Abstract
The immune system has remarkable capabilities to combat disease with exquisite selectivity. This feature has enabled vaccines that provide protection for decades, and more recently, advances in immunotherapies that can cure some cancers. Greater control over how immune signals are presented, delivered, and processed will help drive even more powerful options that are also safe. Such advances will be underpinned by new tools that probe how immune signals are integrated by immune cells and tissues. Biomaterials are valuable resources to support this goal, offering robust, tunable properties. This review highlights the growing role of biomaterials as tools to dissect immune function in fundamental and translational contexts. These technologies can serve as tools to understand the immune system across molecular, cellular, and tissue length scales. A common theme is exploiting biomaterial features to rationally direct how specific immune cells or organs encounter a signal. This precision strategy, enabled by distinct material properties, allows isolation of immunological parameters or processes in a way that is challenging with conventional approaches. The utility of these capabilities is demonstrated through examples in vaccines for infectious disease and cancer immunotherapy, as well as settings of immune regulation that include autoimmunity and transplantation.
Keywords: immunology, nanoparticle and microparticle, immunotherapy and vaccine, organ-on-a-chip and organoid, high content data and big data
Graphical Abstract

The selectivity and memory of the immune system have positioned vaccines and immunotherapies as important clinical advances. New tools to study the interactions of immune signals with immune cells and tissues will help drive the next generation of these technologies. This review highlights emerging ways in which tunable properties of biomaterials are creating new tools to decode and control immunity.
1. Biomaterials are enabling tools across multiple length scales to advance the immunology, vaccine, and immunotherapy fields
Vaccines are unique in their ability to provide specific, long-term protection against widespread infectious diseases. The central premise of these technologies is the ability to safely expose the immune system to a fragment of a pathogen – termed an “antigen”, generating immunological memory that protects the recipient against a future encounter with the same pathogen. Initiating, maintaining, and concluding immune function relies on sophisticated signal integration. This review will discuss how the unique capabilities of biomaterials are being harnessed to create powerful tools to decode and control these immunological processes. We will begin by providing a brief overview of vaccines and immunology before delving into the ways in which materials have been used to understand and manipulate immune function across molecular, cellular, and tissue scales.
Since their advent, vaccines have led to some stellar success stories including dramatic decreases in the prevalence of established pathogens such as diphtheria, polio, and small pox.[1] Advances in immunological understanding have improved vaccine technologies and expanded the range of diseases against which protection is achieved. For example, Dengvaxia was recently approved by the Food and Drug Administration as the first vaccine for preventative protection against Dengue virus in specific endemic regions – a new option to combat the spread of this mosquito-transmitted disease.[2] Further, improved vaccine technologies have enabled the development of multivalent vaccines that provide protection against multiple pathogens. One such example is Vaxelis, a hexavalent vaccine spanning Diphtheria, Tetanus, Polio, Hepatitis B, and Haemophilus Influenzae type B.[3] However, even effective vaccines require ongoing campaigns, as illustrated by the recent re-emergence of measles, a disease that previously was nearly eliminated in several parts of the world.[4]
In addition to the use of vaccines against infectious diseases, fundamental understanding of non-infectious diseases such as cancer – where mutated self-cells divide unconstrained – has catalyzed interest in vaccines and immunotherapies for other diseases. The first cancer vaccines, for example, targeted cancer-associated viruses, such as Human Papilloma Virus linked to cervical cancer,[5] but there have been tremendous advances in immunotherapies that target cancer directly. Some of the most recently-approved therapies include checkpoint blockade and chimeric antigen receptor (CAR) T cell therapies; checkpoint blockade requires the production of monoclonal antibodies, while CAR T cell therapy involves removal, external manipulation, and reinfusion of a patient’s immune cells.[6,7] These immune-modifying therapies have dramatically improved outcomes for a subset of patients with certain types of cancer by limiting the ability of the cancer to suppress the immune response and by generating large numbers of T cells that can more specifically target cancerous cells. Even more broadly, increasing immunological knowledge has enabled new types of immunotherapies for diseases involving excess inflammation, such as allergies or autoimmunity.[8] Autoimmune diseases occur when the body’s immune system malfunctions and attacks self-tissue, as occurs in multiple sclerosis (MS). One recent drug, Ocrevus, is a monoclonal antibody that selectively targets B cells.[9] This biologic was recently approved as the first therapy for patients with primary progressive MS, a disease stage that previously had no specifically-approved drugs.
The successes just outlined also reveal some of the ongoing and emerging challenges for vaccines and immunotherapies. The Dengue virus vaccine mentioned above, for example, is approved for limited age groups and geographic regions owing to ongoing safety concerns.[10] Since vaccines are generally administered to healthy individuals, safety is a central consideration. A related issue is limiting the systemic toxicity that can result from the components included in vaccines to stimulate the immune response, termed “adjuvants”.[11,12] Thus, technologies to minimize the dose of adjuvants or other potent components – or to target vaccines to specific tissues, are important for the future of vaccine and immunotherapy development. Increasingly, difficulties in producing enough clinical grade material are also an issue. The annual U.S. Influenza vaccine is a pertinent case, as both the availability of the vaccine and the effectiveness vary by year.[13] One approach to address this challenge is to develop more robust vaccines that have broader or longer-lasting disease protection. This example also highlights the demands that increasingly complex vaccines have placed on manufacturers and regulatory agencies, who must understand and assess each strategy. The challenges just mentioned are faced in emerging immunotherapies as well. In cancer, for example, isolating and expanding T cells from a patient before reinfusion for the CAR T cell therapy mentioned above is extremely costly and complex. One recently-approved CAR T cell therapy – Yescarta – costs approximately $400,000 per patient.[14] Thus, despite the clinical advances in vaccines and immunotherapies over the past several decades, new tools and technologies that improve the study and control of immune function will be crucial for continued advancement.[15,16]
Moving forward, biomaterials – including synthetic polymers, lipid carriers, metal nanoparticles (NPs), implantable scaffolds, and others – are poised to play a central role in the next generation of advances, not just as delivery agents, but as tools to study immune function to address the challenges outlined above. Broadly speaking, the tunability of biomaterials make them important technologies to study immune cell signaling and interactions with relevant tissues of the immune system and host. This tunability is particularly pertinent to develop new immunological tools, as immune function is driven by the constant integration of soluble, surface-displayed, and tissue-localized signals. Thus, the complexity of immune cell signaling requires analysis at multiple length scales. At a molecular scale, biomaterials such as lipid carriers, polymer scaffolds, and engineered conjugates allow control over the antigen conformation and display.[17,18] This precise control allows for the elucidation of relationships between properties such as antigen density and a particular immunological outcome. For example, the type of antibodies a B cell secretes to neutralize extracellular pathogens depends on the number and affinity of interactions the B cell has with antigen and helper immune cells during activation.[18,19] At a larger length scale, biomaterials allow defined perturbations of cellular interactions or signaling. Some key examples include measuring specific immune functions, while using materials to vary the signals loaded in a material, the relative concentrations of these signals, the duration of signal presentation (e.g., through controlled release), or the direction of signals to specific immune cells or intracellular locations.[20,21] The physicochemical properties of materials – such as size, shape, surface charge, hydrophobicity, and stiffness can also be used as levers to control the interactions just mentioned.[8,22] Together, these opportunities can be exploited to decipher how multiple immune signals are integrated, to determine how the kinetic availability of immune signals impacts vaccines or immunotherapy outcomes, and to engineer systems to evaluate the mechanism of action of immune signals and cues.
Moving to an even greater length scale – tissue scale, biomaterials can be used to recapitulate or manipulate key features of the extracellular matrix (ECM), stromal cells, or other tissue features that are integral in immune function. [23–25] These areas include studies that control properties such as compressive stress, fluid flow, ligand profile on the ECM, and chemotactic gradient. Thus, biomaterials are tools that can be used to design 3D scaffolds that mimic tissue with opportunities to control both mechanical and surface features. For example, matrix stiffness is an influential factor on immune cell migration and specific functions.[26,27] These systems can also be used to study how signaling cues are transported through the lymphatic vessels and immune tissues – such as lymph nodes (LNs), or to sites of disease – such as tumors, and ultimately, the influence these signals have on the immune response.[28] Likewise, controlled delivery or display of immune cues using biomaterials enables the creation of defined chemical gradients to support study of how immune cells migrate in three-dimensional systems.
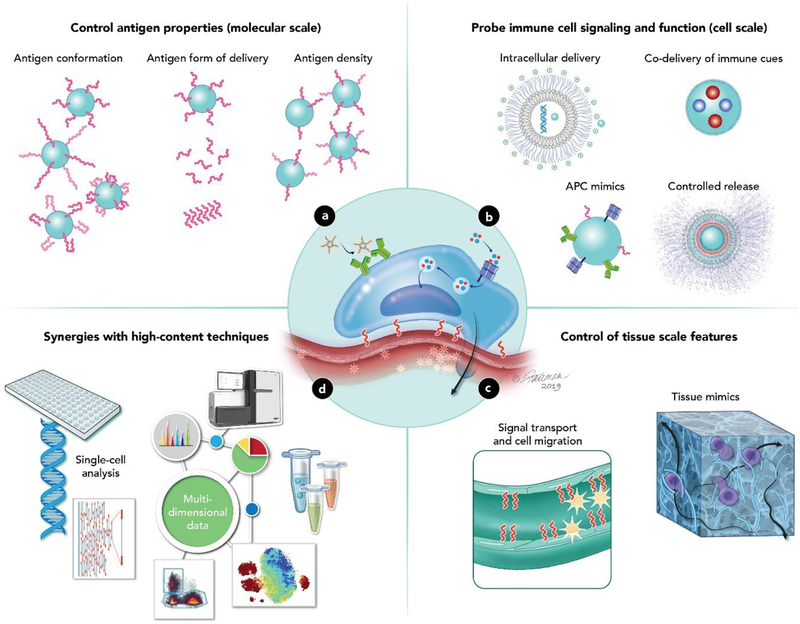
In this review, we illustrate the concepts above using key examples in which biomaterials serve as tools to support immunological advances, both fundamental and translational (Figure 1). We begin by providing a brief immunological background in Section 2. The subsequent three sections describe three length scales over which engineered materials are being used to study important immunological processes. First, in Section 3, we discuss the molecular scale, at which the ability of materials to control antigen structure and density is being exploited to probe antigen processing and presentation (Figure 1a). Focusing on the cellular scale, Section 4 highlights the use of biomaterials to control how cells encounter immune signals as a means to understand the role of specific signals or pathways (Figure 1b). This control has been used in the immune engineering field to support several emerging directions, several of which we use to illustrate this concept: genetic engineering, synergies between innate immune pathways, immunometabolism, and signal presentation by artificial immune cells. Moving to the tissue scale, Section 5 focuses on biomaterials as tools to develop ex vivo immune models – such as organoids and on-a-chip platforms, to study ligand-dependent immune cell trafficking, and to probe the influence of mechanical forces on immune cells and tissues (Figure 1c). We illustrate the concepts just summarized by drawing on cases from the infectious disease, cancer, and autoimmunity literature. We conclude with Section 6, which discusses emerging multi-dimensional and systems level analysis technologies that are being coupled with biomaterials to create powerful new immunological tools (Figure 1d). This review is centered on using biomaterials to generate insight that informs future vaccines and immunotherapies; thus, some other important areas – such as foreign body response to biomaterials -are outside the scope of the review’s focus.
Figure 1. Overview of how biomaterials serve as tools to decipher immune function.
a. Materials can be used to control the molecular features of antigen display (molecular scale) b. Different biomaterial properties can be exploited to study how immune cells respond to different types of stimulus (cellular scale) c. Immune cells interact with the surrounding tissue to develop particular functions (tissue scale). Two areas that materials have been useful in are studying how immune cells respond to physical forces and environmental signal molecules. d. Biomaterials can interface with new high content data techniques to more deeply probe immune function.
2. Immune responses rely on coordinated cell and tissue interactions
Before moving to specific examples of biomaterials being used as tools, we first provide some background to introduce the key immune cell types, tissues, and responses. Broadly speaking, the immune system is composed of cells belonging to two functional groups: innate and adaptive.[29] Innate immune cells are the first line of defense against pathogens. These cells rapidly move to sites of infection or tissue damage. Once there, antigen presenting cells (APCs) collect antigens, then migrate to specialized immune organs – LNs and spleen.[30] These innate cells secrete potent inflammatory mediators to help quickly destroy extracellular pathogens. The adaptive immune cells, T and B cells, complement innate immune cells function. Adaptive cells recognize specific antigens associated with pathogens. Prior to activation, T and B cells reside in LNs and spleen awaiting activation by APCs that display the antigen a T or B cell is specific for – the “cognate” antigen. Following activation, T and B cells proliferate and differentiate. Then, these cells migrate out of immune organs and return to the site of infection to rapidly destroy the pathogen with a high degree of specificity. Critically, some T and B cells exhibit long-lived memory functions that prevent reinfection by a previously encountered pathogen; these cells allow many vaccines to provide protection for decades.[29] The innate and adaptive responses work cooperatively to effectively clear infections and resolve inflammation without damaging host tissues. This section, in particular, emphasizes some of the key functions and connections between innate and adaptive immune cells that will enable understanding of the biomaterial tools discussed in Sections 3–6.
2.1. Innate immune cells are the first line of defense against pathogens
To protect the body from pathogens, immune cells must be able to differentiate between self and foreign protein-based antigens.[31] This differentiation relies on host tissues to display self-molecules on the cell surface that broadly instruct innate immune cells not to attack. At the same time, innate cells must constantly circulate and arrive quickly to sites of infection.[32] At these sites, innate cells then secrete cytotoxic molecules and inflammatory cytokines to destroy pathogens and recruit additional innate immune cells. Many innate immune cells also serve an important role in clearing pathogens or tissue debris through internalization – “phagocytosis” -of these materials and subsequent degradation. While innate immune cells initially respond rapidly, they also have a short half-life and limited proliferative capacity. This capacity enables the cells to rapidly kill pathogens and trigger inflammation at sites of infection, but then quickly subside to prevent damage to host tissue.
APCs are specialized innate cells – including dendritic cells (DCs) and macrophages – that play a role in generating adaptive immunity against pathogens. An important way in which APCs detect pathogens involves pattern recognition receptors (PRRs). These receptors recognize pathogen-associated molecular patterns (PAMPs), which are molecules or structural motifs common in disease causing bacteria and viruses, but uncommon in humans.[33] APCs encountering these PAMPs recognize “danger”, resulting in maturation of the APC; thus PAMPs are signals that cause APCs to express the co-stimulatory signals introduced above. Toll-like receptors (TLRs) are one important family of pathways relevant to many of the examples that will be discussed. TLRs are located on the cell surface and endosomal membranes and detect a range of signals in the cellular locations that specific pathogens are normally processed.[34] For example, extracellular TLRs detect bacterial cell membrane motifs such as lipopolysaccharides. This is because bacteria are generally encountered by immune cells in the extracellular space. In an analogous manner, intracellular TLRs detect intracellular signs of infection – such as the presence of a virus – by sampling endosomes. TLRs are not the only pathogen sensing pathways. Stimulator of interferon genes (STING), and nucleotide-binding oligomerization domain (NOD)-like receptors are additional PRRs that are present in the cell’s cytoplasm and trigger the expression of the three activation signals.[35,36] Triggering combinations of TLR pathways in APCs (or other synergistic immune pathways) is one developing area to improve understanding of the generation and regulation of a range of immune processes, both innate and adaptive.[37–39]. Another key function of APCs is the connection these cells have to adaptive immunity, which is the focus of the next section.
2.2. Adaptive immune cells have specialized functions tailored for specific pathogen types
Adaptive immune cells consist of T and B cells. APCs activate these cells through a series of cell-cell interactions in LNs and spleen.[31] T and B cells initially respond to infection more slowly than innate immune cells because they must first be activated through cooperative interactions with APCs. Prior to encountering foreign antigens, APCs move along the endothelial cells and the ECM. The direction of this movement is established by ligand-receptor interactions that create defined chemical gradients for cells to migrate along. These gradients direct APCs toward event-specific sites. Initially, the migratory molecules – chemokines, cause cells to move toward sites of inflammation. Following exposure to pathogens, different chemokine gradients guide APCs back to LNs where they interact with T and B cells. In the absence of a pathogen, APCs constantly internalize and process (self) antigen with low levels of antigen presentation and without the cues needed to activate T and B cells. In addition to altering migration, pathogens cause additional developmental changes – processes known as activation and maturation – in APCs. In this process, APCs increase the surface presentation of internalized (foreign) antigen and signals that activate T and B cells, while returning to the LNs where T and B cells await activation and expansion.[40]
As just alluded to, the activation of adaptive (i.e., antigen-specific) immunity involves APCs providing several developmental cues to T and B cells: i) cognate antigens, ii) costimulatory molecules, and iii) soluble cytokines.[41,42] The first cue is the presentation of foreign antigen in a surface protein known as the major histocompatibility complex (MHC). Depending on whether the antigen came from an extracellular pathogen like a bacterium or an intracellular pathogen – such as a virus, APCs display antigen in either MHCI or MHCII to stimulate the type of adaptive immune response that is most effective for clearing that class of pathogen.[31] Extracellular pathogens such as bacteria are internalized by APCs and enzymatically degraded in endosomes; the resulting protein fragments (antigens) are then loaded into MHCII molecules to activate helper T cells that support the production of antibodies.[43] Antibodies are proteins that can bind, inactivate, or tag extracellular pathogens for destruction. In contrast, virally infected cells already have antigen (i.e., the virus) in the infected cell’s cytoplasm, which ultimately results in loading of the antigen into MHCI molecules.[44] Whether a T cell is activated following interactions with MHCI or MHCII molecules depends on the cells function. Upon being stimulated with MHCI, cytotoxic T cells (CTLs) mature. These cells directly kill host cells that are infected with virus; this process prevents the further replication of the virus and the spread of infection. In addition to antigen, the second signal APCs provide to T and B cells to initiate the processes just summarized is costimulation; costimulation is communicated by an increase in surface proteins (e.g. CD40, CD80, CD86) that trigger an intracellular signaling cascade ending in T cell activation.[45–47] An example of one of these crucial cascades is the binding of the CD28 surface protein on T cells. Without CD28 binding by costimulatory molecules, T cells become unresponsive to antigen and undergo apoptosis – programmed cell death – since the lack of costimulation suggests to the immune system that the response is no longer needed. Lastly, the third type of signals involved in T and B cell activation are the soluble signals secreted by APCs known as cytokines. These proteins promote – and later regulate, immune cell proliferation and differentiation.[43] Specific cytokines or combinations of cytokines expressed by APCs can influence T or B cells toward the development of specific phenotypes and functions.
