Abstract
Background & Aims:
It is unclear whether a sustained virologic response (SVR) to direct-acting antiviral (DAA) therapy reduces the risk of incident hepatic encephalopathy (HE) in patients with hepatitis C virus (HCV) infection or whether it leads to resolution of pre-existent HE.
Methods:
We identified 71,457 patients who initiated antiviral treatments in the Veterans Affairs Healthcare System from January 1, 1999 through December 31, 2015; 35,871 patients (58%) received only interferon, 4535 patients (7.2%) received DAAs plus interferon, and 21,948 patients received (35%) DAA-only regimens. We collected data from patients through October 31, 2018, for an average of 6.6 years. We evaluated the association between SVR and the development of incident HE or the resolution of pre-existent HE (defined by cessation of pharmacotherapy) as well as the risk of hospitalization with HE for each, after adjusting for potential confounders.
Results:
Compared to no SVR, SVR after DAA therapy was associated with a significantly lower risk of developing HE (0.28 vs 1.39 per 100 person-years; adjusted hazard ratio [AHR] 0.41; 95% CI, 0.32–0.51). This association persisted among patients with co-morbid alcohol-use disorder and diabetes as well as patients with cirrhosis (AHR, 0.36; 95% CI, 0.31–0.43) and model for end-stage liver disease (MELD) scores of 9 or more (AHR, 0.36; 95% CI, 0.30–0.44). SVR was also associated with reduced risk of hospitalization with HE (AHR, 0.59; 95% CI, 0.43–0.81). Among 2396 patients who were receiving pharmacotherapy for HE at the time of antiviral treatment, SVR was associated with a significantly increased likelihood of HE resolution for those with MELD scores below 9 (AHR, 2.26; 95% CI, 1.74–2.93) but not those with MELD scores of 9 or more.
Conclusions:
In a retrospective study of veterans, we found DAA eradication of HCV infection to be associated with a 59% reduction in risk of development of HE and an increased likelihood of resolution of pre-existing HE in all subgroups except patients with MELD scores of 9 or more.
Keywords: Cirrhosis, liver disease, alcohol, diabetes
Introduction
Hepatitis C virus (HCV) is now curable in the vast majority of patients after a short course of direct-acting antiviral (DAA) therapy.(1) Following HCV eradication, patients with cirrhosis can experience dramatic improvements in liver function and short-term outcomes.(2–4) In conjunction with earlier observational data from the pre-DAA era, these short-term improvements suggest that curing HCV may reduce the long-term risk of progressive disease and cirrhosis complications.(5) However, controversy persists. Given the short follow-up of randomized-controlled trials, some have argued that the long-term clinical benefits of antiviral treatment and sustained virologic response (SVR) have not yet been demonstrated.(6) It is therefore imperative to continue to evaluate the long-term benefits of DAA-induced SVR in observational studies.
Clinicians caring for patients with cirrhosis wish to eradicate HCV in order to prevent, ameliorate, or reverse the complications of cirrhosis. Among the complications of cirrhosis, none is more devastating than hepatic encephalopathy (HE).(7) HE increases the risk of mortality, hospitalization, falls, and other injurious accidents while simultaneously diminishing the quality of life for both patients and their caregivers.(8, 9) Interventions that prevent or resolve HE would offer substantial value in improving the morbidity and public health footprint of cirrhosis. Data are lacking regarding the effectiveness of DAA therapy with respect to either the prevention of incident HE or the resolution of pre-existent HE.
We aimed to determine the associations between HCV eradication and the development of incident HE or the resolution of pre-existent HE and to investigate factors such as disease severity and comorbidities that modify these associations in the Veterans Affairs Healthcare System (VAHS).
Methods
Data Source
The VAHS is the largest integrated healthcare provider of HCV antiviral treatment in the United States.(10) The VA uses a single comprehensive electronic healthcare information network which integrates all care applications into a single, common database. We obtained data on all patients who initiated antiviral therapy for chronic HCV in the VA system using the VA Corporate Data Warehouse (CDW), a national, continually updated repository of all aspects of healthcare data.(11) The study was approved by the Institutional Review Board of the Veterans Affairs Puget Sound Healthcare System.
Study Population
We identified all HCV antiviral regimens (n=105,362 regimens in 78,940 patients) initiated in the VA during 17 calendar years from 1/1/1999 to 12/31/2015. We defined sustained virologic response (SVR) as a serum HCV RNA viral load test below the lower limit of detection performed at least 12 weeks after the end of HCV treatment.(12) We excluded 6,071 patients (7,821 regimens) with missing SVR data, and 1,412 patients (2,452 regimens) with a prior liver transplant. The remaining 71,457 patients (95,089 regimens) were included in the study, including 2,396 patients (2,815 regimens) who were receiving HE pharmacotherapy at the time of antiviral treatment and 3,627 patients (4,813 regimens) who developed HE after antiviral therapy. The antiviral regimens are detailed in Supplementary Table 1.
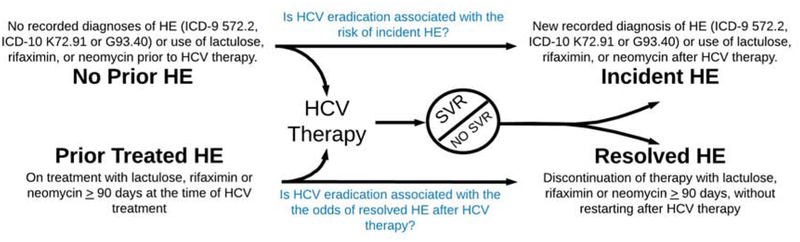
Outcome Measures (Figure 1)
Figure 1:
Study Outcomes and Definitions
HCV = hepatitis C virus, HE = hepatic encephalopathy, SVR = sustained virologic response
We explored 3 outcomes:
Development of incident HE during follow-up after antiviral treatment, among patients without evidence of HE prior to antiviral treatment.
Resolution of HE, among patients who were receiving HE pharmacotherapy at the time they underwent antiviral treatment.
Hospitalization for HE. We evaluated the risk of hospitalization with HE and the number of hospitalizations for HE in the 3 years following therapy for both those without baseline HE and those with treated HE at the time of HCV therapy.
