Abstract
Amyloidosis is a group of disorders characterized by a misfolded protein that deposits in organs and compromise their function. Clinician should have a high index of suspicion because in most cases, the clinical picture is non-specific. Typing of amyloid is of utmost importance and should be an integral part of accurately diagnosing a patient
AL amyloidosis is the most common systemic amyloidosis in the western world in which the misfolded proteins are immunoglobulin light chains secreted by clonal plasma cells. New data about prognostication of AL amyloidosis patients are accumulating. The treatment goal is to eradicate the amyloidogenic plasma cell clone, by using high dose melphalan and/or novel agents (proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies against CD38). Early diagnosis is important for effectively treating the patient as late diagnosis hampers chances for organ recovery.
ATTR amyloidosis is less recognized but is increasingly seen due to better recognition and improved diagnostic tools. New data about treatment options (patisiran, inotersen and tafamidis) have recently been published and are discussed.
Keywords: amyloidosis, light chains, risk-adapted, ATTR, daratumumab
1. Overview
Amyloidosis is a heterogenous group of disorders in which proteinaceous fibrils accumulate in certain organs and compromise their structure and their function. The diagnosis is based on histological documentation of amyloid deposits. In hematoxylin and eosin staining, an amorphous eosinophilic deposit is detected, and when viewed under polarized light after Congo red stain, green birefringence is seen (1). Additionally, it is important to characterize the amylogenic protein subunit by using immuno-electron microscopy (IEM), mass spectrometry or immunohistochemistry. Accurate typing of systemic amyloidosis is of paramount importance for assessing the prognosis and tailoring the appropriate treatment.
There are over 30 subtypes of proteins that can cause amyloidosis but only 14 of them cause a systemic disease (2). The most common types of systemic amyloidosis are immunoglobulin light chain (AL), reactive (AA), mutant or wild type transthyretin (ATTR), fibrinogen (AFib) and apolipoprotein A-I (AApoAI) (3). AL amyloidosis usually presents with nonspecific symptoms (weight loss, fatigue, dyspnea, paresthsias) and the signs that are highly specific such as macroglossia and periorbital purpura are quite insensitive. AL amyloidosis is the most common type of systemic amyloidosis with a reported incidence of 9–14 cases per million-person years in the USA (4). A recently published real-world study using claim database suggested that the prevalence of AL amyloidosis in the US increased significantly between 2007 and 2015 (5). In contrast, an updated report showed that the incidence of AL amyloidosis in Olmsted County, Minnesota, remained stable between 1990 and 2015 and was 12 per 1,000,000 person-years (6). Due to under-diagnosis of this disease, it reasonable to speculate that the prevalence may be higher. All MGUS patients should be routinely screened for amyloidosis by asking directed questions and performing a focused physical examination. Some experts recommend routine assessment of NT-proBNP and urine albumin at each visit (7) although there are no prospective clinical trials to support these recommendations.
The therapies for AL amyloidosis are often derived from therapies used in multiple myeloma with widening treatment options in recent years. Tailoring treatment is the goal for these clinically complicated patients and this can be achieved by improving risk and response assessments. Recent advances have been made in improving risk stratification and efforts are made to choose patients for whom autologous stem cell transplantation (ASCT) would be safe in an attempt to minimize treatment-related mortality (TRM).
In newly diagnosed patients, treatment is usually bortezomib based and for highly selected patients high-dose melphalan and ASCT (8, 9). In the relapse setting, data is lacking about the appropriate timing to reinstitute treatment and novel agents are being tested in clinical trials (10). Daratumumab in combination or as monotherapy appears to be a very interesting treatment option, with rapid achievement of hematological responses and a tolerable safety profile (11)
Over the past few decades, earlier diagnosis, better risk stratification, improvement in supportive care and availability of new treatments have significantly improved overall survival (12).
ATTRm amyloid is an autosomal dominant, multisystemic, progressive disease. Various mutations in the gene encoding transthyretin (TTR) result in defective protein production in the liver. The defective protein accumulates in the heart, nerves and GI tract (13). In ATTRwt amyloidosis, normal TTR protein misfolds and forms amyloid fibrils. Until recently, the treatment options were limited and included orthotopic liver transplantation and transthyretin tetramer stabilizers (tafamidis or diflunisal). In 2018 the results of two large clinical trials for the treatment of ATTRm neuropathy were published (14, 15), representing a change in the treatment paradigm for this life-threatening disease. Tafamidis, inotersen and patisiran are now approved in the US.
The goal of this review is to provide hematologists with an overview of the available data regarding the diagnosis, risk stratification, treatment and response assessment in AL amyloidosis and in ATTRm and ATTRwt amyloidosis.
2. Clinical manifestations, diagnosis and typing
The diagnosis of AL Amyloidosis is difficult because no single blood test or imaging test is pathognomonic (16). The extent and number of organs involved determines the clinical picture, which in most cases is not specific and subtle, and includes fatigue, weight loss, dyspnea and peripheral edema. The signs of amyloid which are highly specific are insensitive. For example, soft tissue involvement that raises suspicion of AL amyloidosis is uncommon (10%). The most commonly involved organs are the heart, kidneys, liver, nervous system, gastrointestinal tract and soft tissue (17–19). Most patients have several involved organs while approximately a third of the patients have one affected organ (20).
2.1. Clinical manifestation
2.1.1. Cardiac Involvement
Cardiac involvement occurs in 60–75% of AL patients and is of utmost importance in determining the prognosis (21). Patients can present with heart failure (dyspnea on exertion, orthopnea, fatigue, peripheral edema, jugular venous distension, and pleural effusion), arrhythmia, syncope, sudden cardiac death, and rarely myocardial infarction (MI) caused by amyloid deposition in the coronary arterioles. Rapid clinical deterioration in a patient that was previously stable might be caused by pulmonary emboli or atrial fibrillation.
Natriuretic peptides (NT-proBNP and BNP) are the most sensitive indicator of myocardial involvement in light-chain amyloidosis and are a useful tool for response evaluation (22). Electrocrdiogram (ECG) demonstrates a low voltage in more than 50% of the patients with heart involvement (23). Other common patterns of abnormal ECG in AL amyloidosis include atrial fibrillation, a pseudo-infarct pattern (poor R-wave progression in chest leads without a known previous MI) and conduction disturbances (24).
Cardiac amyloidosis is mainly a disease of diastole; therefore, ejection fraction (EF) is commonly preserved. Echocardiography may demonstrate left ventricular hypertrophy, thickening of the interventricular septum, pericardial effusion, reduced EF and reduced global longitudinal strain (GLS) (25). The characteristic “granular sparkling” of the myocardium is seen in approximately half of the patients and is no longer used for the diagnosis of AL amyloidosis (26). A recently published retrospective study found that the prevalence of AL amyloidosis in patients with congestive heart failure was only 0.5%. The authors suggested that in order to increase pretest probability for AL cardiac amyloidosis in unselected heart failure patients, serum FLC assay as screening tool should be considered in patients with high NT-pro-BNP (≥5000 pg/mL) and increased posterior wall thickness (≥13 mm) (27). We would also add serum electrophoresis/immunofixation and urine electrophoresis/immunofixation to this screening.
Endomyocardial biopsy is the gold standard for diagnosing cardiac amyloidosis, but it is an invasive procedure and we avoid it if possible. Cardiac MRI is a noninvasive procedure with high sensitivity (80–100%) and specificity (80–94%) (28, 29). The finding of late gadolinium enhancement is highly suggestive of the diagnosis of amyloidosis, however, is unable to classify the amyloidosis.
Scintigraphy with Tc-99m-pyrophosphate (99mTc-PYP) is widely available in the USA, is simple and non-invasive method that helps differentiate ATTR from AL amyloidosis (30). It should be noted that the PYP scan can be positive in some AL cases. In a patient who does not have an M protein but has a positive PYP scan, only then can the treating physician assume that this is ATTR and not AL. If a patient has an M protein and a positive PYP scan, a diagnosis of TTR cannot be based on the PYP alone and tissue is required for typing. Cardiac retention can be assessed by two methods: The semi-quantitative visual score assesses the uptake in relation to bone uptake (0-no cardiac uptake to 3- diffuse uptake higher than bone). The second method is a quantitative analysis by drawing a region of interest (ROI) over the heart corrected for contralateral counts and calculating the heart to contralateral (H/CL) ratio (31). The degree of cardiac tracer retention in the heart correlated with left ventricular wall thickness and mass and was found to be a prognostic marker for survival in ATTR amyloidosis (32).
2.1.2. Renal involvement
Renal involvement occurs in about 50–70% of AL amyloidosis patients and may present as nephrotic syndrome or as nonselective proteinuria. Kidney involvement can be determined by proof of amyloid deposits in the renal parenchyma, although is rarely needed. Alternatively, in the presence of alternate site amyloid deposit it is defined as a urinary protein excretion of above 0.5 g per 24 hours, predominantly albumin (33). Enlarge kidneys might rarely be seen in ultrasound or CT scan. Renal amyloidosis might progress to end-stage renal disease if ineffectively treated or diagnosed late. The risk of progression to end-stage renal disease is predicted by the level of proteinuria at presentation, the estimated glomerular filtration rate (eGFR) (34) and by response to treatment (35).
2.1.3. Nerve Involvement
Nerve involvement may include sensory (paresthesia, numbness and pain), autonomic (gastroparesis, bladder or bowel dysfunction and orthostatic hypotension) or motor neuropathy.
2.1.3.1. Sensory neuropathy
Electromyography (EMG) is an accessible and useful test for evaluating peripheral neuropathy that usually will demonstrate symmetrical axonal sensorimotor polyneuropathy. It should be noticed that EMG might be normal when neuropathy effects only the small unmyelinated C - fibers (5% of amyloidosis patients with peripheral neuropathy) (36). The gold standard for diagnosing small fiber neuropathy is quantification of intraepidermal nerve fibers in a skin biopsy and has a 90% sensitivity and specificity (37). Early onset of polyneuropathy (<50 years) is more typical of ATTRm amyloidosis (38) and bilateral carpal tunnel syndrome (CTS) may antedate ATTR amyloid neuropathy by more than a decade (39). CTS can precede a diagnosis of AL amyloidosis as well (20%).