These activated adaptive immune cells undergo rapid proliferation – exponentially expanding cells specific to the disease-causing pathogen and pathogen infected cells. Different types of T cells require different signals to develop. As previously mentioned, CD8+ T cells require MHCI stimulation for development. These cells are specialized for the secretion of inflammatory cytokines and cytotoxic mediators to kill virally infected cells.[31] Because these cells also cause damage to host tissue, their activation is tightly regulated. In addition to CD8+ CTLs, there are CD4+ T cells know as helper T cells. These helper T cells have several functions. For example, when activated, helper T cells proliferate, differentiate, and migrate to sites of infection, they secrete cytokines to enhance the function of other immune cells at these sites. Additionally, helper T cells have the important role of stimulating B cells in the LNs – providing B cells with “help” – to differentiate into cells that produce specific, long-lasting antibodies.[31] This process occurs when helper T cells and B cells are brought into contact by chemokine gradients in specialized domains of LNs called germinal centers (GCs). As mentioned, antibodies are produced by B cells and can directly neutralize or tag extracellular pathogens for destruction; thus, some types of antibodies are termed “neutralizing antibodies”. In the process of producing these antibodies, activated B cells migrate into the B cell zone and then into GCs, where B cells proliferate and undergo several specialized processes; one of these is called class switching.[48,49] Class switching is the process that produces the different antibody classes, each exhibiting distinct structural features to provide the diversity of immunological functions needed to clear infections. Antibodies produced by B cells exhibit several different classes, such as Immunoglobulin-(Ig) G, though all may bind the same target antigen. For example, one class of antibody- IgA, has the ability to migrate through mucosal membranes more efficiently than other classes, which is a characteristic needed to combat certain pathogens. In addition the production of different classes of antibodies, a process known as affinity maturation occurs in the GCs.[49] Affinity maturation is an iterative process that allows B cells receiving T cell help to generate more potent antibodies by ensuring antibodies are highly selective and optimized to tightly bind to a target antigen. The result is antibodies that can rapidly neutralize infections. Together, these processes ensure the generation of an effective and specific immune response carried out by T and B cells.[50] However, the pathways just described also highlight the complexity of the immune system and necessity for multiple signals, cell types, and tissues to work cooperatively. Below we describe the exciting approaches emerging to exploit biomaterials in helping to decipher this complexity.
3. Biomaterials provide control over the molecular properties of antigens to study antigen processing and presentation
As discussed in Section 2.2, antigens are internalized and processed by APCs, then displayed on the APC surface to activate T and B cells. Some pathogens interfere with this key process through structural features of antigens that hinder internalization and presentation of antigen. Viruses, for example, often exhibit structural regions with high mutation rates. This constant change can limit the immune system’s ability to display and produce neutralizing antibodies that bind critical portions of the virus. In this context, biomaterials are useful as systems to retain native antigen structures, or to display antigen with control over specific molecular characteristics such as antigen conformation, whether antigen is delivered in soluble or particular form, or the density at which antigen is displayed. Biomaterials often achieve this control through linkage of antigen to the surface or interior of particles, scaffolds, on other substrates.[51] This approach ensure immune cells encounter antigen in a form or density of interest, allowing the role of the impact of the parameter to be isolated. The resulting insight can improve understanding of how antigen is processed and presented by APCs, and ultimately, how these antigen characteristics link to T and B cell function. The insight from such studies is useful in settings where potent immunity is desired (e.g., vaccines, cancer immunotherapy), as well as in settings of immune regulation, such as autoimmune therapies. This section - focuses on the molecular scale – discusses some of the emerging efforts leveraging biomaterial to study the roles of antigen conformation, form of delivery, and display density.
3.1. Biomaterials allow for analysis of how antigen conformation and form of antigen delivery impact immune response
Compared to attenuated or live vaccines that can have safety risks, NPs, scaffolds, and other engineered materials provide opportunities to display antigen in defined conformations without other pathogenic or replicative components. Equally important, however, is the ability to preserve this naturally-occurring antigen orientation for immunogenicity. Since antibodies binding to a pathogen rely not just on antigen sequence but also the structure, controlling conformation helps ensure antibodies produced against a given antigen can still bind to the target pathogen during an infection. This subsection will focus on examples using liposomes, polymer particles, and self-assembly to study the effects of antigen conformation and the role of delivering antigen in a soluble form compared with a particulate form. Scaffolds have also been used for immunomodulation and have shown promise for clinical applications against infectious disease, cancer, and autoimmune disease.[52]
One area where using biomaterials to control antigen conformation may be important is in the development of vaccines with complex antigen characteristics, such as Human Immunodeficiency Virus (HIV). Virus-neutralizing antibodies are thought to be important in an eventual HIV vaccine because of the ability of these molecules to bind virus particles to limit replication.[53] However, vaccines that elicit antibodies that neutralize HIV virus have been difficult to develop. Two reasons for this challenge are the low availability of antigen targets that have been identified for these antibodies, and the high degree of variability in antibody binding sites that occur across different strains of HIV.[54] One of the neutralizing antibody targets that is currently known is the HIV surface envelope spike. This structure is comprised of two subunit proteins that assemble into trimer molecules. A recent approach targeting this spike involved a recombinant protein engineered to retain the native trimer structure by modifying the sequence of one of the trimer subunits. This strategy relied on disulfide bonds to stabilize the subunit interactions.[55] Immunization with the recombinant protein prevented viral replication in non-human primates, highlighting the importance of antigen conformation in eliciting a protective immune response.
Because such recombinant approaches are complex, synthetic platform technologies- such as NPs – could help accelerate vaccine design by identifying immunogenic or conserved conformation properties. Tokatlian et al. recently designed a liposomal system that directed the orientation of HIV envelope trimers.[56] These liposomes used interactions between maleimide-functionalized lipids, nickel nitrilotriacetic acid (Ni-NTA)-functionalized lipids, and engineered protein antigens to study antigen presentation. This approach allowed the trimer to be displayed on the liposomes in a discrete orientation, creating unique advantages compared to the soluble trimer. In this system, the ability to tune the strength of the protein trimer’s association with the liposome membrane allowed binding affinity to be probed, and to improve the stability of the trimer-liposome association. Loading the recombinant protein onto liposomes reduced the number of antibodies generated against the artificial, disease-irrelevant parts of the protein, while modestly increasing the concentration of trimer spike-specific antibodies. This result is an example of using biomaterials to maintain antigens in orientations that faithfully represent the conformations needed to generate selective, productive immune responses (i.e., neutralizing antibodies). The result is also relevant because it demonstrates that using liposomes to control the trimer conformation impacts the range of antigens that the resulting antibodies can bind to.
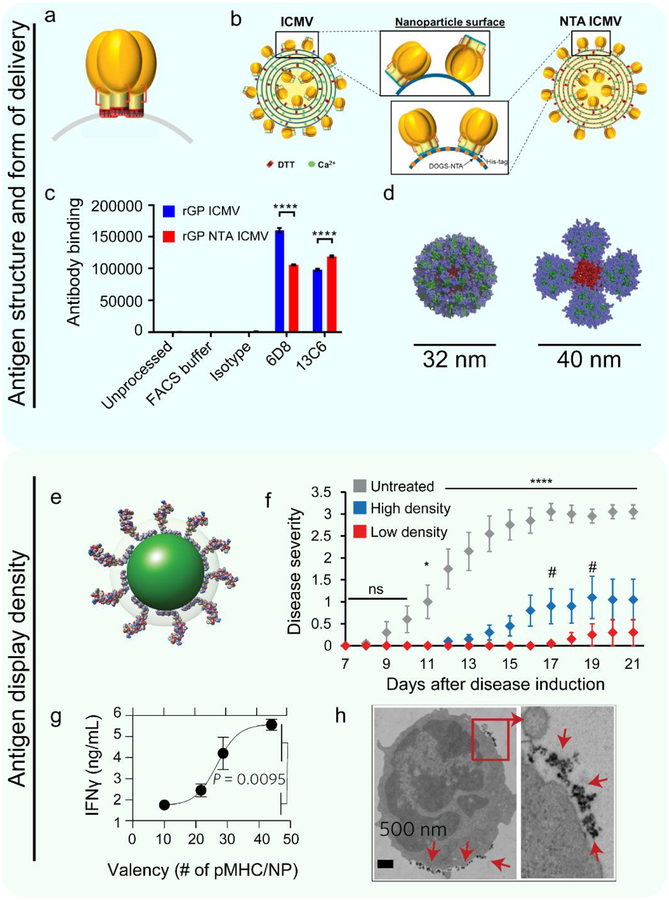
A similar approach has been to study other emerging pathogens. For example, in Ebola virus, past studies have revealed a single surface-exposed protein known as Ebola Virus (EBOV) envelope glycoprotein (GP). This protein is required for the virus to bind to and infect host cells. This role makes antibodies targeting this antigen very effective at preventing infection. Bazzill et al. developed lipid-based NPs called interbilayer crosslinked multilamellar vesicles (ICMVs).[57] These ICMVs were engineered to display EBOV GP in a controlled orientation (Figure 2a). To study antigen orientation, ICMVs were formulated with and without NTA-functionalized lipids. ICMVs without the NTA-functionalized lipids bind to any one of five sites in the protein. Thus, this design results in EBOV GP antigen loaded onto the ICMVs without controlling for orientation. In contrast, by including NTA-functionalized lipids, the EBOV GP can be loaded in an orientation-controlled manner because the lipids interact with a protein feature present only in the selected location on the ICMVs (Figure 2b). To determine how the oriented and non-oriented designs impact antigen recognition by immune molecules, EBOV GP-specific antibody binding was measured. This analysis revealed the orientation-controlled EBOV GP increased antibody binding by 30% (Figure 2c). This result illustrates that biomaterials that control antigen orientation can be used to understand the relative importance of this parameter for a specific vaccine or immunotherapy, or to optimize binding location or level. This is important because antibodies must bind an antigen on a pathogen that is immunogenic to elicit a beneficial effect, whereas many other antigen fragments will lead to irrelevant (i.e., non-functional) immune responses.
Figure 2. Biomaterials can control antigen conformation, form of delivery, and display density.
a. Trimer proteins self-assemble as on the surface of a virus in the native orientation. Reproduced with permission.[57] Copyright 2019, Elsevier. b. These capsids can be loaded onto ICMVs in a random fashion without NTA-functionalized lipids, or in a controlled fashion by using NTA-functionalized lipids at the base of the construct. Reproduced with permission.[57]Copyright 2019, Elsevier. c. The amount of fluorescence signal generated by fluorescently labeled antibodies that were incubated with antigen loaded ICMVs. The x-axis indicates the clone of the antibody the ICMV was treated with. Different antibody clones are reactive against different features of the antigen. This figure demonstrates that directed antigen orientation causes changes in antibody binding capability. Reproduced with permission.[57] Copyright 2019, Elsevier. d. A computational model of the self-assembled 60-mer eOD HIV antigen NP structure (left) or the MD39–8-mer of the self-assembled HIV antigen NP structure (right). The blue color depicts glycans. The green color depicts antigen (eOD – left, MD39 – right). The red color is the material core that facilitates self-assembly. These constructs have distinct physical structures which can impact the development of the immune response. Reproduced with permissions.[58] Copyright 2018, American Association for the Advancement of Science. e. QDs can be used as a platform to control the molecular density of antigen displayed to APCs. The green sphere depicts the core/shell of the quantum dots. The loaded molecule is a myelin antigen. Adapted with permissions.[66] Copyright 2017, Wiley-VCH. f. QDs with low densities of myelin antigen significantly reduced disease-induced paralysis as measured using a disease severity scale in a mouse model of MS. Reproduced with permissions.[66] Copyright 2017, Wiley-VCH. g. Increasing the density of pMHC on NPs revealed a range in which valency causes an exponential increase in the amount of an inflammatory cytokine (IFNγ) produced by T cells. Reproduced with permissions.[67] Copyright 2017, Springer Nature. h. The functional fate of cells encountering pMHC NPs is controlled in part by the pMHC density, which, above a threshold density, induce microclustering of the NPs on the T cell membrane as indicated with red arrows. Reproduced with permissions.[67] Copyright 2017, Springer Nature.
The previous studies discussed how anchoring protein antigens to the surface of lipids has allowed for the study of antigen orientation. A related idea centers on controlling molecular antigen properties using self-assembly of antigen into protein-based NPs.[58] This strategy has enable study of the distinct immune effects driven by either soluble or particulate forms of antigen. In this system, two formulations of self-assembled NPs containing known HIV antigens were recently synthesized. One particle was comprised of a protein from the outer layer of the virus. This protein was fused to a bacterial protein that self-assembled into NPs containing 60 protein copies (Figure 2d, left). Another NP fusion formulation resulted in NPs containing 8 protein copies (Figure 2d, right). This strategy allowed comparison of immune responses resulting from antigens presented in several different particulate forms and those resulting from the soluble antigen. The particle forms of antigen increased IgG levels up to 90 times, increased IgG affinity, and increased the number of B cells in antibody-producing GCs, as compared to treatment with soluble antigen. These studies also revealed the change in antibody production was a result of NPs being rapidly transported into B cell zones and GCs by specialized DCs in LNs. In contrast to the rapid transportation of NPs, soluble antigen did not accumulate in the GCs. Broadly, these findings demonstrate an emerging theme that particulate antigen often generates more potent responses due to changes in antigen transportation, processing, and display. Using this concept to understand the effects of antigen form may help engineer antigens that generate potent virus-neutralizing treatments against infections that are currently not curable.
One difficulty in studying how antigen structure impacts the immune response is that many antigens do not stimulate a strong immune response. Lassa virus is one example of a pathogen with antigens that do not naturally drive strong immune responses. Antibodies against the Lassa virus envelope glycoprotein - 1 (LASV GP1) in soluble form have a low affinity for this target. However, this is an important protein for antibodies to strongly bind because this interaction can prevent the virus from infecting cells. [59]
As already mentioned in this section, biomaterials allow delivery of multiple copies of antigen to promote or augment a developing response. This control is important because it allows for analysis of how other factors such as antigen structure alter the development of the immune response by delivering multiple copies of the antigen. This strategy can stimulate greater numbers of antibodies, enabling the study of other antibody features – such as structure – owing to the greater availability of the antibody of interest. In the context of Lassa virus, several studies have loaded LASV GP1 into polymersomes (PS). [59,60] One important finding from these studies is that the antigens the antibodies bind after vaccinating mice are different, depending on whether the LASV GP1 was administered in a soluble form or in the PS. In particular, delivery of multiple copies of the protein in the PS increased the development of antibodies that bind to an important site known to neutralize Lassa virus, whereas the soluble vaccines did not.[59] The investigators discovered this may be a result of valency effects resulting from having many copies of the protein antigens concentrated within the PS. More broadly, this demonstrates a link between the form antigen is delivered in and the site at which the resulting antibodies bind an antigen. This information is importance since this binding contributes to how effectively pathogens are cleared.
The use of biomaterials to study the role of antigen conformation and the form of delivery is not limited to infectious disease. In cancer for example, spherical nucleic acid NPs have been investigated to study how the form of antigen delivery impacts uptake, display, and the resulting anti-tumor immune response.[61] Likewise, other classes of materials have been used to control antigens that self-assemble into distinct structures, such as nanofibers.[62] Depending on the specific combinations of protein antigen used to build the nanofibers, these structures elicit immune responses that differ from one another, and also from soluble antigen. Thus, the examples in this subsection demonstrate that materials can be used to control antigen conformation and the form of delivery, parameters which each play important roles in determining the nature of the immune response against the antigen.