Prior HE: We defined any history of HE prior to antiviral treatment by the presence of diagnostic codes for HE (ICD-9 code 572.2 or ICD-10 code K72.91 or G93.40) recorded at least twice or use of lactulose, rifaximin, or neomycin (for a duration of >90days) at any point prior to antiviral therapy or up to 90 days after initiation of antiviral therapy,
Incident HE was defined among patients without prior HE (defined as above) if identified for the first time at least 90 days after initiation of antiviral treatment based on ICD-9 code 572.2 or ICD-10 code K72.91 or G93.40 recorded at least twice or the prescription of lactulose, rifaximin, or neomycin for a duration ≥ 90 days (less if death or transplantation occurred before 90 days), whichever came first. The specificity for HE of the ICD-9 code 572.2 is 95–99%.(13) As previously studied,(14) we maximized sensitivity for incident HE using pharmacy linkage for the prescription of medications that are specific for HE therapy. Whereas chronic rifaximin or neomycin use has limited-to-no indications other than HE, lactulose is only rarely used for constipation.
Resolved HE: In order to test the effect of antiviral therapy on resolution of HE, we focused exclusively on patients with prior HE who were receiving pharmacological therapy for HE at the time of their antiviral treatment, defined by prescriptions for lactulose, rifaximin, or neomycin covering any time of the period 90 days before or after antiviral initiation, for durations ≥ 90 days. Resolved HE was defined as the cessation of prescription fills for HE therapy for ≥ 90 days without re-initiation of HE therapy at some point after antiviral treatment.
Baseline Patient Characteristics
We collected baseline data including age, sex, race/ethnicity, diabetes, body mass index (BMI), HCV genotype, HCV viral load and receipt of prior antiviral treatment. We extracted all clinical factors and laboratory tests prior to treatment and recorded the value of each test closest to the treatment starting date within the preceding 6 months. We defined HBV coinfection by positive HBV surface antigen or viral load. We also determined the presence of cirrhosis, decompensated cirrhosis (ascites, encephalopathy, gastroesophageal varices and hepatorenal syndrome), type 2 diabetes mellitus, alcohol use disorders, substance use disorders, based on appropriate, validated ICD-9 or ICD-10 codes recorded at least twice prior to treatment initiation in any inpatient or outpatient encounter.(15) The model for endstage liver disease (MELD) was calculated as previously described.(16)
Statistical Analysis
a. Association between SVR and incident HE risk
We used Cox proportional hazards regression to compare patients who achieved SVR to those who did not achieve SVR with respect to the risk of developing HE. We performed multivariable adjustment and, in the supplement, a both a propensity-matched analysis (using inverse probability weighting for SVR), and a Fine-Gray model to account for the competing risk of death. We also performed multiple Landmark analyses to account for immortal-time bias, varying cohort entry from 0-90-365 days and end-of-therapy. Adjustment for potential confounders that may be associated with both SVR and the risk of progressive liver disease and HE included type of antiviral treatment, demographics, hepatic and extrahepatic comorbidities, and liver disease severity (laboratory values and decompensations). Continuous variables were categorized and modeled as dummy categorical variables.
Follow-up for HE incidence extended until 10/31/2018 so that even the patients treated in late 2015 (i.e. the most recent in our cohort) would have substantial follow-up. Patients without incident HE were censored at the time of death or last follow-up in the VA. We presented subgroup analyses according to baseline variables that were associated with progressive liver disease including markers of disease severity (MELD score, in increments of 3), alcohol use disorders, diabetes, treatment regimens, and the era of anti-viral therapy.
Survival analyses are stratified by the Veterans Affairs facility at which the antiviral treatment was administered. All treatments received by a patient during the study period were analyzed. A significant proportion (23.8%) of patients received more than one antiviral treatment. Patients who did not achieve SVR were censored at initiation of a subsequent regimen that led to SVR, if applicable. The intragroup correlation induced by clustering within patient was accounted for by using robust variance estimation. Hospitalizations after HCV treatment were modelled as time-to-event (Cox-models) and hospitalizations within 3 years of HCV therapy (negative binomial regression).
b. Association between SVR and Resolution of HE
