Heijden GW, Bortvin A. Optimized flow cytometry isolation of murine spermatocytes. Cytom Part A 2014; 85:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Gely-Pernot A, Hao C, Becker E, Stuparevic I, Kervarrec C, Chalmel F, Primig M, Jégou B, Smagulova F. The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine. BMC Genomics 2015; 16:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Soper SFC, van der GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, Bortvin A, Maelstrom M. A component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell 2008; 15:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Margoshes M, Valiee BL. A cadmium protein from equine kidney cortex. J Am Chem Soc 1957; 79:4813–4814. [Google Scholar]
- [24]. Ziller A, Fraissinet-Tachet L. Metallothionein diversity and distribution in the tree of life: a multifunctional protein. Metallomics 2018; 10:1549–1559. [DOI] [PubMed] [Google Scholar]
- [25]. Sharan SK. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development 2003; 131:131–142. [DOI] [PubMed] [Google Scholar]
- [26]. Smagulova F, Brick K, Pu Y, Sengupta U, Camerini-Otero RD, Petukhova GV. Suppression of genetic recombination in the pseudoautosomal region and at subtelomeres in mice with a hypomorphic Spo11 allele. BMC Genomics 2013; 14:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Li X, Schimenti JC. Mouse pachytene checkpoint 2 (Trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet 2007; 3:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem 2000; 275:9377–9384. [DOI] [PubMed] [Google Scholar]
- [29]. Saydam N, Georgiev O, Nakano MY, Greber UF, Schaffner W. Nucleo-cytoplasmic trafficking of metal-regulatory transcription factor 1 is regulated by diverse stress signals. J Biol Chem 2001; 276:25487–25495. [DOI] [PubMed] [Google Scholar]
- [30]. Beall EL, Manak JR, Zhou S, Bell M, Lipsick JS, Botchan MR. Role for a drosophila Myb-containing protein complex in site-specific DNA replication. Nature 2002; 420:833–837. [DOI] [PubMed] [Google Scholar]
- [31]. Jiang J, Benson E, Bausek N, Doggett K, White-Cooper H. Tombola, a tesmin/TSO1-family protein, regulates transcriptional activation in the drosophila male germline and physically interacts with always early. Development 2007; 134:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Deplancke B, Mukhopadhyay A, Ao W, Elewa AM, Grove CA, Martinez NJ, Sequerra R, Doucette-Stamm L, Reece-Hoyes JS, Hope IA, Tissenbaum HA, Mango SE et al. A gene-centered C. elegans protein-DNA interaction network. Cell 2006; 125:1193–1205. [DOI] [PubMed] [Google Scholar]
- [33]. Schmit F, Korenjak M, Mannefeld M, Schmitt K, Franke C, Von Eyss B, Gagrica S, Hänel F, Brehm A, Gaubatz S. LINC, a human complex that is related to pRB-containing complexes in invertebrates regulates the expression of G2/M genes. Cell Cycle 2007; 6:1903–1913. [DOI] [PubMed] [Google Scholar]
- [34]. Schmit F, Cremer S, Gaubatz S. LIN54 is an essential core subunit of the DREAM/LINC complex that binds to the cdc2 promoter in a sequence-specific manner. FEBS J 2009; 276:5703–5716. [DOI] [PubMed] [Google Scholar]
- [35]. Matsuo T, Kuramoto H, Kumazaki T, Mitsui Y, Takahashi T. LIN54 harboring a mutation in CHC domain is localized to the cytoplasm and inhibits cell cycle progression. Cell Cycle 2012; 11:3227–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