3.2. Biomaterials can be used to study the link between antigen display density and immune response.
The density at which antigen is displayed to immune cells is important in many immunological processes spanning activating, resolving, and regulating responses. For example, the density at which antigen is displayed impacts the generation of an antibody response and the affinity of these molecules. Likewise, for T cell function, the valency of interactions play a role in balancing T cell functional biases between pro-immune function and immunological tolerance. The former – immunity – is crucial to promote the inflammatory functions needed to fight pathogens; the latter is needed to keep excess or unwanted immune responses in check. For example, self-tolerance is important to prevent the development of autoimmune disease such as MS and type 1 diabetes. These diseases occur when the immune system malfunctions and attacks self-antigens, leading to inflammation and host tissue destruction. In MS, for example, myelin lining the central nervous system is mistakenly attacked. Many materials-based approaches have revealed nano- or microparticles containing myelin or other self-antigens ameliorated disease symptoms in mouse models of MS, diabetes, or other diseases.[8,63–65] These discoveries reveal self-antigen alone can drive not only inflammation, but also tolerance to self-antigens. The specific outcome is dependent upon the specific contexts in which the antigen is displayed, including parameters such as density and the other signals present during immune cell differentiation.
Hess et al. investigated the role of antigen density in driving immune tolerance using quantum dots (QDs) as a materials platform to display defined numbers of myelin self-peptide antigens (Figure 2e).[66] QDs offer uniform diameters and intrinsic fluorescence, features that make them useful tools for studying the immune system. QDs were synthesized with different antigen densities such that a fixed number of peptides were distributed among either a larger number of particles at a lower density, or a smaller number of particles at a higher density. These treatments were administered during a mouse model of MS (EAE) that results in the development of disease-driven paralysis. Strikingly, it was found that treating mice with the QDs displaying the lowest antigen density – distributed among the largest number of QDs – attenuated disease severity and delayed time of paralysis onset relative to the same dose of antigen distributed among a smaller number of QD (i.e., high density display) (Figure 2f). This was indicated by a reduction in the disease severity according to a standard clinical scale on which increasing scores indicate an increasing degree of paralysis. These findings add new insight into the role of antigen density in promoting tolerance. More broadly, the studies demonstrate the concept of exploiting a material to precisely vary a property – such as ligand density – to link the parameter to an immunologic process of interest – tolerance, in this case.
While the examples just presented study the interactions between antigen display density on a particle to an APC, the Santamaria lab has used biomaterials to study downstream effects. These investigators employed iron oxide NPs displaying peptide in MHC molecules to decipher how the density of antigen displayed to a cognate T cell impacts proliferation and function.[67] These NPs were functionalized with peptide antigen-MHC (pMHC) complexes to create a library of NPs with defined numbers of pMHC molecules. Using multiple ligand densities revealed a range in which increasing ligand density results in an exponential increase in the amount of an inflammation-associated cytokine produced by T cells (Figure 2g). Intriguingly, when pMHC were displayed at or above a minimum threshold density on the NPs, T cells encountering the NPs formed a high number of pMHC-NP microclusters, increasing the interaction time with antigen (Figure 2h). This clustering caused T cells to adopt a more tolerogenic function, while less-densely decorated NPs did not cause this polarization, nor were they associated with significant microclustering. Insight such as this could help design rules to drive specific types of pro-immune or tolerogenic responses. Thus, this work illustrates the crucial role of biomaterials in allowing a relationship to be developed between signal density and cellular activation, since the NPs allowed antigen density to be varied without impacting other antigen features.
The previous examples discussed the importance of antigen density in the context of autoimmune disease, but this feature is also important in mounting immune responses against infectious diseases. As one illustration, recent studies have exploited liposomes to control how malaria antigens are presented.[68] These liposomes are designed to load antigens into the membrane stably by coordinating interactions with metal ions located in the particles. This approach allows for ligands to be loaded into liposomes individually or in combination. Again, in a theme already emerging from the discussed work, using materials – liposomes, in this case, was useful to study the role of antigen density. These studies revealed a sensitivity to antigen content, whereby a 5 percent decrease in antigen decoration from the maximum density led to a ten-fold decrease in antibody production. This subsection has highlighted the importance of antigen density on immune function, but this is only one parameter of antigens that is important in understanding and tuning the immune response.
4. Biomaterials enable analysis of immune signaling through controlled cell interactions
Immune cells can adopt either inflammatory or tolerogenic functions depending upon the signaling pathways activated. The pathways regulating the balance between tolerance and inflammation are thus key areas of research. One theme already emerging from the previous section is the ability of biomaterials to link specific properties of immune signals (e.g., antigen) to the immunological processes they trigger. Along analogous lines, Section 4 focuses on the ability of biomaterials to control how immune signals are received or processed by immune cells. This cellular-scale control is enabled by unique material capabilities, including i) directing the delivery of immune signals to specific cells or intracellular locations, ii) controlling the relative combinations of signals that immune cells encounter, iii) tuning the duration over which signals persist (i.e., controlled release), and iv) recapitulating the natural display of signals using artificial immune cell mimics. Below we illustrate these concepts with recent studies using biomaterial tools to investigate ubiquitous cellular processes in the immunology field: gene expression and genetic engineering, synergies between innate immune pathways such as TLRs, immunometabolism, and cell-cell signaling.
4.1. Using biomaterials to guide the delivery of immune signals to specific cell locations expands knowledge of the genetic systems regulating immune cell functions
Nucleic acid delivery is a natural area where the ability to guide signals to specific intracellular locations is important because DNA and RNA are decoded at distinct locations (e.g., nucleus, cytoplasm). In the immune field, biomaterials are used to deliver a range of vectors that either encode immune signals or bind complementary nucleic acid sequences involved in immune function; alternatively, the cargo are nucleic acid-based ligands – such as TLR agonists – that trigger innate immune pathways, but do not directly encode genetic information (see Section 4.2). Thus, precision delivery of DNA, messenger RNA (mRNA), microRNA (miR), small interfering RNA (siRNA), and other genetic molecules offer multiple levels to probe how the trafficking and processing of immune signals impacts immunity and regulation.[69–73] The delivery of these different classes of nucleic acid will be discussed in this section. Along these lines, innate immune cells have historically proven difficult to successfully transfect because of the efficiency with which these cells degrade nucleic acid and the limited proliferative capacity of these cells. While other technologies – such as viral vectors and virus like particles – have had some success, pathogenic reactivation, excess inflammatory responses, and limited expression of the delivered nucleic acid remain as challenges.[74] The properties of biomaterials have been exploited broadly in the drug delivery field to protect nucleic acids from degradation, improve uptake, and ultimately, to promote endosomal escape without toxicity in traditional cell targets; these same ideas are now expanding to immune cell targets. Irrespective of the targeted type of cell, the goal of these approaches is to deliver intact genetic material to the target cell for expression as functional proteins.
One important area gene expression impacts is immune cell migration through the dynamic regulation of surface proteins. Immune cell migration is an important topic to understand because the movement of these cells impacts the cells functional abilities. These cues guide cells along protein gradients between immune tissues and sites of infection and cancer, depending on the maturation state of the cells. For example, one protein gradient that immune cells follow into LNs is CCL19 and CCL21. These proteins cause immune cells expressing CCR7, such as DCs, to migrate toward regions with higher CCL19/CCL21 concentrations found in LNs. In light of this, biomaterials have been used to deliver DNA encoding CCR7 and protein antigens to DCs to promote more efficient migration of these cells back to LNs as they internalized antigens.[75] In this study, protein antigen and CCR7 encoding DNA was delivered in micelles formed from chitosan – a naturally occurring polymer – combined with stearic acid to create a biocompatible delivery vehicle. The modular nature of the system allowed for tuning of how tightly the DNA was bound to the carrier, as well as the inclusion of a DC targeting molecule (mannose) that enabled the cargo to be delivered specifically to DCs. In the micelle approach, delivery of the plasmid DNA encoding CCR7 increased DC migration by 3.5-fold as compared to a commercially available gene delivery system. Since tumors suppress the ability of APCs to sample tumor antigens and return to LNs – processes important in mounting anti-tumor responses – the ability to increase DC migration back to LNs to present antigen is one way to improve immunotherapies. Other approaches have used electrostatically assembled materials to study the relative importance of tightly condensing immune cargo to promote uptake by immune cells, while maintaining interactions that are weak enough to still allow interactions with intracellular immune receptors such as TLRs.[76]
While the previous approach delivered DNA, another method to control gene expression is by delivering mRNA. mRNA is the template cells generate from DNA to support protein production. One locality distinction of mRNA compared with DNA is that mRNA is processed in the cell’s cytoplasm. This is useful for nucleic acid delivery and genetic regulation because the cargo is active in the cytoplasm, circumventing hurdles related to ensuring DNA cargo reaches the nucleus. Biomaterial features such as charge, polymer buffering capacity, and polymer chain length can be used to control the efficiency of mRNA delivery into the cell. Oberli et al. created a system using lipid NPs to deliver mRNA to APCs.[77] These NPs were composed of ionizable lipids to bind to the delivered mRNA, polyethylene glycolated (PEGylated) lipids to prevent NP aggregation, and phospholipids and cholesterol to enhance NP stability and escape from APC endosomes. Thus, as a tool, such multi-component designs allow the distinct impact of each material component to be studied with respect to mRNA stability, uptake levels, and concentration in the cytoplasm. These formulations also allowed the amount of mRNA delivered to the cytoplasm to be connected to the resulting functional changes in the APC and to the ability of these cells to improve induction of cytotoxic T cells.
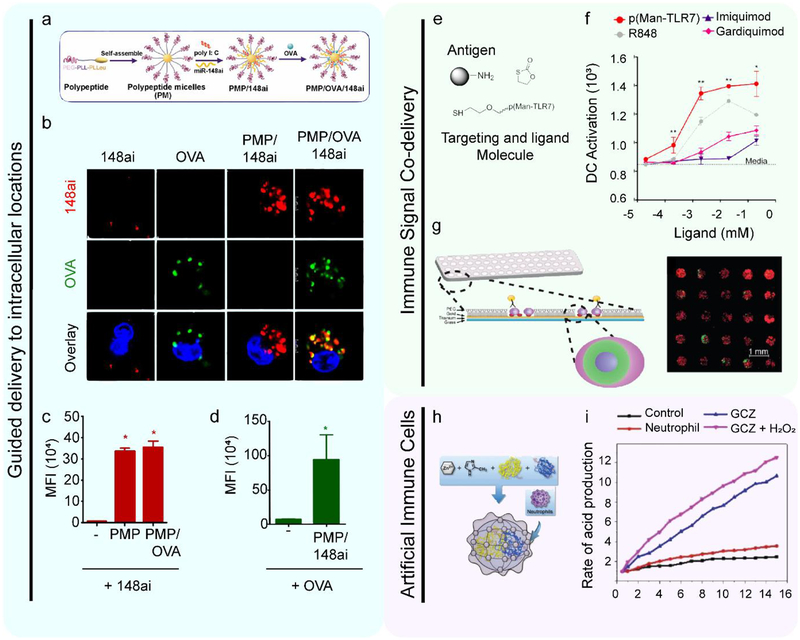
Another type of RNA where biomaterials are enabling new levels of control of delivery to cells is miR. MiR are short RNAs that do not encode a protein product. Instead, these molecules inhibit translation and promote the degradation of mRNA to help regulate cell function. However, tumors exploit these regulatory molecules in the immunosuppressive tumor microenvironment to inhibit the function of immune cells trying to destroy the tumor. For example, many tumor-associated DCs (TADCs) are unable to become activated after exposure to inflammatory stimulus because of the suppressive environment, hindering the development of an adaptive immune response. One such family of miR reported to limit APC activation is miR148a. In particular, this class of molecules has recently been shown to inhibit TLR-induced activation in DCs, and promote regulatory function in T cells.[78] Thus, the authors tested if inhibiting miR148a would restore DC activation in the tumor microenvironment. To study this pathway, polymeric micelles (PMs) were synthesized to coencapsulate defined combinations of TLR3-ligand (Poly(I:C)), miR148a antagonist (miR148ai), and a model antigen (OVA) (Figure 3a).[79] Because the PMs allowed control over which signals were included in the structures, the investigators were able to determine the uptake of and relative importance of each of these components – including miR148a – on the immune response during a mouse model of cancer (Figure 3b). One observation was that PMs containing miR148ai resulted in a 70-fold increase in miR148ai delivered to TADCs compared to soluble miR148ai (Figure 3c), while PMs containing a model antigen (OVA) increased delivery to TADCs by 10-fold as compared to soluble OVA (Figure 3d). Using the PMs to define the combinations of signals delivered, the authors discovered PMs containing miR148ai and poly(I:C) reactivated TADC, compared to PMs not containing Poly(I:C), or PMs in which miR148ai was replaced with a control miR. This is one example where using a materials platform to control uptake can be used to understand how the delivery of each cargo impacts the immune response.
Figure 3. Biomaterials can be used to study immune cell signaling and processing by exploiting material features to guide interactions with cells.
a. A schematic depicting the design of PMs. PMs were first synthesized without any cargo. Then, miR antagonist and Poly(I:C) were added to the formulation. Finally, a model antigen OVA incorporated into these structures. Reproduced with permissions.[79] Copyright 2016, American Association of Immunologists, Inc. b. The cellular uptake of miR148ai (red) and OVA (green) are shown four hours post treatment using confocal microscopy. In these images, cells were treated with either soluble miR-148ai, soluble OVA, polypeptide micelles encapsulating miR-148ai, and polypeptide micelles with OVA and miR-148ai. These images demonstrate that only the cells treated with polypeptide micelles with OVA and miR-148ai have both signals present in the cytoplasm. Reproduced with permissions.[79] Copyright 2016, American Association of Immunologists, Inc. c. Uptake was quantified at 0.5 hours post treatment using flow cytometry. This panel indicates miR-148ai-positive cells as a measure of median fluorescence intensity. Reproduced with permissions.[79] Copyright 2016, American Association of Immunologists, Inc. d. As in panel c, uptake was quantified at 0.5 hours post treatment using flow cytometry to measure the median fluorescently intensity of fluorescently-labeled OVA in live cells. Reproduced with permissions.[79] Copyright 2016, American Association of Immunologists, Inc. e. The antigen, p(Man-TLR7), and biproduct molecules that are released in the endosome as the delivered molecule is degraded by the immune cell. Reproduced with permissions.[84] Copyright 2019, Springer Nature. f. Commercially available TLR7 agonists(gray, pink, and purple) activated DC less than the synthesized p(Man-TLR7) agonist (red). Reproduced with permissions.[84] Copyright 2019, Springer Nature. g. A schematic of the cell-based assay and representative images of DCs colocalized with particles. Bone marrow derived DCs (red – rhodamine phalloidin; blue - nucleus) were cultured with PLGA particles (green). These DCs are cultured with particles then analyzed for changes in surface protein expression or soluble cytokine secretion. Reproduced with permissions.[94] Copyright 2017, Royal Society of Chemistry. h. A schematic showing the formation of artificial neutrophils. Enzymes were embedded in an organic Zn framework. These frameworks were then encapsulated in cell membranes isolated from activated neutrophils. Reproduced with permissions.[106] Copyright 2019, Wiley-VCH. i. Artificial neutrophils generated inflammatory molecules more quickly than neutrophils isolated from mice with a model of cancer. Reproduced with permissions.[106] Copyright, Wiley-VCH.
Another area where RNA delivery has been important is the development of new technologies to target specific genetic sequences, including clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9). CRISPR enables targeted probing of immune cell signaling. This technique uses specific DNA sequences encoding a DNA cut site for the DNA cleaving enzyme, Cas9, to edit DNA. The CRISPR/Cas9 system offers a high level of specificity and efficiency, creating simplicity in introducing mutations, gene deletions/insertions, or other manipulations. This system requires the delivery of the DNA and a short-guide (sg) RNA to the same cell for a specific gene to be edited. Biomaterials have the ability to support the loading of nucleic acids of disparate sizes, stabilize delivery vehicles, and deliver multiple signals simultaneously. These capabilities could make CRISPR/Cas9 more accessible for future use in immune studies. In one recent study, a type of lipid-like material – coined zwitterionic amino lipids – were developed to deliver the long Cas9 encoding mRNAs to produce the necessary enzymes and sgRNAs to target desired DNA sequences.[80] Zwitterionic amino lipids were synthesized to support the loading of both nucleic acids by capturing features of both cationic lipids and phospholipids, ensuring both the stability of the NPs and efficient cargo delivery. This approach could simplify CRISPR/Cas9 because it adds modularity to more easily and rapidly test new short guide RNA sequences.
While this approach is exciting, the work did not incorporate a means to target specific cell types for editing. The ability to probe specific cell types could be very useful in understanding immune cell regulation. Toward this goal, Luo et al. applied targeted CRISPR/Cas9 by engineering a macrophage-targeting cationic lipid based on PEG-b-PLGA NPs that were able to encapsulate a plasmid.[81] A cell type-specific promotor was included to regulate the cell type that Cas9 would be expressed in. The promoter is the region of DNA that initiates transcription. This system thus allows for the efficient delivery of plasmid DNA to all immune cells, but by using a promoter only expressed in macrophages, the approach ensures that only macrophages express the delivered gene. While this approach does not employ cell selectivity at the biomaterial level, future work could combine the capability of biomaterials to target specific cell types with this type of biological advance to further improve cell targeting. In this section, we have discussed some of the ways that material properties can be exploited in gene delivery and genetic engineering in immune cells. In the next subsection, we highlight the ability of materials to provide combinatorial control over the delivery of multiple immune signals using recent work in the area of innate immune signaling as one important illustrative setting.