Among patients with pharmacologically treated HE at baseline (defined as above), we used multivariable Cox proportional hazards regression to determine the association between SVR and resolution of HE (i.e. cessation of pharmacotherapy) following antiviral treatment after adjusting for potential confounders, as described above.
Results
Characteristics of study population
All demographics and clinical characteristics are detailed Table 1. Compared to patients treated with IFN-only, those treated with DAA-only were older, more likely to have cirrhosis, HCC, and alcohol-use or substance-use disorders. Overall, patients who achieved SVR were less likely to have diabetes, cirrhosis or decompensated cirrhosis.
Table 1.
Baseline characteristics of HCV-infected patients who received their first antiviral treatment from 1999–2015 according to whether they achieved SVR
| All Patients (N=71,457) | IFN-ONLY | DAA+IFN | DAA-ONLY | ||||
|---|---|---|---|---|---|---|---|
| No SVR (n=26,406) | SVR (n=14,457) | No SVR (n=1860) | SVR (n=2883) | No SVR (n=2658) | SVR (n=22,193) | ||
| Age, yrs (mean [SD]) | 55.8 [7.8] | 52.4 [6.4] | 52.1 [6.8] | 57.7 [5.9] | 57.3 [6.7] | 60.5 [6.9] | 61.0 [6.7] |
| BMI, (mean [SD]) | 28.2 [5.3] | 28.4 [5.2] | 28.2 [5.2] | 28.6 [5.3] | 28.3 [5.0] | 28.5 [5.8] | 27.9 [5.4] |
| Male (%) | 96.6 | 96.9 | 95.7 | 95.6 | 96.4 | 98.2 | 96.6 |
| Race/Ethnicity (%) | |||||||
| White, non-Hispanic | 55.6 | 52.1 | 67.2 | 50 | 60 | 52.7 | 52.6 |
| Black, non-Hispanic | 26 | 26 | 12.5 | 36.3 | 25.8 | 31.2 | 33 |
| Hispanic | 5.9 | 6.8 | 5.9 | 6.1 | 4.4 | 6.7 | 4.9 |
| Other | 1.7 | 1.7 | 1.9 | 1.5 | 1.4 | 2 | 1.7 |
| Declined to answer/missing | 10.8 | 13.4 | 12.5 | 6.1 | 8.4 | 7.4 | 7.8 |
| Non-Genotype 1 (%) | 27.9 | 27.2 | 57.3 | 1.3 | 4.9 | 27.9 | 15.4 |
| HBV coinfection(%) | 1 | 0.6 | 1 | 1.7 | 1.8 | 0.9 | 1.3 |
| Cirrhosis (%) | 16.5 | 12.7 | 7 | 28.6 | 21.1 | 36.2 | 22.8 |
| Decompensated cirrhosis (%) | 4.3 | 3.6 | 1.8 | 6.6 | 3.8 | 13.2 | 5.6 |
| Ascites (%) | 0.5 | 0.7 | 0.4 | 0.1 | 0.1 | 0.6 | 0.4 |
| Varices (%) | 3.5 | 2.2 | 0.9 | 6.6 | 3.4 | 12.9 | 5.4 |
| Hepatocellular Carcinoma | 1.2 | 0.3 | 0.3 | 1.7 | 1 | 6.3 | 2.2 |
| Diabetes (%) | 21.4 | 19.2 | 13.4 | 25.3 | 20.4 | 31.9 | 27.4 |
| Alcohol Use Disorder (%) | 38.6 | 34.7 | 33.8 | 41.9 | 40.7 | 50.8 | 44.2 |
| Substance Use Disorder (%) | 31.4 | 27.1 | 26.2 | 34.8 | 32.7 | 41.5 | 37.9 |
| Laboratory Results (mean [SD]) | |||||||
| Alpha Fetoprotein, ng/mL | 5.8 [4.1] | 6.1 [4.2] | 4.6 [3.2] | 7.8 [4.8] | 6.0 [4.1] | 7.1 [4.6] | 6.0 [4.2] |
| Hemoglobin, g/dL | 14.8 [1.5] | 15.0 [1.5] | 15.1 [1.4] | 14.9 [1.4] | 15.0 [1.4] | 14.3 [1.7] | 14.5 [1.6] |
| Platelet Count, k/μL | 192.2[72.4] | 197.4 73.6] | 210.9[69.2] | 174.0[64.6] | 187.9[63.5] | 159.0[74.2] | 181.1 [70.7] |
| Creatinine, mg/dL | 1.0 [0.6] | 1.0 [0.7] | 1.0 [0.4] | 1.0 [0.7] | 0.9 [0.3] | 1.0 [0.4] | 1.0 [0.5] |
| Bilirubin, g/dL | 0.7 [0.5] | 0.7 [0.5] | 0.6 [0.4] | 0.7 [0.4] | 0.6 [0.4] | 0.8 [0.7] | 0.7 [0.5] |
| Albumin g/dL | 4.0 [0.5] | 4.0 [0.4] | 4.1 [0.4] | 3.9 [0.5] | 4.0 [0.4] | 3.7 [0.6] | 3.9 [0.5] |
| INR | 1.1 [1.0] | 1.1 [0.9] | 1.1 [1.0] | 1.2 [1.3] | 1.2 [1.1] | 1.2 [1.0] | 1.2 [0.9] |
| MELD | 8.0 [3.1] | 7.9 [3.0] | 7.6 [2.7] | 7.9 [3.4] | 7.6 [3.1] | 8.6 [3.4] | 8.3 [3.4] |
DAA = direct acting antivirals, HBV = hepatitis B virus, IFN = interferon, INR =international normalized ratio, MELD = model for endstage liver disease, SVR = sustained virologic response
Association between SVR and Incident HE
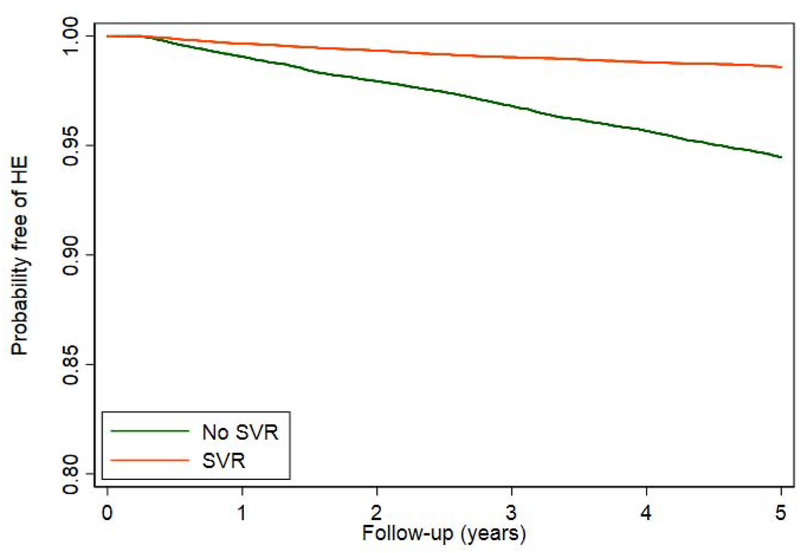
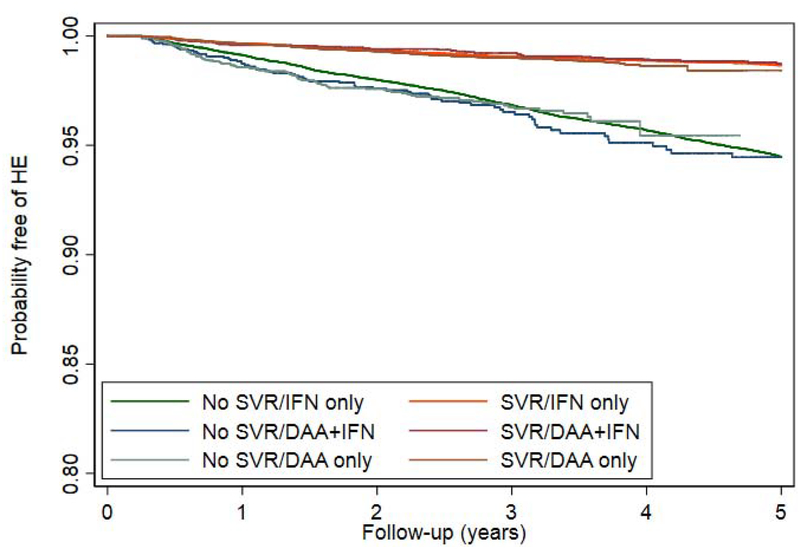
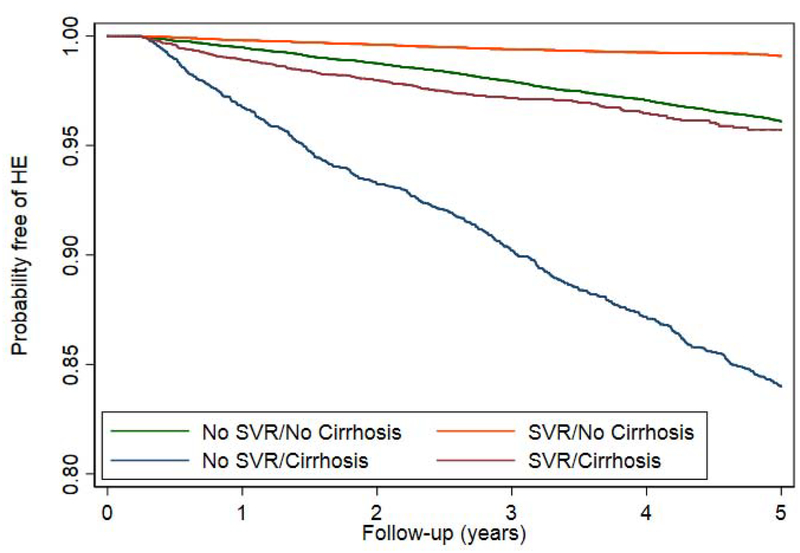
Restricting the treated population to patients without prior HE, we evaluated the impact of SVR on the incidence of HE. During a mean follow-up of 6.6 years after antiviral treatment, 3627 out of 71,457 patients developed HE (incidence 0.77 per 100 patient-years). The timing of HE with respect to treatment initiation is described in Supplementary Table 2. The cumulative incidence of HE was lower in patients who achieved SVR compared to those who did not (Figure 2a), irrespective of treatment regimen (Figure 2b). Although SVR is associated with a lower cumulative incidence and adjusted risk of HE for our 16,395 patients with cirrhosis (AHR 0.36, 95% CI 0.31–0.43), they still experience a substantial residual risk of HE.(Figure 2c). The reduced risk of HE was present for all regimen types. Effect estimates were similar independent of sex, comorbid liver diseases such as diabetes and alcohol-use disorder, as well as all baseline MELD scores.(Table 2) These results are also robust to multiple landmark analyses, varying cohort entry from 0-90-365 from the end-of-therapy, propensity matching, and competing-risks analysis.(Supplementary Tables 3–5) These analyses are further illustrated in cumulative incidence curves using the matched populations in Supplementary Figure 1a–c.
