Abstract
Rationale:
Parental drug use around or before conception can have adverse consequences for offspring. Historically, this research has focused on the effects of maternal substance use on future generations but less is known about the influence of the paternal lineage. This study focused on the impact of chronic paternal morphine exposure prior to conception on behavioral outcomes in male and female progeny.
Objectives:
This study sought to investigate the impact of paternal morphine self-administration on anxiety-like behavior, the stress response, and memory in male and female offspring.
Methods:
Adult, drug-naïve male and female progeny of morphine-treated sires and controls were evaluated for anxiety-like behavior using defensive probe burying and novelty-induced hypophagia paradigms. Hypothalamic-pituitary-adrenal (HPA) axis function was assessed by measuring plasma corticosterone levels following a restraint stressor in male and female progeny. Memory was probed using a battery of tests including object location memory, novel object recognition and contextual fear conditioning.
Results:
Paternal morphine exposure did not alter anxiety-like behavior or stress-induced HPA axis activation in male or female offspring. Morphine-sired male and female offspring showed intact hippocampus-dependent memory: they performed normally on the long-term fear conditioning and object location memory tests. In contrast, paternal morphine exposure selectively disrupted novel object recognition in female, but not male progeny.
Conclusions:
Our findings demonstrate that paternal morphine taking produces sex-specific and selective impairments in object recognition memory while leaving hippocampal function largely intact.
Keywords: multigenerational, stress, HPA axis, corticosterone, anxiety
Introduction
Substance use disorders are a growing epidemic, which present a substantial economic and societal burden worldwide (Degenhardt et al. 2014). An increasing number of studies indicate that parental drug use at or around the time of conception can have harmful consequences for offspring, including negative birth outcomes, as well as increased anxiety and cognitive impairments into adulthood (Calhoun et al. 2015; Narkowicz et al. 2013). A recent estimate suggests that the fathers of over 5 million children under the age of 18 meet the criteria for substance use disorder (Calhoun et al. 2015). However, little research into the long-term impact of paternal drug use has been performed to date (He et al. 2006; Killinger et al. 2012; Vassoler et al. 2013; White et al. 2016), with most of this work focusing on maternal drug use and manipulations. Chronic opioid exposure is known to cause cognitive impairments (Baldacchino et al. 2012; Block and Cianfrini 2013; Castellano 1980; Li et al. 2001; Rabbani et al. 2009; Saha et al. 1991) but the impact of chronic opioid exposure on the next generation remains largely unstudied.
Transmission of paternal experience is thought to occur via epigenetic reprogramming of the germline (Bale 2015; Lim and Brunet 2013; Pierce et al. 2018; Yohn et al. 2015). Epigenetic inheritance refers to heritable changes in phenotypes that are independent of changes to the DNA sequence. Environmental insults, such as change in diet (Carone et al. 2010; Dunn and Bale 2011; Watkins et al. 2018; Zhou et al. 2018), exposure to drugs of abuse (Le et al. 2017; Vassoler et al. 2013; Yohn et al. 2015), and stress (Bale 2015; Morgan and Bale 2011; Rodgers et al. 2013b; Rodgers et al. 2015) have been shown to cause epigenetic changes in male germ cells. Exposure to drugs of abuse changes several components of the epigenetic landscape, including acetylation of histones and DNA methylation in sperm of both rodents (Le et al. 2017; Vassoler et al. 2013) and humans (Chorbov et al. 2011). Paternal drug exposure causes disruptions in a multitude of behaviors, including drug sensitivity and reward, anxiety, and memory in offspring (Goldberg and Gould 2018; Pierce et al. 2018; Vassoler et al. 2018a; Yaw et al. 2019; Yaw et al. 2018; Yohn et al. 2015). Paternal cocaine exposure produced increased anxiety in offspring whether cocaine was experimenter-administered (Fischer et al. 2017) or self-administered by the sires (White et al. 2016). Furthermore, paternal cocaine self-administration elicited memory formation deficits in male offspring, coupled with blunted hippocampal long-term potentiation (Wimmer et al. 2017). Some of the aforementioned studies have investigated the impact of paternal cocaine taking on progeny, but much of the work studying the multigenerational influences of opioid exposure has focused on the maternal lineage and little is known about the consequences of paternal opioid exposure on offspring.
The majority of research investigating the multi- or transgenerational impact of paternal opioid experience has been performed in conjunction with maternal opioid experience or relied on an experimenter-administered drug exposure paradigm. Maternal and parental opioid exposure has been shown to affect drug-induced locomotor sensitization (Byrnes et al. 2013), memory (Akbarabadi et al. 2018; Moulaei et al. 2018; Sepehri et al. 2014), anxiety (Byrnes 2005; Byrnes et al. 2011; Li et al. 2014), corticosterone response (Vassoler et al. 2018b), and drug-induced reward (Vassoler et al. 2017; Vassoler et al. 2016) in offspring. It has been well-established that the method of drug delivery can dramatically affect behavioral outcomes (Donny et al. 2006; Ploense et al. 2018; Twining et al. 2009; Weise-Kelly and Siegel 2001), and much of the previous work investigating the effect of parental drug experience on offspring has relied on forced experimenter-administered drug delivery. These factors make it difficult to conclude the effect of voluntary paternal opioid consumption on behavioral outcomes in offspring. Here, we used a paternal self-administration model of drug exposure solely in male rats in order to more translationally model the human condition of voluntary paternal opioid consumption.
This study focuses on the effect of paternal morphine self-administration on anxiety-like behavior, the stress response, and memory in offspring. Morphine- or saline-exposed male rats (sires) were bred with drug-naïve females (dams), giving rise to the first (F1) generation of morphine-sired or saline-sired offspring. We then tested drug-naïve, male and female offspring for changes in anxiety-like behavior, stress responsivity and memory in adulthood.
Methods
Animals and housing
Male and female Sprague-Dawley rats were obtained from Taconic Laboratories weighing 250–300g. Animals were maintained on a 12-hour/12-hour light/dark cycle with the lights off at 8:30am in a temperature and humidity controlled animal care facility. All experiments were conducted in the dark phase. Food and water were available ad libitum. All animals were pair-housed whenever possible and unless otherwise noted in the methods. All animals were handled daily for 2–5 minutes each for at least 5 days prior to the start of any behavioral procedure or test. The Institutional Animal Care and Use Committee of Temple University approved all animal care and experiments.
Drugs
Morphine sulfate was obtained from Spectrum Chemical (Gardena, CA) and dissolved in sterile 0.9% saline.
Jugular Catheterization Surgery
Male rats were anesthetized using an i.p. injection of 80 mg/kg ketamine and 12 mg/kg xylazine prior to surgery. An indwelling silastic catheter was threaded subcutaneously over the shoulder blade, inserted in the jugular vein and sutured in place. The catheter routed to a mesh back mount platform (Strategic Applications Inc, Lake Villa, IL) which was sutured below the skin between the shoulder blades. Catheters were flushed daily with 0.2mL of timentin (0.93 mg/mL) dissolved in heparinized saline and sealed with plastic obturators when not in use.
Morphine Self-Administration
Rats recovered from surgery for at least 7 days prior to morphine self-administration. Sires were placed in operant chambers and allowed to lever press on a fixed ratio 1 (FR1) schedule for morphine infusions (0.75mg/kg/infusion over 5s). Infusions of morphine were accompanied by a 5-second light cue and followed by a 20-second timeout period during which the house light turned off and lever presses were recorded but were not reinforced by drug infusions. Animals had daily 3-hour access to morphine self-administration. Control animals underwent the same catheterization surgeries and self-administration protocol but only had access to saline and were never exposed to morphine.
Breeding
After sixty continuous days of morphine self-administration, naïve female rats were placed in a cage with each male rat. Sires continued to self-administer morphine for three hours a day during a five day mating period. Paternal stress has been shown to have long lasting consequences for offspring, therefore sires continued to self-administer during mating to avoid withdrawal-related stress as a confounding factor (Morgan and Bale 2011; Rodgers et al. 2013b; Rodgers et al. 2015).Sires were then removed and dams reared pups independently until post-natal day (PND) 21, when pups were weaned and group housed with littermates. Female rats were housed individually during gestation, and for rearing of the first generation (F1) progeny. Male and female offspring were pair-housed with same sex littermates upon weaning and remained pair-housed throughout behavioral testing. F1 behavioral testing was conducted when offspring were 2–6 months old. 1–2 animals from each litter were randomly selected for behavioral test, such that no litter was over-represented in any particular experiment.