4.2. Biomaterials allow isolation of distinct or synergistic roles during delivery of ligands for PRRs
As discussed in Section 2.1, PRRs are key pathways APCs use to detect foreign pathogens. TLRs, for example, each bind distinct pathogen-associated motifs to provide the appropriate warning signals connected to the most suited immune response. Biomaterials have been able to improve understanding of TLR signaling by enabling the co-delivery or the presentation of defined combinations of TLR ligands to the same APC. This approach allows for the isolation of the role of individual signals, as well as the study of how TLRs signal synergistically or antagonistically to shape APC activation. Importantly, TLRs are not the only PRRs present on innate immune cells. Some other important classes include STING and NOD pathways, which can both help induce inflammation. These classes of PRRs have also been the focus of several recent biomaterial studies. In this section, we explain how biomaterials are being used to understand the action of TLR ligands, and to improve the action of combinations of ligands for different PRRs.
Stimulating TLRs can be useful in generating strong responses against viruses, bacteria, or cancerous cells by increasing costimulatory molecule expression and the secretion of inflammatory cytokines. As introduced in Section 4.1 for DNA and RNA, biomaterial properties can also be exploited to target TLR molecules to their biologically active location. For example, TLR7 is a TLR located in intracellular compartments called endosomes. This receptor recognizes viral-associated single stranded RNAs and synthetic imidazoquinolines (IMDQ) to induce a strong anti-viral immune response. The use of soluble TLR7 agonists has been limited due to the induction of strong systemic inflammation. For this reason, TLR7 agonists have been an ideal target to pair with biomaterials. Biomaterials enable TLR7 agonists to be covalently attached to large molecules or cell targeting molecules to alter the transportation of the molecule within the body. In one recent example, a nanogel was made of two polymers: a pH responsive polymer block and hydrophilic polymer block.[82] These polymers displayed a TLR7 agonist bound before nanogel synthesis. The use of a pH responsive polymer enabled the TLR7 agonist to only be released under endosomal pHs, preventing systemic inflammation. These nanogels reduced viral load during challenges with a respiratory virus relative to standard adjuvants, and other control antigen formulations. Subsequent studies also demonstrated an analogous approach for cancer vaccination.[83] This work highlights the modular nature of biomaterials to deliver signals to desired immune locations to polarize the resulting immune responses for specific applications.
Because some TLRs are differentially expressed on APCs, and because APCs have distinct functions, using biomaterials to guide these signals provides more precise control of signaling. In one example, a complex – coined p(Man-TLR7) – was designed that involved an antigen, a TLR7 agonist, and a molecule to target DCs (mannose).[84] The TLR agonist and mannose molecules were bound to the protein antigen by a self-immolative linker that allows the antigen to be released from the DC targeting molecule and adjuvant once in the endosome (Figure 3e). This release is important because it promotes antigen display and DC activation, providing two of the necessary signals for adaptive immune cell activation. Of note, p(Man-TLR7) significantly increased DC expression of surface activation markers and inflammatory cytokines relative to commercially-available TLR7 agonists (Figure 3f). This DC activation was only observed with p(Man-TLR7), not other forms of the components. Thus, these studies also allude to the importance of combinatorial signals delivered, the topic we will now focus on.
Simple mixtures of immune signals do not offer the ability to control the combinations or concentrations with which signals reach immune cells. Using biomaterials to achieve this capability can drive new understanding of how TLR or other PRR ligands are processed by APCs, the types of immune reactions they generate, and how to improve the immunogenicity of these ligands; signal combinations may also generate strong responses with lower total doses. One interesting area for combinatorial delivery of immune signals is self-assembly.[71] In one system, nanostructured materials are assembled entirely from immune signals using electrostatic interactions to create immune polyelectrolyte multilayers (iPEMs).[85,86] In the context of autoimmunity, iPEMs have been used to investigate whether restraining TLR9 signaling while codelivering a myelin self-antigen prevents the development of inflammation in a mouse model of MS.[87] These iPEMs were assembled from a cationic myelin peptide and a TLR9 antagonist – GpG – that is a negatively-charged oligonucleotide. Because the signals are complexed, this strategy ensures that immune cells process myelin antigen and the regulatory GpG together. This feature allows for the study of how combinations of immune signals can shape or reshape the immune response, for example, to redirect the response to myelin away from inflammation and toward immune tolerance. In co-culture studies, these complexes increased the development of myelin-specific regulatory T cells compared to complexes that incorporated a control oligonucleotide. During a mouse MS model, treatment eliminated disease. Further, treatment of immune cells isolated from human MS patients with these myelin/GpG iPEMs decreased the production of inflammatory cytokines compared to cells treated with iPEMs containing myelin and control oligonucleotides. In similar studies, the response of immune cells to soluble signals relative to equivalent iPEMs were compared. These studies demonstrated that iPEMs increased signal colocalization within DCs and elicited more potent effects compared to dose matched soluble components.[88] In addition to forming carrier free complexes, these same principles can be applied to build and release iPEM surfaces for delivery using other administration routes, such as microneedle vaccines.[89,90] Further, these complexes could be synthesized to contain defined ratios of each signal, creating a platform to reveal the relative importance or synergies between pathways. This highlights another useful feature of materials, in that fundamental properties of a specific system can be applied to different settings, such as an injectable or device formulation.
The discussion of self-assembly of immune signals also suggests some trade-offs between soluble signals and signals delivered in particulate form, encapsulated or adsorbed using other biomaterials. First, there is sometimes a balance between signal accessibility and internalization. For example, soluble signals may be more accessible for immune processing than DNA or RNA molecules tightly-condensed by a carrier. However, ultimately the biomaterial formulation may enter cells more readily – because of the particulate nature, generating a greater overall response. Soluble components can also sometimes be more potent because all of the signal is available for processing upon administration, as compared to a signal encapsulated or condensed in a particle. This accessibility of soluble signals may result in side effects because of non-targeted or systemic distribution. These ideas reinforce the importance of considering not just efficacy observed with a biomaterial formulation, but aspects such as the kinetics of delivery or function, and selectivity to target specific cells or tissues.
In addition to using materials engineering to co-deliver TLR ligands with antigen (or self-antigen) as just discussed, other strategies have used biomaterials to study the role that the combination and relative concentration of multiple adjuvants have on immune function. For example, combinations of TLR ligands have been shown to alter APC function and polarize T cells toward specific phenotypes by stimulating distinct sets of APC signaling pathways. Along these lines, helper T cells adopt phenotypes optimized to combat specific classes of pathogens: TH1 cells help clear intracellular pathogens like viruses and bacterium, while TH2 cells help clear parasites and pathogens outside the cell. Ebrahimian et al. developed a novel signal delivery method using a cationic polymer polyethylenimine (PEI) conjugated to poly(lactic-co-glycolide) (PLGA) NPs co-encapsulating either a TLR7/8 agonist – resiquimod, or a TLR4 agonist -monophophoryl lipid A (MPLA).[37] These components were co-assembled with a TLR9 agonist, CpG, to form NP formulations containing defined combinations of two TLR agonists. CpG is a DNA motif common in bacteria that binds to endosomal TLR9 molecules causing APC activation, while MPLA is a TLR4 agonist that mimics warning signals commonly in bacterial polysaccharides; Resiquimod is an artificial TLR7 agonist; TLR7 is located in the endosome where it senses single stranded RNA triggering an anti-viral immune response.
Because these NPs contained two distinct TLRs within a single particle, the internalization of particles ensured cells received each signal simultaneously. This allowed for analysis of the effects of delivering multiple TLR agonists or matched doses of a single TLR agonist. In mice, codelivery of TLR7/8 and TLR9 agonists increased expression of TH1-associated cytokines, including IFNγ, relative to delivery of a single TLR agonist. In contrast, NPs coencapsulating TLR4 and TLR9 agonists stimulated greater TH2-associated cytokines – such as IL4, compared to the NPs coencapsulating TLR7/8 and TLR9 agonists. Thus, the NPs containing TLR4 and TLR9 favored TH2 responses, while codelivery of TLR7/8 and TLR9 agonists favored a TH1 response.[37] Similar strategies have also been used to deliver coencapsulated ligands and antigens for generating pathogen clearing immune responses.[91–93]
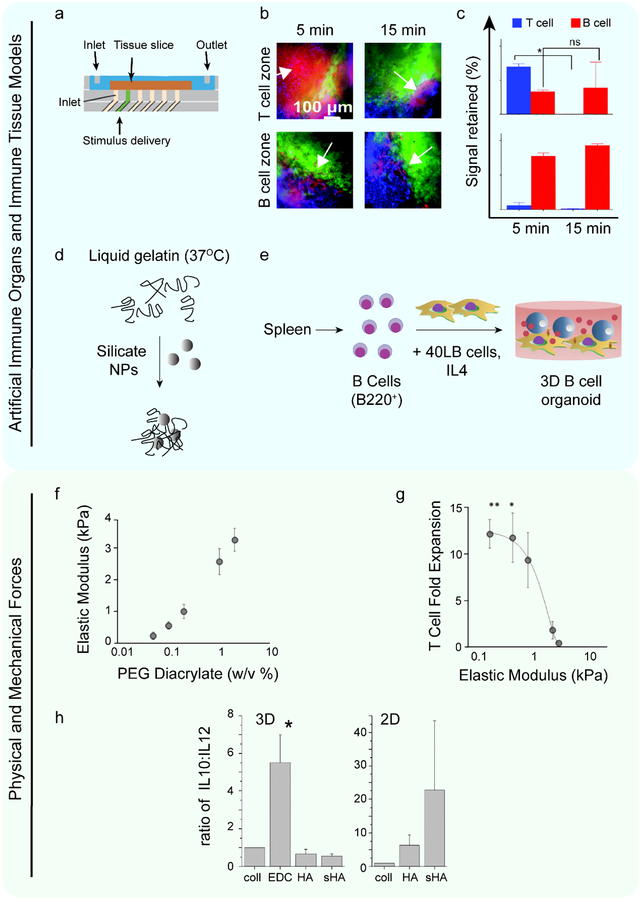
Simultaneously stimulating multiple TLRs may recapitulate the natural response APCs mount against pathogens. However, this idea could be further developed by deciphering how the relative concentrations of each TLR ligand impacts upstream signaling and downstream responses. Biomaterials can facilitate this analysis by controlling the loading concentration and subsequent codelivery of multiple encapsulated cargos. In one such example, to study dose dependent TLR interactions, microparticles (MPs) encapsulating TLR3, TLR4, and TLR9 ligands either individually or in combination were printed onto coverslips before seeding with DCs to create a cell-based microarray (Figure 3g).[94] Using this platform, more than 200 combinations of ligands were assayed for their impact on cytokine levels and activation marker expression. This approach revealed that a relative ratio of 1 TLR9 agonist: 2 TLR4 agonists: 2 TLR3 agonists stimulated the greatest proinflammatory DC responses, as measured by increased surface activation marker expression and decreased regulatory cytokine production. Further, analysis of cell responses to individual TLR ligands revealed differential patterns of upregulation among the activation markers. For example, TLR9 and TLR3 agonists led to the greatest increases in CD86 – a costimulatory marker, while TLR4 agonists led to the greatest increase in MHCII expression – which is a key complex for antigen presentation. Future studies leveraging biomaterials for screening or multiplexing analysis to control the extent to which specific sets of immune pathways are activated could help support rationale design rules for vaccines and immunotherapies.
In addition to TLRs, agonists for several other important classes of “danger” signals have been combined with biomaterials, including a cytoplasmic DNA-sensing pathway called cyclic GMP-AMP Synthase (cGAS)-STING. This pathway has recently gained particular attention for the ability to elicit potent anti-cancer immune responses.[95] In this context, one study leveraging biomaterials compared the STING pathway to TLR signaling and other environmental sensing pathways during the generation of an anti-tumor response.[96] This strategy used acetalated dextran MPs encapsulating either: a TLR7 agonist, a TLR3 agonist, a STING agonist, or a nucleotide-binding oligomerization domain-2 (NOD2) agonist; these pathways have in common the ability to sense cytoplasmic nucleic acids. In another approach, when tumors were injected with these different formulations, mice that received the MPs encapsulating the STING agonist experienced the most significant reduction in tumor burden in a mouse-model of melanoma. Knowledge about which of these pathways most potently stimulates an anti-tumor immune response was previously difficult to achieve due to toxicity because in their soluble form all of these molecules cause systemic inflammation. Other work with STING agonists has leveraged designer materials to tailor bioavailability within specific cell destinations (e.g., endosome).[97] Likewise, some of these approaches are now being extended to still other PRR families, including the retinoic acid-inducible gene I (RIG-I) pathway; this pathway triggers interferon-based immune response useful in driving potent anti-viral immune response.[98] While these approaches have not yet been used to study combinations of agonists from these TLRs, STING, RIG-I, or other families, the modular nature of biomaterials clearly offer exciting possibilities to understand how these distinct immune detection mechanisms can be exploited for different pathogens or diseases. More broadly, this section has highlighted the theme that biomaterials can be used to study how different signaling pathways interact and to probe how the connections shape the resulting immune response. These concepts can also be coupled with new screening and multiplex technologies, an interface we will return to later in Section 6.
4.3. Controlled release from biomaterials can be used to study immune cell function
Many biomaterials offer controlled release of cargo through degradation of carrier components, porosity, or in response to environmental cues such as pH. This feature is useful in vaccines and immunotherapies to both control the kinetics of a particular functional outcome and to limit systemic exposure through regulation of the maximum signal concentration present at a particular time. Immunometabolism is one area where these controlled release capabilities have great potential to probe immune function. The cellular processes underlying immune cell activation, proliferation, and differentiation require energy. This energy comes from highly regulated metabolic processes. Delivering metabolic modulators at controlled rates allows for the study of how the underlying metabolic processes impacts inflammatory or regulatory immune function. This is important because these processes exert large impacts on both the downstream immune function and the extent of off-target effects.
One important drug studied in the context of metabolism is rapamycin - an example of an immunosuppressant; these are drugs used to maintain immunosuppression, for example, to prevent organ rejection following transplantation. However, most existing therapies involve systemic administration of these drugs, which can also make patients susceptible to infection. Rapamycin is an inhibitor of a key metabolic pathway, mammalian target of rapamycin (mTOR). Thus, there is significant interest in understanding the diverse roles of this drug. For example, lipoprotein nanocarriers have been used to deliver rapamycin to reduce the inflammatory function that macrophages direct against heart grafts in mice.[99] This change was correlated with a decrease in aerobic glycolysis as measured by an increase in lactate production. Numerous other studies have explicitly leveraged biomaterials for controlled release as a means of modulating mTOR for tolerance.[63,90,100] Yet, other approaches have used controlled release of rapamycin to modulate the mTOR pathway in a different manner, to enhance pro-immune function. For example, Gammon et al. used PLGA NPs to deliver small, extended release doses of rapamycin to lymph nodes during vaccination with melanoma antigens and TLR agonists.[101] This approach altered mTOR signaling to promote T cells that were antigen specific, but exhibiting a central memory phenotype useful for long-lasting vaccination or high rates of T cell proliferation to combat tumors. These examples demonstrate a key theme for modulating metabolism with biomaterials: the same pathway – mTOR, in this case – can be exploited to direct divergent immunological outcomes such as inflammation, tolerance, or memory functions. Controlled release is an important variable in this context because both the concentration and duration of exposure to metabolic cues impacts the outcome.
Glutamate metabolism is another immunologically relevant metabolic pathway that influences cell function, including proliferation and energy production.[102] Of note, high concentrations of glutamate in the central nervous system of patients with MS contributes to inflammation and neurodegeneration. The metabotropic glutamate receptor (mGluR) family helps direct the metabolism of glutamate, and some of these receptors are expressed by immune cells.[103] In particular, the mGluR4 pathway can be inhibited with the drug N-Phenyl-7-(hydroxyimino) cyclopropa[b]chromen-1a-carboxamide (PHCCC) to promote regulatory function. While PHCCC can direct T cells to differentiate into these regulatory T cells to control inflammation, this drug is poorly water soluble, has a short half-life, and exhibits systemic toxicity. Because of these limitations, several biomaterial strategies – including degradable polymer NPs and PEGylated liposomes – have been explored to probe how the duration of exposure to PHCCC impacts control of a mouse model of MS, and PHCCC toxicity.[104,105] For example, encapsulation of PHCCC in NPs reduced toxicity by 36-fold compared to free drug.[104] These particles slowly released PHCCC into solution over 3 days. Treating APCs with the NPs, followed by co-culture with myelin-reactive T cells, shifted T cell function away from inflammatory phenotypes and toward regulatory function. Critically, in mice, treatment with the controlled release NPs every 3 days reduced disease severity relative to dose and interval-matched treatments with free PHCCC. The examples demonstrate how controlled release features of biomaterials can be used to understand links between signal exposure kinetics and immune response.
4.4. Biomaterials can be designed as artificial immune cell mimics to study immune signal processing and response.
One theme already discussed in Section 4 is the interconnected nature of immune signaling, and how biomaterials can be used to control the combinations and relative concentrations of multiple signals (Section 4.2). This is one dimension of the broader theme of Section 4, using materials to study immune cell interactions. Not surprisingly, the physical features of cells, particles, and other biomaterials also play an important role in these interactions. To leverage these different aspects, biomaterials are also now being used to create artificial immune cell mimics. Using a blend of synthetic materials, cellular features such as the plasma membrane, physical characteristics (e.g., aspect ratio), and defined surface-displayed or secreted activation signals, these mimics allow simplified opportunities to study the combinations of these parameters.
