Abstract
Activity-induced pain is common in those with chronic musculoskeletal pain and limits participation in daily activities and exercise. Our laboratory developed a model of activity-induced pain and shows that depletion of muscle macrophages prevents development of hyperalgesia. Adenosine triphosphate (ATP) is released from fatiguing muscle, activates purinergic receptors (P2X), and P2X4 receptors are expressed on macrophages. We hypothesized that exercise releases ATP to activate P2X4 receptors on muscle macrophages, which subsequently release interleukin-1β (IL-1β) to produce hyperalgesia. In an animal model of activity-induced pain, using male and female C57BL6/J mice, we show increased expression of P2X4 on muscle macrophages, and blockade of P2X4 receptors in muscle prevented development of hyperalgesia. Using a lentivirus expressing an artificial micro-RNA to P2X4 under the control of a CD68 promoter, we decreased expression of P2X4 mRNA in cultured macrophages, decreased expression of P2X4 protein in muscle macrophages in vivo, and prevented development of activity-induced hyperalgesia. We further show that macrophages primed with LPS differentially released IL-1β when treated with ATP in neutral or acidic pH. Lastly, blockade of IL-1β in muscle prevented development of hyperalgesia in this model. Thus, our data suggest that P2X4 receptors could be a valid pharmacological target to control activity-induced muscle pain experienced by patients with chronic musculoskeletal pain.
Keywords: chronic musculoskeletal pain, P2X4, ATP, IL-1β, fatigue
Introduction
Chronic pain is a major cause of disability and costs over $600 billion annually in medical costs and lost wages [16,50,56]. While exercise is an effective non-pharmacological treatment to chronic musculoskeletal pain [4,14,24], fatiguing exercise significantly increases pain and limits participation in physical activities [1,10,28]. Further, people with chronic pain conditions such as low back pain, osteoarthritis, and fibromyalgia (FM) are less physically active when compared to healthy controls, and lower physical activity is associated with disability and reduced function [23,30,38,39,50,60]. Effective treatment of movement-evoked pain could increase participation in activity and exercise. Thus, understanding mechanisms underlying movement-evoked pain could identify new therapeutic targets.
During fatiguing muscle contraction, muscles release metabolites including adenosine triphosphate (ATP), protons and lactate, which synergistically produce pain in human subjects [32,54,58,66]. In animals, these metabolites enhance neuron activity and produce muscle hyperalgesia [5,22,27,34]. Extracellular ATP activates purinergic (P2X) cation channels and has been implicated in a variety of pain models [37,48,68,70]. Of particular interest, P2X4 mRNA is increased after exercise in people with fibromyalgia [35], and P2X4 receptor knockout mice do not develop hyperalgesia after peripheral inflammation [69,70]. P2X4 receptors are expressed on immune cells including tissue-resident macrophages [72] suggesting immune cells could be involved in the effects of P2X4. In keeping with this, our recent data demonstrate increases in the number of muscle macrophages after fatiguing stimuli, and removal of muscle macrophages prevents development of activity-induced hyperalgesia [19].
Activated immune cells release cytokines that can subsequently activate nociceptors to produce pain and hyperalgesia. Pro-inflammatory cytokines, interleukin (IL)-1β, tumor necrosis factor-〈 (TNF) and IL-6, can all activate nociceptors and produce hyperalgesia [11,18,59,73]. Prior studies show higher doses of ATP when given alone are required to induce release of IL-1β from macrophages, but after priming macrophages with the Toll-like receptor (TLR)-4 agonist lipopolysaccharide (LPS), lower doses of ATP are sufficient to induce IL-1® release [52]. It is unclear if there is a synergistic interaction between acidic pH and ATP to enhance release, or if cells need to be primed with a TLR agonist to see increases in inflammatory cytokines. Thus, we hypothesized that fatiguing exercise releases ATP to activate P2X4 receptors on muscle macrophages to enhance release of IL-1β and produce hyperalgesia (Fig. 1a). We tested this hypothesis using pharmacological, genetic, and immunochemical techniques in a mouse model of activity-induced hyperalgesia.
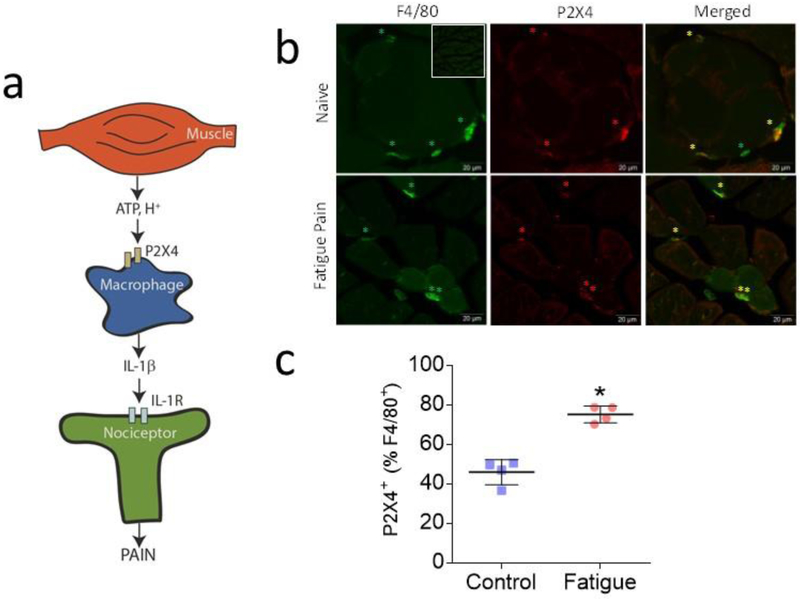
Fig 1. Increases in P2X4 in muscle macrophages after induction of activity-induced hyperalgesia.
a. Schematic graphic showing cross talk between muscle, local macrophages, and neurons. We suggest that fatigue metabolites, ATP and hydrogen ions, are released from contracting muscle that subsequently activate receptors on macrophages. Activation of macrophages by ATP would then increase release of the pro-inflammatory cytokine, interleukin-1β, which will subsequently activate receptors located on nociceptors to increase input to the central nervous system and produce pain. The current study is consistent with this hypothesis by showing blockade of the ATP receptor P2X4 in muscle, and downregulation of P2X4 in macrophages, prevents development of activity-induced hyperalgesia. We further show that ATP applied to macrophages increases release of IL-1β that is potentiated by acidic pH, and blockade of IL-1β receptors prevents development of hyperalgesia. b. Representative images of double-labeling for P2X4 (red) and the macrophage marker F4/80 (green) in muscle tissue from animals 24h after induction of the activity-induced pain model and naïve mice. Yellow star on merged imaged highlights double-labeled cells. Inset shows a lower power image of control immunohistochemical staining without the primary antibody (anti-P2X4). Bar = 20μm c. Quantification of the staining showed an increase in the percentage of P2X4+ on macrophages in muscles 24h after induction of the activity-induced pain model (n=4) when compared to muscles from naive animals (n=4)(B). * P <0.0001. Bar represents 50μm. Data are represented as scatterplots with mean and SEM.
Materials and Methods
Animal care and use
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Iowa and were performed in accordance with the National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals. Male and female C57BL6/J mice from Jackson Laboratories, age 4–9 weeks, were used in all procedures.
Development of Lentivirus
Development of lentiviral vector.
Synthetic oligonucleotides for pre-miRNA sequences against mouse P2X4 were selected by the proprietary BLOCK-iT software (Invitrogen, Carlsbad, CA). Three candidate oligonucleotide sequences were purchased and cloned into the plasmid pcDNA6.2-GW EmGFP-miR (Invitrogen). The DNA sequence of each construct was verified by bidirectional DNA sequencing. The target sequences on P2X4 mRNA and their respective locations are listed on Table 1. As a negative control, a miRNA sequence not predicted to target any known mammalian gene was also inserted into pcDNA6.2-GW EmGFP-miR (Invitrogen). Preliminary studies examined the effectiveness of the three candidate pre-miRNA sequences by knockdown of heterologous P2X4 expression in CHO cells. Of these three sequences, miR-869 produced the greatest knockdown of P2X4 protein, examined by Western Blot (93%) and was chosen for subsequent experiments (unpublished results).