Novelty-Induced Hypophagia
Animals were singly housed for these experiments and handled daily for at least 5 days prior the start of the study. Food and water were removed from the home cage 90 minutes prior to training and testing. Rats were habituated to the testing room in their home cages for 15 minutes. During 8 days of training and a post-test on day 10, animals had access to peanut butter chips (Reese’s Peanut Butter Chips, 5 g; H.B. Reese Candy Co., Hershey, PA, USA) placed in a glass bowl for 15 minutes in their home cages. On day 9, animals were given access to the peanut butter chips in a novel environment located in the same room. The novel environment was a black polycarbonate cage (76 cm L × 76 cm W × 40 cm H) lined with bedding and sprayed with diluted dishwashing liquid (Lemon Joy, Procter & Gamble, Cincinnati, Ohio, USA). Peanut butter chips were placed in the middle of the cage in the same glass bowl used for training. On day 10, animals underwent a post-test where they were given access to the peanut butter chips in their home environment in the same manner as training. Across training and testing, the latency to consume the peanut butter chips was recorded. Latency was determined as the time to start eating peanut butter chips.
Defensive Probe Burying
Rats were placed into a clear polycarbonate cage 48 cm L × 26 cm W × 20 cm H, with a 1-cm diameter hole located 7 cm from the base of one end of the cage to accommodate the shock probe. Fresh bedding lined the cage to a depth of approximately 5 cm and the shock probe extended 8 cm into the cage. The probe was attached to a shock generator (SGS-004, BRS-LVE, Laurel, MD, USA) set to deliver 0.5 mA of current when the probe was contacted. The 15-minute test period began when the rat was placed in the arena containing the probe. Rats that did not make contact with the probe within 180 seconds of the onset of the test were excluded. The probe remained electrified for the duration of the session and time spent burying was recorded.
Restraint Stress
Acute restraint stress was chosen in order to elicit a hypothalamic pituitary adrenal (HPA) axis response (Spencer and Deak 2017). Male and female F1 rats were individually placed in a restraint tube for 15 minutes. Serial blood samples were obtained from the saphenous vein at baseline (i.e. before the restraint), and then 15-minute, 30-minute, and 90-minute time points. Blood samples were collected into lithium heparin coated tubes (Starstedt Inc, Newton, NC) and centrifuged for 10 minutes at 2000g to obtain plasma. The plasma was stored at −80 degrees Celsius until analysis. The plasma corticosterone levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Enzo, Cat. No. ADI-900–097) according to manufacturer’s instructions. This kit detected plasma corticosterone in the 32 – 20,000 pg/mL range. This corticosterone antibody cross-reacted 100% with corticosterone, 28.6% with deoxycorticosterone, 1.7% with progesterone, and 0.13% with testosterone. The intra-assay variability was 8.4% and the inter-assay variability was 8.2%.
Contextual Fear Conditioning
Male and female F1 rats were handled for 2 minutes per day for a minimum of 5 days leading up to fear conditioning. Animals were introduced to fear conditioning chambers (Med-Associates) on day 1 where they received two unsignaled footshocks (1.0mA, 2s) beginning 180 seconds after being placed in the chamber. There was a 60 second inter-shock interval, and the animals remained in the chamber for 60 seconds following the last footshock presentation. Twenty-four hours after the training trial, animals were reintroduced to the same context for 5 minutes. Both sessions were recorded with the ANY-Maze videotracking system (Wood Dale, IL) and total immobility per minute was scored by a trained observer blind to siring condition.
Object Location Memory
Male and female F1 rats were handled for 2 minutes per day for a minimum of 5 days preceding the onset of behavioral procedures. All experiments were conducted beginning at lights off. Animals were habituated to the training environment and cues, but no objects, during two 5-minute sessions on days 1 and 2. On the day of training, animals were placed in the training environment with two identical objects for a total of three 5-minute sessions, with an intersession interval of 1–2 minutes, during which the animal was returned to their home cage. The objects used were a glass Erlenmeyer flask and an aluminum L-bracket and they were counterbalanced across experiments. The objects were secured to the floor using double sided tape. The training environment was a 30” square, constructed of plexiglass. Bedding was cleaned and smoothed between sessions. Testing took place 24-hours after training in the same environment used for habituation and training. One of the two objects in each box was moved to a new position relative to the cue, and animals were allowed to explore for 5 minutes. Training and testing sessions were recorded and time spent exploring the objects was scored by a trained observer blind to experimental group. Exploration of objects was defined as actively sniffing or otherwise interacting with the objects with the nose of the animals within 1 cm of the object. The preference index was calculated as follows: (time exploring displaced object/total time exploring both objects) × 100.
Novel Object Recognition Memory
Male and female F1 rats were handled for 2 minutes per day for a minimum of 5 days preceding the onset of behavioral procedures. All experiments were at lights off and in the same testing environment described above but with no spatial cues inside of the arenas. During habituation, animals were placed into the testing environment without objects present for 10 minutes per day, for 5 days. 24 hours after the last habituation session, animals were returned to the chamber, which now contained two identical objects, for 10 minutes. Testing took place 24 hours after the training session. One of the training objects was removed, and a novel object was placed in the same position. Training and testing sessions were recorded and time spent exploring the objects was scored by a trained observer blind to siring conditions. Exploration of objects was defined as actively sniffing or otherwise interacting with the objects, with the nose of the animals within 1 cm of the object. Preference index was calculated as follows: (time exploring novel object/total time exploring both objects) × 100.
Data Analysis
Two-way repeated-measures ANOVAs were used to compare latency to feed in saline- and morphine-sired animals. Session (day 1 vs day 2 vs day 3) was used as the within-subjects factor, and siring (saline- vs morphine-sired) was used as the between-subjects factor. For growth curves of F1 progeny and analyses of corticosterone levels following an acute restraint stress, mixed models ANOVAs were used with time or day as a within-subject factor and sex and sires as between-subject factors. Time spent burying and time spent freezing were compared using two-tailed t-tests. For both object location memory and novel object recognition tests two-way repeated-measures ANOVAs were used to analyze the percent preference for the displaced or novel object of saline- and morphine-sired animals. In all cases, if a significant interaction was found after performing ANOVAs, post-hoc comparisons were made using Bonferroni corrections.
Results
Chronic paternal morphine self-administration does not affect insemination rates
Naïve male Sprague Dawley rats self-administered either morphine or saline 3 hours per day, for 60 consecutive days. The germline epigenome is an essential carrier of environmental information across generations (Bale 2015; Goldberg and Gould 2018; Pierce et al. 2018; Yohn et al. 2015). Methylation of DNA and the action of small non-coding RNAs can be transmitted through the father’s sperm to his offspring and play an essential role in neurodevelopmental programming (Jenkins and Carrell 2012). Our study design ensured that morphine exposure encompassed the sensitive windows of spermatogenesis, during which these highly dynamic processes can be modified by environmental insults (Bale 2015; Jenkins and Carrell 2012). First generation (F1) progeny were generated by breeding saline- or drug-exposed sires to drug-naïve females (Figure 1A). Sires that had daily access to morphine earned more infusions than sires receiving saline (Figure 1B; effect of drug type: F(1,77)=8.801, p=0.0040). Previous studies have found that paternal stress can produce deleterious effects in progeny (Morgan and Bale 2011; Rodgers et al. 2013b; Rodgers et al. 2015). Here, we aimed to circumvent the potentially confounding effects of stress related to acute withdrawal symptoms following chronic morphine self-administration in sires. Sires continued to self-administer morphine after drug-naive females were added to the home cage during the mating period, which lasted 5 days. Total morphine intake in the days prior to and after drug-naïve females were introduced to the home cage were similar (before=104.7±7.059 infusions; after=112.4±6.348 infusions; effect of day: F(2,101)=0.7524, p=.5088; Effect of breeding: F(1,38)=3.184, p=.0824, interaction: F(3,128)=1.341, p=.2620).
Figure 1. Experimental Design, Sire Self-Admin.