Figure 2.
Kaplan-Meier curves comparing the cumulative incidence of HE development in patients who achieved SVR versus those who did not, among all patients or clinically-relevant subgroups.
a. ALL PATIENTS
b. ACCORDING TO ANTIVIRALREGIMEN
c. ACCORDING TO PRESENCE/ABSENCE OF CIRRHOSIS
DAA = direct acting antivirals, IFN = interferon, SVR = sustained virologic response
Table 2.
Association between SVR and the Risk of Developing Incident HE
| Number of patients (%) | Mean Follow-up(Years) | Number who developed HE (%) | HE incidence per 100 patient-years | Crude hazard ratio (95% CI) | Adjusted* hazard ratio (95%CI) | ||
|---|---|---|---|---|---|---|---|
| IFN-ONLY regimens | No SVR | 34,006(66.7) | 8.6 | 3,613(10.6) | 1.24 | 1 | 1 |
| SVR | 16,973(33.3) | 10.6 | 508(3.0) | 0.28 | 0.23(0.21–0.26) | 0.26(0.23–0.30) | |
| DAA+IFN regimens | No SVR | 3,198(42.4) | 3.3 | 132(4.1) | 1.26 | 1 | 1 |
| SVR | 4,345(57.6) | 5.2 | 74(1.7) | 0.33 | 0.27(0.20–0.37) | 0.31(0.22–0.43) | |
| DAA-only regimens | No SVR | 3,336(10.2) | 2.7 | 124(3.7) | 1.39 | 1 | 1 |
| SVR | 29,414(89.8) | 3.2 | 362(1.2) | 0.39 | 0.28(0.22–0.35) | 0.41(0.32–0.51) | |
| Cirrhosis | No SVR | 6,940(42.3) | 5.2 | 1,302(18.8) | 3.6 | 1 | 1 |
| SVR | 9,455(57.7) | 4.2 | 419(4.4) | 1.05 | 0.29(0.25–0.32) | 0.36(0.31–0.43) | |
| No Cirrhosis | No SVR | 33,600(44.9) | 8.2 | 2,567(7.6) | 0.93 | 1 | 1 |
| SVR | 41,277(55.1) | 6.2 | 525(1.3) | 0.2 | 0.23(0.20–0.25) | 0.25(0.22–0.28) | |
| Men | No SVR | 39,311(44.6) | 7.7 | 3,771(9.6) | 1.25 | 1 | 1 |
| SVR | 48,862(55.4) | 5.8 | 905(1.9) | 0.32 | 0.25(0.23–0.28) | 0.26(0.23–0.28) | |
| Women | No SVR | 1,209(39.5) | 8.2 | 91(7.5) | 0.91 | 1 | 1 |
| SVR | 1,855(60.5) | 6.4 | 39(2.1) | 0.33 | 0.34(0.22–0.51) | 0.35(0.20–0.62) | |
| Diabetes | No SVR | 8,800(42.9) | 6.9 | 936(10.6) | 1.55 | 1 | 1 |
| SVR | 11,731(57.1) | 4.7 | 288(2.5) | 0.52 | 0.33(0.29–0.39) | 0.36(0.30–0.44) | |
| No diabetes | No SVR | 31,740(44.9) | 7.9 | 2,933(9.2) | 1.17 | 1 | 1 |
| SVR | 39,001(55.1) | 6.2 | 656(1.7) | 0.27 | 0.23(0.21–0.26) | 0.26(0.23–0.30) | |
| Alcohol use disorder | No SVR | 14,647(42.0) | 7.0 | 1,393(9.5) | 1.36 | 1 | 1 |
| SVR | 20,226(58.0) | 5.3 | 406(2.0) | 0.38 | 0.27(0.24–0.31) | 0.31(0.27–0.37) | |
| No alcohol use disorder | No SVR | 25,893(45.9) | 8.1 | 2,476(9.6) | 1.19 | 1 | 1 |
| SVR | 30,506(54.1) | 6.2 | 538(1.8) | 0.28 | 0.24(0.22–0.27) | 0.27(0.23–0.31) | |
| Pre 2009 | No SVR | 27,308(68.5) | 9.4 | 3,216(11.8) | 1.25 | 1 | 1 |
| SVR | 12,583(31.5) | 11.8 | 407(3.2) | 0.27 | 0.22(0.20–0.25) | 0.25(0.21–0.29) | |
| 2009–2015 | No SVR | 13,232(25.8) | 4.1 | 653(4.9) | 1.19 | 1 | 1 |
| SVR | 38,149(74.2) | 3.9 | 537(1.4) | 0.36 | 0.29(0.26–0.33) | 0.30(0.26–0.34) | |
| MELD < 9 | No SVR | 28,555(43.0) | 7.6 | 2,348(8.2) | 1.08 | 1 | 1 |
| SVR | 37,820(57.0) | 5.7 | 552(1.5) | 0.25 | 0.24(0.22–0.27) | 0.27(0.24–0.30) | |
| MELD ≥9 | No SVR | 4,759(42.4) | 6.2 | 765(16.1) | 2.6 | 1 | 1 |
| SVR | 6,455(57.6) | 4.7 | 246(3.8) | 0.82 | 0.29(0.25–0.34) | 0.36(0.30–0.44) | |
| MELD ≥ 12 | No SVR | 1,881(39.8) | 5.5 | 303(16.1) | 2.92 | 1 | 1 |
| SVR | 2,849(60.2) | 4.2 | 126(4.4) | 1.06 | 0.34(0.27–0.42) | 0.39(0.29–0.52) | |
| MELD ≥ 15 | No SVR | 860(38.6) | 5.8 | 100(11.6) | 2.02 | 1 | 1 |
| SVR | 1,366(61.4) | 4.3 | 47(3.4) | 0.8 | 0.39(0.26–0.58) | 0.48(0.30–0.76) | |
| MELD ≥ 18 | No SVR | 591(42.1) | 6.0 | 66(11.2) | 1.87 | 1 | 1 |
| SVR | 812(57.9) | 4.4 | 19(2.3) | 0.53 | 0.29(0.17–0.49) | 0.30(0.14–0.61) | |
| MELD ≥ 21 | No SVR | 304(41.7) | 6.3 | 31(10.2) | 1.63 | 1 | 1 |
| SVR | 425(58.3) | 4.8 | 8(1.9) | 0.39 | 0.25(0.11–0.56) | 0.34(0.09–1.26) |
Adjusted for regimen type, cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, body mass index, HBV co-infection, type 2 diabetes mellitus, ascites, varices, hepatocellular carcinoma, alcohol use disorders, substance use disorder, platelet count, serum bilirubin, serum creatinine, serum albumin, INR and blood hemoglobin levels. The laboratory tests were categorized into quartiles and modeled as dummy categorical variables.