Table 1.
The target sequences for the miRNA against P2X4
| miRNA | Pre-miRNA Sequence | Target on P2X4 RNA |
|---|---|---|
| 160 | TGCTGCACAGAGTCCGTTTCCTGGTAGTTTTGG CCACTGACTGACTACCAGGACGGACTCTGTG |
GUGUCUCAGGCAGGACCAU |
| 422 | TGCTGTTCCAGTCCCAATTCCACTGCGTTTTGG CCACTGACTGACGCAGTGGATGGGACTGGAA |
AAGGUCAGGGUAGGUGACG |
| 869 | TGCTGAATTGTAGCCAGGAGACACGTGTTTT GGCCACTGACTGACACGTGTCTTGGCTACAATT |
UUAACAUCGGUUCUGUGUG |
A lentivirus was created with a cell-specific promotor for macrophage CD68, GFP and an artificial miRNA to the P2X4 receptor [13,51]. Lentiviruses are not retrogradely transported from peripheral terminals of sensory neurons to the cell body, and thus do not affect gene transcription in this cell type [49]. Recombinant lentiviruses were constructed by cloning miR-869 into miRNA expression cassettes (Invitrogen) using the pDRIVE-mCD68 plasmid (Invitrogen, San Diego, CA), which contains a CD68-specific promoter. Virus particles were pseudotyped using a VSV-G envelope protein. The resulting viruses were produced in 293T cells, concentrated by high-speed centrifugation, purified by further centrifugation through 20% sucrose/PBS-D and stored in 10% sucrose/PBS-D at −80° C. Virus particle concentrations were determined by quantitative real-time PCR for proviral DNA 24 hours following transduction of 293T cells and are expressed as transducing units per microliter (tu/μl).
Transfection of cultured macrophages with lentivirus.
Elicited macrophages were obtained from the peritoneal cavities of mice using standard techniques. Briefly, 1% thioglycollate (Becton Dickinson, Sparks, MD) was injected into the peritoneal cavity of mice (9 wk old, n=5: 3M, 2F) to elicit macrophage influx 5 days prior to harvesting macrophages for culture. Mice were sacrificed (CO2 inhalation). Fetal bovine serum (3%, FBS) was injected into the peritoneum, the abdomen was massaged for 5 min, and fluid retrieved and centrifuged (1500 RPM, 10 min, 4°C). Cells were resuspended (in RPMI-1640, 10% FBS, P-S (penicillin-streptomycin); Life Technologies, Carlsbad, CA), and then cells from each mouse were split into 3 T25 flasks at 6 × 106 cells/flask and incubated for 48h (37°C, 5% CO2).
Cultured macrophages were transduced with lentiviral constructs applied at multiplicity of infection (MOI) of 2 (in RPMI-1640, P-S, 2.5% FBS, 2 μg/ml polybrene, 24h, 37°C, 5% CO2). Three matched sets for each mouse were treated as follows: untreated, control virus, and P2X4 miRNA virus. After 24h, the medium was removed, and cells were incubated in fresh medium for 6 days. On the 6th day 2 μg/ml puromycin was added to the cultures to select for transduced macrophages; 2 days later cells were prepared for RNA isolation and analysis.
Quantitative PCR from cultured macrophages
RNA was isolated from treated macrophages with RNA-STAT60 (Tel-test, Friendswood, TX) according to the manufacturer’s directions, with the following specifications. Samples were stored at −80°C for less than two weeks before processing. Total RNA concentrations were measured on a Nanodrop One (Thermo Scientific, Wilmington, DE) and showed an average 260/280=1.94. Concentration of RNA was confirmed and quality assessed with an Agilent Bioanalyzer (Model 2100, Santa Clara, CA) at the Iowa Institute of Human Genomics (University of Iowa). All RNA samples showed an RNA integrity number between 9.5–10. Reverse transcription, with 17.5, 35, or 1400 ng RNA per 20 μl reaction, was carried out using SuperScript VILO cDNA synthesis kit (Life Technologies, Carlsbad, CA) in a MyCycler thermocycler (BioRad, Hercules, CA) programmed as follows: 25°C 10’, 42°C 60’, 85°C 5’. cDNA was stored at −20°C.
qPCR was performed with 25 μl/well reactions using 2X Taqman Mastermix and 20X Taqman Gene Expression Assays (Life Technologies, Carlsbad, CA) for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Mm99999915_g1, 109 bp of amplicon length), β-actin (Actb, Mm02619580_g1, 143 bp of amplicon length), P2X3 (Mm00523699_m1, 74 bp of amplicon length), P2X4 (Mm0051787_m1, 66 bp of amplicon length), P2X5 (Mm00473677_m1, 104 bp of amplicon length) and P2X7 (Mm01199500_m1, 80 bp of amplicon length).
Assays contained One 6-FAM™ dye-labeled TaqMan® MGB probe, and either 2.5 ng/well (P2X4, P2X7) or 100 ng/well (P2X3, P2X5). GAPDH and β-actin internal controls were assayed at both 2.5 and 100 ng/well. qPCR was run on either an Applied Biosystems Prism7900 (Efficiency determination, reference gene validity) or Applied Biosystems QuantStudio-7 FLEX (ΔΔCt of non-treated, control virus, RNAiP2X4 virus samples) at the University of Iowa Institute of Human Genetics Genomics Division. All samples were assayed in triplicate.
Controls included removal of the cDNA from the assay (no template control), and not performing reverse transcription - no amplification was observed in any control. Efficiencies for each Gene Expression Assay were determined through serial dilution curves on cDNA transcribed from RNA isolated from non-transduced macrophages; efficiency of amplification ranged from 89.3–96.8 and R2 values were ≥0.990. The validity of the reference genes chosen, GAPDH and β-actin, were determined by observing comparable relative cycle thresholds (Cts) for equivalent input cDNA transcribed from RNA isolated from non-transduced and transduced macrophages. The Cts for both reference genes were comparable between groups.
Measurement of cytokines from peritoneal macrophages
Cytokines were measured using multiplex from supernatants from cultured macrophages treated as follows. On day one, resident peritoneal macrophages were collected by lavage (n=6) as described above, but without thioglycollate elicitation, and resuspended in MEM-alpha complete instead of RPMI complete (10% FBS, Pen-Strep, 1X glutamax, Life Technologies, Carlsbad). Macrophages from a single mouse (n=3) or pooled from 2 mice (n=3) were used to obtain a sufficient number of macrophages for plating. Macrophages (600,000 cells/well) were plated in 24-well non-treated tissue culture plate (Nest Biotechnology #702011) for 24h. On day 2, cells were incubated with or without 10ng/ml LPS (Chondrex, Redmond, WA, E.Coli) for 4h, and then treated with pH 7.4 media (26 mM NaHCO3, 25 mM HEPES) or pH 6.5 media (26 mM NaHCO3, 20 mM MES, 5 mM HEPES) with or with 1mM ATP for 24h. Supernatants were collected and stored at −80°C until analysis. Supernatants were run using multiplex for the primary cytokine of interest, IL-1β, and secondary cytokines IL-6, TNFα, IFN©, GM-CSF, IL-10, IL-5, and IL-4. Multiplex plates were processed using standard procedures provided by the manufacturer and read on a BioRad Bio-Plex (Luminex 200) at the University of Iowa Flow Cytometry Core Facility. Two standards were used to generate a standard curve across the detectable range. Standard curves for the multiplex were run using pH 6.5 media and pH 7.4 media to address any effects of pH on antibody binding, and correlated accordingly. Samples were run in duplicate and coefficient of variation was assessed for each cytokine. All samples showed a coefficient of variation less than 20%.