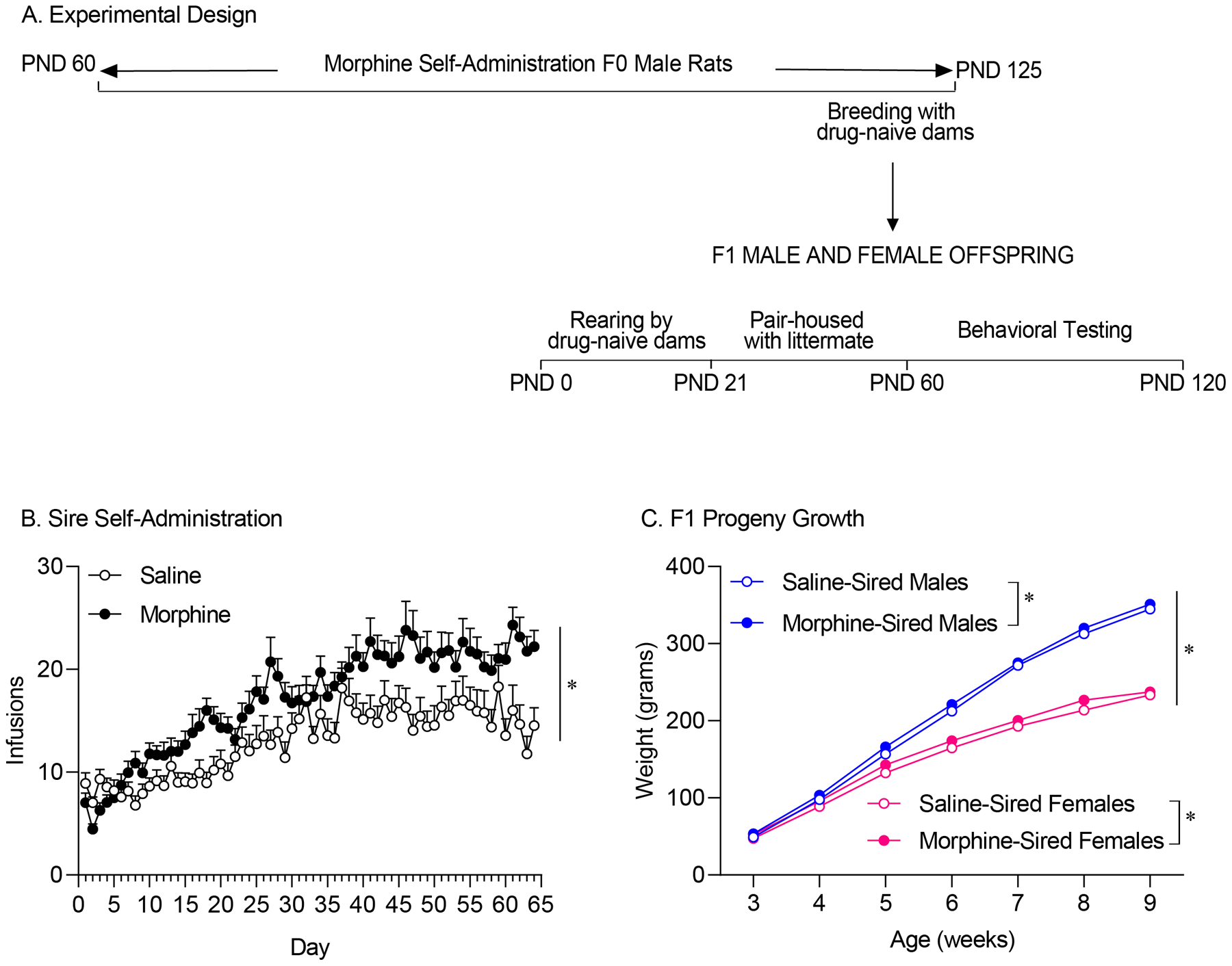
A. Experimental Design. Sires self-administered saline or morphine for 60 continuous days before being paired with drug-naïve females for breeding. Dams were separated from sires and raised pups independently. F1 progeny were pair-housed with littermates throughout adolescence and behavioral testing. All F1 behavioral testing occurred in adulthood. B. Total infusions of either morphine or saline earned by sires prior to mating. Animals that had access to morphine earned more infusions than animals receiving saline. C. Male and female progeny were weighed weekly after weaning. Paternal morphine exposure resulted in a slight increase in body weight in both male (saline, n=11; morphine, n=14) and female (saline, n=11; morphine, n=14) litters. Overall male progeny gained more weight than female offspring.). Data shown mean ± SEM; * p<0.05
In contrast to previous literature reporting diminished fertility following chronic morphine exposure (Cicero et al. 2002; Cicero et al. 1995), the insemination rates were comparable between morphine-treated and saline-exposed sires bred to drug-naïve females (Table 1). The number of pups per litter and the sex ratio of the litters were also unaffected by paternal drug use (Table 1). We measured body weight weekly across development starting at weaning (3 weeks of age) for male and female F1 progeny. Overall, males gained more weight than females (Figure 1C; effect of sex: F(1,46)=209.4, p<.0001). Paternal morphine exposure resulted in a slight but significant increase in body weight in both males and females (Figure 1C; main effect of sire, F(1,46)=4.142, p=.0476; sex × sire interaction F(1,46)=0.0249, p=.8754). In both males and females, the differences in weight gain dissipated by week 9 of age (Tukey post-hoc tests, adjusted p values females=.9585, males=.4334). Taken together, these data indicate that insemination rates were not impacted by chronic morphine self-administration and that differences in growth curves driven by paternal morphine exposure dissipated by adulthood in first generation male and female progeny.
Table 1: Breeding Outcomes.
| Saline | Morphine | p-value | |
|---|---|---|---|
| Total # of Sires | 40 | 39 | - |
| Total # of Dams Paired | 55 | 58 | - |
| Litters Produced | 49 | 51 | - |
| Insemination Rate | 0.890909091 | 0.879310345 | 0.8204 |
| Average Litter Size (mean +/−standard deviation) | 12.12 +/− 2.04 | 11.63 +/− 2.70 | 0.3045 |
| Sex Ratio (mean +/−standard deviation) | 0.5124 +/− 0.1392 | 0.5127 +/− 0.1390 | 0.9935 |
Paternal morphine exposure did not affect anxiety-like behavior in male or female progeny
We used the novelty induced hypophagia paradigm (NIH) to assess the effect of paternal morphine exposure on anxiety-like behaviors in adult male and female offspring. Rats were first trained to consume peanut butter chips in their home cage and were then exposed to the peanut butter chips in a novel environment. All animals displayed increased latency to feed in a novel environment compared to the home cage and there was no effect of paternal morphine exposure on latency to feed in a novel environment in drug-naïve male F1 progeny (Figure 2A; effect of environment: F(2, 34)=29.11, p<.0001; effect of sire: F(1, 17)=.9317, p=.3479). Paternal morphine exposure did not have any impact on female progeny, evidenced by the fact that both saline- and morphine-sired female F1 progeny all showed increased latencies to consume the peanut butter chips in the novel environment, compared to home cage (Figure 2B; effect of environment: F(2, 22)=20.92, p<.0001; effect of sire: F(1, 11)=.00437, p=.9485). Taken together, these results suggest that paternal history of morphine use does not affect anxiety in drug-naïve male and female offspring.
Figure 2. Paternal morphine exposure does not affect anxiety in male or female F1 offspring.