One example of a type of immune cell that biomaterials have been used to mimic is neutrophils.[106] As innate immune cells, neutrophils are the first defense against infiltrating pathogens and even malignant cancers. Neutrophils rapidly sense inflammatory mediators and migrate to sites of infection, then employ phagocytosis, degranulation, and reactive oxygen species to destroy pathogens. In addition to inducing the inflammatory response, neutrophils also regulate the function of other immune cells through the secretion of soluble signaling molecules.[107] In recent studies, biomaterials were designed to mimic two key neutrophil features: the ability to generate inflammatory mediators and the ability to migrate quickly to sites of infection.[106] In this strategy, two enzymes that contribute to the production of hypochlorous acid – which promotes inflammation and kills pathogens – were embedded into a zeolitic framework (Figure 3h). By controlling the relative abundance of each enzyme, it was possible to determine the impact that the relative enzyme ratio has on maximizing the rate of production of a pathogen-killing molecule, hypochlorous acid. Further, these mimics retain the ability to migrate to sites of infection by encapsulating the enzyme-loaded scaffolds in neutrophil membranes. These membranes were prepared by loading scaffolds with neutrophil membranes containing common surface-expressed proteins displayed on activated neutrophils. This approach thus used naturally-occurring cell membranes to maintain migratory capacity along signaling gradients, and biomaterials to provide control of the concentrations of the enzymes needed for production of hypochlorous acid. Demonstrating the power of this approach, more chloroperoxidase – one of the enzymes that produce hypochlorous acid – could be loaded into the scaffolds than would naturally occur in neutrophils. This resulted in neutrophil mimics that generated hypochlorous acid – to drive the destruction of pathogens, at a rate 8 times that of normal activated neutrophils (Figure 3i). This type of quantitative study using mimics could support more efficient vaccine and immunotherapy designs to combat infections or tumors.
Neutrophil mimics are not the only immune cells that have been explored using biomaterials. Artificial APCs (aAPCs) have been developed to study how T cells develop in response to the arrangement of immune signals that are provided by APCs (e.g. antigen or costimulatory molecules).[51] These cells have been evaluated for use in diseases such as cancer, where the tumor microenvironment contains inhibitory molecules that suppress the host immune response. In addition, aAPCs have been explored in basic research to establish relationships between physical parameters and cellular functions. For example, these systems have been used to investigate how the relative length and width (i.e. aspect ratio) of an aAPC impacts T cell activation.[108] This strategy used PLGA particles that were stretched to control the aspect ratio. EDC chemistry was then used to covalently bind T cell-stimulating molecules to either spherical or ellipsoidal particles. Studies comparing these two aAPC designs revealed ellipsoidal particles reduced the volume of tumors to a greater extent than spherical particles. These results again demonstrate the possibility of identifying natural features of immune cells, then manipulating these parameters – such as aspect ratio – to understand how immune cells perceive the physical features of these molecular signals. Ultimately, this approach can be used to generate stronger, more specific immune responses. Section 4 has highlighted the features of biomaterials that can be used to probe immune cell interactions, using gene delivery, PRRs, metabolism, and immune cells mimics as representative immunological topics. In Section 5, we move beyond the cellular scale to tissue and microenvironment interactions.
5. Biomaterials enable studies at the interface of immune cells and extracellular environments.
This section focuses on how biomaterials support studies at a greater length scale – the immune tissues and immune cell migration throughout these tissues and the host. This discussion will highlight the use of biomaterials as tools to study how the interactions immune cells have with the ECM can influence immune function. One of the key themes is the ability of biomaterials to create three-dimensional culture systems. Three-dimensional structure can advance immunological understanding by introducing controlled perturbation of mechanical stimulation, tissue level signaling gradients, and protein ligand display.[109] Hydrogels, microfluidics, and other polymer scaffolds enable properties such as porosity, stiffness, and signaling gradients to be defined. As discussed in the subsections below, these capabilities create valuable tools to program mechanical forces or other environment-specific parameters that mimic distinct tissues or disease states in the body; these cues directly influence the nature of the immune response that develops.
5.1. Biomaterials mimic cellular and tissue properties to elicit a targeted immune response
This subsection draws motivation from the developmental processes that occur in specific domains of immune tissues, such as LNs. In this context, biomaterials have been used to develop on-a-chip and organoid culture models that mimic aspects of the three-dimensional environments of immune tissues.[23,110] These material-enabled mimics have the capability to model processes that occur in specific sub-domains of immune organs, such as those that develop in B and T cell zones of LNs during B and T cell activation. B and T cells differ in their ability to fight infection; however, accurate systems to model how the respective zones process soluble signals, such as those that would be received from the lymph would be of great value. Traditionally immune cells are isolated by disrupting tissues and preparing single cell suspensions for culture studies, which prevents the analysis of functional differences between tissue domains, the underlying role of stromal cells, migration, and other tissue-level effects. Microfluidics systems provide a useful tool to study tissue level structures and functions because microfluidics use defined channels through which fluid flow recapitulates that occurring in the body. Biomaterials, scaffolds, and microfluidic devices could allow for delivery of “probe” signals to specific locations of immune tissue integrated into these devices. For example, since microfluidic systems provide control over the flow rate, concentration of stimulus (e.g., antigens, adjuvants, cytokines), and location (i.e., defined inlet channel) that stimulus is applied to, these technologies are well-suited to study how signals are processed at the tissue level. To investigate specific functions in LN sub-domains, one recent example used a microfluidic device to deliver signals precisely to the T or B cell zones within a live LN slice.[111] This system employed a polydimethylsiloxane (PDMS, silicone) device with a chamber that accommodates a thin slice of live LN tissue. The chamber also integrated multiple inlets to allow for the controlled delivery of signals with a high level of spatial resolution. This control allowed for delivery of cues specifically to T or B cell zones (Figure 4a). A fluorescently-labeled glucose molecule was delivered as a model to determine that signals delivered to the B cell zone are retained longer than when the same signals are delivered to T cell zones (Figure 4b, 4c). Such insight could help in the design of the best ligands or most appropriate doses for future vaccines and immunotherapies.
Figure 4. Biomaterials can be used to study how tissue level processes impact immunological outcomes.
a. A schematic of the microfluidics device to deliver signals to different tissue locations. The green port indicates the controlled delivery of stimulus through a specific port to the tissue slice located above. Reproduced with permissions.[111] Copyright 2017, Royal Society of Chemistry. b. These are image depicting the local stimulation of LN slices labeled with Alexa Fluor 647-glucose-BSA (red), nucleus (blue), and B cell zone (green). White arrows point to the site at which signal was delivered. In the top images, fluorescent glucose was delivered to the T cell zone of the LN. In the bottom images, fluorescent glucose was delivered to the B cell zone. Reproduced with permissions.[111] Copyright 2017, Royal Society of Chemistry. c. Quantification of images in b. Reproduced with permissions.[111] Copyright 2017, Royal Society of Chemistry. d. A schematic demonstrating the synthesis of silicate NPs encapsulated within a gelatin hydrogel. Adapted with permissions.[113] Copyright 2015, Elsevier. e. A schematic of how the 3D B cell follicle organoids are formed. These organoids contain primary B cells isolated from spleens, cultured with 40LB stromal cells and soluble signals to support growth into the hydrogel depicted in panel c. Adapted with permissions.[114] Copyright 2017, Springer Nature. f. By altering the percent weight by volume of PEG diacrylate it was possible to tune the hydrogel stiffness as measured by elastic modulus. Reproduced with permissions. [27] Copyright 2019, Wiley-VCH. g. T cells proliferated more with less stiff hydrogels as measured by a lower elastic modulus. Reproduced with permissions.[27] Copyright 2019, Wiley-VCH. h. Collagen (coll) hydrogels, collagen hydrogels functionalized with hyaluronan (HA), or functionalized with sulfate hyaluronan (sHA) were created. Hydrogels were crosslinked with EDC had greater stiffness than the other formulations. A higher IL10:IL12 ratio indicates immune cells with more regulatory functions. This figure demonstrates two-dimensional and three-dimensional culture systems differentially impact immune cell function. Reproduced with permissions.[116] Copyright 2017, Wiley-VCH.
An alternate approach has created synthetic scaffolds that mimic processes occurring within specific LN domains, rather than analyzing live tissue slices in a device as just discussed. This idea has been used to create a biomaterial-based culture system to study GCs.[50] As mentioned in Section 2.2, GCs are microdomains in LNs where antibodies change function and develop increasing affinity for their antigen. These processes are difficult to study because the cells within GCs have a great degree of heterogeneity and do not undergo GC processes in traditional cell culture conditions, which lack structural organization and guided interactions with APCs and helper T cells. Thus, these new three-dimensional culture systems utilize the ability of biomaterials to provide cellular interactions, such as those that immune cells experience when interacting with the ECM or stromal features in LNs. One such system uses gelatin-based hydrogels that encapsulate cells.[112,113] These hydrogels were formed by ionically linking silicate NPs and a gelatin solution (Figure 4d) containing B cells, as well as cells expressing necessary B cell activating signals (Figure 4e). These hydrogels were then submerged in solution containing soluble immune signals to drive specific developmental processes. Since this system allows for control over the types of cells encapsulated, the signaling molecules present, and the interactions with ECM ligands, these materials can be designed to mimic a range of conditions B cells experience in the body. The B cells in these GC-like culture systems underwent a greater degree of class-switching – the process of altering the type of antibody produced by B cells – as compared to B cells in two dimensional cultures. One research front that these immune organoids have already advanced is transcriptional analysis within GCs: analysis of B cells in these culture systems appear to have some of the same developmental signatures as GCs in animal models. These similarities may allow for analysis of the regulatory processes governing GCs in culture conditions, reducing reliance on animal models. In particular, by encapsulating B cells lacking specific signaling pathways, the investigators were able to use this system to discover a new pathway that regulates development of GCs at a cellular level.[114] This pathway helps control the level of B cell division occurring in GCs by suppressing specific molecules that regulate cell division, and is relevant to several blood cancers. These findings demonstrate the power of carefully controlling the signals immune cells are exposed to in structured environments that mirror naturally-occurring environmental conditions.
5.2. Biomaterials can present ECM-like cues to control the physical and mechanical forces experienced by immune cells.
The ECM is able to provide an abundance of cues to developing immune cells. Some of these cues are dependent upon the identity of the proteins,[115] while other cues are physical properties of the ECM that regulate immune cell development. The previous section focused on using biomaterials as frameworks to mimic immune tissue processes. In this section, approaches are highlighted that use biomaterials to study how specific properties of the ECM -such as stiffness – impact immune cell function. These features can be controlled or mimicked by altering material properties such as crosslinking density, polymer composition or molecular weight, and porosity.[115] These links are important because immune cells are constantly subjected to internal and external stress, but traditional culture methods require that cells grow in a two-dimensional plane; this setting does not reflect the three-dimensional forces cells are exposed to in the body.[109] Understanding the relationships between function and mechanical properties or multi-dimensional structure could support vaccines and therapies that leverage these interactions, as well as more accurate tissue-engineered models or immune organs.
One type of material that has been instrumental in studying how mechanical forces impact immune cells are hydrogels. The stiffness of hydrogels can easily be tuned by changing parameters such as the degree of crosslinking. One area where substrate stiffness has been evaluated is T cell proliferation.[27] This is important because many commercial products – from cell culture systems, to human CAR T cell therapies – rely on the efficient expansion of T cells. With respect to stiffness, one type of hydrogel has been designed with hyaluronic acid – an ECM component, and PEG diacrylate.[27] Varying the amount of cross-linker supplied during gel formation allows tuning of the viscoelasticity, resulting in hydrogels with elastic moduli that could be tuned between 0.2 and 3 kPa (Figure 4f). To mimic in vivo conditions, these hydrogels were created with a low elastic modulus similar to that of LNs. Without biomaterials, it would not be possible to control the stiffness of the ECM without altering the ligands presented to a cell. These studies revealed that T cells cultured on stiff substrates proliferated at low levels, while the same conditions using a softer hydrogel substrate caused an 80 percent increase in T cell proliferation (Figure 4g). Further, the greatest amount of proliferation occurred in substrates with less than 1kPa of elastic modulus. These gels contained the same concentration and density of signaling molecules, allowing isolation of the impact of matrix stiffness on cellular functions. This system also allowed probing of how matrix stiffness impacts T cell receptor clustering, which is an important regulator of T cell activation. For this parameter, softer hydrogels were found to significantly increase T cell receptor clustering, which also improves T cell activation. This example highlights the importance of creating accurate and robust in vitro culture systems to study important processes such as T cell proliferation and differentiation.
With respect to dimensionality, one recent study used hydrogels to demonstrate how immune cells respond differently to two-dimensional and three-dimensional environments.[116] These hydrogels were composed of either pure collagen gels or gels containing hyaluronic acid or high-sulfated hyaluronan, and crosslinked with EDC. The inclusion of these natural polymers mimics the components present in the native tissue, but allows programed control over dimensionality and hydrogel stiffness. The lack of three-dimensional structure in traditional cell culture models also create significant shortcomings in structured interactions between immune cells and the ECM. Immune cells express integrins to interact with the ECM and other immune cells. The formation of three-dimensional structures around these cells influences the strength of the interactions. In addition, three-dimensional cultures preserve a porosity parameter that is present in native extracellular matrices. In this hydrogel system, macrophages responded differently to stimulus depending on whether the culture system was two- or three-dimensional (Figure 4h).[116]
As mentioned previously, porosity is another area where biomaterials can support studies to generate new insight. As one example, pore size has been investigated in the context of DC migration and activation.[117] To study this link, hydrogels with defined pore size were prepared from poly (2-hydroxyethyl methacrylate) (pHEMA) and PDMS. Using a templating method to define pore size, hydrogels were synthesized with interconnected pore templates exhibiting diameters of 20, 40, or 90 μm. Scaffolds of each pore size and material were then implanted in mice. These studies revealed that regardless of the polymer used to synthesize the hydrogel, hydrogels with smaller pores improved the retention of DCs and expression of costimulatory molecules in response to stimulus compared to hydrogels with larger pores.
A similar study was performed in which microporous PLGA scaffolds encapsulating a DC growth factor were formed with different pore sizes. This allowed for analysis of how pore volume and surface porosity influence the ability of DCs to infiltrate these scaffolds in animals and in culture conditions.[118] Surprisingly, the trends that were observed in culture and those observed following implantation in a mouse were not the same. Following scaffold implantation in mice, the scaffold with the lowest porosity contained the greatest number of cells recovered seven days after implantation. Further, within each scaffold, the percentage of cells differed depending on the porosity. For example, the samples with the greatest number of pores exhibited the highest percentage of macrophages, while scaffolds with the lowest level of porosity contained the greatest percentage of DCs. In addition, the size of the pore played a role with the most immune cells recruited to scaffolds with pores between 10 and 32μm. Together, the examples in this section highlight how materials can be used to probe and establish relationships between physical factors – such as stiffness or pore size – and immune outcomes.
5.3. Biomaterials have been exploited to reveal immune cell interactions with lymphatic and vascular tissue.
The previous subsection focused on the relationship between mechanical properties of the ECM and immune cell function. Another important area is how vasculature and lymphatic vessels work with immune cells to support communication and immune signaling. Lymphatic and blood vessels provide a means for the body to transport immune cells, fluids, and signaling molecules across great distances.[119,120] These lymphatic vessels contain lymphatic endothelial cells (LECs) that regulate immune cell functions by secreting cytokines, expressing ligands that activate immune cells, and displaying molecules that control cellular migration.[121] These interactions allow immune cells to communicate with the ECM and vice versa. For example, immune cells can secrete enzymes to reshape the ECM, and the ECM can display receptors that alter dynamic processes in immune cells (e.g., adhesion). The local development of new lymphatic vessels – a process known as lymphangiogenesis, is one important area to study because alterations to the tissues immune cells can cause differences in cytokine and growth factor profiles that directly regulate matrix remodeling. However, lymphatic development and the impact it has on immune cell trafficking has historically been difficult to study because of the rapid diffusion of soluble proteins following injection into animals, as well as safety concerns associated with repeated injection of lymphatic growth factors such as vascular endothelial growth factor C (VEGF-C). One new alternative is a three-dimensional culture system in which the growth factors present such as VEGF-C are bound to hydrogels made of naturally occurring fibrin proteins.[121] When ECM proteins are cleaved by enzymes produced by immune cells during the process of ECM remodeling, VEGF-C is released over 10–12 days. This extended release of growth factors enabled the study of how sustained VEGF-C signaling impacts the development of local lymphatic vessels during the wound healing process. In this system, VEGF-C only impacted local lymphatic capillaries, without systemic VEGF-C administration or alterations to downstream lymphatic vessels. When the hydrogels were implanted at wound sites in mice, these sites exhibited a significant increase in lymphatic formation as compared to mice receiving hydrogels that did not contain VEGF-C. Because an increase in lymphatic vessel formation occurred, the number and types of cells arriving at the wound were measured. When compared to untreated wounds, mice that were implanted with gels containing VEGF-C exhibited a greater number of DCs migrating into the LN that drained the hydrogel implant site. This increase in DCs demonstrates the real-time recruitment of immune cells to support the development of lymphatic vessels. This study demonstrates that biomaterials can be used to induce and monitor the formation of three-dimensional structures such as lymphatic vessels.