Animal model of activity-induced pain
Muscle hyperalgesia was induced by combining low-intensity muscle insult with 6 min of fatiguing contractions as previously described [21]. On day 0, mice were anesthetized with 2–4% isoflurane and an intramuscular (i.m.) injection of 20μl normal saline adjusted to pH 5.0 or 7.2 (control group) were given. On day 5, mice were anesthetized with 2–4% isoflurane, needles electrodes were placed in the gastrocnemius muscle belly and 6 min of submaximal fatiguing contractions were given through a Grass stimulator (7V intensity, 40 Hz for 3.75s, 4.25 s between contractions; Grass Technologies, WesWarwick, RI). Immediately after the end of fatiguing muscle contractions, a second i.m. injection of 20 μl pH 5.0 or 7.2 normal saline were given. The hindpaw was attached to a force plate connected to a force transducer to quantify force output (iWorx, Dover, NH). Fatigue was quantified by examining the % decline in maximum force to three 100 Hz stimuli at 7 V by comparing forces before and after the fatiguing stimuli. We previously show that unbuffered pH 5.0 saline injections reduce muscle pH to approximately 6.9 [64] and neither two injections of pH 5.0 alone nor fatigue alone produce hyperalgesia [21,76].
Measurement of Muscle Withdrawal Thresholds
Muscle withdrawal thresholds (MWT) were measured by applying force-sensitive tweezers to the belly of the gastrocnemius muscle as previously described [63]. Mice were placed in a gardener’s glove, the hind limb was held in extension, and the muscle was squeezed until the animal withdrew its hind limb. A total of 3 measurements were taken and averaged. A decrease in withdrawal thresholds was interpreted as muscle hyperalgesia. The tester was blinded to groups in all behavioral measurements.
Antagonism of P2X4 receptors in the Activity-Induced Pain Model
For behavioral pharmacology experiments, mice were pretreated with the P2X4 receptor antagonist 5-(3-Bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one [2,7] (5-BDBD; 5, 50 or 500 μM, 20μl, i.m., n=8: 4M, 4F, per each condition) or vehicle (0.9 % saline, 20μl, n=8: 4M, 4F) 5 min before muscle fatigue on day 5. Twenty-four hours after the second pH 5.0 saline injection, animals were tested for muscle withdrawal threshold. In addition, another group were pretreated with 5-BDBD (500 μM, i.m., 20μl, n=8: 4M, 4F) or vehicle (0.9 % saline, n=8: 4M, 4F) 5 min before the first pH 5.0 saline injection, on day 0. For IL-1β antibody blocking experiments, an anti-mouse antibody to IL-1β (BioXCell, BE0246, West Lebanon, NH; .67 μg/μl, 20μl, n=6; 3M, 3F; 7.71 μg/μl, 20μl, n=6, 3M, 3F) was injected 30 minutes prior to fatiguing stimuli on Day 5. On day 5, muscle fatigue was induced and, immediately after the end of contractions, the second pH 5.0 injection was given. Twenty-four hours after, animals were tested for muscle withdrawal threshold. Animals were divided into multiple groups tested across several weeks. Each testing group consisted of vehicle control and multiple doses of 5-BDBD. The change from baseline 24h after induction of the model was calculated and presented for each testing condition.
Injection of lentivirus in vivo
Mice were anesthetized with 2–4% isoflurane and the virus injected into the muscle as previously described [19]. The skin overlying the left gastrocnemius muscle was incised, 4 × 5μl of miR_869 (12.1 × 109 vg/ml) or control miRNA (2.0 × 109 vg/ml) was injected into exposed muscle at a flow rate of 5 μl/min with a 30-gauge needle connected to a Hamilton syringe by a PE-10 tubing. After injection, the wound was covered by sterile gauze, and the tissue allowed to absorb the solution for 10 min. The wound was sutured and mice recovered for 4 weeks prior to behavioral tests.
Immunofluorescence of muscle macrophages
Mice were anesthetized with an intraperitoneal injection of pentobarbital (100 mg/kg) and transcardially perfused with heparinized saline followed by either 1) 4% paraformaldehyde (Fig. 1b) or 2) Periodate-Lysine-Paraformaldeyde (PLP) fixative (2% paraformaldehyde; 75mM Lysine; 10mM periodate; 0.05M Phosphate Buffer) (Fig. 3d). For PLP-fixed tissue, the muscle was post-fixed overnight with PLP at 4°C. The next day the muscle was washed with 0.1M Sorenson’s buffer and sequentially incubated in 15% sucrose in PBS for 24 h at 4°C, 30% sucrose in PBS for 24 h at 4°C, and 1:1 mixture of 30% sucrose and OCT mounting media for 24 h at 4°C. Finally, tissues were snap frozen in OCT using gentle Jane and stored at −80°C until cryosectioned. For 4% paraformaldehyde-fixed tissue, muscles were cryoprotected overnight in 30% sucrose and frozen at −80°C in OCT until cryosectioned. Muscle tissues were cut at 20μm, placed on slides and immunostained simultaneously to minimize experimental variability.
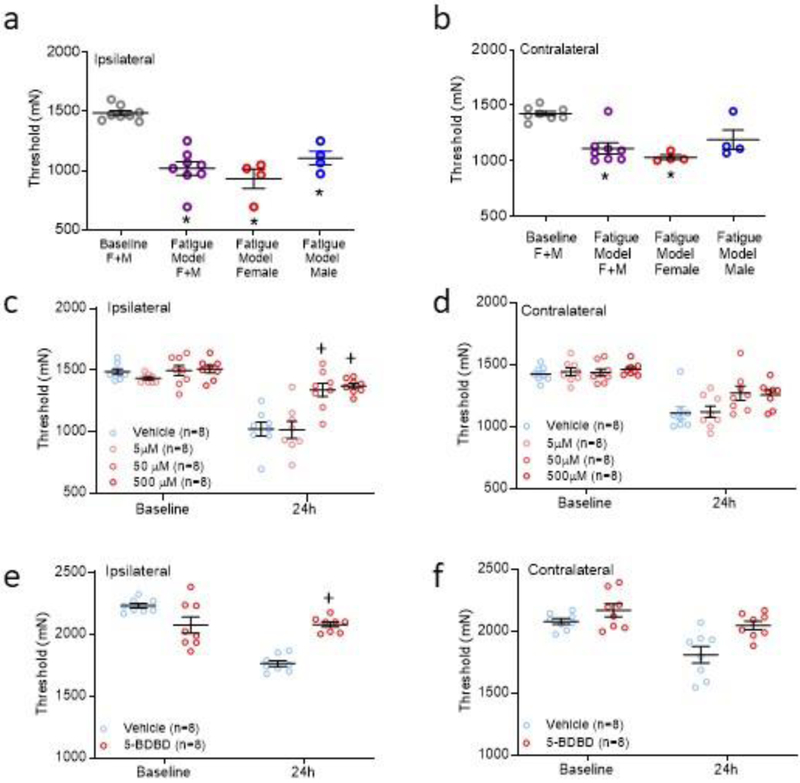
Fig 2. Blockade of P2X4 receptors in muscles in the activity-induced pain model.
a,b. Scatter plots with mean+S.E.M. showing withdrawal thresholds in the vehicle-treated group (n=8, 4M, 4F) ipsilaterally (a) and contralaterally (b) prior to induction of the model and twenty-four hours after the second injection of acidic saline. Withdrawal thresholds were significantly decreased bilaterally in female (F) and ipsilaterally in male (M) mice. *, p<0.05 compared to baseline. c,d. Scatter plots with mean+S.E.M. showing withdrawal thresholds of the muscle ipsilaterally (c) and contralaterally (d) for groups of animals treated with vehicle or 5, 50, or 500 μM 5-BDBD in muscle on Day 5 prior to the fatiguing contraction and second intramuscular saline injection. +, p<0.05 compared to vehicle control. e,f. Scatter plots with mean+S.E.M. showing withdrawal thresholds of the muscle ipsilaterally (e) and contralaterally (f) for groups of animals treated with vehicle or 500 μM 5-BDBD in prior to the first intramuscular saline injection. +P < 0.05 compared to vehicle control.