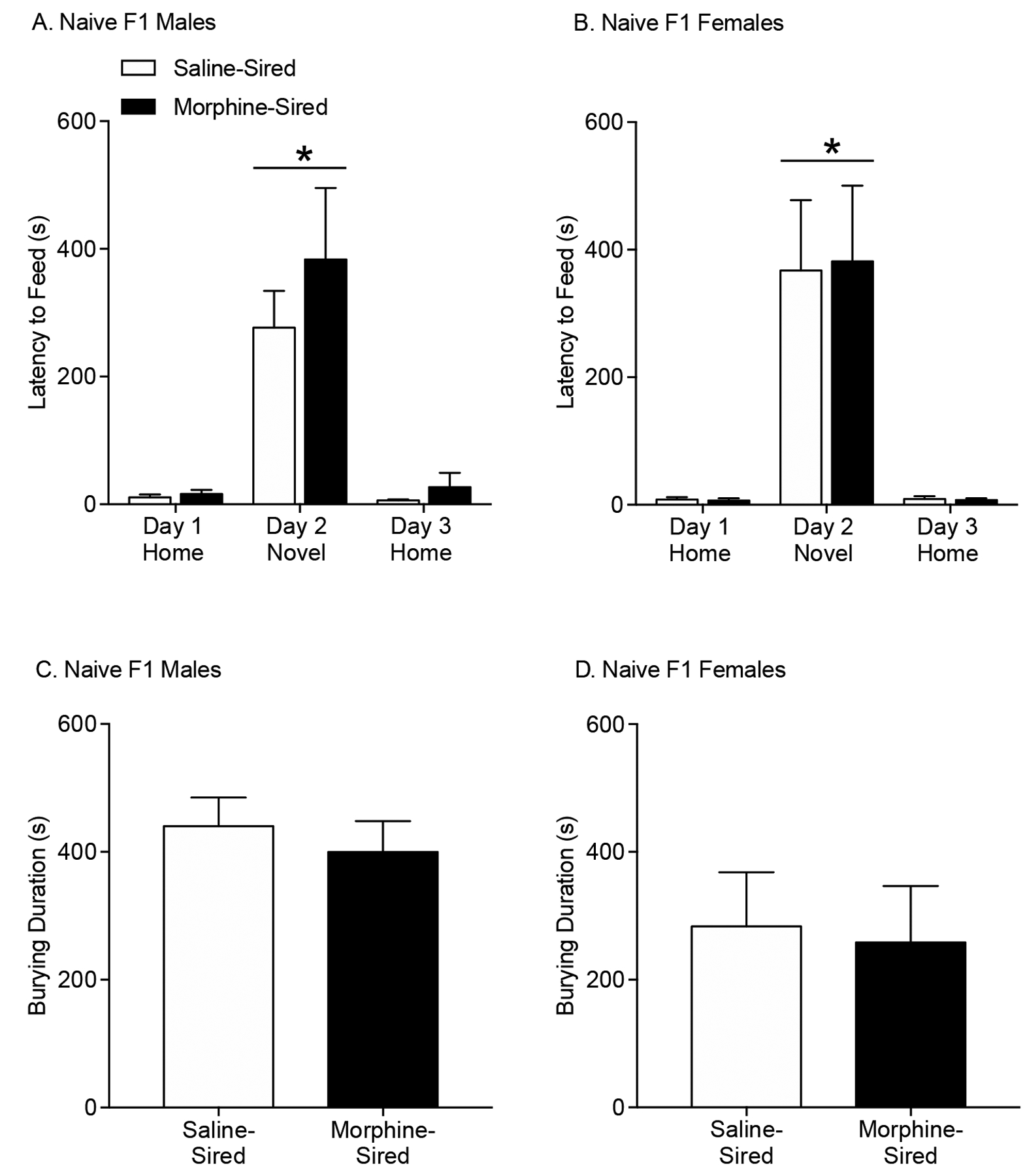
A. Both morphine-sired male rats (n = 10, from 10 sires) and saline-sired male rats (n= 10, from 10 sires) show an increase in the latency to approach and consume peanut butter chips in a novel environment. B. Morphine-sired female rats (n = 7, from 7 sires) and saline-sired female rats (n= 6, from 6 sires) show a similar increase in latency to consume peanut butter chips in a novel environment. C. Morphine-sired male rats (n = 12, from 6 sires) do not show a difference in the amount of time spent burying compared to saline-sired male rats (n = 12, from 6 sires). D. Morphine-sired female rats (n = 8, from 4 sires) do not show a difference in the amount of time spent burying compared to saline-sired female rats (n = 8, from 4 sires). Data shown mean ± SEM. * p<0.05
Anxiety-like behavior is difficult to assess in rodents. To complement our findings using the appetitive NIH task, we turned to an aversive defensive probe burying paradigm. Animals were introduced to an electrified probe and the time spent burying the probe was measured. Paternal morphine experience was not found to affect burying duration in males (Figure 2C; t21=0.6094, p=.5488) or females (Figure 2D; t14=0.208, p=.8382). Taken together, these results demonstrate similar levels of anxiety in saline- and morphine- sired male and female progeny.
Hypothalamic-pituitary-adrenal (HPA) axis response to restraint stress was unaltered by paternal morphine self-administration.
We monitored corticosterone response to an acute restraint stress as a measure of HPA axis activity in saline- and morphine-sired progeny. Blood was collected from the saphenous vein at four time points: before stress (t=0), immediately after a 15 minute-restraint stress (t=15), 15 minutes after the end of restraint (t=30), and 75 minutes after the end of restraint (t=90). Corticosterone concentrations in the blood were measured by ELISA. The concentration of corticosterone changed significantly over the time course for both males (Figure 3A) and females (Figure 3B; effect of time: F (3, 96) = 18.80, p<.0001). However, there was no difference in corticosterone response to restraint stress between saline- and morphine-sired progeny at any of the time points in both male and female (Figure 3; effect of sire: F(1, 32)=0.3104, p=.5813) rats. The corticosterone levels were higher in females than in males (effect of sex: F(1,32)=63.32, p<.0001), which is consistent with previous reports (Kudielka and Kirschbaum 2005). Overall, these data suggest that the HPA axis response to acute stress is unaffected by paternal morphine self-administration in male or female progeny.
Figure 3. Paternal morphine consumption does not affect HPA axis response to stress in F1 male or female animals.
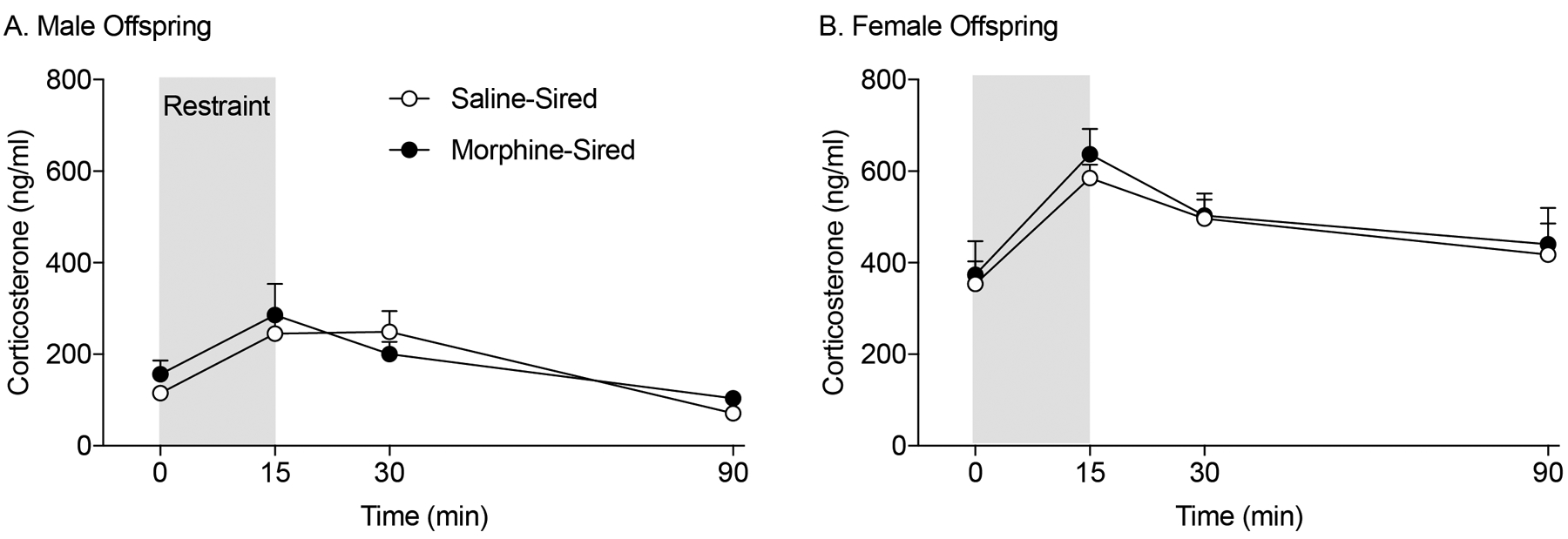
A. Morphine-sired male rats (n = 8, from 6 sires) do not show altered corticosterone response to a 15-minute restraint stress compared to saline-sired male rats (n = 9, from 6 sires). B. Morphine-sire female rats (n = 11, from 7 sires) do not show altered corticosterone response to a 15-minute restraint stress compared to saline-sired female rats (n = 8, from 8 sires).
Hippocampus-dependent memory was not affected by paternal morphine exposure
After establishing that paternal morphine consumption does not affect anxiety or stress response in offspring, we used contextual fear conditioning to evaluate the effect of paternal morphine consumption on hippocampus-dependent memory in adult male and female progeny. Rats received footshocks in a novel context, and were returned to the same context 24 hours later. Both sessions were recorded and time spent freezing was interpreted as an association between the context and the footshock. All animals displayed increased time spent freezing during the 24-hour memory retrieval test compared to baseline freezing and freezing behavior was not impacted by paternal morphine history (Figure 4A and 4B, main effect of day: F(1,33)=276.2, p<0.0001; effect of sire: F(1,33)=1.403, p=.2447). Taken together, these results suggest that paternal morphine consumption does not alter hippocampus-dependent fear memory in adult drug-naïve male or female progeny.
Figure 4. Paternal morphine use does not affect hippocampal-dependent memory in male or female F1 offspring.