In AL and ATTRm amyloidosis, the EMG finding of slow conduction with prolonged distal motor latencies may lead to a misdiagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) (40).
2.1.3.2. Autonomic neuropathy
Patients with amyloidosis may have signs of autonomic dysfunction such as upper intestine dysmotility with pseudo-obstruction causing vomiting or lower intestine dysmotility causing alternating constipation and diarrhea. Erectile dysfunction and recurrent syncope due to orthostatic hypotension may also be present (41).
2.1.4. GI Involvement
Symptomatic gastrointestinal involvement occurs in about 10% of AL amyloidosis patients (42). The clinical manifestations are diverse and may include diarrhea, constipation, nausea and vomiting caused by gastroparesis (43, 44), weight loss, malabsorption, dyspepsia and GI bleeding (45, 46). Liver involvement occurs in nearly 20% of AL amyloidosis patients and can manifest as early satiety and weight loss, enlarged liver, an elevation of alkaline phosphatase and abnormal clotting tests. FibroScan® may demonstrate increased liver stiffness (> 17.3 kPa) (47).
2.1.5. Other manifestations of amyloidosis
Purpura is usually above the nipple line and classical periorbital purpura is seen in less than 20% of patients (48). Macroglossia is considered highly suggestive of AL amyloidosis (49) and the tongue is typically stiff and has signs of dental indentations. It is present in around 10% of AL amyloidosis patients and might severely impair quality of life by causing sleep apnea, dry mouth and difficulty swallowing. There are other possible causes for tongue enlargement that should be excluded, such as hypothyroidism, acromegaly and tongue cancer.
Lung involvement may manifest as dyspnea and dry cough with an interstitial radiographic pattern. Jaw, calf or buttock claudication may occur due to infiltration of small vessel (50). The shoulder pad sign results from periarticular deposits of amyloid and is rare. Bleeding diathesis in AL amyloidosis may be attributed to factor X deficiency, acquired von Willebrand disease, decreased synthesis of coagulation factors in advanced liver disease, and amyloid infiltration of blood vessels.
The clinical presentation of systemic non-AL amyloidosis is indistinguishable from AL amyloidosis. Nonetheless, AL patients have a wider repertoire of organ involvement. ATTRwt amyloidosis patients with cardiac involvement are 90% male (51). Half have a history of CTS (51). Kidney involvement is rare. Biceps rupture and peripheral neuropathy are seen.
2.2. Diagnosis and typing
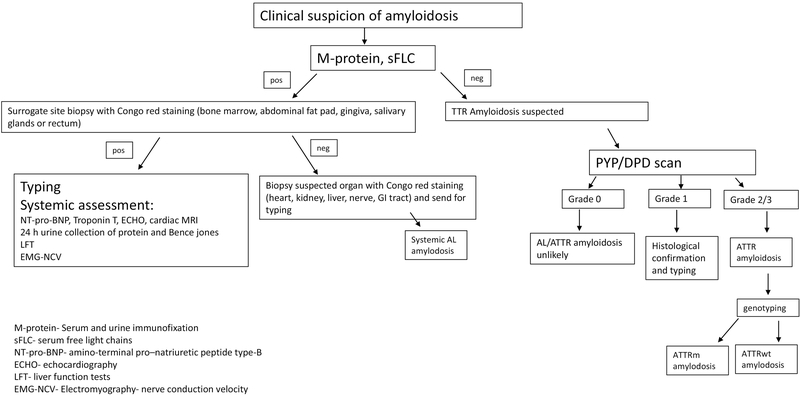
Hematologists have a key role in properly diagnosing amyloid patients. The diagnostic algorithm for AL and ATTR amyloidosis is shown on figure 1.
Figure 1-.
The diagnostic algorithm for AL and ATTR amyloidosis.
The diagnostic criteria of AL amyloidosis include the presence of systemic syndrome (e.g. renal, liver, heart, gastrointestinal tract or peripheral nerve involvement), a histological documentation of amyloid by Congo-red stain, evidence of a monoclonal plasma cell disorder (in the serum, the urine or in a bone marrow biopsy) and protein typing that supports the diagnosis (52).
Localized production of light chains in situ like skin, urinary tract, orbit, colon (53) and airways (54) is defined as localized AL amyloidosis (55). In these patients, serum and urine is negative for monoclonal immunoglobulins. This entity should not be treated systemically and therefore it is important to differentiate it from systemic amyloidosis (33). The clinical course is usually benign and long-term prognosis of localized amyloidosis treated with radiation (56) or surgery is good (57)
Amyloid fibrils may be demonstrated in tissue samples taken from a surrogate site (bone marrow, abdominal fat pad, gingiva, salivary glands or rectum) or from a suspected organ (heart, kidney, liver, nerve, GI tract). The abdominal fat aspirate is a minimally invasive simple procedure with a much higher sensitivity for AL amyloidosis (80%) than for ATTR amyloidosis (12%) (58). Up to 40% of symptomatic multiple myeloma patients have amyloid deposits in their bone marrow without evidence of systemic organ involvement (59). However only 15% of MM patients have systemic AL amyloidosis. The sensitivity of fat pad aspiration is 78%, higher for lambda -AL than kappa -AL (60). Moreover, specificity of fat pad aspiration is 93%, underscoring that substantial false positives occur (61). The sensitivity of fat pad for ATTRm is 50% and for ATTRwt 15% (62). Fat pad aspiration is best performed by experienced operators. Excisional biopsy with a goal of obtaining (>700 mm3) or 50 mg of tissue will increase the likelihood of making a diagnosis. Other surrogate sites that can be used if experience with fat aspiration is lacking are salivary gland (89% sensitivity) (63) and bone marrow (70%) (64). If surrogate site biopsies are negative and the clinical suspicion is high, the suspected organ should be biopsied.
Typing of amyloid is an essential part of the diagnosis of amyloidosis because misdiagnosis can lead to treatment with chemotherapy that is not indicated. The prevalence of light chain monoclonal gammopathy of undetermined significance (MGUS) above the age of 50 is 4.2% (65), so monoclonal proteins in the serum, urine or clonal bone marrow plasma cells are not diagnostic for AL type and might be misleading. There are three methods of amyloid typing: immunohistochemistry (IH), Immunoelectron microscopy (IEM) and mass spectrometry. Table 1 summarizes the characteristics of each method (60, 66, 67). Mass spectrometry is not yet available in most centers. However, it is strongly recommended to send tissue samples to one of several specialized laboratories. We certainly prefer proteomic analysis over immunohistochemistry despite the lack of availability.
Table 1-.
The characteristics of the three AL amyloidosis typing method.
| Immunohistochemistry | Immunoelectron microscopy | mass spectrometry | |
|---|---|---|---|
| Accessibility | Widely available, | Not available in most centers | Not available in most centers |
| Sensitivity (%) | 75–80 | 75–80 | 95 |
| Specificity (%) | 80 | 100 | 100 |
| Comments | Should be done by highly specialized pathologist, nonstandardized, | The gold standard for AL amyloidosis typing |
Genetic tests for the exclusion of hereditary amyloidosis should be performed, especially for patients with a family history of amyloidosis and for patients with no paraprotein. Serum amyloid P component (SAP) scintigraphy may be helpful in diagnosing and monitoring response to treatment, but it is less useful in diagnosing and monitoring cardiac involvement and is not widely available. The sensitivity of SAP scintigraphy is high for AA and AL amyloidosis (90% each) and much lower for ATTR amyloidosis (48%) and the specificity is 93% (68). The main use of SAP scintigraphy is to rule out systemic involvement if localized disease is found.
3. Risk Stratification
The clinical course of patients with AL amyloidosis is heterogenous and depends on which organs are involved and to what extent.
Three prognostic models are used for risk stratification in AL amyloidosis: the Mayo AL amyloidosis 2004 model (69), the Mayo AL amyloidosis 2012 model (21) and the European model, which is a modification of the Mayo 2004 (70). Table 2 shows the components of those three prognostic models and the recently validated Boston prognostic model (71). A recent trial aimed to compare the three prognostic models and showed that increased stage correlated well with shorter OS. The best prognostic models for one year and three-year OS were the European model and the Mayo 2012 model, respectively (72). The Boston University biomarker scoring system was published recently and is expected to provide a risk model for centers lacking accessibility to NT-pro- BNP. This scoring system used BNP and troponin I to create three stages that are concordant with the Mayo AL amyloidosis 2004 model (73).
Table 2-.
The three prognostic models of AL amyloidosis.
| The prognostic model | Criteria | Survival |
|---|---|---|
| Mayo model 2004 | TnT<0.035 microgram/L NT-pro-BNP < 332 ng/L BNP < 81 ng/L TnI <0.1 microgram/L |
stage 1- median 26.4 months stage 2- median 10.5 months stage 3- median 3.5 months |
| Mayo model 2012 | TnT<0.025 ng/ml NT-pro-BNP < 1800 pg/ml Serum dFLC<180 mg/dL BNP < 400 ng/L TnI- No data |
stage 1- median 94.1 months stage 2- median 40.3 months stage 3- median 14 months stage 4- median 5.8 months |
| European model 2015 | TnT<0.035 ng/ml NT-pro-BNP < 332 pg/ml BNP < 81 ng/L TnI <0.1 microgram/L Mayo stage 3 is subclassified using NT-pro-BNP < 8500 pg/ml |
stage 1- no death cases stage 2– 3 years 52% stage 3a- 3 years 55% stage 3b- 3 years 19% |
| Boston university score 2019 | TnT <0.1 ng/ml BNP < 81 pg/mL |
stage 1- median not reached stage 2- median 9.4 years stage 3a- median 4.3 years stage 3b- median 1 year |
TnT-Troponin T, NT-pro-BNP -N- terminal pro-brain natriuretic peptide, TnI- Troponin I, BNP-brain natriuretic peptide.
Cardiac magnetic resonance (CMR) late gadolinium enhancement (LGE) is prognostic of all-cause mortality (74–76). Two retrospective studies showed that global LGE was associated with all-cause mortality irrespective of cardiac biomarkers (77, 78). The finding of non-sustained ventricular tachycardia and atrial fibrillation on Holter monitor were prognostic of inferior OS at 3 and 6 months after the Holter test was preformed (79).