In addition to lymphatics, immune cells also influence vascular development through the secretion of growth factors and cytokines that impact the function of endothelial cells that form vessels. Like lymphatics, vascular tissue and cells are involved in transporting cells, soluble signaling molecules, and establishing communication pathways throughout the body. Biomaterials can enable analysis of different immune cell types that impact vascular tissue development and the link to infiltration by migrating immune cells by controlling the signals present on material or tissue surfaces. For example, helper T cells can enhance artery and muscle development during peripheral artery disease (PAD) through the secretion of combinations of inflammatory cytokines.[122] Understanding how these T cell types impact angiogenesis and vascular remodeling during healthy or disease states could be useful for both vaccines and immunotherapies, as well as the management of PAD and related conditions. To study the process of vascular development, endothelial cells have been seeded into collagen gels in the presence of endothelial growth factors.[123] Media that different types of T cells had been cultured in was then used to treat these matrices. These studies revealed TH2 and TH17-conditioned media increased endothelial processes that promote angiogenesis compared to basal media. TH2 cells contribute to the clearance of extracellular pathogens, while TH17 cells serve to recruit other immune cells to disease sites. In contrast, TH1 conditioned media was found to inhibit vascular formation as well as endothelial cell migration and proliferation. TH1 cells contribute to the clearance of intracellular pathogens. These findings reveal distinct T cell types can differentially support ECM remodeling and angiogenesis, processes that also create feedback that promotes or limits immune cell migration to sites of injury or disease.
While blood vessels circulate blood throughout the body, the lymphatic vessels enable movement of lymph throughout the body. This organization allows immune cells in the LN to sample the antigens and other signals present throughout the body transported in lymph fluid. For example, APCs can sample protein antigens and respond to PRR agonists draining from peripheral sites of infection to the immune organs where adaptive immune cells are stimulated. Thus, the transportation of cells and immune signaling molecules is fundamental to the development of immunity.[124] Biomaterials are useful tools to study the properties necessary for molecules to be transported through lymphatics. The ability to tune chemical and physical factors such as charge and hydrophobicity allows isolation of factors that influence effective transportation of immune molecules. One such approach used lipophilic molecules with linkers to bind albumin – a protein that naturally shuttles cargo to LNs – to increase the trafficking of desired cargos to LNs. By integrating antigen into these carriers, the increase in LN transport of the cargo resulted in a 20-fold increase in antigen-specific T cells compared to vaccines involving soluble antigen.[20] Moynihan et al. expanded this approach to a range of albumin binding moieties.[21] For example, domains that bind α-tocopherol and cyclized-albumin conjugated to a PEG sequence each bound albumin and significantly increased antigen presence in LNs compared to unmodified antigen. Once in the LN, these antigens were taken up by APCs. To analyze the downstream impact, T cell development was analyzed. In these studies, mice were vaccinated with soluble antigen and an activating stimulus, albumin binding antigen with an activating stimulus, or activating stimulus alone. Relative to mice receiving soluble antigen, mice injected with the albumin-binding antigen exhibited greater T cell proliferation and activation. These studies demonstrate that materials can be used to exploit natural transport pathways by modifying immune signals – such as antigens – to direct delivery to specific sites, and to understand how features such as binding moiety and linker length impact this transport. This subsection has highlighted the idea that biomaterials can be used to study how immune cells and vasculature communicate to influence the transportation of signaling molecules, as well as examples of how biomaterials have been used to exploit these natural signal transportation mechanisms.
5.4. Biomaterials can be used to study how altering disease microenvironments impacts immune response
This section focuses on using biomaterials to study microenvironments that undergo changes as a result of dysfunction or disease. Such examples include the suppressive microenvironment that tumors establish to blunt immune function, or LNs generating inflammatory responses against self-antigens during autoimmune disease or tissue grafts during transplantation. These are examples where a local environment is created that supports a particular immune outcome, whether inflammation or tolerance/suppression. As demonstrated in many of the past examples, biomaterials have the ability to target specific locations or cells, ensure the delivery of multiple cues, and control how or for how long molecules are displayed or delivered. Along these lines, one feature that impacts tumor-immune cell interactions that has been difficult to capture with conventional models is the role of fluid flow. To address this limitation, a multi-compartment microfluidic chip was recently developed that continuously circulates a small volume of fluid between two tissue samples.[125] In one setup, fluid passes across a tumor slice and subsequently, across a LN slice to mimic the natural process of signals leaving tumors and draining to LNs. By engineering the system with microscopy-compatible materials, it was possible to image the tissue interactions and fluid flow in real-time. This system established constant flow rates and shear stresses representative of the in vivo environment to prevent changes in cell function due to alterations in these mechanical forces. The control afforded by this system makes it a useful tool to dissect communication between distant in vivo sites with established communication pathways (e.g., lymphatics).
Cancer cells nucleate primary tumors in part by releasing exosomes and other soluble factors that bias the local immune setting toward tolerance. These and other factors – such as the role of fibroblasts in the ECM – help establish a tumor-supportive microenvironment known as the premetastatic niche (PMN); these sites have the stromal and cellular infrastructure to help a tumor develop.[126] Excitingly, biomaterials have been used to create synthetic PMNs to sample tumor and immune cells. This has been accomplished using a microporous PLGA scaffold that can be implanted into mice.[127,128] In this system, the scaffolds are designed to be microporous to support infiltration of tumor cells. These studies revealed that tumor cells accumulated in scaffolds, while analogous sites in other mice without scaffolds did not accumulate tumor cells; this result indicates the scaffolds attract the tumor cells. The authors postulate that the relative protection and porosity afforded by the implanted matrix creates a permissive environment for tumor cells to develop. The impact that physical components of such scaffolds might have on infiltrating immune cells is a topic already introduced in Section 5.2. In another set of studies, mice were injected with cancer cells to establish a primary tumor.[128] One week later the scaffolds were implanted at a site distant from the primary tumor. Analysis of scaffolds removed 28 days later revealed the presence of cancer cells in the scaffolds. This approach demonstrates the ability to sample tumor cells over time, a technique that could be very useful in analyzing how tumor cells change over time or respond to changes in the surrounding environment (e.g., during a therapy). Further, immune cells were also present in the scaffolds, and the analysis of these populations revealed new insight into how specific immune cell populations contributed to the development of the PMN. Thus, these scaffolds serve as artificial PMNs that allow for analysis of how cancer and immune cells contribute to the development and resolution of metastases.
In addition to using the innate features of biomaterials to attract cells to a new location, biomaterials can include signaling molecules that specify the delivery destination of the material construct. For example, certain peptides like placental growth factor-2 (PGF-2) have a high affinity for the ECM.[129] By combining ECM-targeting peptides and immune modifying signals, such as monoclonal antibodies, it is possible to study how immune signaling molecules contribute to the development of the tumor-permissive microenvironment. To develop a localized delivery method, PGF-2 was attached to anti-CD40α antibodies. CD40α is a signaling molecule that stimulates costimulatory marker expression by APCs and inflammatory cytokine production. By combining ECM targeting with anti-CD40α, this inflammatory signal could be delivered directly to the tumor site by peri-tumoral injection and retained at the site through the programmed ECM interactions. This enabled analysis of how an increase in immune activation at the site of a tumor influences anti-tumor response and the tumor cell behaviors. Following injection in mice, PGF-2 anti-CD40α conjugates were localized to the tumor for up to 96 hours after injection; however, soluble anti-CD40α was not detectable at the tumor site 48 hours after injection. During the same period of time, analysis of serum revealed that mice treated with soluble anti-CD40α exhibited higher levels of anti-CD40α than mice treated with PGF-2 anti-CD40α. Retention at the injection site resulted in differences in T cell development. In these studies, PGF-2 anti-CD40α significantly increased the frequency of T cells that kill tumor cells at the tumor site, while soluble anti-CD40α did not. Thus, the ability to retain signals at injection site or at the tissue of interest allows for assessment of how receiving multiple signals impacts the immune response to a particular cargo.
Cancer is not the only setting in which the local environment alters the disease outcome. Organ transplantation is another field where the local environment – the transplant site – impacts the outcome. While tumors establish suppressive microenvironments to block immune function, transplantation can result in undesirable inflammation caused by the body recognizing the transplanted tissue as foreign. Currently, patients receive soluble treatments of immunosuppressive molecules to limit the number of transplant-reactive T cells that become activated. However, systemic administration of immunosuppressive agents – such as rapamycin – cause patients to develop susceptibility to infection because these drugs also suppress healthy immune function needed to combat pathogens. One approach to prevent susceptibility to infection is by targeting the delivery of immunosuppressive drugs to activated T cells in proximity to the transplanted organ.[130,131] Using hydrogels to reengineer the environment around the transplant site provides a means to probe how this local change alters the activity of graft-reactive T cells. In this report, the hydrogels were composed of self-assembled amphiphilic peptides encapsulating an immunosuppressive drug. The peptides composing the hydrogel were designed to include a cleavage site for a cell produced enzyme, protein tyrosine kinase (PTK). When T cells bind to APCs and become activated, T cells increase the secretion of PTK. Thus, upon T cell activation, PTK phosphorylates the structural peptides comprising the hydrogel, inducing a transition from a gel to a solution. This change releases the immunosuppressive drug that was encapsulated. Because these hydrogels were implanted at transplant sites, most of the activated T cells were reactive against the transplanted organ; thus the therapy has potential to block these graft-reactive T cells while leaving normal T cell function intact.
While signaling molecules can be included to alter a local tissue environment, biomaterials themselves are also often able to recruit, activate, or even suppress immune cells. For example, as already discussed, properties such as stiffness and pore size impact the immune response. The dimensionality effects (e.g., two-dimensional, three-dimensional) discussed in Section 5.2 also play important roles by supporting cellular infiltration, function, and migration. The development of new vasculature following a transplant enables the new tissue to communicate with the rest of the body; however, it also exposes the tissue to immune surveillance, which can result in organ rejection. One strategy that examines both the immune response to new tissue and the tissue function focuses on treatment of diabetes using transplanted pancreatic islet cells. During type 1 diabetes, self-reactive immune cells destroy pancreatic islet cells that produce insulin; this results in the loss of control of blood glucose. Transplanting functional islets can restore the ability to regulate blood glucose in animal models; however, these cells are rapidly killed by immune cells following transplantation. Recently, studies have been done to examine how different types of hydrogels – microporous and cell encapsulating – impact the survival of the transplanted cells.[132] One of the factors related to transplant survival is the type of immune cell that are present at the site following the transplant. The two formulations of hydrogels caused different kinds of cells to accumulate when implanted in mice. One hydrogel formulation contained cells that were seeded atop of the hydrogel after formation, while the other formulation had cells mixed in with the hydrogel precursor to encapsulate the cells within the hydrogel. The scaffolds seeded with islets attracted significantly more neutrophils than the hydrogels encapsulating the islet cells. Interestingly, when diabetic mice received microporous hydrogels with seeded islet cells, normal blood glucose levels were observed within 6 days of transplantation; however, mice that received hydrogels with encapsulated islets did not experience a decrease in blood glucose until 17 days after transplant. These findings demonstrate that the way cells are added to hydrogels impacts not only the immune cells attracted to the hydrogel but also the responsiveness of transplanted islet cells within the hydrogel.
In sum, Section 5 has highlighted the ability to use material features such as stiffness, porosity, ligand inclusion, and matrix composition to probe the development and function of immune cells in culture models, lymphatics, vasculature, and in vivo tissue environments. This ability to alter these features in a controlled manner makes biomaterials powerful tools to study the factors that impact how immune cells interact with the ECM. Throughout this section, there was a unifying theme that material properties can be used to manipulate the types of cells that develop.
6. Materials have properties that synergize with emerging single cell and multidimensional analysis techniques
While the previous sections focused on biomaterials as frameworks to study immunological signals, cells, and tissues, these materials are also useful in directly developing analytical tools. In a different arena, powerful new techniques are emerging for single cell analysis and for the generation of multi-dimensional or high-content data. In this section, we present some of the ways in which biomaterials are being coupled with these new technologies. One current area of research is the development of multiplexing technologies that allow for the measurement of many genes or proteins produced by individual cells or cell populations. In this section, we also highlight opportunities to combine biomaterials with technologies to which materials have not yet been applied; doing so could increase assay sensitivity and selectivity. Lastly, we discuss how biomaterials can support new sampling techniques to study target populations of immune cells. For example, analysis of the individual immune cells that are present in a tumor could lead to the development of a more effective gene-based therapy to be delivered using lipids that support uptake by the most receptive cell types identified using a high throughput screen.
Recently, single cell analysis techniques have gained momentum for their ability to capture the heterogeneity present among immune cells. By measuring individual cells, new functional and developmental stages of immune cells have been identified.[133–135] It would otherwise not have been possible to detect these cells due to subtle variations in the expression of surface proteins between related cell populations. Single cell approaches have been instrumental in establishing new regulatory pathways and disease-associated gene and protein signatures.[136,137] One biomaterial-based approach to single cell analysis has leveraged microfluidic devices to create three-dimensional hydrogel droplets that encapsulate individual cells. These droplets can then be used to characterize the genomic and proteomic content of the encapsulated cells.[138,139]
One pitfall of conventional single cell analysis techniques is that they do not capture rare events, such as when an adaptive immune cell is specific for a given antigen.[140,141] This limits the ability to detect the rare T cells that are specific for a desired antigen such as in cancer immunotherapies. To create a real-time system to study this specificity in the context of T cell activation, Segaliny et al. used soft lithography to create a PDMS microfluidic system to form droplets.[142] Droplets were formed using a flow focusing process in which fluorocarbon oil and surfactant intersect at a right angle with an aqueous solution containing cells (Figure 5a). In this system, two solutions flowed side-by-side, with one solution containing cancer cells and the other containing a heterogeneous T cell population. Thus, this approach allowed for control of the identity of cells experiencing exactly one cell-cell interaction by encapsulating exactly one cancer cell and one T cell in each droplet. T cell activation was monitored using T cells that contained a fluorescent reporter in response to T cells recognizing a cancer antigen expressed by the coencapsulated cancer cells. The resulting fluorescence signal was used to sort droplets containing T cells specific for the tumor antigen (Figure 5b), and these T cell receptors could be further analyzed through sequencing to reveal the specific antigen binding sequence. These results demonstrate that droplet based microfluidic platforms can be used to monitor individual cell functions like T cell activation and characterize individual ligands promoting these interactions. This approach could be further combined with material applications to study how material features previously explored such as antigen structure, gene delivery, and matrix stiffness alter how T cells become activated.
Figure 5. Coupling biomaterials with new high content data and cellular analysis techniques creates synergistic opportunities.
a. A schematic depicting how droplets are formed that encapsulate T cells and cancer cells. Briefly, T cells and cancer cells flow alongside each other before passing through oil that creates a droplet containing one cell of each type. Reproduced with permissions.[142] Copyright 2018, Royal Society of Chemistry. b. Cell containing droplets can be sorted on the basis of cellular processes such as T cell activation. This process allows for more detailed downstream analysis of cells with characteristics of interest. Reproduced with permissions.[142] Copyright 2018, Royal Society of Chemistry. c. A schematic depicting how droplet encapsulation can be combined with single cell RNA sequencing. This allows for cells to be coencapsulated with unique RNA molecules that allow for separation of RNA from each cell.[147] d. Single cell RNA sequencing was able to differentiate between the types of cells the RNA was isolated from in pure (left) or mixed (right) cultures. Reproduced with permissions.[147] Copyright 2017, Springer Nature. e. A schematic depicting microneedles that are capable of sampling the fluid and cells that are present in the interstitial space and epidermis. These microneedles are covered in a hydrogel layer that swells in interstitial fluid and capture infiltrating immune cells. Reproduced with permissions.[149] Copyright 2018, American Association for the Advancement of Science. f. Following immunization, the percent of antigen specific cells detected using sampling microneedles was the same as in traditional blood measurements. This demonstrates that microneedles have the ability to capture the same trends as traditional sampling methods such as blood draws. Reproduced with permissions.[149] Copyright 2018, American Association for the Advancement of Science.
In addition to analysis of surface-bound molecules, high throughput systems have been developed to interrogate the secretion of soluble signaling molecules. A process called microengraving, for example, uses a dense array of wells of nanoliter volumes in a PDMS mold.[143] One type of functional cellular analysis this technique has been used to study is cytokine secretion.[144] To achieve this, the wells of the molds were coated with T cell activating antibodies, then T cells were added to the arrays. To measure cytokine secretion, “microengraving” was carried out by contacting a glass slide containing antibodies against cytokines produced by T cells atop the T cell-containing wells. This design enabled analysis of two cytokines across hundreds of thousands of individual T cells monitoring the expression of these signals over time and in combination with stimulatory T cell ligands. This technique revealed a great degree of heterogeneity among the cytokine secreting profiles among T cells with the same antigen specificity, and enabled analysis of the secretion of two cytokines from individual cells. The ability to detect cytokine secretion on an individual cell level is a powerful tool to understand how each cell responds to programmed or defined stimuli.