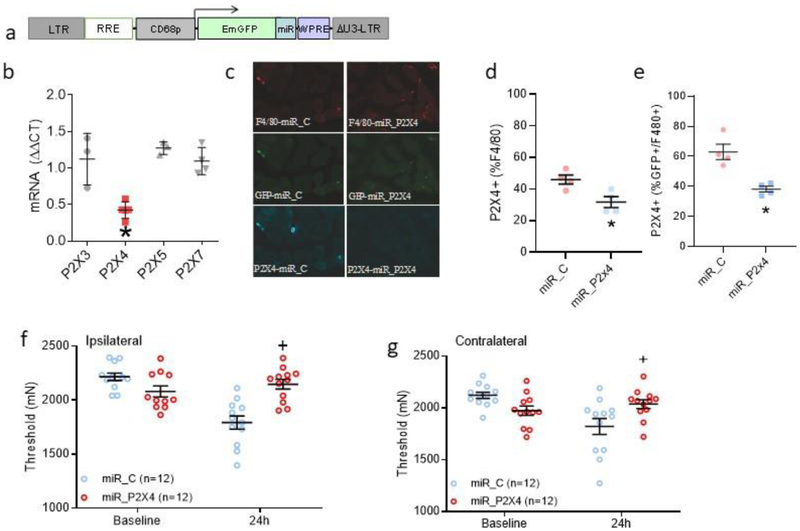
Fig 3. Genetic downregulation of P2X4 on muscle macrophages selectively downregulates P2X4 mRNA and protein in macrophages and prevents activity-induced hyperalgesia.
a. Schematic representation of the lentivirus vector design. The lentivirus developed in the current study incorporated a CD68 promoter to selectively express an artificial miRNA to P2X4, miR_869, in macrophages. b. Scatter plots with means and SEM showing cultured peritoneal macrophages treated with miRNA_896 showed a decrease in P2X4 mRNA (n=5), but not of P2X3 (n=3), P2X5 (n=3), or P2X7 (n=4) mRNA. *, P<0.05 compared to control virus. c. Representative images from muscle showing F4/80+, GFP+ and P2X4+ cells from animals injection with a lentivirus expressing a control miRNA (miR_C) or an artificial miRNA expressing P2X4 (miR_P2X4). d. The percentage of immunohistochemically stained macrophages (F4/80+) that express P2X4 from mice injected with the lentivirus expressing miRNA_869 (miR_P2X4, n=4) decreased the percentage of GFP+ cells expressing P2X4 relative to GFP+ cells in muscle from mice injected with control lentivirus (miR_C, n=4) *, P<0.05 e. The percentage of GFP+ macrophages (F4/80+) that express P2X4 in muscle tissue from mice injected with the lentivirus expressing miRNA_869 (miR_P2X4) or control lentivirus (miR_C) *, P<0.05. f.g. Bar graphs showing the change in ipsilateral (f) and contralateral (g) withdrawal thresholds of the muscle before (Baseline) and 24h after induction of the model from mice treated with a lentivirus expressing miR_C or miR_P2X4. Pretreatment with the Lentivirus expressing miRNA_869 (miRNA_P2X4)(n=12: 6M, 6F) prevented activity-induced pain ipsilaterally in male (e) and bilaterally in female mice (e,f) when compared to those injected with control miRNA (miR_C) (n=12: 6M, 6F). +P<0.05 compared to miR_C. Data are mean with SEM.
The sections from Figure 1b were post-fixed in PLP, and Figures 1b and 3d were blocked using Fc receptor blocker (Innovex Biosciences, Richmond, California) and 5% normal goat serum with background buster prior to incubation in primary antibody. For Figure 1b, we examined expression of P2X4 in muscle macrophages in the activity-induced pain model using double-staining for P2X4 and F4/80, a macrophage-specific marker (n=4: 2M, 2F per group). Sections were incubated with 1:400 rabbit anti-P2X4 (Alomone, Jerusalem, Israel) plus 1:500 rat anti-F4/80 (AbD serotec, Bio-Rad Co, USA) overnight at 4°C. The next day, sections were incubated with 1:500 dilution of Alexa568-conjugated goat anti-rat-IgG (Life Technologies, Grand Island, NY) plus 1:500 dilution of Alexa 647-conjugated goat anti-rabbit IgG (Life Technologies, Grand Island, NY) for 1h at room temperature. Examination of P2X4 expression after virus injection (Fig. 3d) was done by double-staining for P2X4 and F4/80 and examining for GFP-positive fluorescence. After performing a dilution series for both P2X4 and F4/80 we stained the tissue with a 1:2500 dilution rabbit anti-P2X4 antibodies (Alomone, Jerusalem, Israel) and a 1:500 dilution of anti-F4/80 antibody (AbD serotec, Bio-Rad Co, USA) overnight at 4°C. The next day, sections were incubated in a 1:500 dilution of Alexa 568-conjugated goat anti-rat IgG (Life Technologies, Grand Island, NY) plus 1:500 dilution of Alexa 647-conjugated goat anti-rabbit IgG (Life Technologies, Grand Island, NY) for 1h at room temperature. As a control, incubation of tissue without the primary antibody showed no immunoreactivity (Figure 3). Coverslips were mounted to slides using Prolong Diamond anti-fade mountant (Life Technologies, Grand Island, NY).
Muscle sections were imaged with a Zeiss LSM 710 Confocal fluorescence microscope (Fig. 1a) or using a BX-61 fluorescent microscope (Fig. 3d) connected with a spot camera in the Central Microscopy Research Facility at the University of Iowa. All images were taken under the same conditions on the same microscope and stored for later analysis. For Figure 2a, the number of F4/80+ macrophages that were also labeled for P2X4 were counted from saved images using Image J software (National Institutes of Health) from animals 24h after induction of the model. For quantification of macrophages in Figure 4d, we examined the 1) the percentage of F4/80+ macrophages that were positive for P2X4, and 2) the percentage of number of GFP+/F4/80+ macrophages that were P2X4-positive using Image J software.
Fig 4. Release of cytokines in LPS-primed macrophages culture treated with ATP in combination of neutral or acid pH.
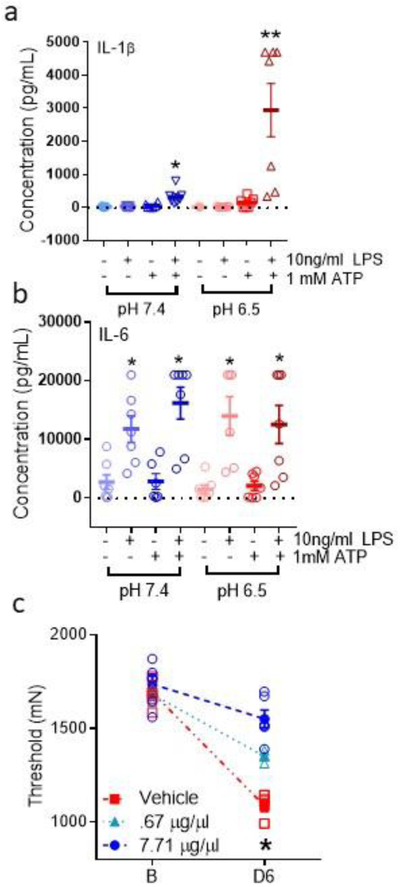
a. Scatter plots with mean and SEM showing IL-1β release from cultured macrophages. LPS (10ng/ml)-primed macrophages treated with ATP (1mmol) show an increase in IL-1β in supernatants compared with control conditions in pH 7.4, and an additional increase in pH 6.5 compared with control conditions or ATP and pH 7.4 conditions (n=6 per condition, except n=5 for LPS-/ATP+/pH 7.4 and (LPS+/ATP-/pH 6.5). *, P<0.05 compared to LPS-/ATP-/pH; **p<0.05, compared to LPS+/ATP+/pH 7.4 condition. b. Scatter plots with mean and SEM showing IL-1β release from cultured macrophages. IL-6 show increases with LPS-priming but do not show additional increases with ATP with or without pH 6.5 *, P<0.05 compared to LPS-/ATP-/pH. c. Scatter plots with mean and SEM from animals treated with an IL-1β immunizing antibody or vehicle prior to induction of the model. Behavior tests were performed 24h after induction of model (D6). Pretreatment of the muscle with an immunizing antibody to IL-1β (7.71 μg/μl, n=6; 3M, 3F) prevented the activity-induced pain compared to vehicle (n=6; 3M, 3F). *, P<0.05 compared to vehicle control.
Experimental Design and Statistical Analysis
In the present study we hypothesized that exercise releases ATP to activate P2X4 receptors on muscle macrophages, which subsequently release interleukin-1β (IL-1β) to produce hyperalgesia. To address this hypothesis we used pharmacological, genetic, and immunochemical techniques in a mouse model of activity-induced hyperalgesia.