Additional factors considered as significant are host related factor such as age (80, 81), systolic blood pressure (82), performance status (81) and the number of involved organs (81). Factors related to disease biology that were found to have prognostic significance are cytogenetic abnormalities (83, 84), having more than 10% plasma cells in the bone marrow (85), difference between the involved to uninvolved light chains (dFLC) (86) and immunoparesis (87, 88). Similar OS was reported for κ-AL and λ-AL. However, lack of an identifiable serum intact immunoglobulin conferred shorter median OS (86).
Interphase fluorescence in situ hybridization (iFISH) is of importance in untreated AL amyloidosis. t (11; 14) is present in nearly half of AL amyloidosis patients (89, 90) and is associated with worse outcome in patients treated with bortezomib-based regimens and immunomodulatory-based treatments. These patients achieved VGPR or better less frequently and had inferior OS (83). If eligible, it is reasonable to consider these patients for ASCT based on a study that showed improved CR rates that translated into better hematologic event free survival (90). Trisomies are present in 26% of AL amyloidosis patients and their presence correlated with inferior OS in patients treated with high dose mephalan (83). In patients treated with bortezomib, t (4;14), t (14;16), gain 1q21 and del17p are rare and did not correlate with inferior OS, as reported in multiple myeloma, but analysis was underpowered (84). In patients treated with high dose melphalan, these cytogenetic abnormalities did not correlate with inferior OS, but were present only in nine patients, so this could be the result of small sample size (90). A retrospective study reported that > 50% del 17p in iFISH correlated with shorter median survival (91).
At diagnosis, multiparametric flow cytometry (MFC) can be used as another tool for prognostication and may play a role in defining hematological response. MFC detects clonality in the vast majority of patients with AL amyloidosis (92). A shorter OS and PFS were reported when ≥2.5% monotypic plasma cells compared with patients with <2.5% monotypic plasma cells. Moreover, a polytypic plasma cell to clonal plasma cells ratio of ≤5% correlated with shorter PFS compared with patients with a ratio above 5%. At the end of first line of treatment, ≥0.1% monotypic plasma cells correlated with a shorter PFS and OS compared with patients with <0.1% residual monotypic plasma cells (93).
4. Treatment
Treatment should be guided by experienced centers using a multidisciplinary approach, involving specialized hematologists, nephrologists, cardiologists, neurologists and gastroenterologists. Few randomized phase III trials have been conducted, so whenever possible, patients should be treated in the context of clinical trials (94).
Symptom management is an integral part of therapy and includes diuretics, antiarrhythmic drugs, agents that control bowel habits and medications used to control neuropathic pain. Diuretics reduce peripheral edema but might cause serious side effects such as hypotension, electrolyte disturbances and creatinine elevation. Midodrine can help in the management of orthostatic hypotension as well as the use of compression stockings (95). Regarding the choice of antiarrhythmic therapy, amiodarone is used for atrial fibrillation, beta blockers need to be avoided and digoxin can be safely used for rate control in amyloidosis
Cardiac amyloidosis (CA) patients are at increased risk of sudden cardiac arrest. However, implantable cardioverter- defibrillators (ICDs) are not routinely recommended because their efficacy in improving OS has not been demonstrated prospectively. In a retrospective study of 33 AL amyloidosis patients and 20 ATTR amyloidosis patients, there was a high rate of appropriate ICD shocks in the AL amyloidosis subgroup but no OS benefit (96). Pacemaker placement failed to salvage patients with bradyarrhythmias in a study of three patients with symptomatic cardiac AL amyloidosis (97).
Doxycycline was effective in interfering with amyloid fibril formation in a transgenic mouse model of AL amyloidosiss (98). In ASH 2012 the results of a cohort of patients with systemic AL amyloidosis treated with doxycycline as antimicrobial prophylaxis post autologous stem cell transplantation were published. There was a significantly improved survival compared to controls who were given penicillin (99). There is currently a randomized Phase II/III trial evaluating the role of doxycycline vs. standard supportive therapy in Newly-diagnosed cardiac AL Amyloidosis patients undergoing bortezomib-based Therapy ( NCT03474458). We consider adding doxycycline for at least one year in both transplant eligible and transplant ineligible patients.
4.1. AL amyloidosis
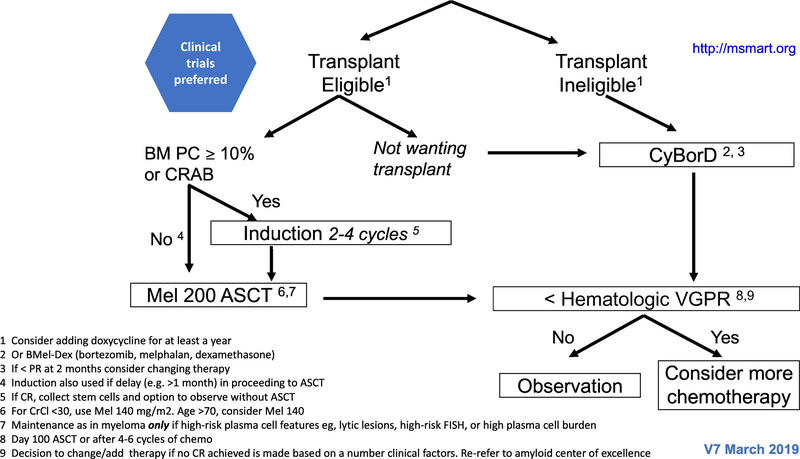
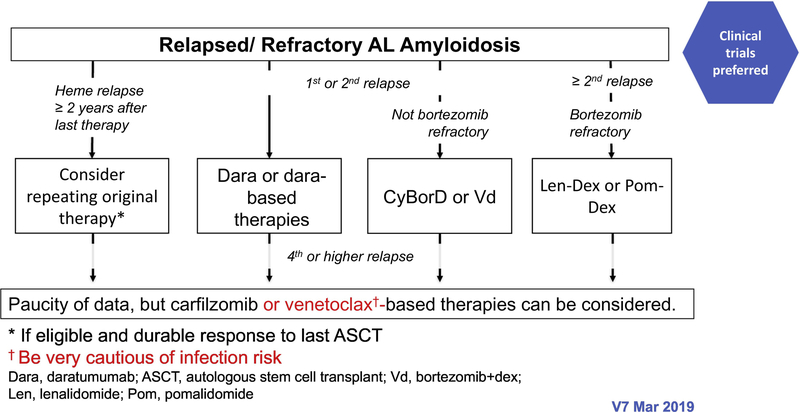
The treatment paradigm in AL amyloidosis is mainly based on phase II studies, retrospective comparisons and case series. The regimens used are similar to those that are used for multiple myeloma, although are oftentimes modified due to treatment intolerance. In patients with IgM-related AL amyloidosis, the regimens used are the same as those used in Waldenstrom macroglobulinaemia (100). The mSMART algorithms for treatment of newly diagnosed and relapsed AL amyloidosis are shown in figures 2 and 3, respectively (101).
Figure 2-.
The mSMART algorithms for treatment of newly diagnosed AL amyloidosis.
Figure 3-.
The mSMART algorithms for treatment of relapsed AL amyloidosis.
4.1.1. Autologous stem cell transplantation
The first step in deciding how to treat a patient is to assess the eligibility for ASCT. The high rate of treatment related mortality (TRM) in AL amyloidosis are mainly attributed to cardiac involvement (10–12%) (102). Therefore, most AL amyloid patients are not eligible for ASCT. The eligibility criteria set by the Mayo group include biological age ≤70 years, troponin T < 0.06 microgram/L (hs-troponin T <75 ng/L) (103), systolic blood pressure ≥90 mm Hg, creatinine clearance ≥30 mL/min (unless on chronic stable dialysis), Eastern Cooperative Oncology Group (ECOG) performance status ≤2, New York Heart Association (NYHA) functional status class I or II, no large pleural effusions, and no dependency on oxygen therapy (101).
Table 3 summarizes the data regarding the outcomes of high dose melphalan followed by ASCT (102, 104–107). The outcomes were consistently better for patients achieving complete hematological responses and for patients receiving full dose melphalan (200mg/m2) (108, 109). When selecting patients properly, ASCT is associated with a high rate of durable hematologic and organ responses. Only one randomized study concerning the role of ASCT was published to date. It randomized 100 patients to ASCT vs standard dose melphalan and dexamethasone. OS was significantly worse in the ASCT arm, but patient selection was different than it is today, mainly in terms of cardiac involvement (110). A Mayo clinic study included 89 patients that could select treatment with standard dose melphalan and dexamethasone or ASCT. Patient that chose the transplant arm were generally more fit (younger, lower ECOG, less cardiac involvement). The hematological response rates were similar between the groups (56% vs 70%, p=0.2) and the ASCT has better 3 years OS rates (59% in the no transplant group vs 84% in the ASCT arm) (111).
Table 3-.
The studies about the outcome of high dose melphalan followed by autologous stem cell transplantation.
| The study | Number of patients | Cardiac involvement % | Complete Hematological response % | Organ response % | Treatment related mortality % | Median PFS/OS |
|---|---|---|---|---|---|---|
| Gertz 2010 | 434 | Not rported | 39 | 47 | 10 | Complete response >120 m Partial response 107m No response 32 m |
| Sanchorawala Blood 2011 | 421 | 45 | 34 | 53 | 11.4 | Med OS 75m |
| Sanchorawala 2014 | 607 | 53 | 34 | NR | 9 | Med OS 80m |
| Parmar 2014 | 80 | 23 | 19 | 39 | 12.5 | 5 years OS 72% |
| Jaccard 2007 | 100 - randomized to ASCT or MP | 73 | No significant difference between the two treatment groups (47% Vs 61%) | No significant difference between the two treatment groups | 24 | Med OS was 56.9 months in the MP arm and 22.2 months in the ASCT arm (P=0.04) |
| Gertz 2016 | 89 – randomized to ASCT (55) or Mel dex (39) | 41 in mel dex arm and 15 in ASCT arm | 20, No significant difference between the two treatment groups | 30 No significant difference between the two treatment groups | 5.6 | 3-year PFS of 29.1% in mel dex arm and 51.7% in the ASCT arm. 3y OS was 58.8% in mel dex arm vs 83.6% in ASCT arm. |
A phase II prospective multicenter reported the outcome of 69 newly diagnosed patients that received three courses of vincristine, doxorubicin and dexamethasone induction follow by ASCT. The cohort has a median OS of 10 years but the TRM was 12% and 18% died during induction (112). A recently published phase II prospective study reported the outcome of 50 patients with newly diagnosed AL amyloidosis treated with four cycles of bi-weekly bortezomib and dexamethasone followed by ASCT. The overall hematological response was 80% with 58% of the patients achieving VGPR/CR (113). In both studies approximately 30% of the patients did not proceed to ASCT, mostly due to treatment related toxicity.