One of the challenges associated with characterizing the secretion of proteins on a single cell level is the limit of detection. Frequently, immunoassays such as Enzyme-linked immunosorbent assays (ELISA) have a limit of detection of about 10−9 M, which is too restrictive to quantify protein secretions from individual cells. Biomaterials provide a means to improve the limit of detection. For example, immune cells have been encapsulated individually in droplets that also contain a hydrogel particle that functions as an immunosensor.[145] Poly (N-isopropylacrylamide-co-acrylic acid) p(NIPAM-co-AAC) was chosen as the hydrogel material because of the porosity – which increasing the available surface area for proteins to bind to detection antibodies; this design also shrinks when heated, effectively concentrating the secreted proteins within the hydrogel. By increasing the molecular weight of PEG included in the formulation, it was possible to tune the surface porosity of the hydrogel. In addition, the amount of capture antibody covalently linked to the hydrogel could be controlled by varying the relative amount of carboxylic acid copolymer. Studies with these hydrogels revealed the hydrogel contraction significantly increased signal, effectively reducing the limit of detection for cytokines.
The idea of encapsulating cells in droplets can also be combined with genomic analysis. A method to analyze total RNA – RNA sequencing – has been combined with single cell analysis to better identify rare populations or events that would be missed by analyzing the bulk. As described previously, biomaterials can be used to isolate cells in individual droplets with other necessary cargo – a process that is not possible without the use of materials. For single cell RNA sequencing, this isolation can be achieved by crossing an aqueous stream containing immune cells that will be sequenced and functionalized beads with an oil stream.[146] By controlling the flow rate, this approach ensures that each droplet contains a single cell and a single bead. In one approach, the beads in droplets were functionalized with nucleotides to amplify and differentiate the information in each droplet (Figure 5c).[147] This system allowed for over 130,000 droplets per minute to be generated with beads present in approximately 80 percent of droplets. One of the nucleic acids present in the bead was a DNA molecule that serves as a “barcode” for the RNA being probed; this approach allowed for differentiation between which cell each molecule originated in. Once barcoded, such systems can be used to characterize the amount and function of RNA molecules expressed by individual cells. The ability to determine the cell that a particular set of nucleic acids came from can also be used to analyze mixed cell populations. For example, tumors often have higher mutational rates than other cells present in the body. The ability to differentiate between genes expressed in tumor and healthy cells can lead to better understanding of what dysregulations are associated with diseases like in cancer and autoimmunity. As a result, in the system just discussed it was possible to differentiate between immune and cancer cells in a 99:1 mixed cell population (Figure 5d).[147] This information can be used to establish regulatory networks for how cells respond to stimulus.
Another example of a developing technology using single cell and multidimensional analysis is mass cytometry.[148] Mass cytometry uses antibodies labeled with different metal isotypes to label proteins expressed by immune cells. These cells are then vaporized, and the ion intensities associated with the metal labels in the antibodies are analyzed by time-of-flight mass spectrometry. This method can be used to analyze thousands of cells each second, and the sensitivity is controlled by the number of metal ions bound to an antibody. This method is advantageous over traditional flow cytometry because it requires small volumes and allows for the analysis of single cells and the use of greater numbers of surface markers than fluorescence due to reduced background between labels. While this technique is powerful, it has not been extensively used with biomaterials. Of note, however, in one example, mass cytometry analysis used 38 biomarkers to analyze the subpopulations present within human DCs.[135] This protein measurement approach was combined with genetic analysis for a more complete picture of what is occurring in the immune cells. The analysis revealed a new stage of pre-DCs during which cells are capable of stimulating T cells and secreting inflammatory cytokines; however, these functions were previously incorrectly attributed to other types of DCs. This finding demonstrates the importance of being able to analyze cells using higher dimension approaches. Biomaterials could be integrated into this technique in future studies to characterize how immune cells respond to the material properties explored in Sections 3–5. Some of the themes highlighted previously, such as control of the location and ratio of signals delivered, could be exploited to unearth new cell types and processes. These types of analyses would yield a more sophisticated understanding of how immune cells are regulated by specific stimuli.
When using the technologies discussed above, it is important to be able to sample the appropriate immune cell population. As an illustration, despite the importance of immune cells residing in peripheral tissue, most analyses techniques for immune cells either examine the blood or are invasive. These aspects limit the study of tissue-resident APCs by preventing long-term analyses and constraining the number of cells retrieved. By directly sampling peripheral tissue, it may be possible to create a more complete picture of the immune processes that are occurring. For example, the skin contains multiple APC populations that are not present in the blood and therefore would not be characterized using traditional sampling techniques. One biomaterial-based approach to study the resident immune cells in the skin is the use of microneedles. Microneedles are micron scale needles that have been more commonly used to deliver cargo to the skin without the challenges associated with using needles. However, this length scale also makes them well poised to study the populations and functions of immune cells present in the skin. To this end, solid polylactide microneedle arrays have recently been coated with an alginate hydrogel using electrostatic adhesion.[149] The inclusion of the alginate layer – the composition of which was optimized to allow cell infiltration, allowed for these microneedles to sample immune cells. When these microneedles penetrate the skin, the hydrogel swells as it is exposed to interstitial fluid. Simultaneously, APCs migrate toward the wound site due to the damage to the skin caused by the penetration of the microneedle and infiltrate the alginate layer on the microneedle (Figure 5e). Following sampling, the alginate layer could be dissolved, the cells recovered, and the cell phenotype analyzed. This is one area where use of the mass cytometry assay or single cell analysis could expand the information collected from these microneedles. In order to compare the ability of this tool to sample immune cells and molecules, mice were immunized against a model antigen then blood was collected, and microneedle patches were applied to sample immune cells. This study revealed that microneedle-collected samples contained the same percentage of antigen specific T cells as blood samples (Figure 5f). In addition, the level of antigen-specific antibodies collected in microneedles was significantly greater than those present in unimmunized mice; these levels were lower than that measured in serum. Taken together, these findings demonstrate that microneedles allow for the study of immune cells and molecules that are present in the peripheral tissues and demonstrated trends similar to those established using conventional methods. Furthermore, these microneedles can be used to selectively sample immune cells of interest by including signaling molecules within the hydrogel layer. These microneedles are a powerful tool to sample different populations of immune cells and subsequently, explore the processes that regulate their functions.
This section has discussed ways that biomaterials can be used to improve biological assays, both traditional and emerging. The development of single cell technologies such as droplet encapsulation and microengraving provide distinct approaches to analyze individual cells. The analysis of individual cells can be used to improve understanding of heterogeneity among immune cell functions such as T cell activation and cytokine secretion. When these types of techniques become more holistic, it may be possible to model individual cellular processes more completely. These advances have allowed for the development of multidimensional techniques such as RNA sequencing and mass cytometry to identify novel developmental pathways and functional populations of immune cells.
7. Conclusion
Engineering immune cells and tissues with a desired function or reactivity is an important capability for next generation of vaccines and immunotherapies in infectious disease, cancer, and autoimmune disease alike. Biomaterials provide greater control over the “details” of presentation relative to traditional soluble approaches. Leveraging the tunability of biomaterials in this context will enable greater understanding of immune function. This new insight will support the development of more accurate in vitro assays that capture relevant, accurate information more rapidly and with reduced use of animal models. Likewise, combining the precision capabilities or modularity of materials with new multidimensional analysis techniques will further advance understanding. Together, all of these directions will help support the next generation of vaccines and immunotherapies.
Acknowledgements
This work was supported in part by the United States Department of Veterans Affairs (Award # 1I01BX003690) and the National Institutes of Health (Award # R01EB026896, # R01EB027143, and # R01AI144667). H.B.E. is a trainee of the Cell and Molecular Biology Training Program Fellowship (NIH Award # T32GM080201).
Biographies
Author Biographies
Haleigh B. Eppler is a PhD candidate in the Biological Sciences Training program at the University of Maryland. Her research uses biomaterials and immunology to study how materials influence the immune response. In particular, she is interested in using biomaterials to probe cellular processes occurring during autoimmune disease.

Christopher M. Jewell is the Minta Martin Professor of Engineering and Associate Chair for Research in the Fischell Department of Bioengineering at the University of Maryland. Dr. Jewell is also a Research Microbiologist with the Department of Veterans Affairs. Dr. Jewell’s research spans engineering and immunology to study and manipulate immune function for therapeutic vaccines targeting autoimmunity and cancer. He earned his PhD in Chemical Engineering from the University of Wisconsin, then worked as a consultant at the Boston Consulting Group in the Healthcare Practice. Dr. Jewell completed his postdoctoral training at MIT and Harvard, launching his lab in 2012.

Footnotes
Disclosures
C.M.J. is an employee of the VA Maryland Health Care System. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government. C.M.J. has an equity position in Cellth Systems, LLC.
Contributor Information
Haleigh B. Eppler, Fischell Department of Bioengineering, 8278 Paint Brach Drive, College Park, MD 20742, USA Biological Sciences Training Program, 1247 Biology Psychology Building, College Park, MD 20742, USA.
Christopher M. Jewell, Fischell Department of Bioengineering, 8278 Paint Brach Drive, College Park, MD 20742, USA Biological Sciences Training Program, 1247 Biology Psychology Building, College Park, MD 20742, USA; Robert E. Fischell Institute for Biomedical Devices, 8278 Paint Branch Drive, College Park, MD 20742, USA; United States Department of Veterans Affairs, VA Maryland Health Care System, 10. N Green Street, Baltimore, MD 21201, USA; Department of Microbiology and Immunology, University of Maryland Medical School, 685 West Baltimore Street, HSF-I Suite 380, Baltimore, MD 21201, USA; Marlene and Stewart Greenebaum Cancer Center, 22 South Greene Street, Baltimore, MD 21201, USA.
References
- [1].Ehreth J, Vaccine 2003, 21, 596. [DOI] [PubMed] [Google Scholar]
- [2].Flasche S, Jit M, Rodríguez-Barraquer I, Coudeville L, Recker M, Koelle K, Milne G, Hladish TJ, Perkins TA, Cummings DAT, Dorigatti I, Laydon DJ, España G, Kelso J, Longini I, Lourenco J, Pearson CAB, Reiner RC, Mier-y-Terán-Romero L, Vannice K, Ferguson N, PLoS Med 2016, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Obando-Pacheco P, Rivero-Calle I, Gómez-Rial J, Rodríguez-Tenreiro Sánchez C, Martinón-Torres F, Vaccine 2018, 36, 5485. [DOI] [PubMed] [Google Scholar]
- [4].Paules CI, Marston HD, Fauci AS, Engl N. J. Med 2019, NEJMp1905099. [Google Scholar]
- [5].Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S, PLoS One 2013, 8, DOI 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wei SC, Duffy CR, Allison JP, Cancer Discov 2018, 8, 1069. [DOI] [PubMed] [Google Scholar]
- [7].June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC, Science (80-. ) 2018, 359, 1361. [DOI] [PubMed] [Google Scholar]
- [8].Gammon JM, Jewell CM, Adv. Healthc. Mater 2019, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mulero P, Midaglia L, Montalban X, Ther. Adv. Neurol. Disord 2018, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Halstead SB, Vaccine 2017, 35, 6355. [DOI] [PubMed] [Google Scholar]
- [11].Coffman RL, Sher A, Seder RA, Immunity 2010, 33, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thompson EA, Loré K, Curr. Opin. Immunol 2017, 47, 1. [DOI] [PubMed] [Google Scholar]
- [13].Rambhia KJ, Rambhia MT, Clin. Infect. Dis 2019, 68, 1235. [DOI] [PubMed] [Google Scholar]
- [14].Hay AE, Cheung MC, J. Med. Econ 2019, 0, 1. [DOI] [PubMed] [Google Scholar]
- [15].Rosenberg SA, Restifo NP, Science (80-. ) 2015, 348, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pardoll DM, Nat. Rev. Cancer 2016, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stuart MAC, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S, Nat. Mater 2010, 9, 101. [DOI] [PubMed] [Google Scholar]
- [18].Zhang K, Gao H, Deng R, Li J, Angew. Chemie - Int Ed. 2019, 58, 4790. [DOI] [PubMed] [Google Scholar]
- [19].Bazzill JD, Ochyl LJ, Giang E, Castillo S, Law M, Moon JJ, Nano Lett 2018, 18, 7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Nature 2014, 507, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moynihan KD, Holden RL, Mehta NK, Wang C, Karver MR, Dinter J, Liang S, Abraham W, Melo MB, Zhang AQ, Li N, Le Gall S, Pentelute BL, Irvine DJ, Cancer Immunol. Res 2018, 6, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andorko JI, Hess KL, Jewell CM, AAPS J 2015, 17, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gosselin EA, Eppler HB, Bromberg JS, Jewell CM, Nat. Mater 2018, 17, DOI 10.1038/s41563-018-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gao S, Tang G, Hua D, Xiong R, Han J, Jiang S, Zhang Q, Huang C, J. Mater. Chem. B 2019, 7, 709. [DOI] [PubMed] [Google Scholar]
- [25].Langhans SA, Front. Pharmacol 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley M, Bader JS, Pandey A, Pardoll D, Elisseeff JH, Nat. Methods 2016, 12, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hickey JW, Dong Y, Chung JW, Salathe SF, Pruitt HC, Li X, Chang C, Fraser AK, Bessell CA, Ewald AJ, Gerecht S, Mao H-Q, Schneck JP, Adv. Mater 2019, 1807359, DOI 10.1002/adma.201807359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Swartz MA, Hubbell JA, Reddy ST, Semin. Immunol 2008, 20, 147. [DOI] [PubMed] [Google Scholar]
- [29].Clem AS, J. Glob. Infect. Dis 2011, 3, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cella M, Sallusto F, Lanzavecchia A, Curr. Opin. Immunol 1997, 9, 10. [DOI] [PubMed] [Google Scholar]
- [31].Abbas AK, Lichtman AHH, Pillai S, Cellular and Molecular Immunology, Saunders, 2014. [Google Scholar]
- [32].Kolaczkowska E, Kubes P, Nat. Rev. Immunol 2013, 13, 159. [DOI] [PubMed] [Google Scholar]
- [33].Kapsenberg ML, Nat. Immunol 2003, 3, 984. [DOI] [PubMed] [Google Scholar]
- [34].Akira S, Takeda K, Nat. Rev. Immunol 2004, 4, 499. [DOI] [PubMed] [Google Scholar]
- [35].Tartey S, Kanneganti TD, Immunology 2019, 156, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen Q, Sun L, Chen ZJ, Nat. Immunol 2016, 17, 1142. [DOI] [PubMed] [Google Scholar]
- [37].Ebrahimian M, Hashemi M, Maleki M, Hashemitabar G, Abnous K, Ramezani M, Haghparast A, Front. Immunol 2017, 8, 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kuai R, Sun X, Yuan W, Ochyl L, Xu Y, Hassani Najafabadi A, Scheetz L, Yu M-Z, Balwani I, Schwendeman A, Moon JJ, Control J. Release 2018, 282, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Song H, Huang P, Niu J, Shi G, Zhang C, Kong D, Wang W, Biomaterials 2018, 159, 119. [DOI] [PubMed] [Google Scholar]