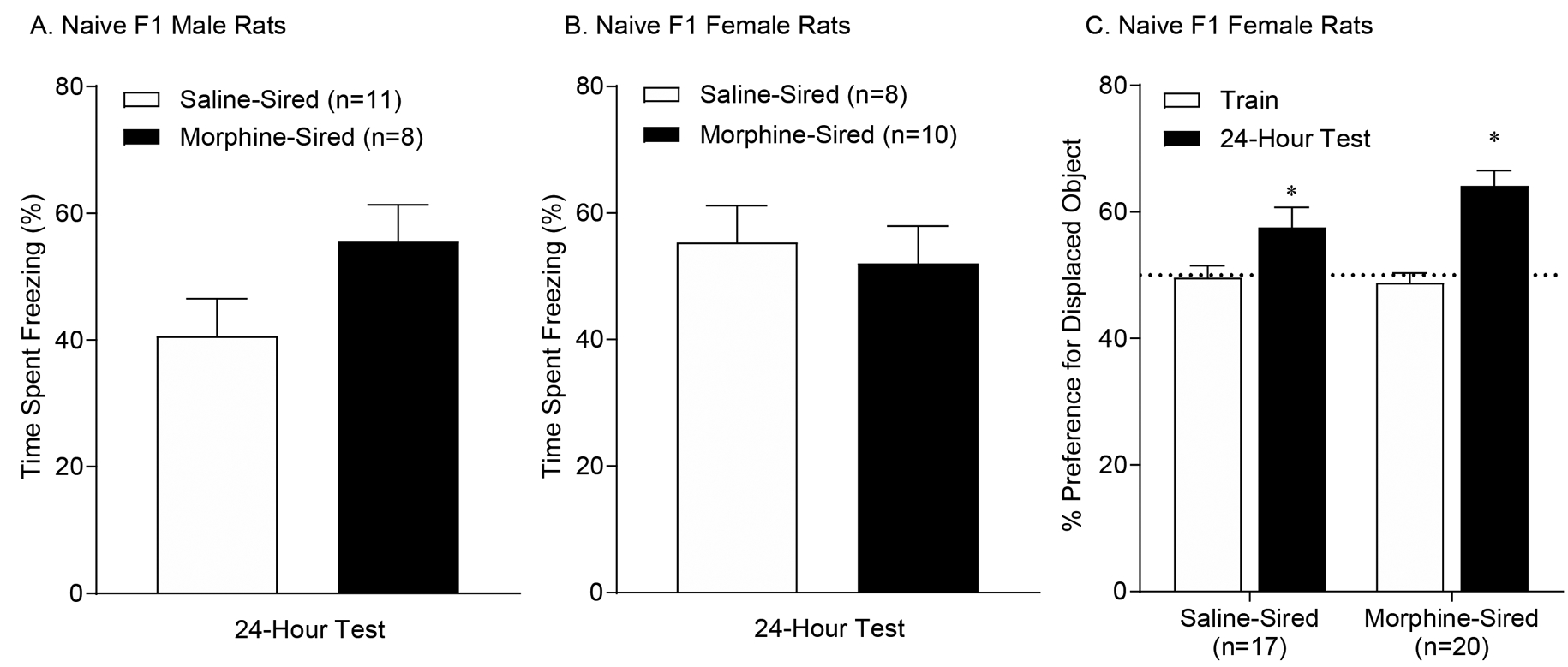
A. Male morphine-sired rats (n = 8, from 5 sires) do not show any difference in time spent freezing during the 24-hour memory test compared to male saline-sired rats (n = 11, from 7 sires). B. Female morphine-sired rats (n = 10, from 7 sires) do not show any difference in time spent freezing during the long-term contextual fear conditioning memory test compared to female saline-sired rats (n = 8, from 5 sires). Data are expressed as time spent freezing (s, mean ± SEM) during a test 24-hours following fear conditioning. C. Both saline- and morphine-sired female offspring show a preference for the displaced object during the 24-hour memory test. For morphine-sired female rats (n = 20, from 10 sires) and saline-sired female rats (n = 17, from 10 sires). *p<0.001.
Recent evidence suggests that female rats are prone to exhibiting an active fear response in addition to freezing (Gruene et al. 2015). Thus we sought to complement these findings by using another hippocampus-dependent task: object location memory. Animals were allowed to explore a chamber containing two objects positioned equidistant from a cue mounted on one side. Twenty-four hours later, one of the original objects was moved to a different position in the chamber, and the rats were allowed to explore both objects. Sessions were recorded and the time spent interacting with the objects was measured. Both saline- and morphine-sired females demonstrated increased preference for the displaced object 24-hours following training and there was no effect of paternal morphine consumption on time spent investigating the displaced object (Figure 4C; effect of object displacement: F(1,33)=22.95, p<.0001; effect of sire: F(1,33)=1.672, p=.2050; interaction: F(1,33)=2.288, p=.1399). Taken together, these data suggest that paternal morphine consumption does not alter hippocampal-dependent memory in adult drug-naïve male or female progeny.
Paternal morphine exposure impaired novel object recognition memory in female, but not male offspring
We used novel object recognition testing in order to assess hippocampal-independent memory of morphine-sired and saline-sired female rats (Winters et al. 2004). Rats were habituated to the testing chamber for 5 days prior to training and spatial cues were removed from the training arena in order to minimize hippocampal involvement for this task (Forwood et al. 2005; Mumby et al. 2002; Oliveira et al. 2010). Two identical objects were then placed in the chamber and the amount of time spent exploring the objects was measured. 24 hours later, one of the objects was replaced with a novel object, and the amount of time spent exploring each object was recorded. For male progeny, two-way ANOVA (within subject factor=trial; between factor=sire) revealed that both groups showed an increase in preference for the novel object during the 24-hour memory test (Figure 5A; effect of trial (F(1,26)=6.458; p=.0174; sire F(1,26)=0.1568, p=.9654; interaction F(1,26)=0.1141, p=.733). These results suggest that paternal morphine history did not impact long-term object recognition memory in male offspring. In sharp contrast, only saline-sired females showed a preference for the novel object during the long-term memory test (Figure 5B; effect of sire: F1,25=1.199, p=.2839; effect of trial: F1,25=2.564, p=.1219; interaction: F1,25=7.492, p=.0112). Bonferoni post hoc tests demonstrated a significant increase in the amount of time saline-sired females interacted with the novel object, while morphine-sired female progeny spent equal amount of time with the familiar and novel object. These data indicate that paternal morphine exposure disrupts hippocampal-independent memory selectively in female offspring.
Figure 5. Paternal morphine self-administration impairs long-term object recognition memory in female but not male progeny.
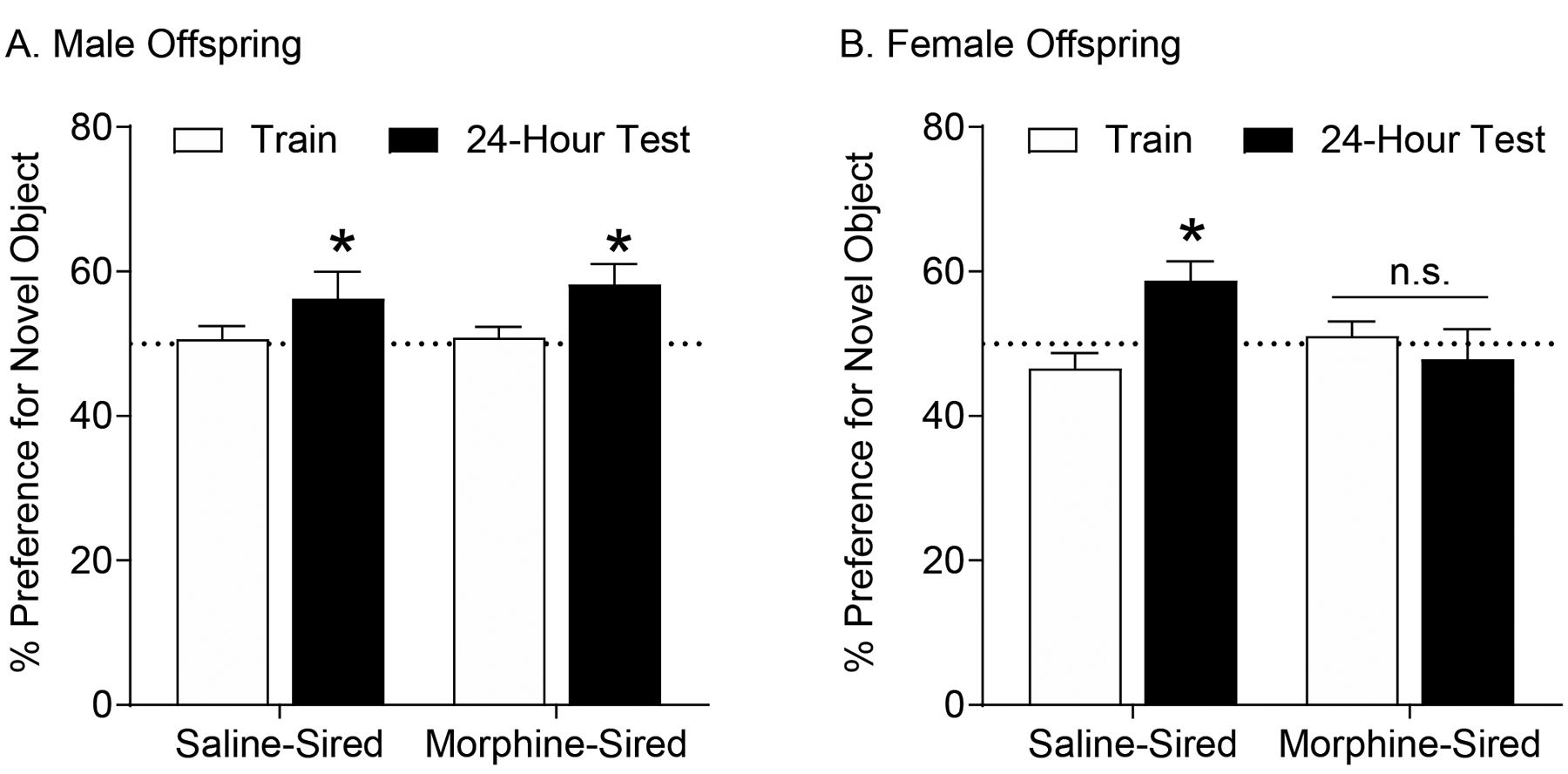
A. Saline-sired (n=14, from 9 sires) and morphine-sired male rats (n=14, from 8 sires) both show a preference for the novel object during the 24-hour object recognition memory test compared to their initial preference during the training trial. (* p<0.05) B. Saline-sired female rats (n = 14, from 9 sires) spent equal time exploring both objects during training, and showed a preference for the novel object during a test 24-hours after training. In contrast, morphine-sired female rats (n = 13, from 9 sires) spent equal time exploring both objects during training and during the 24-hour memory test. (*p<0.01 comparing training preference to preference during 24-hour test using Bonferroni post-hoc correction).