Early post-transplant mortality has substantially decreased over the past few decades (114) presumably due to carful patient selection (115) and better supportive care. During stem cell collection patients might accumulate fluid (116) so close monitoring is essential. Patients undergoing stem cell transplant are at particularrisk for significant fluid retention, atrial fibrillation, worsening of renal function and gastrointestinal hemorrhage
The role of induction treatment before ASCT is unclear. Studies evaluating the role of induction before ASCT are listed in table 4 (117–119). At Mayo Clinic a bortezomib-based induction is given to patients with 10% or more plasma cells in the bone marrow biopsy and/or for those with CRAB features.
Table 4-.
Studies that evaluate the role of induction treatment before ASCT in AL amyloidosis.
| The study | Number of patients | Treatment arms | Cardiac involvement % | Hematological response % | TRM % | Median PFS/OS | Comments |
|---|---|---|---|---|---|---|---|
| Scott 2014 | 56 | 2 Vd Vs no induction | 48 | Higher CR in the induction arm | 9.6 | 3yOS 73% | Retrospective trial. The median time to maximum hematologic response after ASCT was reduced in the induction arm (3 vs. 14 months). |
| Sanchorawala 2004 | 100 | 2 MP Vs No induction | 46 | Not different between the groups | NR | Not different between the groups | Prospective trial. |
| Afrough 2018 | 128 | MP (25 pt) vs Novel agent (83 pt) Vs no induction (20 pt) | 29 | HR at 100 d 62% in MP arm 87% in novel agent arm and 60% in no induction arm | NR | 2y PFS not different between the groups. 2y OS were 76% in MP, 87% in novel agent arm and 73% in no induction | Retrospective |
Novel agents = thalidomide, lenalidomide, or bortezomib
4.1.2. Proteasome inhibitors
Proteasome inhibitors (PI) as a monotherapy and in combinations have an important role in the frontline therapy of MM and amyloidosis. In a phase two trial of 18 patients, bortezomib and dexamethasone administration resulted in median time to hematological response of 0.9 months and an overall hematological response of 94% (CR 44%). The overall organ response was 28% with a median time to organ response of 4 month (120). A trial of 174 patients that compared bortezomib, melphalan and dexamethasone to melphalan and dexamethasone was reported. Patients were matched for age, cardiac and renal function and free light chain burden. The addition of bortezomib resulted in higher rates of CR (42 vs 19%) but there was no OS benefit (121). A phase III trial comparing melphalan and dexamethasone (MDex) to bortezomib melphalan and dexamethasone (BMDex) was completed ( NCT01277016. The study enrolled 110 patient and above 70% in each arm had cardiac involvement). The study was not finally analyzed and published. Preliminary results showed that after three cycles, overall hematological response was 78% in the BMDex arm and 51% in the MDex arm (p=0.001) with doubling of the CR/VGPR rates in the BMDex arm (122). In a study that evaluated bortezomib (1.5 mg/m2 once weekly) with cyclophosphamide and dexamethasone (VCD) in 70 patients with AL amyloidosis, the overall hematological response rate was 94% (CR 71%). The median time to response was 2 months (123). Another study investigated VCD in 230 newly diagnosed AL amyloidosis patients and showed overall hematological response in 60% (VGPR 43%). Cardiac and renal responses were 17 and 25%, respectively (70). The results with a median follow-up of 77 months of 35 patients treated with bortezomib induction followed by ASCT were recently reported. Overall hematological response was 100% at 6 months post-SCT and median OS and PFS were not reached. At 5 years post-transplant, renal and cardiac responses occurred in 65% and 88%, respectively (9).
Bortezomib is also effective in the relapsed setting. In a prospective trial of 70 relapsed AL patients, bortezomib was given once (1.6 mg/m2) or twice weekly (1.3 mg/m2). The overall hematological response was 50% (CR 20%). The median time to first response and to best response was shorter in the twice weekly group. However, the twice weekly group had a higher rate of discontinuation and grade ≥ 3 toxicities (124).
Carfilzomib (dosage 20/36 mg/m2) is a second-generation PI that is effective in relapsed AL amyloidosis but has considerable cardiovascular side effects. In ASH 2016 the results of 28 patients with relapsed AL amyloidosis treated with carfilzomib monotherapy were reported. Overall hematological response rate was 63% had and 21% organ responses. Fourteen patients hade grade 3 or 4 cardiac or pulmonary events (125). Recently, a dose finding study aimed to evaluate the safety and determine the maximum tolerated dose of carfilzomib in patients with previously treated systemic AL amyloidosis was closed and results are awaited ( NCT01789242).
Ixazomib is an oral PI that has received FDA breakthrough therapy designation in 2014 for relapsed AL amyloidosis because it has demonstrated high efficacy with a manageable toxicity profile. In a trial of 27 relapsed AL amyloid patients, the overall hematological response rate was 52% (100% in patients that did not receive bortezomib in prior lines). Kidney and cardiac responses were 18% and 50%, respectively (126). Recently, the study TOURMALINE- AL1 that aimed to evaluate the role of ixazomib and dexamethasone in relapsed AL amyloidosis was discontinued due to failure to demonstrate a significant improvement in overall hematologic response compared with standard therapy.
4.1.3. Immunomodulatory drugs
Immunomodulatory drugs are given orally and are active in AL amyloidosis. However, their tolerability in AL amyloidosis is low.
Thalidomide was evaluated in a phase I/II trial in 16 patients at doses higher than those used today (up to 300 mg daily). Neurotoxicity and fatigue were important causes of dose reductions and discontinuation (25% of the patients discontinued thalidomide due to side effects). No complete hematologic responses were reported (127). Similar results emphasizing the tolerability issue of thalidomide were reported in a phase II trial of 12 patients, all of whom discontinued study medication due to side effects (128).
Lenalidomide in AL amyloidosis is used in lower doses than in MM due to lower tolerability. Lenalidomide might raise natriuretic peptides and cause cardiac decompensation in AL amyloidosis. Assessing cardiac response using NT-pro-BNP may not be valid in lenalidomide treated patients and assessing cardiac response with troponin may be more reasonable (129). Lenalidomide showed activity in combination with dexamethasone with 10 out of 23 patients responding to treatment (130). The all oral combination of lenalidomide, melphalan and dexamethasone was evaluated in 25 newly diagnosed AL amyloidosis patients, most of whom had cardiac involvement (92%). 40% died early in the study due to cardiac decompensation. 1-year OS and overall hematological response was both 58% and only 8% had documented organ response (131). A retrospective study evaluated 55 relapsed/refractory AL amyloidosis patients, 77% had Mayo stage 2/3. 26% withdrew lenalidomide due to toxicity. The hematologic response rate was 51% and organ response was achieved in 16% of the patients (132).
Pomalidomide in combination with dexamethasone was evaluated in a phase 2 trial of 28 patients with relapsed AL amyloidosis. Fluid retention and infection were frequently reported. The overall hematological response was 68% and 30% achieved VGPR or better. The response was rapid with a median time to response of one month. The median OS and PFS were 26 and 16 months, respectively (133). A similar phase II study that enrolled 33 patients (92% of them presented with cardiac involvement) showed an overall hematological response of 48% with a median time to response of 1.9 month. The 1-year OS and PFS were 76% and 59%, respectively (134). In a phase I/II trial of 27 previously treated AL amyloidosis patients, treatment with pomalidomide and dexamethasone resulted in an overall hematologic response of 50% and with a median follow-up of 17.1 months the median OS has not yet been reached (135). Pomalidomide can cause paradoxical increase in NT-pro-BNP though without clinical symptoms of heart failure (136).
4.1.4. Monoclonal antibodies
Daratumumab is an intravenously administered monoclonal antibody. The target glycoprotein for daratumumab, CD38, is expressed on clonal plasma cells. Daratumumab is given in the same schedule as in MM (137) with similar median time to response of 1 month (138, 139). Daratumumab showed rapid and deep responses in previously treated AL amyloidosis with an excellent tolerability profile and lower rates of infusion-related reactions than in MM. It should be noted that daratumumab is not approved for AL amyloidosis and only limited data is currently available regarding its efficacy.
The first 2 cases of response to daratumumab were reported in 2016 (137). In a case series of 25 previously treated AL patients, 72% had cardiac involvement. Daratumumab was given as monotherapy and patients received a median of three prior lines of therapy. The overall hematologic response rate was 76% (CR 36%, VGPR 24%). Daratumumab was very well tolerated, even among patients with cardiac involvement and grade 1–2 infusion-related reactions were reported in 60% of the patients (no grade 3 or 4 reactions were reported) (138). In a retrospective trial of 20 patients (50% had cardiac involvement) treated with daratumumab monotherapy, the overall hematological response was 86% (CR 33%, VGPR 53%). The OS at a median follow-up of 10 months was 80% (139). In ASH 2017 the results of a retrospective trial of 32 previously treated patients were reported. The overall hematologic response rate was 63% (CR 10%, VGPR 43%). NT-Pro-BNP progression under therapy was reported in 42% of the assessed patients (140).
The results of 44 relapsed patients receiving daratumumab were recently reported by the Mayo group. Half received daratumumab monotherapy and half combination-therapy (with bortezomib, pomalidomide or lenalidomide). The overall hematological response was 83% (88% with combination, 78% with daratumumab monotherapy). At a median follow-up of 10 month, PFS and OS were not reached in both groups. The infusion-related reactions were reported in 22% of the patients. None of the patients (out of nine) treated with daratumumab in combination with lenalidomide or pomalidomide, experienced cardiac decompensation (11). A phase III trial is currently enrolling newly diagnosed AL amyloidosis patients and aims to evaluate daratumumab combined with CyBorD versus CyBorD alone ( NCT03201965). In this study daratumumab is being investigated as a subcutaneous injection which is particularly interesting in AL amyloidosis because of the low volume.