The primary endpoints were defined prior to data collection and included the withdrawal threshold of the muscle for behavioral experiments and quantitation of IL-1β by multiplex analysis. Secondary endpoints included confirmation of knockdown using qPCR, immunofluorescence microscopy, and quantitation of additional cytokines by multiplex. For behavioral studies, power analysis determined the minimal sample size for withdrawal thresholds at 80% power and p<0.05 by comparing means for two independent samples (mean 1: 102%; mean 2: 80%; SD: 15) indicate a sample size of 8 mice per group. For IL-1β release from cultured macrophages we used preliminary results (mean 1: 88; mean 2: 10; SD: 27) to estimate a sample size of 4 per group; we increased these to 6–7 per conditions to improve robustness of results. We excluded reporting IL-12 since the limit of detection was altered by the presence of tissue culture media and influenced by pH. All data collected, including outliers, were analyzed. For biological assays, samples were run in duplicate or triplicate and replicated more than once, and the researcher that analyzed the results was blinded to groups. In behavioral assays, an average of 3 trials at each time period was tested, testing was done on multiple days with both controls and experimental groups, and the tester was blinded to groups in all behavioral measurements.
Data are reported as mean ± S.E.M. Muscle withdrawal thresholds and fatigue reductions are reported as mean thresholds. The muscle withdrawal thresholds were analyzed with a repeated measures ANOVA (sex, group) followed by post hoc with a Tukey’s test; sex considered an exploratory analysis. qPCR quantification for each P2X receptor and macrophage quantification were assessed with a one-way ANOVA and post-hoc testing with a paired t-test. Immunofluorescence data compared two groups (control, experimental) with a t-test. Cytokine release was analyzed with repeated measures ANOVA with post-hoc testing with a paired t-test. Data were considered significant if p<0.05.
Results
Increased expression of P2X4 on muscle macrophages after induction of activity-induced pain model
Since our prior study showed that depletion of macrophages in muscle prevents development of activity-induced hyperalgesia [19], and P2X4 receptors are located on macrophages [70], we examined if P2X4 receptors are expressed on muscle macrophages and if they are upregulated in our activity-induced pain model (Fig. 1b,c). There was a significant increase in the percentage of F4/80+ cells that express the P2X4+ receptor in muscles 24h after induction of the model when compared to muscles from control animals (t-test, p<0.0001, Fig. 1c). An average of 256 ± 25.2 F4/80+ macrophages were counted per animal from multiple sections across the muscle.
Blockade of P2X4 receptors in muscle prevents development of activity-induced pain
Average baseline withdrawal latencies in the vehicle-treated group averaged 1486+23 mN ipsilaterally (Fig. 2a) and 1425+21 mN contralaterally (Fig.2b) prior to induction of the model. Twenty-four hours after the second injection of acidic saline, withdrawal thresholds decreased by 68±4% on the ipsilateral muscle (Fig. 2a, ALL: 1018±56 mN, p=0.0001; Male 1106±58 mN; Female 931±80 mN), and an average of 79±4% mN on the contralateral muscle (Fig. 2b, ALL: 1123±48 mN, p=0.002; Male 1189±87 mN; Female 1058±25)(Fig. 2). There was a significant effect for time both ipsilaterally (F1,16=80, p=0.0001) and contralaterally (F1,16=27.3, p=0.002). Similar to our prior publication [21], withdrawal thresholds were significantly decreased bilaterally in female mice (p=0.01 ipsilateral; p=0.0024 contralateral), and ipsilaterally in male mice (p=0.04 ipsilateral; p=0.40 contralateral).
To test if P2X4 receptors in muscle play a significant role in the development of activity-induced pain, we pharmacologically blocked P2X4 receptors with 5-BDBD in muscle before the fatiguing contraction. There was a significant effect for time bilaterally (ipsilateral: F1,24=68.2, P=0.0001; contralateral: F1,24=91.6, P=0.0001) (F3,24=7.7, =0.001). In control animals treated with vehicle, male mice showed a decrease in withdrawal threshold of the muscle ipsilaterally, and female mice show a decrease in withdrawal threshold of the muscle bilaterally, as we previously published [21]. Blockade of P2X4 receptors in the injected muscle by 5-BDBD prior to the fatiguing contraction at the time of the second injection on Day 5 dose-dependently prevented the decrease in muscle withdrawal threshold ipsilaterally (time*dose: F3,24=4.8, p=0.009) but not contralaterally (time*dose: F3,24=2.2, p=0.12). Post-hoc testing showed significant increases in withdrawal thresholds for the 50 (p=0.002) and 500 μM (p=0.0001) dose ipsilaterally (Fig. 2b,c). Exploratory analysis for sex showed a bilateral reversal with 50 (F: p=0.005; M: p=0.001) and 500 μM (F: p=0.005; M: p=0.002) doses in male and female mice ipsilaterally, and a significant reversal with 50 (p=0.05) and 500 μM (p=0.03) for female, but not male, mice contralaterally (Supplementary Figure 1). These data suggest that activation of P2X4 receptors in the muscle tissue during the fatiguing contractions contributed to muscle hyperalgesia.
To test if activation of P2X4 is important for the priming stage in the activity-induced pain model, we administered 5-BDBD (500μM) to the muscle before the first injection of acidic saline. Blockade of P2X4 prior to the first injection prevented the decreases in withdrawal threshold ipsilaterally (F1,16=43.7, p=0.0001) but not contralaterally (Fig. 2e,f). Exploratory analysis for sex differences showed in male mice 5-BDBD prevented the decrease on the ipsilateral side (p=0.003) and in female mice 5-BDBD prevented the decrease on the on both the ipsilateral (p=0.01) contralateral side (p=0.02) (Supplementary Figure 1).
To examine if blockade of P2X4 altered the amount of muscle fatigue, we examined maximal force output to a suprathreshold electrical stimulation. The force of muscle contractions decreased significantly across time in all groups, but there were no significant differences between groups immediately after the fatiguing stimulation (61 ± 10 Vehicle; 63 ± 5 5-BDBD; p>0.05).
Knockdown of P2X4 receptors on muscle macrophages prevents development of activity-induced hyperalgesia
To test whether P2X4 receptors expressed on local macrophages are required for the development of hyperalgesia in the activity-induced pain model, we developed a cell-specific lentivirus to downregulate P2X4 in macrophages in vivo. This lentivirus utilized a CD68 promotor to express a miRNA to P2X4, miR_869, specifically in macrophages (Fig. 3a). To show selectivity of the miR_869 to P2X4, we infected cultured peritoneal macrophages with the lenti-P2X4 or control virus and examined mRNA expression for P2X3 (n=3), P2X4 (n=5), P2X5 (n=3) and P2X7 (n=4) using qPCR. We show that P2X4 mRNA was significantly decreased in the cells treated with miR_869 (one-way ANOVA with Tukey’s post hoc test, p=0.003), whereas mRNAs for P2X3, P2X5, or P2X7 were unaltered (Fig. 3b). Next, we tested the ability of the lentivirus expressing miR_869 to downregulate P2X4 protein in muscle macrophages. Immunofluorescent staining of muscle from mice injected with the lentivirus expressing miR_869 (n=4) showed that the number of F4/80+ cells and GFP+/F480+ cells expressing P2X4 was significantly reduced as compared to F4/80+ (p=0.02) and GFP+/F4/80+ cells (p=0.004) in muscle from mice injected with control lentivirus (Fig. 3c,d). Thus, these data confirm that lenti-miR_869 significantly decreases P2X4 in macrophages, and this decrease in P2X4 occurred in muscle macrophages in vivo.
We then injected the lentivirus into muscle to downregulate P2X4 on muscle macrophages and examined activity-induced hyperalgesia. Pre-treatment with the lentivirus expressing miRNA_869 (n=12; n=6M; n=6F) prevented the development of hyperalgesia ipsilaterally in male and female mice (Fig. 3e) and contralaterally in female mice (Fig. 3f) when compared to those injected with control miRNA (n=12; n=6M; n=6F) or naïve (n=4; n=2M; n=2F). There was a significant effect for group ipsilaterally (F1,20=29.6, p=0.0001) and contralaterally (F1,20=5.20, p=0.03). Exploratory analysis for sex showed a significant difference between groups in both male (p=0.02) and female (p=0.02) mice ipsilaterally and for female (p=0001) mice contralaterally. The force of contraction decreased significantly in both groups after the fatiguing stimulus, but there were no significant differences between groups in the post-fatigue force (miR_C 48 ± 6; miR_p2X4 49 ± 6; t-test; p>0.05).