Discussion
The present study indicates that morphine self-administration in sires has sex-specific multigenerational consequences on offspring. We found that paternal morphine exposure did not affect anxiety-like behavior or the corticosterone response to acute stress in either male or female progeny. Hippocampus-dependent memory, assessed using either object location memory or contextual fear conditioning, was unaltered in morphine-sired male and female progeny. In sharp contrast, a deficit in novel object recognition was found in morphine-sired female, but not male, progeny.
We chose to use morphine self-administration to chronically expose sires to morphine for several reasons. Firstly, this approach uniquely allows animals to titrate the daily dose of morphine consumed and accounts for the tolerance that develops with repeated exposure to opioids. Accordingly, sires slowly increased the number of infusions earned over the two months of daily access to morphine self-administration. We favored this design over an experimenter-delivered chronic regimen that would have required a pre-determined escalation paradigm that has not been previously deployed over such extended periods of time. Self-administration was also preferable to using morphine pellets, which render exact dosage and time of exposure difficult to tightly control over weeks or months (Yoburn et al. 1985). Secondly, the volitional aspect of self-administration added a more translational element to the study. Multigenerational and transgenerational effects of paternal insults such as stress and diet have been reported in humans (Kaati et al. 2002; Pembrey et al. 2006; Radley et al. 2011; Yehuda et al. 2016; Yehuda and Lehrner 2018) but are intrinsically difficult to study and remain highly debated. The rodent morphine self-administration multigenerational model that we have developed offers an opportunity to explore some of these questions by controlling most environmental factors outside of opioid exposure while still using a translational and volitional method of drug delivery. Lastly, drug self-administration reduces the level of stress associated with repeated experimenter-delivered drug injections. Paternal stress has been shown to produce profound deleterious consequences in future generations (Rodgers et al. 2013a; Rodgers et al. 2015) and represents a potentially confounding factor in our objective to highlight consequences emanating from paternal morphine exposure. Thus, we aimed to minimize stress in morphine-exposed sires by using morphine self-administration and by allowing sires to self-administer morphine daily during the mating period in order to avoid any withdrawal-mediated effects in this study.
Previous research examining the multigenerational impact of morphine exposure on anxiety-like behaviors in offspring have yielded conflicting results. In a study where male and/or female rats consumed morphine orally for 21 days, followed by naloxone-precipitated withdrawal and a ten day withdrawal period prior to mating, maternal and parental exposure elicited increased anxiety-like behaviors in male offspring, while paternal use did not cause a change in anxiety-like behavior (Sabzevari et al. 2018). Here, we did not find differences in anxiety-like behaviors following paternal morphine exposure by intravenous self-administration without a withdrawal period prior to mating. On the other hand, studies in rats using experimenter-delivered drug with a three-week withdrawal period prior to mating found increased anxiety in male and female offspring, regardless of which parent was drug-exposed prior to mating (Li et al. 2014). This effect was attenuated by the presence of environmental enrichment during adolescence in the offspring (Li et al. 2014), or during parental morphine withdrawal (Pooriamehr et al. 2017). It has been demonstrated that the effects of drugs of abuse vary with the mode of delivery (Donny et al. 2006; Ploense et al. 2018; Twining et al. 2009; Weise-Kelly and Siegel 2001) therefore, it is plausible that difference in anxiety-like behaviors following parental opioid exposure is attributable, at least in part, to different drug delivery methods. The duration of the withdrawal period or the precipitation of withdrawal prior to mating could also influence whether anxiety-like phenotypes emerge in offspring of drug-exposed sires and dams.
Chronic morphine exposure has been shown to modulate HPA axis function (Bali et al. 2015; Zhou et al. 2010). The present study found no effect of paternal morphine self-administration on restraint-induced corticosterone plasma levels in male or female offspring. It is worth noting that all of the experiments in this study were conducted during the active, dark period. Thus the baseline levels of corticosterone in all subjects were noticeably higher that those expected during the rest, light period (Moore and Eichler 1972). The possibility that more subtle differences in the corticosterone levels elicited by an acute stressor could emerge during the light phase cannot be completely excluded. However, the fact that our experimental design revealed the well-established sex difference in corticosterone elevations following an acute restraint stress (Handa et al. 1994; Kudielka and Kirschbaum 2005; Lu et al. 2015), with females showing a more robust change in corticosterone overall should minimize the concerns associated with this potential caveat.
Few studies have investigated the effect of paternal morphine exposure on hippocampal and cognitive function with the majority of this work focused on maternal or prenatal influences (Ahmadalipour et al. 2018; Lin et al. 2009; Nasiraei-Moghadam et al. 2013; Sepehri et al. 2014; Sithisarn et al. 2011; Tan et al. 2015; Yang et al. 2006). Both maternal and paternal oral morphine administration caused impairments in passive avoidance memory in both male and female offspring (Akbarabadi et al. 2018). Here we found hippocampal function to be intact in male and female progeny. The differences with the aforementioned study are likely tied to methodological considerations including mode of delivery and time of exposure in sires. In contrast, we found that morphine-sired female, but not male progeny had a deficit in long-term object recognition memory. Previous evidence suggests that when object recognition occurs in a familiar environment with limited spatial cues, the task becomes hippocampus-independent, and largely driven by the perirhinal cortex (Barker and Warburton 2011; Forwood et al. 2005; Piterkin et al. 2008; Winters et al. 2004). We used multiple habituation sessions without the presence of spatial cues, in an attempt to disengage the hippocampus and probe perirhinal cortex function (Oliveira et al. 2010). Lesion studies indicate that the perirhinal cortex is also involved in object location memory (Liu and Bilkey 1998; 2001; Wiig and Bilkey 1994), which we found to be intact in our manipulations. It is possible however that paternal morphine exposure produces more subtle changes in perirhinal cortex function compared to lesions, which would be consistent with our observations and the selectivity of paternal morphine treatment on object recognition memory in female progeny. Interestingly, the results reported here are drastically different than reports of memory deficits following paternal cocaine exposure, which produce male-specific deficits in hippocampal function, while sparing object recognition memory (Wimmer et al. 2017). These differences suggest that the germline reprogramming events elicited by cocaine and morphine are distinct and produce divergent developmental trajectories. The exact mechanisms underlying the transmission of drug exposure remain largely unexplored and our results suggest that these processes are drug-specific.