- [40].Lutz MB, Front. Immunol 2012, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kapsenberg ML, Nat. Rev. Immunol 2003, 3, 984. [DOI] [PubMed] [Google Scholar]
- [42].Jain A, Pasare C, J. Immunol 2017, 198, 3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Macri C, Pang ES, Patton T, O’Keeffe M, Semin. Cell Dev. Biol 2018, 84, 11. [DOI] [PubMed] [Google Scholar]
- [44].Melief CJM, Eur. J. Immunol 2003, 33, 2645. [DOI] [PubMed] [Google Scholar]
- [45].Lenschow DJ, Sperling AI, Cooke MP, Freeman G, Rhee L, Decker DC, Gray G, Nadler LM, Goodnow CC, Bluestone JA, J. Immunol 1994, 153, 1990. [PubMed] [Google Scholar]
- [46].Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA, Immunity 2016, 44, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bevan MJ, Nat. Rev. Immunol 2004, 4, 595. [DOI] [PubMed] [Google Scholar]
- [48].Stavnezer J, Amemiya CT, Semin. Immunol 2004, 16, 257. [DOI] [PubMed] [Google Scholar]
- [49].Victora GD, Nussenzweig MC, Annu. Rev. Immunol 2012, 30, 429. [DOI] [PubMed] [Google Scholar]
- [50].Purwada A, Shah SB, Béguelin W, August A, Melnick AM, Singh A, Biomaterials 2018, 198, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eggermont LJ, Paulis LE, Tel J, Figdor CG, Trends Biotechnol 2014, 32, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Singh A, Peppas NA, Adv. Mater n.d., 26, 6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Richman DD, Wrin T, Little SJ, Petropoulos CJ, Proc. Natl. Acad. Sci 2003, 100, 4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wibmer CK, Moore PL, Morris L, Curr. Opin. HIV AIDS 2015, 10, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Peña A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR, Immunity 2017, 46, 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tokatlian T, Kulp DW, Mutafyan AA, Jones CA, Menis S, Georgeson E, Kubitz M, Zhang MH, Melo MB, Silva M, Yun DS, Schief WR, Irvine DJ, Sci. Rep 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bazzill JD, Stronsky SM, Kalinyak LC, Ochyl LJ, Steffans JT, van Tongeren SA, Cooper CL, Moon JJ, Nanomedicine Nanotechnology, Biol. Med 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Change YHJ, Steichen JM, Dane EL, Liguori A, Sangesland M, Lingwood D, Crispin M, Schief WR, Irvine DJ, Science (80-. ) 2018, 363, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Galan-Navarro C, Rincon-Restrepo M, Zimmer G, Ollmann Saphire E, Hubbell JA, Hirosue S, Swartz MA, Kunz S, Virology 2017, 512, 161. [DOI] [PubMed] [Google Scholar]
- [60].Scott EA, Stano A, Gillard M, Maio-Liu AC, Swartz MA, Hubbell JA, Biomaterials 2012, 33, 6211. [DOI] [PubMed] [Google Scholar]
- [61].Wang S, Qin L, Yamankurt G, Skakuj K, Huang Z, Chen P-C, Dominguez D, Lee A, Zhang B, Mirkin CA, Proc. Natl. Acad. Sci 2019, 116, 10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wu Y, Norberg PK, Reap EA, Congdon KL, Fries CN, Kelly SH, Sampson JH, Conticello VP, Collier JH, ACS Biomater. Sci. Eng 2017, 11, 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tostanoski LH, Chiu YC, Gammon JM, Simon T, Andorko JI, Bromberg JS, Jewell CM, Cell Rep 2016, 16, 2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Getts DR, Martin AJ, Mccarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJC, Shea LD, Miller SD, Nat. Biotechnol 2012, 30, 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tasi S, Wang J, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong VW, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P, Nature 2016, 530, 434. [DOI] [PubMed] [Google Scholar]
- [66].Hess KL, Oh E, Tostanoski LH, Andorko JI, Susumu K, Deschamps JR, Medintz IL, Jewell CM, Adv. Funct. Mater 2017, 1700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Singha S, Shao K, Yang Y, Clemente-Casares X, Solé P, Clemente A, Blanco J, Dai Q, Song F, Liu SW, Yamanouchi J, Umeshappa CS, Nanjundappa RH, Detampel P, Amrein M, Fandos C, Tanguay R, Newbigging S, Serra P, Khadra A, Chan WCW, Santamaria P, Nat. Nanotechnol 2017, 12, 701. [DOI] [PubMed] [Google Scholar]
- [68].Huang WC, Deng B, Lin C, Carter KA, Geng J, Razi A, He X, Chitgupi U, Federizon J, Sun B, Long CA, Ortega J, Dutta S, King CR, Miura K, Lee SM, Lovell JF, Nat. Nanotechnol 2018, 13, 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K, Biomaterials 2014, 35, 5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Granot Y, Peer D, Semin. Immunol 2017, 34, 68. [DOI] [PubMed] [Google Scholar]
- [71].Tostanoski LH, Jewell CM, Adv. Drug Deliv. Rev 2017, 114, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Maeda M, Kojima T, Song Y, Takayama S, Adv. Healthc. Mater 2019, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mukalel AJ, Riley RS, Zhang R, Mitchell MJ, Cancer Lett 2019, 458, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bachmann MF, Jennings GT, Nat. Rev. Immunol 2010, 10, 787. [DOI] [PubMed] [Google Scholar]
- [75].Yang X, Lian K, Meng T, Liu X, Miao J, Tan Y, Yuan H, Hu F, ACS Appl. Mater. Interfaces 2018, 10, 33532. [DOI] [PubMed] [Google Scholar]
- [76].Tsai SJ, Andorko JI, Zeng X, Gammon JM, Jewell CM, Nano Res 2018, 11, 5642. [Google Scholar]
- [77].Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D, Nano Lett 2017, 17, 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X, J. Immunol 2010, 185, 7244. [DOI] [PubMed] [Google Scholar]
- [79].Liu L, Yi H, Wang C, He H, Li P, Pan H, Sheng N, Ji M, Cai L, Ma Y, J. Immunol 2016, 197, 1231. [DOI] [PubMed] [Google Scholar]
- [80].Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, Zhu H, Siegwart DJ, Angew. Chemie 2018, 56, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Luo YL, Xu CF, Li HJ, Cao ZT, Liu J, Wang JL, Du XJ, Yang XZ, Gu Z, Wang J, ACS Nano 2018, 12, 994. [DOI] [PubMed] [Google Scholar]
- [82].Nuhn L, Van Hoecke L, Deswarte K, Schepens B, Li Y, Lambrecht BN, De Koker S, David SA, Saelens X, De Geest BG, Biomaterials 2018, 178, 643. [DOI] [PubMed] [Google Scholar]
- [83].Nuhn L, De Koker S, Van Lint S, Zhong Z, Catani JP, Combes F, Deswarte K, Li Y, Lambrecht BN, Lienenklaus S, Sanders NN, David SA, Tavernier J, De Geest BG, Adv. Mater 2018, 30, 1. [DOI] [PubMed] [Google Scholar]
- [84].Wilson DS, Hirosue S, Raczy MM, Bonilla-Ramirez L, Jeanbart L, Wang R, Kwissa M, Franetich JF, Broggi MAS, Diaceri G, Quaglia-Thermes X, Mazier D, Swartz MA, Hubbell JA, Nat. Mater 2019, 18, 175. [DOI] [PubMed] [Google Scholar]
- [85].Chiu YC, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM, ACS Biomater. Sci. Eng 2015, 1, 1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hess KL, Andorko JI, Tostanoski LH, Jewell CM, Biomaterials 2017, 118, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tostanoski LH, Chiu YC, Andorko JI, Guo M, Zeng X, Zhang P, Royal W, Jewell CM, ACS Nano 2016, 10, 9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bookstaver ML, Hess KL, Jewell CM, Small 2018, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zeng Q, Gammon JM, Tostanoski LH, Chiu YC, Jewell CM, ACS Biomater. Sci. Eng 2017, 3, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tostanoski LH, Eppler HB, Xia B, Zeng X, Jewell CM, Biomater. Sci 2019, 7, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhu M, Ding Z, Zhao R, Liu X, Shen H, Cai C, Ferrari M, Wang HY, Wang R-F, Control J. Release 2018, 272, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM, Trends Immunol 2018, 39, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Oakes RS, Froimchuk E, Jewell CM, Adv. Ther 2019, 1800157, 1800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Acharya AP, Carstems MR, Lewis JS, Dologova N, Xia CQ, Clare-Salzler MJ, Keselowsky BG, J. Mater. Chem. B 2016, 4, 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Barber GN, Nat. Rev. Immunol 2015, 15, 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Watkins-Schulz R, Tiet P, Gallovic MD, Junkins RD, Batty C, Bachelder EM, Ainslie KM, Ting JPY, Biomaterials 2019, 205, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shae D, Becker KW, Christov P, Yun D, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, Wilson JT, Nat. Nanotechnol 2019, 14, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jacobson ME, Wang-Bishop L, Becker KW, Wilson JT, Biomater. Sci 2019, 7, 547. [DOI] [PubMed] [Google Scholar]
- [99].Braza MS, van Leent MMT, Lameijer M, Sanchez-Gaytan BL, Arts RJW, Pérez-Medina C, Conde P, Garcia MR, Gonzalez-Perez M, Brahmachary M, Fay F, Kluza E, Kossatz S, Dress RJ, Salem F, Rialdi A, Reiner T, Boros P, Strijkers GJ, Calcagno CC, Ginhoux F, Marazzi I, Lutgens E, Nicolaes GAF, Weber C, Swirski FK, Nahrendorf M, Fisher EA, Duivenvoorden R, Fayad ZA, Netea MG, Mulder WJM, Ochando J, Immunity 2018, 49, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kishimoto TK, Ferrari JD, Lamothe RA, Kolte PN, Griset AP, Neil CO, Chan V, Browning E, Chalishazar A, Kuhlman W, Fu F, Viseux N, Altreuter DH, Johnston L, Maldonado RA, Nat. Nanotechnol 2016, 11, 890. [DOI] [PubMed] [Google Scholar]
- [101].Gammon JM, Gosselin EA, Tostanoski LH, Chiu YC, Zeng X, Zeng Q, Jewell CM, Control J. Release 2017, 263, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wise DR, Thompson CB, Trends Biochem. Sci 2010, 35, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF, Pharmacol. Rev 2011, 63, 35. [DOI] [PubMed] [Google Scholar]
- [104].Gammon JM, Tostanoski LH, Adapa AR, Chiu YC, Jewell CM, Control J. Release 2015, 210, 169. [DOI] [PubMed] [Google Scholar]
- [105].Gammon JM, Adapa AR, Jewell CM, J. Biomed. Mater. Res. - Part A 2017, 105, 2977. [DOI] [PubMed] [Google Scholar]
- [106].Zhang C, Zhang L, Wu W, Gao F, Li R, Song W, Zhuang Z, Liu C, Zhang X, Adv. Mater 2019, 1901179. [DOI] [PubMed] [Google Scholar]
- [107].Braza MS, Conde P, Garcia M, Cortegano I, Brahmachary M, Pothula V, Fay F, Boros P, Werner SA, Ginhoux F, Mulder WJM, Ochando J, Am. J. Transplant 2018, 18, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sunshine JC, Perica K, Schneck JP, Green JJ, Biomaterials 2014, 35, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Griffith LG, Swartz MA, Nat. Rev. Mol. Cell Biol 2006, 7, 211. [DOI] [PubMed] [Google Scholar]
- [110].Sun W, Luo Z, Lee J, Kim HJ, Lee KJ, Tebon P, Feng Y, Dokmeci MR, Sengupta S, Khademhosseini A, Adv. Healthc. Mater 2019, 8, DOI 10.1002/adhm.201801363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ross AE, Belanger MC, Woodroof JF, Pompano RR, Analyst 2017, 142, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Purwada A, Singh A, Nat. Protoc 2017, 12, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, Cerchietti L, Singh A, Biomaterials 2015, 63, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Béguelin W, Rivas MA, Calvo Fernández MT, Teater M, Purwada A, Redmond D, Shen H, Challman MF, Elemento O, Singh A, Melnick AM, Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA, ACS Biomater. Sci. Eng 2015, 1, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon J-C, Pompe T, Franke K, Adv. Healthc. Mater 2017, 6, 1600967. [DOI] [PubMed] [Google Scholar]
- [117].Chen R, Ma H, Zhang L, Bryers JD, Biotechnol. Bioeng 2018, 115, 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kim J, Li WA, Sands W, Mooney DJ, ACS Appl. Mater. Interfaces 2014, 6, 8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Maisel K, Sasso MS, Potin L, Swartz MA, Adv. Drug Deliv. Rev 2017, 114, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schudel A, Francis DM, Thomas SN, Nat. Rev. Mater 2019, 4, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Güç E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, Swartz MA, Biomaterials 2017, 131, 160. [DOI] [PubMed] [Google Scholar]
- [122].Fu X, Xiao J, Wei Y, Li S, Liu Y, Yin J, Sun K, Sun H, Wang H, Zhang Z, Zhang BT, Sheng C, Wang H, Hu P, Cell Res 2015, 25, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kwee BJ, Budina E, Najibi AJ, Mooney DJ, Biomaterials 2018, 178, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Clement CC, Wang W, Dzieciatkowska M, Cortese M, Hansen KC, Becerra A, Thangaswamy S, Nizamutdinova I, Moon J-Y, Stern LJ, Gashev AA, Zawieja D, Santambrogio L, Sci. Rep 2018, 8, 11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shim S, Belanger MC, Harris AR, Munson JM, Pompano RR, Lab Chip 2019, 19, 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Doglioni G, Parik S, Fendt S-M, Front. Oncol 2019, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Azarin SM, Yi J, Gower RM, Aguado BA, Sullivan ME, Goodman AG, Jiang EJ, Rao SS, Ren Y, Tucker SL, Backman V, Jeruss JS, Shea LD, Nat. Commun 2016, 6, 8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Aguado BA, Hartfield RM, Bushnell GG, Decker JT, Azarin SM, Nanavati D, Schipma MJ, Rao SS, Oakes RS, Zhang Y, Jeruss JS, Shea LD, Adv. Healthc. Mater 2018, 7, DOI 10.1002/adhm.201700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ishihara J, Ishihara A, Potin L, Hosseinchi P, Fukunaga K, Damo M, Gajewski TF, Swartz MA, Hubbell JA, Mol. Cancer Ther 2018, 17, 2399. [DOI] [PubMed] [Google Scholar]
- [130].Wu J, Zheng Z, Chong Y, Li X, Pu L, Tang Q, Yang L, Wang X, Wang F, Liang G, Adv. Mater 2018, 30, 1805018. [DOI] [PubMed] [Google Scholar]
- [131].Hua J, Inomata T, Chen Y, Foulsham W, Stevenson W, Shiang T, Bluestone JA, Dana R, Sci. Rep 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rios PD, Skoumal M, Liu J, Youngblood R, Kniazeva E, Garcia AJ, Shea LD, Biotechnol. Bioeng 2018, 115, 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, Sieow JL, Penny HL, Ching GC, Devi S, Adrover JM, Li JLY, Liong KH, Tan L, Poon Z, Foo S, Chua JW, Su IH, Balabanian K, Bachelerie F, Biswas SK, Larbi A, Hwang WYK, Madan V, Koeffler HP, Wong SC, Newell EW, Hidalgo A, Ginhoux F, Ng LG, Immunity 2018, 48, 364. [DOI] [PubMed] [Google Scholar]
- [134].Segura V, Valero ML, Cantero L, Munoz J, Zarzuela E, Garcia F, Aloria K, Beaskoetxea J, Arizmendi JM, Navajas R, Paradela A, Diez P, Degano RM, Fuentes M, Orfao A, Garcia Montero A, Garin-Muga A, Corrales FJ, Sanchez del Pino MM, Proteomes 2018, 6, p ii:E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].See P, Dutertre CA, Chen J, Gunther P, McGovern N, Erdal Irac S, Gunawan M, Beyer M, Handler K, Duan K, Rizal Bin Sumatoh H, Ruffin N, Jouve M, Gea-Mallorqui E, Hennekam RCM, Lim T, Chung Yip C, Wen M, Malleret B, Low I, Binte Shadan N, Foong Shu Fen C, Tay A, Lum J, Zolezzi F, Larbi A, Poidinger M, Chan JKY, Chen Q, Renzia L, Haniffa M, Benaroch P, Schlitzer A, Schultze JL, Newell EW, Ginhoux F, Science (80-. ) 2017, 356, eaag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nirschl CJ, Suárez-Fariñas M, Izar B, Prakadan S, Dannenfelser R, Tirosh I, Liu Y, Zhu Q, Devi KSP, Carroll SL, Chau D, Rezaee M, Kim TG, Huang R, Fuentes-Duculan J, Song-Zhao GX, Gulati N, Lowes MA, King SL, Quintana FJ, suk Lee Y, Krueger JG, Sarin KY, Yoon CH, Garraway L, Regev A, Shalek AK, Troyanskaya O, Anandasabapathy N, Cell 2017, 170, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Korkmaz AG, Popov T, Peisl L, Codrea MC, Nahnsen S, Steimle A, Velic A, Macek B, von Bergen M, Bernhardt J, Frick JS, Proteomics J 2018, 180, 11. [DOI] [PubMed] [Google Scholar]
- [138].Köster S, Angilè FE, Duan H, Agresti JJ, Wintner A, Schmitz C, Rowat AC, Merten CA, Pisignano D, Griffiths AD, Weitz DA, Lab Chip 2008, 8, 1110. [DOI] [PubMed] [Google Scholar]
- [139].Eydelnant IA, Betty Li B, Wheeler AR, Nat. Commun 2014, 5, 1. [DOI] [PubMed] [Google Scholar]
- [140].De Simone M, Rossetti G, Pagani M, Front. Immunol 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Stubbington MJT, Rozenblatt-Rosen O, Regev A, Teichmann SA, Science 2017, 358, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Aude I, Li G, Kong L, Ren C, Chen X, Wang JK, Baltimore D, Wu G, Zhao W, Lab Chip 2018, 18, 3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Love JC, Ronan JL, Grotenbreg GM, Van Der Veen AG, Ploegh HL, Nat. Biotechnol 2006, 24, 703. [DOI] [PubMed] [Google Scholar]
- [144].Song Q, Han Q, Bradshaw EM, Kent SC, Raddassi K, Nilsson B, Nepom GT, Hafler DA, Love JC, Anal. Chem 2009, 377, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hsu MN, Wei SC, Guo S, Phan DT, Zhang Y, Chen CH, Small 2018, 14, 1. [DOI] [PubMed] [Google Scholar]
- [146].Hwang B, Lee JH, Bang D, Exp. Mol. Med 2018, 50, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, Gregory MT, Shuga J, Montesclaros L, Underwood JG, Masquelier DA, Nishimura SY, Schnall-Levin M, Wyatt PW, Hindson CM, Bharadwaj R, Wong A, Ness KD, Beppu LW, Deeg HJ, McFarland C, Loeb KR, Valente WJ, Ericson NG, Stevens EA, Radich JP, Mikkelsen TS, Hindson BJ, Bielas JH, Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ornatsky OI, Lou X, Nitz M, Schäfer S, Sheldrick WS, Baranov VI, Bandura DR, Tanner SD, Anal. Chem 2008, 80, 2539. [DOI] [PubMed] [Google Scholar]
- [149].Mandal A, Boopathy AV, Lam LKW, Moynihan KD, Welch ME, Bennett NR, Turvey ME, Thai N, Van JH, Love JC, Hammond PT, Irvine DJ, Sci. Transl. Med 2018, 10, eaar2227. [DOI] [PMC free article] [PubMed] [Google Scholar]