ATP increases release of IL-1 β in cultured macrophages
To test if ATP increased the release of cytokines, we compared LPS-primed and unprimed cultured macrophages treated with ATP in combination with neutral or acidic pH. We show that LPS-primed macrophages treated with ATP (at pH 7.4) released significantly more IL-1β compared with control, and that acidic pH further increased ATP release (p=0.01) compared with control conditions or exposure to ATP at neutral pH (Fig. 4a)(repeated ANOVA, F7,35=17.9, p=0.008). Significant increases were also observed in secretion of granulocyte macrophage colony stimulating factor (GM-CSF) (F7,35=8.6, p=0.0001) by LPS-primed macrophages treated with ATP at pH 7.4 (p=0.005) or pH 6.5 (p=0.001) (Supplementary Fig. 1). In contrast, IL-6, IL-4, and IL-5 secretion increased significantly after LPS-priming (p=0.007–0.04; IL6: F7,35=11.2, p=0.0001; IL-4 F7,35=8.4, p=0.0001; IL-5 F7,35=7.4, p=0.001) but was not further influenced by ATP with or without pH 6.5 (p=0.12) (Fig. 4b, Supplementary Fig. 1e). LPS-primed macrophages in pH 7.4 increased IFN-© release, as expected (F7,35=6.8, p=0.0001), but this response was slightly blunted by ATP or acidic pH and under these conditions, secretion of INF© was not statistically significant (Supplementary Fig. 2). Secretion of TNF and IL-10 were not significantly altered under any of the conditions tested (F7,35=4.1, p=0.001 and F7,35=0.5, p=0.83, respectively) (Supplementary Fig. 2). Thus, differential release of cytokines from macrophages was observed. ATP and acidic pH elicited a synergistic release of IL-1β in primed macrophages, whereas LPS followed by ATP-induced secretion of GM-CSF.
Blockade of IL-1β in muscle prevents development of activity-induced hyperalgesia
Since IL-1β was significantly increased by ATP as well as ATP in combination with pH 6.5, we tested if blockade of IL-1β during the fatigue task would prevent the development of hyperalgesia. Pretreatment of the muscle with a neutralizing antibody to IL-1β significantly prevented the development of hyperalgesia 24h after induction of the model (time*dose effect: F2,9=27.1, p=0.001) at the highest concentration tested 7.71 μg/μl (p=0.0001 compared to IgG control)(4c).
Discussion
To our knowledge, the findings of the present study demonstrated, for the first time, the involvement of P2X4 receptors expressed on muscle macrophages in the development of activity-induced hyperalgesia. Specifically, we show that P2X4-expressing macrophages were more abundant in muscle after induction of the model, consistent with prior studies showing expression of P2X4 on tissue resident macrophages [70]. We also show that pharmacological blockade of peripheral P2X4 receptors in muscle during the first or the second injection of acidic saline prevents development of activity-induced hyperalgesia, and that downregulation of P2X4 in muscle macrophages prevents development of activity-induced hyperalgesia. These data identify important roles for ATP and macrophages in activity-induced hyperalgesia.
Macrophages are essential for innate immunity and play a critical role in tissue repair and restoration of homeostasis after infection and injury; and as such are located in all tissues, including muscle [40]. The current data are consistent with our prior studies demonstrating that local depletion of macrophages in muscle prevents development of hyperalgesia in animal models of muscle pain [18,19]. Similarly, systemic depletion of monocytes/macrophages prevents development of inflammatory hyperalgesia, neuropathic, incisional pain, formalin-induced pain behaviors and delays resolution of inflammatory hyperalgesia [17,62,74], and hyperalgesia associated with IL-1β and angiotensin II [61,74]. We previously showed a small increase in muscle macrophages after the fatiguing stimulation [19] that is substantially less than an acute inflammatory stimuli [9,18]. Prior studies show activation and proliferation of resident macrophages occurs after mild pathology as well as early after tissue injury; monocyte-derived infiltration only occurs after more severe pathology [41–43]. These data suggest that any changes in macrophages in response to the fatigue and acid stimuli are likely a result of local proliferation as there is no pathology or neutrophilic infiltration in the muscle in this model [21].
Fatiguing muscle contractions decrease muscle pH and release ATP [3,19,25,32,58]. When the combination of fatigue metabolites, low pH, lactate, and ATP are injected into muscle of human subjects, low doses produce a fatigue sensation and higher doses produce pain [54]. Similarly, the combination of ATP with low pH produces a synergistic response as shown by enhanced muscle hyperalgesia in animals, release of calcium from dorsal root ganglia cells, and acid currents in dorsal root ganglia cells [5,22,27,34,45,75]. Our current data show a similar synergism in release of IL-1β from cultured resident macrophages. Specifically, IL-1β and GM-CSF were released from LPS-primed cultured macrophages treated with ATP, and the addition of acidic pH enhanced the release of IL-1β. This enhanced release was specific for IL-1β and did not occur for other measured cytokines. The concentrations of ATP used in the current study could activate either P2X4 or P2X7 [47,67], and thus future experiments should examine the role of P2X4 and P2X7 in release of IL-1β. We further showed that blockade of IL-1β in muscle prevented development of activity-induced hyperalgesia, thereby demonstrating a functional role for IL-1β which is consistent with prior studies showing detectable levels of IL-1β mRNA and protein in skeletal muscle [46,71], increased release of IL-1β in muscle after a single exercise task [6,46], injection of IL-1β into peripheral tissues produces hyperalgesia [12,15] and blockade of IL-1β prevents development of hyperalgesia in animal models of inflammatory pain [8,57]. While prior studies show ATP-induced release of IL-1β from cultured macrophages [52,53], our studies are the first to demonstrate synergistic interactions between acidic pH and ATP in non-neuronal tissues. The underlying mechanisms for this synergism between acidic pH and ATP could be similar to that observed in neurons and may involve interactions between purinergic receptors and acid sensing ion channels [5,34].
P2X4R-deficient mice do not develop acute inflammatory pain induced by intraplantar carrageenan, intraplantar complete Freund’s adjuvant or nerve injury [69,70]. Prior studies show that macrophages are the main cell type expressing P2X4 in tissue and that this receptor is constituently expressed [70], and our data suggest increased expression of P2X4 in muscle macrophages in the activity-induced pain model. Whereas sensory neurons can express P2X4 [29], P2X4−/− mice show reduced ATP-induced calcium currents in macrophages [70] and the present study shows that downregulation of P2X4 in muscle macrophages prevents development of activity-induced hyperalgesia. These data are consistent with a functional role of P2X4 in resident macrophages contributing to activity-induced hyperalgesia.
The lack of sex-specific effects for P2X4 was surprising given prior studies showing sex-specific mechanisms in the dorsal horn microglia for P2X4 [36,65]. Whereas our model shows sex-specific development of hyperalgesia with males producing shorter-lasting and unilateral hyperalgesia compared to females [21], the underlying mechanisms for these sex differences are unclear. Ovariectomy had no effect on the behavioral hyperalgesia, and there were no sex differences in phosphorylation of the NR1 subunit of the NMDA receptor in the brainstem, a marker of neuronal excitability increased in chronic muscle pain models [21]. Nevertheless, we previously identified decreases in intramuscular pH in male but not in female mice during the fatiguing contractions [21], which might suggest that acidic pH contributes to muscle hyperalgesia induced by fatiguing exercise in male mice and that other metabolites released during muscle fatigue may play a more significant role in females [19,20]. However, pharmacological blockade of ASIC3 or ASIC1, endogenous pH sensors, in muscle prevents development of activity-induced hyperalgesia in both male and female mice [19]. Thus, future studies will need to investigate mechanisms that might contribute to the sex differences in the model.