4.1.5. Venetoclax
Fifty percent of AL amyloidosis patients harbor t (11; 14), making venetoclax an effective treatment for this patient population. In a case report of AL patient with t (11; 14) that was treated with bortezomib cyclophosphamide and dexamethasone, the patient achieved partial response but later progressed and cyclophosphamide was substituted with venetoclax (400 mg daily). This change resulted in complete hematological response. However, the duration of response was short and three months after venetoclax treatment was discontinued the patient progressed. Interestingly the patient responded rapidly (two months) to venetoclax upon reintroduction (141). Recently, the FDA stopped the trials that evaluated the roll of venetoclax in MM due to higher mortality related to infections in the venetoclax arm. Venetoclax is not approved for AL amyloidosis in the US.
4.2. ATTR
Therapies for ATTR amyloidosis aim to stabilize the TTR tetramer (diflunisal, tafamidis, doxycycline) or to reduce the production of the mutant protein (liver transplantation, antisense therapies and small interfering RNA).
Diflunisal is a nonsteroidal anti-inflammatory drug (NSAID) and is given orally at a dose of 500mg/d. In a placebo-controlled trial that included 130 patients with ATTRm and polyneuropathy, diflunisal reduced the progression rate of the neuropathy and preserved quality of life (QOL) (142). Tafamidis a benzoxazole derivative lacking nonsteroidal antiinflammatory drug activity and binds the same site as diflunisal on the TTR tetramer. In a phase III placebo-controlled trial 441 patients received 80 mg of tafamidis, 20 mg of tafamidis, or placebo for 30 months. Cardiovascular-related hospitalizations and all-cause mortality were significantly lower in patients that received tafamidis at both dose levels (143). The toxicity profile was manageable and did not differ from placebo. Tafamidis has recently licensed in USA and Japan for cardiac ATTR amyloidosis.
The combination of doxycycline and tauroursodeoxycholic acid was evaluated in a phase 2 trial that enrolled 20 patients, three of them had ATTRwt. No progression in cardiac or neurological involvement was noted (144).
Inotersen and patisiran are two new treatments that were recently granted the Food and Drug Administration (FDA) approved for the treatment of ATTRm amyloidosis patients with neuropathy. The approval was based on two large phase 3 clinical trials (14, 15). Patisiran is a double-stranded interfering ribonucleic acid molecule (RNAi) that binds to the TTR messenger RNA (mRNA) and impairs protein translation in the liver for both ATTRm and ATTRwt (14). It is administered intravenously every 21 days and the main safety issue was mild-to-moderate infusion-related reactions. The APOLLO trial is a recently published randomized, placebo-controlled, phase 3 trial that included 225 patients with ATTRm amyloidosis related polyneuropathy. Patients with prior treatment with tetramer stabilizer were included. The primary endpoint was a change from baseline in modified NIS + 7 (mNIS + 7) between groups. At 18 months, the least-squares mean improvement in mNIS + 7 from baseline was – 6.0 in the patisiran group and 28.0 in the placebo arm. The difference was observed among all subgroups, including genotype, age, clinical severity, presence of cardiac involvement and previous use of tetramer stabilizer. An 80% decrease in serum TTR from baseline was shown in the patisiran arm (14). Inotersen is a single-stranded deoxynucleotide analogue (DNA) complementary to the sequence of TTR pre-mRNA that inhibits the production of the TTR protein in the liver. It is administered as a weekly subcutaneous injection and the main safety issue is thrombocytopenia. The NEURO-TTR trial is a recently published randomized, placebo-controlled, phase 3 trial that included 172 patients with mATTR amyloidosis related polyneuropathy. The patients receiving the drug experienced significantly less decline in mNIS + 7 and QOL (15).
5. Response Assessment
The response criteria in AL amyloidosis are divided into hematological response and organ response.
The hematological response is divided into four groups: complete response defined as normal serum FLC ratio and negative serum/urine immunofixation, VGPR defined as dFLC < 40 mg/L, partial response defined as a reduction of dFLC of 50% or more, and no response if dFLC reduction is less than 50% (33, 145).
An organ response is usually delayed. Kidney response is defined as reduction of proteinuria by ≥30% without an increase in serum creatinine of 25% over baseline (146), while liver response is defined as a 50% decrease in the abnormal alkaline phosphatase and a decrease in liver size of at least 2 cm. Usually nerve improvement is not seen on EMG.
The prognostic effect of the extent of heart involvement on OS are very well established (21, 69). Cardiac biomarkers (troponins and NT-ProBNP) have been used to stratify patients into risk stages (69). A retrospective study evaluated the prognostic effect of troponin and NT-pro-BNP in 171 patients. The definition of response and progression of NT-pro-BNP was a change of more than 30% and more than 300 ng/L. Six months post treatment initiation, an increase of more than 75% in troponin T and the response and progression of NT-pro-BNP were predicative of survival (147). In this study, high-sensitivity troponin T at baseline was found to be better in predicting survival than NT-pro-BNP and cardiac troponin I. Another study that evaluated 271 patients undergoing ASCT, showed that troponin T was an important prognostic marker for treatment-related mortality. The authors suggested a cutoff of 0.06 microgram/L for excluding patients for ASCT (148).
The depth of organ response correlates with OS in patients with cardiac, liver and kidney involvement (149), but this has not yet been validated as a consensus response criteria. Grading the depth of cardiac, hepatic and renal responses can allow a better discrimination of patient outcomes. In a retrospective study, the authors defined four groups with distinct organ response: Non responders (≤30% reduction in organ response parameter), partial responders (31–60% reduction), very good partial responders (>60% reduction not meeting complete organ response definition) and complete organ responders (nadir NT-pro-BNP ≤400 pg/mL; nadir proteinuria ≤200 mg per 24 h; nadir alkaline phosphatase ≤×2 lower limit of normal) (149).
MRD (minimal residual disease) might prove to be a crucial factor for preventing deterioration of organ function or promoting organ response despite conventional hematological CR. A study evaluated the presence of MRD using Next-generation flow cytometry (NGF) with high sensitivity levels approaching 10−6 in 20 patients with AL amyloidosis who had achieved a CR. 40% were MRD negative and 60% were MRD positive (150). In ASH 2016, the results of a study evaluating the role of NGF in 17 patients with AL amyloidosis was presented (151).
6. Future perspectives
Delayed diagnosis is a major obstacle to effective treatment and efforts are underway to raise awareness of clinicians to amyloidosis.
The amyloid deposits are targets of novel therapies. Lately, the development of NEO001 was stopped because the phase II PRONTO trial failed to meet its endpoints. However, a post hoc analysis was recently published showing that an all-cause mortality analysis revealed a potential survival benefit favoring NEOD001 in Mayo stage IV patients (high risk).
Additionally, the phase III VITAL trial was stopped due to futility. Serum amyloid P component (SAP) is a protein that protects the amyloid fibrils protecting from degradation. The anti-SAP antibodies were currently suspended. 11–1F4 is an antibody that targets the amyloid deposits and organ responses have been reported in phase Ia trial ( NCT02245867).
The role of ixazomib maintenance is also under active investigation ( NCT03618537). Elotuzumab with revlimid and dexamethasone are currently evaluated in the relapsed setting ( NCT03252600). We expect that more data will accumulate about daratumumab in combination, which is currently being tested for newly diagnosed combined with CyBorD versus CyBorD alone in a phase III trial ( NCT03201965).
PRX004 is an investigational monoclonal antibody given intravenously and aimed to target and clear the misfolded forms of the TTR protein. It is currently being tested in a phase 1 open-label study ( NCT03336580).
7. Conclusions
Early and accurate diagnosis is very important since the current treatments are less affective for advanced stage disease. ASCT is the preferred treatment strategy for the newly diagnosed patient but careful selection of patients is of utmost importance due to high rates of TRM in patients that have extensive cardiac involvement, are not fit or have very extensive disease. Treatment options for AL amyloidosis have broadened and continue to evolve (daratumumab, ixazomib). For ATTR amyloidosis patients, three phase 3 trials have recently been published and inotersen, patisiran and tafamidis are expected to change outcomes for this patient population.
Practice Points.
Amyloidosis should be considered in all patients with proteinuria, heart failure with preserved ejection fraction, unexplained hepatomegaly, peripheral autonomic neuropathy or atypical MGUS or smoldering myeloma.
Screening for the disease involves immunofixation of serum in urine, immunoglobulin free light chain assay, and Tc pyrophosphate scanning of the heart
The diagnosis may be obtained noninvasively by examination of the bone marrow or subcutaneous fat aspiration
Therapy usually involves bortezomib based chemotherapy and selected patients autologous stem cell transplantation. There are 3 approved agents for the treatment of TTR amyloidosis
Research Agenda.
Explore the utility of monoclonal antibodies capable of dissolving existing amyloid deposits
Developed techniques for earlier diagnosis of cardiac amyloidosis before the development of end-stage cardiac disease
Investigated anti misfolding agents for the management of AL amyloidosis
Footnotes
Conflict of interests
# Dr. Gertz reports personal fees from Ionis/Akcea, personal fees from Alnylam, personal fees from Prothena, personal fees from Celgene, personal fees from Janssen, grants and personal fees from Spectrum, personal fees from Annexon, personal fees from Appellis, personal fees from Amgen, personal fees from Medscape, personal fees from Physicians Education Resource, personal fees for Data Safety Monitoring board from Abbvie, personal fees from Research to Practice, speaker fees from Teva, Speaker fees from Johnson and Johnson; Speaker fees from Medscape, Speaker fees DAVA oncology; Advisory Board for Pharmacyclics Advisory Board for Proclara outside the submitted work; Development of educational materials for i3Health.
Educational Program development i3Health.
Royalties from Springer Publishing.
Grant Funding Amyloidosis Foundation; International Waldenstrom Foundation.
NCI SPORE MM SPORE 5P50 CA186781-04.