When the risk of HE-related hospitalization was examined,(Supplementary Table 6) we found substantially reduced risk of a first-hospitalization after SVR for all treatment types. For example, DAA-alone was associated with an AHR 0.59 (95%CI 0.43–0.81). In Supplementary Table 7, we examine the total number of HE-related hospitalizations in the 3 years following HCV therapy. Again SVR is associated with reduce risk of hospitalization, adjusted incidence-rate ratio (IRR) 0.70 (95%CI 0.52–0.94)
Association between SVR and Resolution of HE
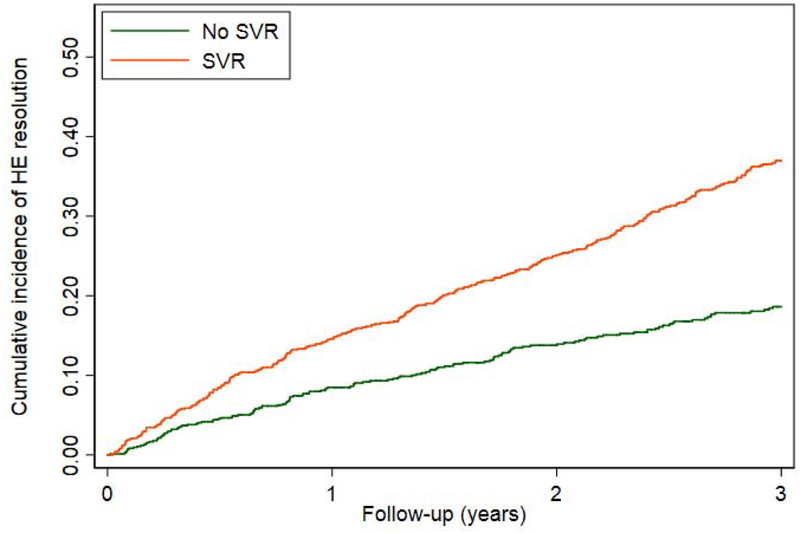
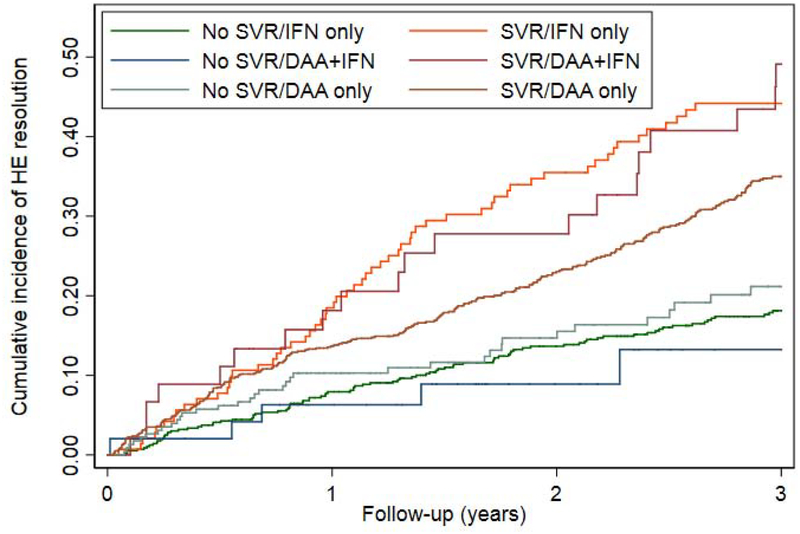
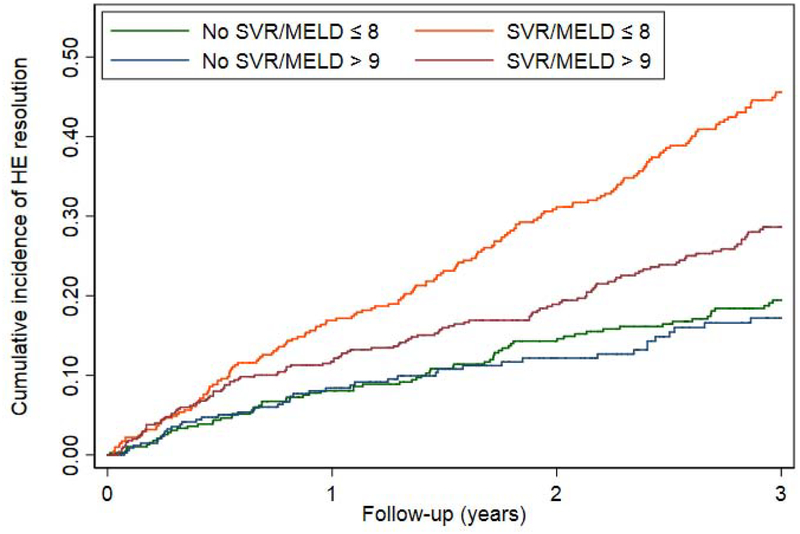
Among 2,396 patients who were receiving HE pharmacotherapy at the time of antiviral treatment, 881 (36.8%) achieved HE resolution (defined as cessation of pharmacotherapy) during a mean follow-up of 3.1 years after antiviral treatment. Patients who achieved SVR were significantly more likely to experience resolution of HE than patients who did not achieve SVR (AHR 1.61 95% CI 1.24–2.10).(Table 3, Figure 3a) SVR was associated with a higher likelihood of HE resolution among many clinically relevant subgroups, such as patients with and without diabetes and alcohol-use disorder. These data were consistent irrespective of the specific treatment used for HE. IFN-induced SVR appeared to be more strongly associated with HE resolution (AHR 2.10 95% CI1.57–2.82) than DAA+IFN-induced SVR (AHR 1.39 95%CI 0.60–3.18) or DAA-induced SVR (AHR 1.39 95% CI 1.03–1.87).(Table 3, Figure 3b) SVR was also associated with a higher likelihood of HE resolution among patients with MELD<9 (AHR 2.26 95%CI 1.74–2.93) but, importantly, not among patients with MELD≥9 (AHR 1.16 95%0.84–1.60).(Figure 3c) However, we suspect that DAA-induced SVR is associated with a lower effect estimate because these therapies were used in a sicker population. Indeed, when the subset of patients with MELD<9 who received DAA was examined, SVR was associated with an AHR for the resolution of HE of 2.20 (95%CI 1.36–3.57). After propensity matching in Supplementary Table 8, SVR remained associated with resolved HE after IFN, however the confidence intervals widened for DAA-alone, HR 1.28 (95%CI 0.95–1.74). Conversely, when accounting for the competing risk of death in Supplementary Table 9, the association between SVR and resolved HE strengthened, AHR 1.51 (95%CI 1.13–2.01). These relationships are further illustrated in the cumulative incidence curves in Supplementary Figure 2a–c.