The sex specificity of the impact of paternal morphine exposure on object recognition memory is intriguing. Estrogen signaling in the hippocampus has been shown to modulate novel object recognition memory in both male and female rats via estrogen receptor alpha (ERα) and beta (ERβ) and G-protein coupled estrogen receptor (GPER) signaling (Jacome et al. 2010; Kim et al. 2016; Lymer et al. 2017; Pereira et al. 2014; Phan et al. 2011; Phan et al. 2015; Tuscher et al. 2016). In the hippocampus, the downstream mechanisms of estrogen-mediated modulation of object memory are distinct between male and female rodents. In the hippocampus of females, 17β-estradiol (E2) is thought to mediate object recognition memory via phosphorylation of the cell signaling kinase extracellular signal-related kinase (ERK) (Fan et al. 2010; Fernandez et al. 2008; Kuroki et al. 2000; Pereira et al. 2014). In the hippocampus of males, E2 does not increase ERK phosphorylation and increases in phosphorylated ERK in the hippocampus are not necessary for memory enhancements following E2 treatment (Koss et al. 2018). While there is a body of literature examining the role of estrogen in the hippocampus on object memory, the role of sex hormones in the perirhinal cortex is not as well defined. Previous studies have found that intra-perirhinal cortex infusions of E2 or an ERβ agonist improved novel object recognition memory in both males and females (Gervais et al. 2016; Gervais et al. 2013). However, it is unclear whether the sex-specific differences in downstream mechanisms of estrogen receptor activation in the perirhinal cortex are similar to the ones reported in the hippocampus. Indeed, fewer studies have examined the impact of estrogen signaling in the perirhinal cortex of females (Mitchnick et al. 2019). Overall, it is clear that estrogen signaling can modulate object memory in rodents via sex-specific downstream molecular cascades. It is tempting to posit that the sex specificity of our observed phenotypes may be related to the unique downstream mechanisms related to estrogen signaling in the perirhinal cortex of males and females, but more research is needed to fully address this possibility.
Our results indicate that paternal morphine self-administration elicits sex-specific effects on hippocampus-independent object location memory in offspring, without affecting spatial memory, anxiety, or the stress-induced corticosterone response. This work adds to a growing literature showing that parental experiences can have profound effects on offspring, and highlights the importance of experimental design, as well as the distinct effects of paternal and maternal experience on outcomes in offspring. As the number of opioid-exposed fathers rises, these data have potentially profound implications for children of fathers that were chronically exposed to opioids.
Table 2.
Time spent investigating objects during object memory tasks (TSI reported in seconds, average ± standard deviation)
| TASK | Figure | Saline-Sired Time (s) | Morphine-Sired Time (s) | p-value |
|---|---|---|---|---|
| Object place memory F1 females | Figure 4B | 103.44 +/− 6.87 | 100.34 +/− 10.47 | 0.8087 |
| Novel object memory F1 males | Figure 4A | 75.60 +/− 8.24 | 71.36 +/− 7.33 | 0.6926 |
| Novel object memory F1 females | Figure 4B | 89.32 +/− 9.21 | 102.83 +/− 7.17 | 0.2631 |
Funding:
This work was supported by the following grant from the National Institutes of Health: DP1 DA046537 (MEW), K01 DA039308 (MEW), T32 DA007237 (ABT; Unterwald EM, PI)
Abbreviations:
- HPA axis
Hypothalamic-pituitary-adrenal axis
- i.p.
intraperitoneal
- FR
fixed ratio
- PND
post-natal day
- NIH
Novelty-induced hypophagia
- n.s.
non-significant
- F1
first generation (offspring)
- F2
second generation (i.e. grand-offspring)
Footnotes
Conflict of interest statement: The authors have no conflict of interest to report.
References:
- Ahmadalipour A, Ghodrati-Jaldbakhan S, Samaei SA, Rashidy-Pour A (2018) Deleterious effects of prenatal exposure to morphine on the spatial learning and hippocampal BDNF and long-term potentiation in juvenile rats: Beneficial influences of postnatal treadmill exercise and enriched environment. Neurobiol Learn Mem 147: 54–64. [DOI] [PubMed] [Google Scholar]
- Akbarabadi A, Niknamfar S, Vousooghi N, Sadat-Shirazi MS, Toolee H, Zarrindast MR (2018) Effect of rat parental morphine exposure on passive avoidance memory and morphine conditioned place preference in male offspring. Physiol Behav 184: 143–149. [DOI] [PubMed] [Google Scholar]
- Baldacchino A, Balfour DJ, Passetti F, Humphris G, Matthews K (2012) Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev 36: 2056–68. [DOI] [PubMed] [Google Scholar]
- Bale TL (2015) Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 16: 332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali A, Randhawa PK, Jaggi AS (2015) Stress and opioids: role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neurosci Biobehav Rev 51: 138–50. [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC (2011) When is the hippocampus involved in recognition memory? J Neurosci 31: 10721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block C, Cianfrini L (2013) Neuropsychological and neuroanatomical sequelae of chronic non-malignant pain and opioid analgesia. NeuroRehabilitation 33: 343–66. [DOI] [PubMed] [Google Scholar]
- Byrnes EM (2005) Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 182: 537–44. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM (2011) Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res 218: 200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, Byrnes EM (2013) Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 227: 263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun S, Conner E, Miller M, Messina N (2015) Improving the outcomes of children affected by parental substance abuse: a review of randomized controlled trials. Subst Abuse Rehabil 6: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ (2010) Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143: 1084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C (1980) Dose-dependent effects of heroin on memory in two inbred strains of mice. Psychopharmacology (Berl) 67: 235–9. [DOI] [PubMed] [Google Scholar]
- Chorbov VM, Todorov AA, Lynskey MT, Cicero TJ (2011) Elevated levels of DNA methylation at the OPRM1 promoter in blood and sperm from male opioid addicts. J Opioid Manag 7: 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Davis LA, LaRegina MC, Meyer ER, Schlegel MS (2002) Chronic opiate exposure in the male rat adversely affects fertility. Pharmacol Biochem Behav 72: 157–63. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O’Connor L, Adams M, Meyer ER (1995) Adverse effects of paternal opiate exposure on offspring development and sensitivity to morphine-induced analgesia. J Pharmacol Exp Ther 273: 386–92. [PubMed] [Google Scholar]
- Degenhardt L, Whiteford H, Hall WD (2014) The Global Burden of Disease projects: what have we learned about illicit drug use and dependence and their contribution to the global burden of disease? Drug Alcohol Rev 33: 4–12. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL (2006) Comparing the physiological and subjective effects of self-administered vs yoked cocaine in humans. Psychopharmacology (Berl) 186: 544–52. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Bale TL (2011) Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152: 2228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM (2010) Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci 30: 4390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM (2008) Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28: 8660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DK, Rice RC, Martinez Rivera A, Donohoe M, Rajadhyaksha AM (2017) Altered reward sensitivity in female offspring of cocaine-exposed fathers. Behav Brain Res 332: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ (2005) Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 15: 347–55. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Hamel LM, Brake WG, Mumby DG (2016) Intra-perirhinal cortex administration of estradiol, but not an ERβ agonist, modulates object-recognition memory in ovariectomized rats. Neurobiol Learn Mem 133: 89–99. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Jacob S, Brake WG, Mumby DG (2013) Systemic and intra-rhinal-cortical 17-β estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm Behav 64: 642–52. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Gould TJ (2018) Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA (1994) Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28: 464–76. [DOI] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS (2006) Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol 28: 198–209. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V (2010) Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem 94: 488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TG, Carrell DT (2012) The sperm epigenome and potential implications for the developing embryo. Reproduction 143: 727–34. [DOI] [PubMed] [Google Scholar]
- Kaati G, Bygren LO, Edvinsson S (2002) Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet 10: 682–8. [DOI] [PubMed] [Google Scholar]
- Killinger CE, Robinson S, Stanwood GD (2012) Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse 66: 902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM (2016) 17β-Estradiol and Agonism of G-protein-Coupled Estrogen Receptor Enhance Hippocampal Memory via Different Cell-Signaling Mechanisms. J Neurosci 36: 3309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Haertel JM, Philippi SM, Frick KM (2018) Sex Differences in the Rapid Cell Signaling Mechanisms Underlying the Memory-Enhancing Effects of 17β-Estradiol. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C (2005) Sex differences in HPA axis responses to stress: a review. Biol Psychol 69: 113–32. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y (2000) Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol 400: 205–9. [DOI] [PubMed] [Google Scholar]
- Le Q, Yan B, Yu X, Li Y, Song H, Zhu H, Hou W, Ma D, Wu F, Zhou Y, Ma L (2017) Drug-seeking motivation level in male rats determines offspring susceptibility or resistance to cocaine-seeking behaviour. Nat Commun 8: 15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CQ, Luo YW, Bi FF, Cui TT, Song L, Cao WY, Zhang JY, Li F, Xu JM, Hao W, Xing XW, Zhou FH, Zhou XF, Dai RP (2014) Development of anxiety-like behavior via hippocampal IGF-2 signaling in the offspring of parental morphine exposure: effect of enriched environment. Neuropsychopharmacology 39: 2777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wu CF, Pei G, Xu NJ (2001) Reversal of morphine-induced memory impairment in mice by withdrawal in Morris water maze: possible involvement of cholinergic system. Pharmacol Biochem Behav 68: 507–13. [DOI] [PubMed] [Google Scholar]