# Dr Angela Dispenzieri - Research dollars from Alnylam, Prothena, Celgene, Takeda, Janssen, and Pfizer.
# Dr Eli Muchtar - Nothing to declare.
# Dr Iuliana Vaxman - Advisory Board for Celgene; Advisory Board for Takeda; Advisory Board for Promedicao; Speaker fees from Takeda.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fernández de Larrea C, Verga L, Morbini P, Klersy C, Lavatelli F, Foli A, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood 2015;125(14):2239–44. [DOI] [PubMed] [Google Scholar]
- 2.Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, et al. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25(4):215–9. [DOI] [PubMed] [Google Scholar]
- 3.Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol 2011;29(14):1924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota 1950, through 1989. Blood. 1992;79(7):1817–22. [PubMed] [Google Scholar]
- 5.Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Larson DR, Kurtin PJ, Kumar S, Cerhan JR, Therneau TM, et al. Incidence of AL Amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin Proc. 2019;94(3):465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood. 2013;121(26):5124–30. [DOI] [PubMed] [Google Scholar]
- 8.Gertz MA. Immunoglobulin light chain amyloidosis: 2018 Update on diagnosis, prognosis, and treatment. Am J Hemol. 2018;93(9):1169–80. [DOI] [PubMed] [Google Scholar]
- 9.Gupta VK, Brauneis D, Shelton AC, Quillen K, Sarosiek S, Sloan JM, et al. Induction Therapy with Bortezomib and Dexamethasone and Conditioning with High-Dose Melphalan and Bortezomib Followed by Autologous Stem Cell Transplantation for Immunoglobulin Light Chain Amyloidosis: Long-Term Follow-Up Analysis. Biol blood marrow transplantation. 2019;25(5):e169–e173. [DOI] [PubMed] [Google Scholar]
- 10.Sanchorawala V Delay treatment of AL amyloidosis at relapse until symptomatic: devil is in the details. Blood Adv. 2019;3(2):216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abeykoon JP, Zanwar S, Dispenzieri A, Gertz MA, Leung N, Kourelis T, et al. Daratumumab-based therapy in patients with heavily-pretreated AL amyloidosis. Leukemia. 2019;33(2):531–6. [DOI] [PubMed] [Google Scholar]
- 12.Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379(1):11–21. [DOI] [PubMed] [Google Scholar]
- 15.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chee CE, Lacy MQ, Dogan A, Zeldenrust SR, Gertz MA. Pitfalls in the diagnosis of primary amyloidosis. Clin Lymphoma, Myeloma Leuk. 2010;10(3):177–80. [DOI] [PubMed] [Google Scholar]
- 17.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32(1):45–59. [PubMed] [Google Scholar]
- 18.Dispenzieri A, Gertz MA, Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012;26(4):137–54. [DOI] [PubMed] [Google Scholar]
- 19.Shimazaki C, Hata H, Iida S, Ueda M, Katoh N, Sekijima Y, et al. Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan. Intern Med. 2018;57(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muchtar E, Gertz MA, Kyle RA, Lacy MQ, Dingli D, Leung N, et al. A Modern Primer on Light Chain Amyloidosis in 592 Patients With Mass Spectrometry-Verified Typing. Mayo Clin Proc. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palladini G, Campana C, Klersy C, Balduini A, Vadacca G, Perfetti V, et al. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107(19):2440–5. [DOI] [PubMed] [Google Scholar]
- 23.Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM : monthly journal of the Association of Physicians. 1998;91(2):141–57. [DOI] [PubMed] [Google Scholar]
- 24.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95(4):535–7. [DOI] [PubMed] [Google Scholar]
- 25.Pagourelias ED, Mirea O, Duchenne J, Van Cleemput J, Delforge M, Bogaert J, et al. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circulation Cardiovascular imaging. 2017;10(3):e005588. [DOI] [PubMed] [Google Scholar]
- 26.Siqueira-Filho AG, Cunha CL, Tajik AJ, Seward JB, Schattenberg TT, Giuliani ER. M-mode and two-dimensional echocardiographic features in cardiac amyloidosis. Circulation. 1981;6.188–96:(1)3. [DOI] [PubMed] [Google Scholar]
- 27.Chang IC, Dispenzieri A, Scott CG, Lin G, Jaffe AS, Klarich KW, et al. Utility of the Serum Free Light Chain Assay in the Diagnosis of Light Chain Amyloidosis in Patients With Heart Failure. Mayo Clin Proc. 2019;94(3):447–54. [DOI] [PubMed] [Google Scholar]
- 28.Bhatti S, Watts E, Syed F, Vallurupalli S, Pandey T, Jambekar K, et al. Clinical and prognostic utility of cardiovascular magnetic resonance imaging in myeloma patients with suspected cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2016;17(9):970–7. [DOI] [PubMed] [Google Scholar]
- 29.Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51(10):1022.30– [DOI] [PubMed] [Google Scholar]
- 30.Papantoniou V, Valsamaki P, Kastritis S, Tsiouris S, Delichas Z, Papantoniou Y, et al. Imaging of cardiac amyloidosis by (99m)Tc-PYP scintigraphy. Hell J Nucl Med. 2015;18 Suppl 1:42–50. [PubMed] [Google Scholar]
- 31.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc imaging. 2013;6(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis. JAMA cardiol. 2016;1(8):880–9. [DOI] [PubMed] [Google Scholar]
- 33.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79(4):319–28. [DOI] [PubMed] [Google Scholar]
- 34.Kastritis E, Gavriatopoulou M, Roussou M, Migkou M, Fotiou D, Ziogas DC, et al. Renal outcomes in patients with AL amyloidosis: Prognostic factors, renal response and the impact of therapy. Am J Hemol. 2017;92(7):632–9. [DOI] [PubMed] [Google Scholar]
- 35.Pinney JH, Lachmann HJ, Bansi L, Wechalekar AD, Gilbertson JA, Rowczenio D, et al. Outcome in renal Al amyloidosis after chemotherapy. J Clin Oncol. 2011;29(6):674–81. [DOI] [PubMed] [Google Scholar]
- 36.Wang AK, Fealey RD, Gehrking TL, Low PA. Patterns of neuropathy and autonomic failure in patients with amyloidosis. Mayo Clin Proc. 2008;83(11):1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol. 2017;16(11):934–44. [DOI] [PubMed] [Google Scholar]
- 38.Waddington-Cruz M, Ackermann EJ, Polydefkis M, Heitner SB, Dyck PJ, Barroso FA, et al. Hereditary transthyretin amyloidosis: baseline characteristics of patients in the NEURO-TTR trial. Amyloid. 2018;25.180–8:(3) [DOI] [PubMed] [Google Scholar]
- 39.Youngstein T, Gilbertson JA, Hutt DF, Coyne MRE, Rezk T, Manwani R, et al. Carpal Tunnel Biopsy Identifying Transthyretin Amyloidosis. Arthritis & rheumatology (Hoboken, NJ). 2017;69(10):2051. [DOI] [PubMed] [Google Scholar]
- 40.Lozeron P, Mariani LL, Dodet P, Beaudonnet G, Theaudin M, Adam C, et al. Transthyretin amyloid polyneuropathies mimicking a demyelinating polyneuropathy. Neurology. 2018;91(2):e143–e52. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama A, Asahina M, Takeda Y, Shiojiri T, Sano K, Ikeda S, et al. Isolated autonomic failure without evident somatic polyneuropathy in AL amyloidosis. Amyloid. 2014;21(3):218–20. [DOI] [PubMed] [Google Scholar]
- 42.Menke DM, Kyle RA, Fleming CR, Wolfe JT 3rd, Kurtin PJ, Oldenburg WA. Symptomatic gastric amyloidosis in patients with primary systemic amyloidosis. Mayo Clin Proc. 1993;68(8):7.63–7 [DOI] [PubMed] [Google Scholar]
- 43.Hoscheit M, Kamal A, Cline M. Gastroparesis in a Patient with Gastric AL Amyloidosis. Case Rep Gastroenterol. 2018;12(2):317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee ASY, Lee DZQ, Vasanwala FF. Amyloid light-chain amyloidosis presenting as abdominal bloating: a case report. Journal of medical case reports. 2016;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franck C, Venerito M, Weigt J, Roessner A, Malfertheiner P. Recurrent diffuse gastric bleeding as a leading symptom of gastrointestinal AL amyloidosis. Z Gastroenterol. 2017;55(12):1318–22. [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, Moon SB, Lee SK, Hong SK, Kim YH, Chae GB, et al. Light-chain amyloidosis presenting with rapidly progressive submucosal hemorrhage of the stomach. Asian J Surgery. 2016;39(2):113–5. [DOI] [PubMed] [Google Scholar]
- 47.Loustaud-Ratti VR, Cypierre A, Rousseau A, Yagoubi F, Abraham J, Fauchais AL, et al. Non-invasive detection of hepatic amyloidosis: FibroScan, a new tool. Amyloid. 2011;18(1):19–24. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal A, Chang DS, Selim MA, Penrose CT, Chudgar SM, Cardones AR. Pinch Purpura: A Cutaneous Manifestation of Systemic Amyloidosis. Am J Med. 2015;128(9):e3–4. [DOI] [PubMed] [Google Scholar]
- 49.Tsourdi E, Darr R, Wieczorek K, Rocken C, Ehehalt F, Conrad K, et al. Macroglossia as the only presenting feature of amyloidosis due to MGUS. Euro J Haematol. 2014;92(1):88–9. [DOI] [PubMed] [Google Scholar]
- 50.Audemard A, Boutemy J, Galateau-Salle F, Macro M, Bienvenu B. AL amyloidosis with temporal artery involvement simulates giant-cell arteritis. Joint, bone, spine : revue du rhumatisme. 2012;79(2):195–7. [DOI] [PubMed] [Google Scholar]
- 51.Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol:(12)15;2014e538–48. [DOI] [PubMed] [Google Scholar]
- 53.Kyle RA, Gertz MA, Lacy MQ, Dispenzieri A. Localized AL amyloidosis of the colon: an unrecognized entity. Amyloid. 2003;10(1):36–41. [DOI] [PubMed] [Google Scholar]
- 54.Dominguez S, Wienberg P, Claros P, Claros A, Vila J. Primary localized nasopharyngeal amyloidosis. A case report. Int J pediatr otorhinolaryngol. 1996;36(1):61–7. [DOI] [PubMed] [Google Scholar]
- 55.Hamidi Asl K, Liepnieks JJ, Nakamura M, Benson MD. Organ-specific (localized) synthesis of Ig light chain amyloid. J Immunol (Baltimore, Md : 1950). 1999;162(9):5556–60. [PubMed] [Google Scholar]