Table 3.
Association between SVR and Resolution of Hepatic Encephalopathy
| Number of patients (%) | Mean Follow-up (Years) | Number with HE resolution (%) | HE resolution per 100 patient-years | Crude hazard ratio (95% CI) | Adjusted* hazard ratio (95%CI) | ||
|---|---|---|---|---|---|---|---|
| IFN-ONLY regimens | No SVR | 811(82.4) | 4.8 | 289(35.6) | 7.47 | 1 | 1 |
| SVR | 173(17.6) | 4.1 | 113(65.3) | 15.76 | 2.08(1.62–2.68) | 2.10(1.57–2.82) | |
| DAA+IFN regimens | No SVR | 114(57.6) | 2.4 | 24(21.1) | 8.68 | 1 | 1 |
| SVR | 84(42.4) | 3.0 | 41(48.8) | 16.27 | 1.88(1.12–3.16) | 1.39(0.60–3.18) | |
| DAA-only regimens | No SVR | 366(22.4) | 1.9 | 63(17.2) | 9 | 1 | 1 |
| SVR | 1,267(77.6) | 2.4 | 432(34.1) | 14.26 | 1.59(1.21–2.09) | 1.39(1.03–1.87) | |
| MELD < 9 | No SVR | 768(51.1) | 4.4 | 253(32.9) | 7.56 | 1 | 1 |
| SVR | 734(48.9) | 2.7 | 357(48.6) | 17.99 | 2.27(1.89–2.72) | 2.26(1.74–2.93) | |
| MELD ≥ 9 | No SVR | 670(42.7) | 3.2 | 164(24.5) | 7.66 | 1 | 1 |
| SVR | 898(57.3) | 2.6 | 274(30.5) | 11.74 | 1.49(1.20–1.84) | 1.16(0.84–1.60) | |
| DAA-only MELD < 9 | No SVR | 120(20.1) | 2.1 | 21(17.5) | 8.18 | 1 | 1 |
| SVR | 476(79.9) | 2.3 | 212(44.5) | 19.27 | 2.35(1.49–3.70) | 2.20(1.36–3.57) | |
| DAA-only MELD ≥ 9 | No SVR | 224(23.7) | 1.8 | 40(17.9) | 10.02 | 1 | 1 |
| SVR | 722(76.3) | 2.4 | 199(27.6) | 11.34 | 1.13(0.79–1.61) | 0.98(0.66–1.45) | |
| Diabetes | No SVR | 410(41.7) | 3.5 | 115(28.0) | 8 | 1 | 1 |
| SVR | 574(58.3) | 2.5 | 208(36.2) | 14.71 | 1.80(1.39–2.34) | 1.95(1.27–2.97) | |
| No diabetes | No SVR | 881(48.1) | 3.9 | 261(29.6) | 7.66 | 1 | 1 |
| SVR | 950(51.9) | 2.7 | 378(39.8) | 14.63 | 1.86(1.56–2.22) | 1.75(1.37–2.24) | |
| Alcohol use disorder | No SVR | 654(43.2) | 3.6 | 186(28.4) | 8 | 1 | 1 |
| SVR | 859(56.8) | 2.6 | 297(34.6) | 13.28 | 1.63(1.33–2.00) | 1.43(1.08–1.90) | |
| No Alcohol use disorder | No SVR | 637(48.9) | 4.0 | 190(29.8) | 7.54 | 1 | 1 |
| SVR | 665(51.1) | 2.6 | 289(43.5) | 16.4 | 2.10(1.71–2.58) | 2.29(1.71–3.06) | |
| Pre 2009 | No SVR | 631(85.0) | 5.2 | 232(36.8) | 7.11 | 1 | 1 |
| SVR | 111(15.0) | 4.5 | 71(64.0) | 14.14 | 1.95(1.43–2.65) | 2.17(1.49–3.15) | |
| 2009–2015 | No SVR | 660(31.8) | 2.4 | 144(21.8) | 9.09 | 1 | 1 |
| SVR | 1,413(68.2) | 2.5 | 515(36.4) | 14.73 | 1.59(1.30–1.94) | 1.71(1.38–2.12) | |
| Men | No SVR | 1,253(46.0) | 3.7 | 364(29.1) | 7.8 | 1 | 1 |
| SVR | 1,470(54.0) | 2.6 | 568(38.6) | 14.81 | 1.85(1.59–2.14) | 2.06(1.73–2.46) | |
| Women | No SVR | 39(41.9) | 4.7 | 13(33.3) | 7.02 | 1 | 1 |
| SVR | 54(58.1) | 3.0 | 18(33.3) | 11 | 1.80(0.85–3.82) | 11.3(0.78–164.5) | |
| Ascites ± varices ± HRS | No SVR | 447(40.2) | 2.7 | 79(17.7) | 6.43 | 1 | 1 |
| SVR | 665(59.8) | 2.5 | 188(28.3) | 11.28 | 1.75(1.32–2.32) | 1.82(1.18–2.80) | |
| All Regimens, lactulose or rifaximin, but not neomycin | No SVR | 1,268(45.5) | 3.8 | 7.77 | 1 | 1 | |
| SVR | 1,521(54.5) | 2.6 | 585(38.5) | 14.65 | 1.84(1.59–2.13) | 1.78(1.44–2.20) | |
| All Regimens, lactulose | No SVR | 1,044(51.6) | 4.1 | 8.1 | 1 | 1 | |
| SVR | 980(48.4) | 2.6 | 467(47.7) | 18.09 | 2.13(1.82–2.49) | 1.84(1.47–2.29) | |
| All Regimens, rifamixin |
No SVR | 51(27.1) | 2.1 | 8(15.7) | 7.5 | 1 | 1 |
| SVR | 137(72.9) | 2.3 | 59(43.1) | 18.89 | 2.57 (1.14–5.78) | 1.85 (0.44–7.85) |
Adjusted for regimen type, cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, body mass index, HBV co-infection, type 2 diabetes mellitus, ascites, varices, hepatocellular carcinoma, alcohol use disorders, substance use disorder, platelet count, serum bilirubin, serum creatinine, serum albumin, INR and blood hemoglobin levels. The laboratory tests were categorized into quartiles and modeled as dummy categorical variables. †Portal hypertension is defined by the presence of varices or ascites. DAA = direct acting antivirals, HRS = hepatorenal syndrome, IFN = interferon, MELD = model for endstage liver disease, SVR = sustained virologic response
Figure 3.