- Lim JP, Brunet A (2013) Bridging the transgenerational gap with epigenetic memory. Trends Genet 29: 176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Tao PL, Jong YJ, Chen WF, Yang CH, Huang LT, Chao CF, Yang SN (2009) Prenatal morphine alters the synaptic complex of postsynaptic density 95 with N-methyl-D-aspartate receptor subunit in hippocampal CA1 subregion of rat offspring leading to long-term cognitive deficits. Neuroscience 158: 1326–37. [DOI] [PubMed] [Google Scholar]
- Liu P, Bilkey DK (1998) Excitotoxic lesions centered on perirhinal cortex produce delay-dependent deficits in a test of spatial memory. Behav Neurosci 112: 512–24. [DOI] [PubMed] [Google Scholar]
- Liu P, Bilkey DK (2001) The effect of excitotoxic lesions centered on the hippocampus or perirhinal cortex in object recognition and spatial memory tasks. Behav Neurosci 115: 94–111. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, Zhang QJ, Huang ML, Bao AM (2015) Sex differences in the stress response in SD rats. Behav Brain Res 284: 231–7. [DOI] [PubMed] [Google Scholar]
- Lymer J, Robinson A, Winters BD, Choleris E (2017) Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology 77: 131–140. [DOI] [PubMed] [Google Scholar]
- Mitchnick KA, Mendell AL, Wideman CE, Jardine KH, Creighton SD, Muller AM, Choleris E, MacLusky NJ, Winters BD (2019) Dissociable involvement of estrogen receptors in perirhinal cortex-mediated object-place memory in male rats. Psychoneuroendocrinology 107: 98–108. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–6. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Bale TL (2011) Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci 31: 11748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulaei N, Mondanizadeh M, Salmani ME, Palizvan MR, Khansarinejad B, Sadegh M (2018) Transgenerational consequences of prepregnancy chronic morphine use on spatial learning and hippocampal Mecp2 and Hdac2 expression. Neuroreport 29: 739–744. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H (2002) Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 9: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkowicz S, Płotka J, Polkowska Ż, Biziuk M, Namieśnik J (2013) Prenatal exposure to substance of abuse: a worldwide problem. Environ Int 54: 141–63. [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S, Sherafat MA, Safari MS, Moradi F, Ahmadiani A, Dargahi L (2013) Reversal of prenatal morphine exposure-induced memory deficit in male but not female rats. J Mol Neurosci 50: 58–69. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17: 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J (2006) Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 14: 159–66. [DOI] [PubMed] [Google Scholar]
- Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS (2014) Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor α. Neurobiol Learn Mem 114: 1–9. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E (2011) Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology 152: 1492–502. [DOI] [PubMed] [Google Scholar]
- Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CD, Kow LM, MacLusky NJ, Pfaff DW, Choleris E (2015) Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci U S A 112: 16018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Fant B, Swinford-Jackson SE, Heller EA, Berrettini WH, Wimmer ME (2018) Environmental, genetic and epigenetic contributions to cocaine addiction. Neuropsychopharmacology 43: 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piterkin P, Cole E, Cossette MP, Gaskin S, Mumby DG (2008) A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem 15: 785–91. [DOI] [PubMed] [Google Scholar]
- Ploense KL, Vieira P, Bubalo L, Olivarria G, Carr AE, Szumlinski KK, Kippin TE (2018) Contributions of prolonged contingent and non-contingent cocaine exposure to escalation of cocaine intake and glutamatergic gene expression. Psychopharmacology (Berl) 235: 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooriamehr A, Sabahi P, Miladi-Gorji H (2017) Effects of environmental enrichment during abstinence in morphine dependent parents on anxiety, depressive-like behaviors and voluntary morphine consumption in rat offspring. Neurosci Lett 656: 37–42. [DOI] [PubMed] [Google Scholar]
- Rabbani M, Hajhashemi V, Mesripour A (2009) Increase in brain corticosterone concentration and recognition memory impairment following morphine withdrawal in mice. Stress 12: 451–6. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP (2011) Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress 14: 481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33: 9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL (2015) Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A 112: 13699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabzevari S, Rohbani K, Sadat-Shirazi MS, Babhadi-Ashar N, Shakeri A, Ashabi G, Khalifeh S, Ale-Ebrahim M, Zarrindast MR (2018) Morphine exposure before conception affects anxiety-like behavior and CRF level (in the CSF and plasma) in the adult male offspring. Brain Res Bull 144: 122–131. [DOI] [PubMed] [Google Scholar]
- Saha N, Datta H, Sharma PL (1991) Effects of morphine on memory: interactions with naloxone, propranolol and haloperidol. Pharmacology 42: 10–4. [DOI] [PubMed] [Google Scholar]
- Sepehri G, Parsania S, Hajzadeh MA, Haghpanah T, Sheibani V, Divsalar K, Shekarforoush S, Afarinesh MR (2014) The effects of co-administration of opium and morphine with nicotine during pregnancy on spatial learning and memory of adult male offspring rats. Iran J Basic Med Sci 17: 694–701. [PMC free article] [PubMed] [Google Scholar]
- Sithisarn T, Bada HS, Dai H, Randall DC, Legan SJ (2011) Effects of perinatal cocaine exposure on open field behavior and the response to corticotropin releasing hormone (CRH) in rat offspring. Brain Res 1370: 136–44. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Deak T (2017) A users guide to HPA axis research. Physiol Behav 178: 43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JW, Duan TT, Zhou QX, Ding ZY, Jing L, Cao J, Wang LP, Mao RR, Xu L (2015) Impaired contextual fear extinction and hippocampal synaptic plasticity in adult rats induced by prenatal morphine exposure. Addict Biol 20: 652–62. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM (2016) Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav 83: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS (2009) Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci 123: 913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, Byrnes EM (2017) Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology 113: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Toorie AM, Byrnes EM (2018a) Increased cocaine reward in offspring of females exposed to morphine during adolescence. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Toorie AM, Byrnes EM (2018b) Transgenerational blunting of morphine-induced corticosterone secretion is associated with dysregulated gene expression in male offspring. Brain Res 1679: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013) Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16: 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Wright SJ, Byrnes EM (2016) Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology 103: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Dias I, Tsuro H, Allen D, Emes RD, Moreton J, Wilson R, Ingram RJM, Sinclair KD (2018) Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc Natl Acad Sci U S A 115: 10064–10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise-Kelly L, Siegel S (2001) Self-administration cues as signals: drug self-administration and tolerance. J Exp Psychol Anim Behav Process 27: 125–36. [PubMed] [Google Scholar]
- White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME (2016) Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol 21: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig KA, Bilkey DK (1994) The effects of perirhinal cortical lesions on spatial reference memory in the rat. Behav Brain Res 63: 101–9. [DOI] [PubMed] [Google Scholar]
- Wimmer ME, Briand LA, Fant B, Guercio LA, Arreola AC, Schmidt HD, Sidoli S, Han Y, Garcia BA, Pierce RC (2017) Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry 22: 1653. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ (2004) Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24: 5901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Liu CA, Chung MY, Huang HC, Yeh GC, Wong CS, Lin WW, Yang CH, Tao PL (2006) Alterations of postsynaptic density proteins in the hippocampus of rat offspring from the morphine-addicted mother: Beneficial effect of dextromethorphan. Hippocampus 16: 521–30. [DOI] [PubMed] [Google Scholar]
- Yaw AM, Prosser RA, Jones PC, Garcia BJ, Jacobson DA, Glass JD (2019) Epigenetic effects of paternal cocaine on reward stimulus behavior and accumbens gene expression in mice. Behav Brain Res 367: 68–81. [DOI] [PubMed] [Google Scholar]
- Yaw AM, Woodruff RW, Prosser RA, Glass JD (2018) Paternal Cocaine Disrupts Offspring Circadian Clock Function in a Sex-Dependent Manner in Mice. Neuroscience 379: 257–268. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, Binder EB (2016) Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol Psychiatry 80: 372–80. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Lehrner A (2018) Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry 17: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoburn BC, Chen J, Huang T, Inturrisi CE (1985) Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther 235: 282–6. [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, Blendy JA (2015) Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol 118: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Proudnikov D, Yuferov V, Kreek MJ (2010) Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res 1314: 235–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhu H, Wu HY, Jin LY, Chen B, Pang HY, Ming ZH, Cheng Y, Zhou CL, Guo MX, Huang YT, Yu DQ, Sheng JZ, Huang HF (2018) Diet-Induced Paternal Obesity Impairs Cognitive Function in Offspring by Mediating Epigenetic Modifications in Spermatozoa. Obesity (Silver Spring) 26: 1749–1757. [DOI] [PubMed] [Google Scholar]