While our study shows ATP-induced release of IL-1β from muscle macrophages, we have yet to test if IL-1β in vivo is released during fatiguing exercise or the activity-induced pain model, and if an increased release results from activation of P2X4. While we clearly show a role for P2X4 on muscle macrophages, it is unclear if P2X4 forms as a homomer, or if it forms heteromers with other P2X receptors, like P2X7, to produce the functional effects observed in this study. It is also unclear if P2X4 is functioning as a membrane-bound or lysosomal receptor in muscle macrophages [26,44]. Since ATP was given at mM doses which can activate both P2X4 and P2X7 receptors [47,67], we cannot rule out the role of other purinergic receptors on macrophages for ATP-induced release of IL-1β. Lastly, we showed that acidic pH-alone had no effect on cytokine release but potentiated the effects of ATP agreeing with prior studies showing a potentiated effect of ATP and acidic pH on dorsal root ganglia neuron activity and hyperalgesia [5,22,34]. Prior studies show that the effects of pH on IL-1β release from monocytes is enhanced by LPS and is pH-dependent [55]. This is in direct contrast since acidic pH decreases in pH activate acid sensing ion channels (ASICs), both ASIC1 and ASIC3 play a role in activity-induced hyperalgesia [19,20] and ASIC3 and P2X4 is increased in immune cells with fatiguing exercise in individuals with chronic non-inflammatory pain [35]. Future studies will be designed to determine the role of individual ion channels including individual purinergic and ASICs, as well as the interactions between these receptors for control of the release of IL-1β.
In people with chronic musculoskeletal pain, an acute bout of exercise can exacerbate pain [10,31] and limits participation in exercise and activities of daily living [1]. Consistent with the current data, moderate fatiguing exercise in individuals with widespread pain increases leukocyte expression of a number of ion channels including P2X4 [33,35]. We propose that release of fatigue metabolites from exercising muscle may contribute to the development of activity-induced pain through activation of P2X4 receptors on muscle macrophages (Fig. 1). Clinically, the prescription of exercise to control pain should be done to minimize release of fatiguing metabolites while building strength to reduce fatigability. Future studies could target P2X4 and macrophages in drug development to control activity-induced pain.
Supplementary Material
a,b. Scatter plots with mean+S.E.M. showing withdrawal thresholds of the ipsilateral (a) and contralateral (b) muscle 24h after induction of the model for male (blue) and female (red) mice treated with vehicle or 5, 50, or 500 μM 5-BDBD on Day 5 prior to the fatiguing contraction and second intramuscular saline injection. For comparison withdrawal threshold from the vehicle treated mouse prior to induction of the model are shown (gray, N). +, p<0.05 compared to vehicle. c.d. Scatter plots mean+S.E.M. showing withdrawal thresholds of the ipsilateral (c) and contralateral (d) muscle 24h after induction of the model for male (blue) and female (red) mice treated with vehicle or 500 μM 5-BDBD prior to the 1st intramuscular saline injection. For comparison withdrawal threshold from the vehicle treated mouse prior to induction of the model are shown (gray, N). +, p<0.05 compared to vehicle. e,f. Scatter plots mean+S.E.M. showing withdrawal thresholds of the ipsilateral (e) and contralateral (f) muscle 24h after induction of the model for male (blue) and female (red) mice treated with a lentivirus expressing a control miRNA (miR_C) or an artificial miRNA to P2X4 (miR_P2X4) prior to the 1st intramuscular saline injection. For comparison withdrawal threshold from the vehicle treated mouse prior to induction of the model are shown (gray, N). +, p<0.05 compared to vehicle. Data are represented as the mean and SEM.
Scatter plots with mean and SEM for GM-CSF (a), IL-5 (b), IL-4 (c), IFNγ (d), IL-10 (e), and TNFα (f) released from cultured peritoneal macrophages treated different combinations of LPS, ATP or acidic pH. ATP evoked release of GM-CSF with or without acidic pH, LPS evoked release of IL-5 and IL-4 regardless of treatment with ATP or pH, and there were no consistent changes in IFNγ, IL-10 or TNFα. * P<0.05 compared to LPS-/ATP-/pH 7.4 condition.
Acknowledgments:
Supported by National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) AR061371, the Carver College of Medicine at the University of Iowa, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and by the Sao Paulo Research Foundation (FAPESP) [grant number 2014/01119-4]. The authors would like to thank Dr. Steven P. Wilson, Professor Emeritus, University of South Carolina for help with design, development, and writing of methods for using of the lentiviral vector.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- [1].Bair MJ, Matthias MS, Nyland KA, Huffman MA, Stubbs DL, Kroenke K, Damush TM. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med 2009;10(7):1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balazs B, Danko T, Kovacs G, Koles L, Hediger MA, Zsembery A. Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell Physiol Biochem 2013;32(1):11–24. [DOI] [PubMed] [Google Scholar]
- [3].Bangsbo J, Krustrup P, Gonzalez-Alonso J, Saltin B. ATP production and efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol Endocrinol Metab 2001;280(6):E956–E964. [DOI] [PubMed] [Google Scholar]
- [4].Bidonde J, Busch AJ, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev 2014;10(1):45–79. [DOI] [PubMed] [Google Scholar]
- [5].Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 2010;68(4):739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borghi SM, Zarpelon AC, Pinho-Ribeiro FA, Cardoso RD, Cunha TM, Alves-Filho JC, Ferreira SH, Cunha FQ, Casagrande R, Verri WA Jr. Targeting interleukin-1beta reduces intense acute swimming-induced muscle mechanical hyperalgesia in mice. J Pharm Pharmacol 2014;66(7):1009–1020. [DOI] [PubMed] [Google Scholar]
- [7].Coddou C, Sandoval R, Hevia MJ, Stojilkovic SS. Characterization of the antagonist actions of 5-BDBD at the rat P2X4 receptor. Neurosci Lett 2019;690:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol 2000;130(6):1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol 2015;51:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dailey DL, Keffala VJ, Sluka KA. Do cognitive and physical fatigue tasks enhance pain, cognitive fatigue, and physical fatigue in people with fibromyalgia? Arthritis Care Res 2015;67(2):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience 2008;152(2):521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 1988;334(6184):698–700. [DOI] [PubMed] [Google Scholar]
- [13].Ferron M, Vacher J. Targeted expression of Cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis 2005;41(3):138–145. [DOI] [PubMed] [Google Scholar]
- [14].Fransen M, McConnell S, Harmer AR, van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2015;1:CD004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res 1994;657(1–2):133–140. [DOI] [PubMed] [Google Scholar]
- [16].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13(8):715–724. [DOI] [PubMed] [Google Scholar]
- [17].Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G-myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 2015;112(49):E6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident macrophages in muscle contribute to development of hyperalgesia in a mouse model of non-inflammatory muscle pain. J Pain 2016;17:1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gregory NS, Brito R, Fusaro MCGO, Sluka KA. ASIC3 is required for development of fatigue-induced hyperalgesia. Molecular Neurobiology 2016;53:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gregory NS, Gautam M, Benson CJ, Sluka KA. Acid Sensing Ion Channel 1a (ASIC1a) Mediates Activity-induced Pain by Modulation of Heteromeric ASIC Channel Kinetics. Neuroscience 2018;386:166–174. [DOI] [PubMed] [Google Scholar]
- [21].Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain 2013;154:2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gregory NS, Whitley PE, Sluka KA. Effect of Intramuscular Protons, Lactate, and ATP on Muscle Hyperalgesia in Rats. PLoS One 2015;10(9):e0138576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Heneweer H, Staes F, Aufdemkampe G, van R M, Vanhees L. Physical activity and low back pain: a systematic review of recent literature. Eur Spine J 2011;20(6):826–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hoeger Bement MK, Sluka KA. Exercise-induced hypoalgesia: An Evidence-based review In: Sluka KA, editor. Pain Mechanisms and Management for the Physical Therapist. Philadelphia: Wolters Kluwer, 2016. pp. 177–202. [Google Scholar]
- [25].Hood VL, Chubert C, Keller U, Muller S. Effect of systemic pH on pHi and lactic acid generation in exhaustive forearm exercise. Am J Physiol 1988;255:F479–485. [DOI] [PubMed] [Google Scholar]