Cumulative probability curves comparing the resolution of HE in patients with SVR versus those without SVR among all patients or clinically-relevant subgroups.
a. All patients
b. By antiviral regimen
c. By MELD category
DAA = direct acting antivirals, IFN = interferon, MELD = model for endstage liver disease, SVR = sustained virologic response
We next evaluated the impact of SVR on time-to-hospitalization (Supplementary Table 10) and the total number of hospitalizations after HCV therapy (Supplementary Table 11) for patients with treated HE at baseline. We find that SVR is associated with reduced risk of first-hospitalization for IFN (AHR 0.53 (95%CI 0.35–0.83) but not DAA, AHR 0.79 (95%CI 0.57–1.10). Similar trends are seen for the total burden of hospitalizations with respective IRR for IFN and DAA of 0.28 (95%CI 0.19–0.41) and 0.80 (95%CI 0.60–1.07).
Discussion
Long-term data regarding the impact of HCV eradication on important clinical outcomes are limited among real-world patients. Of particular importance is the risk of HE. HE is a watershed moment in the natural history of chronic HCV, one after which morbidity and mortality sharply rises.(8, 9, 17–19) Accordingly, there are broad societal benefits tied to interventions that can prevent or resolve HE. To evaluate to impact of SVR on the risk of HE, we examined a very large sample from the VAHS (>70,000 patients, including roughly 25,000 who received DAA-only) with long-term follow-up (>6.5 years per-person). We show that SVR after DAA therapy is associated with a 59% reduction in the risk of incident HE and a 41% reduction in the risk of hospitalization with HE. When HE is present at the time of therapy, SVR is associated with a 61% increased rate of HE resolution.
SVR Reduces the Risk of HE
Intensive therapy for HE is associated with inconsistent benefits. Even patients receiving optimal treatment experience breakthrough episodes and diminished QOL.(20, 21) Treating the underlying liver disease in the hopes of forestalling or reducing further progression to cirrhosis and HE is therefore the best option to reduce HE-related risks. Our data suggest that SVR is associated with a dramatic reduction in the risk of developing HE. This includes reductions of 75% for those without cirrhosis at baseline, 64% for those with cirrhosis, and equivalent reductions for those with MELD≥9, comorbid alcohol-use disorder, and diabetes. These data are bolstered further by a marked reduction in the risk and overall burden of hospitalization with HE.
SVR Increases the Likelihood of HE Resolution
After an episode of overt HE, especially after repeated episodes,(22) it is unclear whether it is possible to safely discontinue HE-therapy without risk of recurrence. Guidelines from the American Association for the Study of Liver Disease acknowledge that data are lacking for this important question but suggest that if liver function improves substantially, a trial of treatment discontinuation could be considered.(22) Addressing this gap, we add novel data to show that among patients with treated-HE at the time of HCV-therapy, SVR is associated with a significantly increased likelihood of successfully discontinuing HE-therapy without recurrence, particularly for patients with MELD score<9. SVR is also associated with fewer hospitalizations with HE. Unfortunately, patients with MELD>9 do not experience this benefit after SVR, suggesting that there is a disease severity threshold after which freedom from HE-therapy is unlikely. It is known that SVR is associated with improved quality of life.(23) Minimal HE is associated with and may even be confused for poor patient reported outcomes. Accordingly, the reasons underlying the clinical decision to discontinue HE therapy are challenging to discern even prospectively. It is clear, however, that SVR was associated with durably discontinuing HE therapy.
What is known about the risk of HE after HCV therapy?
Our findings extend the data on the impact of HCV therapy on HE risk in multiple ways. First, we demonstrate a reduced risk of HE after DAA-induced SVR (as well as IFN-induced SVR) in a contemporary dataset with long follow-up, after adjustment for liver disease severity and comorbid liver diseases such as diabetes and alcohol-use disorder. This dataset is also the largest to explore the association of SVR with HE. Previously, van der Meer et al showed that after HCV therapy, 11 (2.7%) patients without SVR developed overt HE in follow up compared to 0 (of 125) matched patients with SVR.(5) Since the decision to use IFN and IFN-associated SVR are both associated with favorable baseline characteristics that may also be associated with lower risk of developing HE, our adjusted DAA-associated outcomes are likely more applicable to today’s patients with HCV. Clinical trials of DAA in compensated patients or those with early liver disease lack sufficient follow-up to determine associations with HE risk. Two clinical trials of DAAs have included patients with decompensated cirrhosis, both of which demonstrated improved MELD and Child-Pugh scores and Child class but suffered for lack of longterm follow-up beyond 24 weeks.(3, 4)
Second, we show that SVR is associated with increased likelihood of a durable long-term resolution of HE defined by cessation of HE-therapy for all patients save for those with MELD scores ≥ 9. These data extend a recently published combined analysis of the trials of Sofosbuvir-based DAA therapy in patients with decompensated cirrhosis. El-Sherif et al showed that subjects with HE were among those least likely to benefit from DAA therapy after 12–24-weeks of follow-up.(2) These authors demonstrated that those with HE at baseline were most likely to experience the suboptimal outcome known as “MELD purgatory” whereby their MELD would improve (to <15) but they would retain persistent HE.(2, 24) Our study’s design – much longer follow-up (3.14 years vs 24 weeks) and sensitive outcome determination (cessation of therapy) - is more broadly applicable to real-world patients. Though SVR was associated with reduced HE-related hospitalizations, the association was not statistically significant when evaluating DAAs separately.