- 56.Cooper CT, Greene BD, Fegan JE, Rovira D, Gertz MA, Marcus DM. External beam radiation therapy for amyloidosis of the urinary bladder. Pract Radiat Oncol. 2018;8(1):25–7. [DOI] [PubMed] [Google Scholar]
- 57.al-Ratrout JT, Satti MB. Primary localized cutaneous amyloidosis: a clinicopathologic study from Saudi Arabia. Inter Dermatol. 1997;36.428–34:(6) [DOI] [PubMed] [Google Scholar]
- 58.Garcia Y, Collins AB, Stone JR. Abdominal fat pad excisional biopsy for the diagnosis and typing of systemic amyloidosis. Hum Pathol. 2018;72:71–9. [DOI] [PubMed] [Google Scholar]
- 59.Petruzziello F, Zeppa P, Catalano L, Cozzolino I, Gargiulo G, Musto P, et al. Amyloid in bone marrow smears of patients affected by multiple myeloma. Ann Hematol. 2010;89(5):469–74. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez de Larrea C, Verga L, Morbini P, Klersy C, Lavatelli F, Foli A, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;1.2239–44:(14)25. [DOI] [PubMed] [Google Scholar]
- 61.Dhingra S, Krishnani N, Kumari N, Pandey R. Evaluation of abdominal fat pad aspiration cytology and grading for detection in systemic amyloidosis. Acta cytologica. 2007;51(6):860–4. [DOI] [PubMed] [Google Scholar]
- 62.Quarta CC, Gonzalez-Lopez E, Gilbertson JA, Botcher N, Rowczenio D, Petrie A, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Euro Heart J. 2017;38(24):1905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki T, Kusumoto S, Yamashita T, Masuda A, Kinoshita S, Yoshida T, et al. Labial salivary gland biopsy for diagnosing immunoglobulin light chain amyloidosis: a retrospective analysis. Ann Hematol. 2016;95(2):279–85. [DOI] [PubMed] [Google Scholar]
- 64.Muchtar E, Dispenzieri A, Lacy MQ, Buadi FK, Kapoor P, Hayman SR, et al. Overuse of organ biopsies in immunoglobulin light chain amyloidosis (AL): the consequence of failure of early recognition. Ann Med. 2017;49(7):545–51. [DOI] [PubMed] [Google Scholar]
- 65.Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ 3rd, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet (London, England). 2010;375(9727):1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–9. [DOI] [PubMed] [Google Scholar]
- 67.Schonland SO, Hegenbart U, Bochtler T, Mangatter A, Hansberg M, Ho AD, et al. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012;119(2):488–93. [DOI] [PubMed] [Google Scholar]
- 68.Hazenberg BP, van Rijswijk MH, Piers DA, Lub-de Hooge MN, Vellenga E, Haagsma EB, et al. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med. 2006;119(4):355.e15–24. [DOI] [PubMed] [Google Scholar]
- 69.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–7. [DOI] [PubMed] [Google Scholar]
- 70.Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612–5. [DOI] [PubMed] [Google Scholar]
- 71.Tomlinson R, Matigian N, Mollee P. Validation of the Boston University staging system in AL amyloidosis. Amyloid: 2019;26(3):125–7. [DOI] [PubMed] [Google Scholar]
- 72.Muchtar E, Therneau TM, Larson DR, Gertz MA, Lacy MQ, Buadi FK, et al. Comparative analysis of staging systems in AL amyloidosis. Leukemia. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133(3):215.23– [DOI] [PubMed] [Google Scholar]
- 74.Austin BA, Tang WH, Rodriguez ER, Tan C, Flamm SD, Taylor DO, et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2.1369–77:(12) [DOI] [PubMed] [Google Scholar]
- 75.White JA, Kim HW, Shah D, Fine N, Kim KY, Wendell DC, et al. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Migrino RQ, Christenson R, Szabo A, Bright M, Truran S, Hari P. Prognostic implication of late gadolinium enhancement on cardiac MRI in light chain (AL) amyloidosis on long term follow up. BMC medical physics. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boynton SJ, Geske JB, Dispenzieri A, Syed IS, Hanson TJ, Grogan M, et al. LGE Provides Incremental Prognostic Information Over Serum Biomarkers in AL Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2016;9(6):680–6. [DOI] [PubMed] [Google Scholar]
- 78.Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation. 2015;132(16):1570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sidana S, Tandon N, Brady PA, Grogan M, Gertz MA, Dispenzieri A, et al. Prognostic Significance of Holter Monitor Findings in Patients With Light Chain Amyloidosis. Mayo Clin proc. 2019. [DOI] [PubMed] [Google Scholar]
- 80.Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93(3):1062–6. [PubMed] [Google Scholar]
- 81.Palladini G, Kyle RA, Larson DR, Therneau TM, Merlini G, Gertz MA. Multicentre versus single centre approach to rare diseases: the model of systemic light chain amyloidosis. Amyloid. 2005;12(2):120–6. [DOI] [PubMed] [Google Scholar]
- 82.Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–7. [DOI] [PubMed] [Google Scholar]
- 83.Muchtar E, Dispenzieri A, Kumar SK, Ketterling RP, Dingli D, Lacy MQ, et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia. 2017;31(7):1562–9. [DOI] [PubMed] [Google Scholar]
- 84.Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33(12):1371–8. [DOI] [PubMed] [Google Scholar]
- 85.Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013;31(34):4319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar S, Dispenzieri A, Katzmann JA, Larson DR, Colby CL, Lacy MQ, et al. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116(24):5126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muchtar E, Dispenzieri A, Kumar SK, Buadi FK, Lacy MQ, Zeldenrust S, et al. Immunoparesis in newly diagnosed AL amyloidosis is a marker for response and survival. Leukemia. 201792–9:(1)31; [DOI] [PubMed] [Google Scholar]
- 88.Sachchithanantham S, Berlanga O, Alvi A, Mahmood SA, Lachmann HJ, Gillmore JD, et al. Immunoparesis defined by heavy+light chain suppression is a novel marker of long-term outcomes in cardiac AL amyloidosis. Br J Haematol. 2017;179(4):575.85– [DOI] [PubMed] [Google Scholar]
- 89.Bochtler T, Hegenbart U, Cremer FW, Heiss C, Benner A, Hose D, et al. Evaluation of the cytogenetic aberration pattern in amyloid light chain amyloidosis as compared with monoclonal gammopathy of undetermined significance reveals common pathways of karyotypic instability. Blood. 2008;111(9):4700–5. [DOI] [PubMed] [Google Scholar]
- 90.Bochtler T, Hegenbart U, Kunz C, Benner A, Kimmich C, Seckinger A, et al. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood. 2016;128(4):594–602. [DOI] [PubMed] [Google Scholar]
- 91.Wong SW, Hegenbart U, Palladini G, Shah GL, Landau HJ, Warner M, et al. Outcome of Patients With Newly Diagnosed Systemic Light-Chain Amyloidosis Associated With Deletion of 17p. Clin Lymphoma Myeloma Leuk:(11)18;2018e493–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puig N, Paiva B, Lasa M, Burgos L, Perez JJ, Merino J, et al. Flow cytometry for fast screening and automated risk assessment in systemic light-chain amyloidosis. Leukemia. 2018;33(5):1256–1267. [DOI] [PubMed] [Google Scholar]
- 93.Muchtar E, Jevremovic D, Dispenzieri A, Dingli D, Buadi FK, Lacy MQ, et al. The prognostic value of multiparametric flow cytometry in AL amyloidosis at diagnosis and at the end of first-line treatment. Blood. 2017;129(1):82–7. [DOI] [PubMed] [Google Scholar]
- 94.Wechalekar AD, Gillmore JD, Bird J, Cavenagh J, Hawkins S, Kazmi M, et al. Guidelines on the management of AL amyloidosis. Br J Haematol. 2015;168(2):186–206. [DOI] [PubMed] [Google Scholar]
- 95.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120(10):841–7. [DOI] [PubMed] [Google Scholar]
- 96.Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiology. 2013;24(7):793–8. [DOI] [PubMed] [Google Scholar]
- 97.Sayed RH, Rogers D, Khan F, Wechalekar AD, Lachmann HJ, Fontana M, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36(18):1098–105. [DOI] [PubMed] [Google Scholar]
- 98.Ward JE, Ren R, Toraldo G, Soohoo P, Guan J, O’Hara C, et al. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118(25):6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar SK, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Dingli D et al. Doxycycline used as post transplant antibacterial prophylaxis improves survival in patients with light chain amyloidosis undergoing autologous stem cell transplantation. Blood 2012;120:3138. [Google Scholar]
- 100.Wechalekar AD, Lachmann HJ, Goodman HJ, Bradwell A, Hawkins PN, Gillmore JD. AL amyloidosis associated with IgM paraproteinemia: clinical profile and treatment outcome. Blood. 2008;112(10):4009–16. [DOI] [PubMed] [Google Scholar]
- 101.MSMART. https//:www.msmart.org/treatment-guidlines
- 102.Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118(16):4346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muchtar E, Kumar SK, Gertz MA, Grogan M, AbouEzzeddine OF, Jaffe AS, et al. Staging systems use for risk stratification of systemic amyloidosis in the era of high-sensitivity troponin T assay. Blood. 2019;133(7):763.6– [DOI] [PubMed] [Google Scholar]
- 104.Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Int Med. 2004;140(2):85–93. [DOI] [PubMed] [Google Scholar]
- 105.Sanchorawala V, Skinner M, Quillen K, Finn KT, Doros G, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood. 2007;110(10):3561–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar SK, Dingli D, et al. Autologous stem cell transplant for immunoglobulin light chain amyloidosis: a status report. Leuk Lymphoma. 2010;51(12):2181–7. [DOI] [PubMed] [Google Scholar]
- 107.Parmar S, Kongtim P, Champlin R, Dinh Y, Elgharably Y, Wang M, et al. Auto-SCT improves survival in systemic light chain amyloidosis: a retrospective analysis with 14-year follow-up. Bone Marrow Transplant. 2014;49(8):1036–41. [DOI] [PubMed] [Google Scholar]