- [26].Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, Murrell-Lagnado R, Dong XP. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem 2014;289(25):17658–17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 2001;4(9):869–870. [DOI] [PubMed] [Google Scholar]
- [28].Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain 2007;11(1):39–47. [DOI] [PubMed] [Google Scholar]
- [29].Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol 2005;481(4):377–390. [DOI] [PubMed] [Google Scholar]
- [30].Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum 2005;52(1):296–303. [DOI] [PubMed] [Google Scholar]
- [31].Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain 1996;64:415–423. [DOI] [PubMed] [Google Scholar]
- [32].Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 2003;95(2):577–583. [DOI] [PubMed] [Google Scholar]
- [33].Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, Light KC. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J Intern Med 2012;271(1):64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 2008;100(3):1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain 2009;10(10):1099–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain 2018;159(9):1752–1763. [DOI] [PubMed] [Google Scholar]
- [37].McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol 2003;140(8):1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc 2011;43(5):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Merriwether EN, Rakel BA, Zimmerman MB, Dailey DL, Vance CG, Darghosian L, Golchha M, Geasland KM, Chimenti R, Crofford LJ, Sluka KA. Reliability and Construct Validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) Instruments in Women with Fibromyalgia. Pain Med 2016;18:1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest 2003;83(2):175–185. [DOI] [PubMed] [Google Scholar]
- [42].Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, Kiefer R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am J Pathol 2001;159(6):2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Muller M, Wacker K, Getts D, Ringelstein EB, Kiefer R. Further evidence for a crucial role of resident endoneurial macrophages in peripheral nerve disorders: lessons from acrylamide-induced neuropathy. Glia 2008;56(9):1005–1016. [DOI] [PubMed] [Google Scholar]
- [44].Murrell-Lagnado RD. A role for P2X4 receptors in lysosome function. J Gen Physiol 2018;150(2):185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res 2005;38(11):1561–1569. [DOI] [PubMed] [Google Scholar]
- [46].Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Muller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol 2003;170(6):3263–3272. [DOI] [PubMed] [Google Scholar]
- [47].North RA. Molecular physiology of P2X receptors. Physiol Rev 2002;82(4):1013–1067. [DOI] [PubMed] [Google Scholar]
- [48].Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain 2009;141(1–2):127–134. [DOI] [PubMed] [Google Scholar]
- [49].Osten P, Dittgen T, Licznerski P. Lentivirus-based genetic manipulation in neurons in vivo In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton, FL: CRC Press, 2006. [PubMed] [Google Scholar]
- [50].Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PloS one 2014;9(3):e90633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008;8(4):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol 2008;180(11):7147–7157. [DOI] [PubMed] [Google Scholar]
- [53].Perez-Flores G, Levesque SA, Pacheco J, Vaca L, Lacroix S, Perez-Cornejo P, Arreola J. The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem Biophys Res Commun 2015;467(3):484–490. [DOI] [PubMed] [Google Scholar]
- [54].Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 2014;99(2):368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rajamaki K, Nordstrom T, Nurmi K, Akerman KE, Kovanen PT, Oorni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem 2013;288(19):13410–13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rice AS, Smith BH, Blyth FM. Pain and the global burden of disease. Pain 2016;157(4):791–796. [DOI] [PubMed] [Google Scholar]
- [57].Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 1995;115(7):1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch 1976;367(2):143–149. [DOI] [PubMed] [Google Scholar]
- [59].Schaible HG, Von Banchet GS, Boettger MK, Brauer R, Gajda M, Richter F, Hensellek S, Brenn D, Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci 2010;1193:60–69. [DOI] [PubMed] [Google Scholar]
- [60].Segura-Jimenez V, Borges-Cosic M, Soriano-Maldonado A, Estevez-Lopez F, Alvarez-Gallardo IC, Herrador-Colmenero M, Delgado-Fernandez M, Ruiz JR. Association of sedentary time and physical activity with pain, fatigue, and impact of fibromyalgia: the al-Andalus study. Scand J Med Sci Sports 2017;27(1):83–92. [DOI] [PubMed] [Google Scholar]
- [61].Shepherd AJ, Copits BA, Mickle AD, Karlsson P, Kadunganattil S, Haroutounian S, Tadinada SM, de Kloet AD, Valtcheva MV, McIlvried LA, Sheahan TD, Jain S, Ray PR, Usachev YM, Dussor G, Krause EG, Price TJ, Gereau RWt, Mohapatra DP. Angiotensin II Triggers Peripheral Macrophage-to-Sensory Neuron Redox Crosstalk to Elicit Pain. J Neurosci 2018;38(32):7032–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shepherd AJ, Mickle AD, Golden JP, Mack MR, Halabi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RWt, Mohapatra DP. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 2018;115(34):E8057–E8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. Journal of Pain 2005;6(1):41–47. [DOI] [PubMed] [Google Scholar]
- [64].Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve 2001;24:37–46. [DOI] [PubMed] [Google Scholar]
- [65].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18(8):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Spriet LL, Soderlund K, Thomson JA, Hultman E. pH measurement in human skeletal muscle samples: effect of phosphagen hydrolysis. J Appl Physiol (1985) 1986;61(5):1949–1954. [DOI] [PubMed] [Google Scholar]
- [67].Stojilkovic SS, Tomic M, He ML, Yan Z, Koshimizu TA, Zemkova H. Molecular dissection of purinergic P2X receptor channels. Ann N Y Acad Sci 2005;1048:116–130. [DOI] [PubMed] [Google Scholar]
- [68].Teixeira JM, Bobinski F, Parada CA, Sluka KA, Tambeli CH. P2X3 and P2X2/3 Receptors Play a Crucial Role in Articular Hyperalgesia Development Through Inflammatory Mechanisms in the Knee Joint Experimental Synovitis. Mol Neurobiol 2017;54(8):6174–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 2009;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J 2010;29(14):2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Valentine RJ, Jefferson MA, Kohut ML, Eo H. Imoxin attenuates LPS-induced inflammation and MuRF1 expression in mouse skeletal muscle. Physiol Rep 2018;6(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 2004;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 1995;63(3):289–302. [DOI] [PubMed] [Google Scholar]
- [74].Willemen HL, Eijkelkamp N, Garza CA, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 2014;15(5):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 2006;99(5):501–509. [DOI] [PubMed] [Google Scholar]
- [76].Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle Fatigue Increases the Probability of Developing Hyperalgesia in Mice. J Pain 2007;8:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a,b. Scatter plots with mean+S.E.M. showing withdrawal thresholds of the ipsilateral (a) and contralateral (b) muscle 24h after induction of the model for male (blue) and female (red) mice treated with vehicle or 5, 50, or 500 μM 5-BDBD on Day 5 prior to the fatiguing contraction and second intramuscular saline injection. For comparison withdrawal threshold from the vehicle treated mouse prior to induction of the model are shown (gray, N). +, p<0.05 compared to vehicle. c.d. Scatter plots mean+S.E.M. showing withdrawal thresholds of the ipsilateral (c) and contralateral (d) muscle 24h after induction of the model for male (blue) and female (red) mice treated with vehicle or 500 μM 5-BDBD prior to the 1st intramuscular saline injection. For comparison withdrawal threshold from the vehicle treated mouse prior to induction of the model are shown (gray, N). +, p<0.05 compared to vehicle. e,f. Scatter plots mean+S.E.M. showing withdrawal thresholds of the ipsilateral (e) and contralateral (f) muscle 24h after induction of the model for male (blue) and female (red) mice treated with a lentivirus expressing a control miRNA (miR_C) or an artificial miRNA to P2X4 (miR_P2X4) prior to the 1st intramuscular saline injection. For comparison withdrawal threshold from the vehicle treated mouse prior to induction of the model are shown (gray, N). +, p<0.05 compared to vehicle. Data are represented as the mean and SEM.
Scatter plots with mean and SEM for GM-CSF (a), IL-5 (b), IL-4 (c), IFNγ (d), IL-10 (e), and TNFα (f) released from cultured peritoneal macrophages treated different combinations of LPS, ATP or acidic pH. ATP evoked release of GM-CSF with or without acidic pH, LPS evoked release of IL-5 and IL-4 regardless of treatment with ATP or pH, and there were no consistent changes in IFNγ, IL-10 or TNFα. * P<0.05 compared to LPS-/ATP-/pH 7.4 condition.