Limitations
These data must be interpreted in the context of the study design. First, patients were derived from a single, national healthcare system with fairly uniform antiviral treatment practices and guidelines across its facilities. Second, since this is by necessity an observational study (patients cannot be randomized to eradication or not and cannot ethically be randomized to antiviral treatment versus no treatment, especially with long-term follow-up) we cannot exclude the possibility that residual confounding may have contributed to the associations we observed between SVR and prevention or resolution of HE. However, the associations persisted after careful adjustment for 20 baseline characteristics known or suspected to be associated with SVR and HE. Furthermore, the associations persisted across almost all subgroups, except for the lack of association between SVR and HE resolution among patients with MELD score ≥9, which is biologically plausible and enhances the internal validity of the study. Third, we cannot determine whether patients who had ‘resolved HE’ lacked persistent cognitive dysfunction that was potentially associated with minimal HE. Given that we defined resolved HE as the cessation of therapy (without re-initiation during follow-up), if overt HE recurred - particularly in patient who previously received HE-therapy – they would likely universally have been re-started on HE-therapy. Fourth and similarly, we only measured diagnosed HE (using diagnostic codes and medical therapy). As we did not assess cognition, these data do not evaluate the risk of minimal HE or changes in cognitive performance after HCV therapy. Finally, the definition of HE was based in part on the use of chronic lactulose use. Some patients may be placed on this medication exclusively to treat constipation and not HE.
Conclusion
These data from a large cohort of patients undergoing HCV therapy, including roughly 25,000 who received DAA-alone, with and without cirrhosis, and who were followed for many years after therapy demonstrates two core benefits associated with SVR. First, patients achieving SVR are significantly less likely to experience incident HE. Second, for patients with actively treated HE at the time of HCV therapy, SVR is associated with significantly improved likelihood of HE resolution for all clinically-relevant subgroups except patients with MELD≥9. Taken together, these data demonstrate a specific benefit of HCV therapy and one that may reduce the national burden of HE and its related complications.
Supplementary Material
Need to Know.
Background:
It is unclear whether a sustained virologic response (SVR) to direct-acting antiviral (DAA) therapy reduces the risk of incident hepatic encephalopathy (HE) in patients with hepatitis C virus (HCV) infection or whether it leads to resolution of pre-existent HE.
Findings:
In a retrospective study of veterans, we found DAA eradication of HCV infection to be associated with a 59% reduction in risk of development of HE and an increased likelihood of resolution of pre-existing HE in all subgroups except patients with MELD scores of 9 or more
Implications for Patient Care:
Patients with HCV infection should receive DAA therapy even if they have alcohol-use disorder, diabetes, cirrhosis, or HE. HCV eradication reduces risk of HE.
Funding:
The study was funded by a NIH/NCI grant R01CA196692 and VA CSR&D grant I01CX001156 to GNI. Dr. Tapper receives funding from the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the US Government.
Declaration of Personal Interests: Elliot Tapper has received grants from Valeant and Gilead; participated in advisory boards for Mallinckrodt, Bausch; consulted for Novartis and Allergan. No other author has a pertinent conflict of interest.
References
- 1.Pawlotsky J-M, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. Journal of hepatology 2015;62:S87–S99. [DOI] [PubMed] [Google Scholar]
- 2.El-Sherif O, Jiang Z, Tapper E, Huang K, Zhong A, Osinusi A, Charlton M, et al. Baseline Factors Associated with Improvements in Decompensated Cirrhosis After Direct-acting Antiviral Therapy for HCV Infection. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 3.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. New England Journal of Medicine 2015;373:2618–2628. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015;149:649–659. [DOI] [PubMed] [Google Scholar]
- 5.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F, Lammert F, Duarte-Rojo A, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama 2012;308:2584–2593. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsen J, Nielsen E, Feinberg J, Katakam K, Fobian K, Hauser G, Poropat G, et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst. Rev 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy In: Mayo Clinic Proceedings; 2015: Elsevier; 2015. p. 646–658. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, Gibson DP, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009;50:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezaz G, Murphy SL, Mellinger J, Tapper EB. Increased Morbidity and Mortality Associated with Falls among Patients with Cirrhosis. The American journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- 10.Moon AM, Green PK, Berry K, Ioannou GN. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Alimentary pharmacology & therapeutics 2017;45:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veterans Affairs Corporate Data Warehouse. Available at: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm Last accessed on 12/19/16.
- 12.Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr., Ratziu V, Ding X, Wang J, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology 2015;61:41–45. [DOI] [PubMed] [Google Scholar]
- 13.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology 2013;47:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapper EB, Parikh N, Sengupta N, Mellinger J, Ratz D, Lok AS, Su GL. A Risk Score to Predict the Development of Hepatic Encephalopathy in a Population-Based Cohort of Patients with Cirrhosis. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 15.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, et al. Increasing Prevalence of HCC and Cirrhosis in Patients With Chronic Hepatitis C Virus Infection. Gastroenterology 2011;140:1182–1188 e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. New England Journal of Medicine 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapper E, Kanwal F, Asrani S, Ho C, Ovchinsky N, Poterucha J, Flores A, et al. Patient Reported Outcomes in Cirrhosis: A Scoping Review of the Literature Hepatology (Baltimore, Md.) 2017. [DOI] [PubMed] [Google Scholar]
- 18.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Digestive diseases and sciences 2003;48:1622–1626. [DOI] [PubMed] [Google Scholar]
- 19.Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients with Cirrhosis: A Multistate Population-based Cohort Study. Clinical Gastroenterology and Hepatology 2016. [DOI] [PubMed] [Google Scholar]
- 20.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. New England Journal of Medicine 2010;362:1071–1081. [DOI] [PubMed] [Google Scholar]
- 21.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall-related injuries in hospitalized patients with cirrhosis. Clinical Gastroenterology and Hepatology 2015;13:1670–1675. [DOI] [PubMed] [Google Scholar]
- 22.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 23.Younossi ZM, Stepanova M, Jacobson I, Muir AJ, Pol S, Zeuzem S, Younes Z, et al. Not Achieving Sustained Viral Eradication of Hepatitis C Virus After Treatment Leads to Worsening Patient-reported Outcomes. Clinical Infectious Diseases 2019. [DOI] [PubMed] [Google Scholar]
- 24.Carrion AF, Khaderi SA, Sussman NL. Model for end-stage liver disease limbo, model for end-stage liver disease purgatory, and the dilemma of treating hepatitis C in patients awaiting liver transplantation. Liver Transplantation 2016;22:279–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.