- 108.Browning S, Quillen K, Sloan JM, Doros G, Sarosiek S, Sanchorawala V. Hematologic relapse in AL amyloidosis after high-dose melphalan and stem cell transplantation. Blood. 2017;130(11):1383–6. [DOI] [PubMed] [Google Scholar]
- 109.Tandon N, Muchtar E, Sidana S, Dispenzieri A, Lacy MQ, Dingli D, et al. Revisiting conditioning dose in newly diagnosed light chain amyloidosis undergoing frontline autologous stem cell transplant: impact on response and survival. Bone Marrow Transplant. 2017;52(8):1126–32. [DOI] [PubMed] [Google Scholar]
- 110.Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357(11):1083–93. [DOI] [PubMed] [Google Scholar]
- 111.Gertz MA, Lacy MQ, Dispenzieri A, Buadi FK, Dingli D, Hayman SR, et al. Stem cell transplantation compared with melphalan plus dexamethasone in the treatment of immunoglobulin light-chain amyloidosis. Cancer. 2016;122(14):2197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hazenberg BP, Croockewit A, van der Holt B, Zweegman S, Bos GM, Delforge M, et al. Extended follow up of high-dose melphalan and autologous stem cell transplantation after vincristine, doxorubicin, dexamethasone induction in amyloid light chain amyloidosis of the prospective phase II HOVON-41 study by the Dutch-Belgian Co-operative Trial Group for Hematology Oncology. Haematologica. 2015;100(5):677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Minnema MC, Nasserinejad K, Hazenberg B, Hegenbart U, Vlummens P, Ypma PF, et al. Bortezomib based induction followed by stem cell transplantation in light chain amyloidosis: results of the multicenter HOVON 104 trial. Haematologica. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Souza A, Dispenzieri A, Wirk B, Zhang MJ, Huang J, Gertz MA, et al. Improved Outcomes After Autologous Hematopoietic Cell Transplantation for Light Chain Amyloidosis: A Center for International Blood and Marrow Transplant Research Study. J Clin Oncol. 2015;33(32):3741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Dingli D, Leung N, et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48(4):557–61. [DOI] [PubMed] [Google Scholar]
- 116.Bashir Q, Langford LA, Parmar S, Champlin RE, Qazilbash MH. Primary systemic amyloid light chain amyloidosis decompensating after filgrastim-induced mobilization and stem-cell collection. J Clin Oncol. 2011;29(4):e79–80. [DOI] [PubMed] [Google Scholar]
- 117.Scott EC, Heitner SB, Dibb W, Meyers G, Smith SD, Abar F, et al. Induction bortezomib in Al amyloidosis followed by high dose melphalan and autologous stem cell transplantation: a single institution retrospective study. Clin Lymphoma Myeloma Leuk. 2014;14(5):424–30.e1. [DOI] [PubMed] [Google Scholar]
- 118.Sanchorawala V, Wright DG, Seldin DC, Falk RH, Finn KT, Dember LM, et al. High-dose intravenous melphalan and autologous stem cell transplantation as initial therapy or following two cycles of oral chemotherapy for the treatment of AL amyloidosis: results of a prospective randomized trial. Bone Marrow Transplant. 2004;33(4):381–8. [DOI] [PubMed] [Google Scholar]
- 119.Afrough A, Saliba RM, Hamdi A, Honhar M, Varma A, Cornelison AM, et al. Impact of Induction Therapy on the Outcome of Immunoglobulin Light Chain Amyloidosis after Autologous Hematopoietic Stem Cell Transplantation. Biol Marrow Transplant. 2018;24(11):2197–203. [DOI] [PubMed] [Google Scholar]
- 120.Kastritis E, Anagnostopoulos A, Roussou M, Toumanidis S, Pamboukas C, Migkou M, et al. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007;92(10):1351–8. [DOI] [PubMed] [Google Scholar]
- 121.Palladini G, Milani P, Foli A, Vidus Rosin M, Basset M, Lavatelli F, et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 2014;28(12):2311–6. [DOI] [PubMed] [Google Scholar]
- 122.Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira MT, Kwok F, et al. A Randomized Phase III Trial of Melphalan and Dexamethasone (MDex) Versus Bortezomib, Melphalan and Dexamethasone (BMDex) for Untreated Patients with AL Amyloidosis. Blood 2016;128:646. [Google Scholar]
- 123.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19):4391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Blade J, et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118(4):865–73. [DOI] [PubMed] [Google Scholar]
- 125.Cohen AD, Scott EC, Liedtke M, Kaufman JL, Landau H, Vesole DH, et al. A Phase I Dose-Escalation Study of Carfilzomib in Patients with Previously-Treated Systemic Light-Chain (AL) Amyloidosis. Blood 2014;124:4741. [Google Scholar]
- 126.Sanchorawala V, Palladini G, Kukreti V, Zonder JA, Cohen AD, Seldin DC, et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130(5):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seldin DC, Choufani EB, Dember LM, Wiesman JF, Berk JL, Falk RH, et al. Tolerability and efficacy of thalidomide for the treatment of patients with light chain-associated (AL) amyloidosis. Clinical lymphoma. 2003;3(4):241–6. [DOI] [PubMed] [Google Scholar]
- 128.Dispenzieri A, Lacy MQ, Rajkumar SV, Geyer SM, Witzig TE, Fonseca R, et al. Poor tolerance to high doses of thalidomide in patients with primary systemic amyloidosis. Amyloid. 20.257–61:(4)10;03 [DOI] [PubMed] [Google Scholar]
- 129.Dispenzieri A, Dingli D, Kumar SK, Rajkumar SV, Lacy MQ, Hayman S, et al. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 2010;85(10):757–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dispenzieri A, Lacy MQ, Zeldenrust SR, Hayman SR, Kumar SK, Geyer SM, et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 200.465–70:(2)109;7 [DOI] [PubMed] [Google Scholar]
- 131.Dinner S, Witteles W, Afghahi A, Witteles R, Arai S, Lafayette R, et al. Lenalidomide, melphalan and dexamethasone in a population of patients with immunoglobulin light chain amyloidosis with high rates of advanced cardiac involvement. Haematologica. 2013;98(10):1593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kastritis E, Gavriatopoulou M, Roussou M, Bagratuni T, Migkou M, Fotiou D, et al. Efficacy of lenalidomide as salvage therapy for patients with AL amyloidosis. Amyloid. 2018;25(4):234–41. [DOI] [PubMed] [Google Scholar]
- 133.Palladini G, Milani P, Foli A, Basset M, Russo F, Perlini S, et al. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129(15):2120–3. [DOI] [PubMed] [Google Scholar]
- 134.Dispenzieri A, Buadi F, Laumann K, LaPlant B, Hayman SR, Kumar SK, et al. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119(23):5397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sanchorawala V, Shelton AC, Lo S, Varga C, Sloan JM, Seldin DC. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016;128(8):1059–62. [DOI] [PubMed] [Google Scholar]
- 136.Sharpley FA, Manwani R, Mahmood S, Sachchithanantham S, Lachmann H, Gilmore J, et al. Real world outcomes of pomalidomide for treatment of relapsed light chain amyloidosis. British J haematol. 2018;183(4):557–63. [DOI] [PubMed] [Google Scholar]
- 137.Sher T, Fenton B, Akhtar A, Gertz MA. First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis. Blood. 2016;128(15):1987–9. [DOI] [PubMed] [Google Scholar]
- 138.Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130(7):900–2. [DOI] [PubMed] [Google Scholar]
- 139.Khouri J, Kin A, Thapa B, Reu FJ, Bumma N, Samaras CJ, et al. Daratumumab proves safe and highly effective in AL amyloidosis. British J Haematol. 2018. [DOI] [PubMed] [Google Scholar]
- 140.Kimmich CM, Schönland SO, Ziehl R, Ho AD, Dittrich T, Müller-Tidow C, et al. Daratumumab Monotherapy in Thirty-Two Heavily Pre-Treated Patients with Advanced Systemic Light-Chain Amyloidosis. Blood 2017;130:1837. [Google Scholar]
- 141.Leung N, Thome SD, Dispenzieri A. Venetoclax induced a complete response in a patient with immunoglobulin light chain amyloidosis plateaued on cyclophosphamide, bortezomib and dexamethasone. Haematologica. 2018;103(3):e135–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. [DOI] [PubMed] [Google Scholar]
- 144.Obici L, Cortese A, Lozza A, Lucchetti J, Gobbi M, Palladini G, et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid. 2012;19 Suppl 1:34–6. [DOI] [PubMed] [Google Scholar]
- 145.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–9. [DOI] [PubMed] [Google Scholar]
- 146.Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325–32. [DOI] [PubMed] [Google Scholar]
- 147.Palladini G, Barassi A, Klersy C, Pacciolla R, Milani P, Sarais G, et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116(18):3426–30. [DOI] [PubMed] [Google Scholar]
- 148.Gertz M, Lacy M, Dispenzieri A, Hayman S, Kumar S, Buadi F, et al. Troponin T level as an exclusion criterion for stem cell transplantation in light-chain amyloidosis. Leuk Lymphoma. 2008;49(1):36–41. [DOI] [PubMed] [Google Scholar]
- 149.Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, et al. Depth of organ response in AL amyloidosis is associated with improved survival: grading the organ response criteria. Leukemia. 2018;32(10):2240–9. [DOI] [PubMed] [Google Scholar]
- 150.Kastritis E, Kostopoulos IV, Terpos E, Paiva B, Fotiou D, Gavriatopoulou M, et al. Evaluation of minimal residual disease using next-generation flow cytometry in patients with AL amyloidosis. Blood Cancer J. 2018;8(5):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Palladini G, Massa M, Basset M, Russo F, Milani P, Foli A, and Merlini G. Persistence of Minimal Residual Disease By Multiparameter Flow Cytometry Can Hinder Recovery of Organ Damage in Patients with AL Amyloidosis Otherwise in Complete Response. Blood 2016;128:3261. [Google Scholar]