Abstract
Background
The Food and Drug Administration is considering a mandated reduction in the nicotine content of cigarettes. Clinical trials have been limited by non-study cigarette use (noncompliance), which could mask compensation. The goal of the present study was to assess whether compensation occurs when smokers provided with very low nicotine cigarettes cannot access normal nicotine cigarettes.
Methods
In a within-subjects, crossover design, current smokers (n=16) were confined to a hotel for two four-night hotel stays during which they were only able to access the research cigarettes provided. The hotel stays offered normal nicotine cigarettes or very low nicotine content (VLNC) cigarettes, in an unblinded design, available for ‘purchase’ via a study bank.
Results
In the context of complete compliance with the study cigarettes (n=16), there was not a significant increase during the VLNC condition for cigarettes smoked per day, expired carbon monoxide, or N-acetyl-S-(cyanoethyl)-L-cysteine (cyanoethyl-MA, metabolite of acrylonitrile). There was a significant nicotine x time interaction on urine N-acetyl-S-(3-hydroxypropyl)-L-cysteine (hydroxypropyl-MA, metabolite of acrolein), driven by an increase in the VLNC condition during the first 24-hrs. By the end of the VLNC condition, there was no evidence of compensation across any measure of smoking or smoke exposure.
Conclusions
Among current smokers who exclusively used very low nicotine content cigarettes for four days, there was no significant compensatory smoking behavior.
Impact
These data, combined with the larger body of work, suggest that a mandated reduction in nicotine content is unlikely to result in an increase in smoking behavior to obtain more nicotine.
Trial Registration: clinicaltrials.gov Identifier: NCT03311646
Introduction
In 2009, the Family Smoking Prevention and Tobacco Control Act provided the United States (U.S.) Food and Drug Administration (FDA) with the authority to regulate tobacco products1. Because nicotine is the primary addictive constituent in cigarettes, an FDA-mandated reduction in the nicotine content of cigarettes could reduce the prevalence of smoking, thereby dramatically improving public health2. In 2018, the FDA released an Advanced Notice of Proposed Rulemaking formally announcing their interest in a mandated nicotine reduction policy3. Evidence from clinical trials investigating nicotine reduction is encouraging. Several studies show that assignment to very low nicotine content (VLNC) cigarettes reduces the number of cigarettes smoked per day (CPD) and nicotine dependence and increases attempts to quit smoking4–8.
A critical concern is that smokers might respond by increasing their smoke intake—through increases in cigarette consumption and/or changes in puffing behavior—to obtain more nicotine (i.e., compensation). If compensation occurs, corresponding increases in smoke and toxicant exposure could undermine the positive effects of nicotine reduction9. Prior studies have shown that when smokers switch to highly ventilated cigarettes that deliver less nicotine (i.e., light cigarettes), they compensate for the reduction in nicotine yield, largely maintaining their nicotine exposure10–13. However, VLNC cigarettes have a much lower nicotine yield than traditional cigarettes due to large reductions in the actual nicotine content of the tobacco. Clinical trials using VLNC cigarettes report increased puff volume across the first few cigarettes14,15, but over a period of several weeks, participants smoke fewer—not more—CPD and have reduced biomarkers of toxicant exposure4–8,16,17.
One possible reason that smokers in clinical trials do not compensate for the loss in nicotine in VLNC cigarettes is the ease of access to normal nicotine content (NNC) cigarettes. In preceding studies of VLNC cigarettes, most participants were not fully compliant, despite being given explicit instructions to only smoke the study cigarettes provided by the trial, with lower rates of compliance in low nicotine groups than in control groups4,18. Non-compliance during clinical trials may mask compensatory smoking that would occur if NNC cigarettes were unavailable, as would be the case within an FDA-mandated reduction. The goal of the present study was to assess compensatory smoking under the context of exclusive access to VLNC cigarettes using an unblinded, within-subjects design in a naturalistic setting. Participants were confined to a hotel with access to only experimental cigarettes (VLNC or NNC).
Materials and Method
Design
In a within-subjects design, all participants completed two four-night hotel stays during which they were only able to smoke research cigarettes provided to them by study staff. The two hotel stays were separated by a one-week period during which participants smoked as normal. During the first stay, the study research cigarettes had 15.8 mg nicotine/g tobacco (normal nicotine content, NNC week, control condition). During the second stay, the study research cigarettes had 0.4 mg nicotine/g tobacco (very low nicotine content, VLNC week). All participants completed the conditions in a fixed order (i.e., NNC week first) because in a mandated policy, all smokers would transition from NNC to VLNC cigarettes. Participants completed the procedures as part of one of two cohorts in 2018 (Cohort 1 n=6, Cohort 2 n=10). In order to better model smoking conditions in the real world, participants “purchased” all cigarettes from a study cigarette store using a study bank. Requiring participants to purchase cigarettes also reduces the likelihood that participants might smoke at their maximum rate during both study weeks if there are no restrictions on smoking (i.e., a ceiling effect). Study cigarettes were matched to participants’ menthol preference. Written consent was obtained from all participants, and the study was conducted in accordance with the Belmont Report and approved by the Medical University of South Carolina Institutional Review Board.
Unblinded design
Neither participants nor research staff were blind to the nicotine content of the cigarettes, consistent with the idea that smokers in the U.S. would not be blind to a regulatory intervention implemented by the FDA. Participants were told the nicotine content of their assigned cigarette when they arrived at the hotel each week. In the NNC condition, participants were told “All of the cigarettes provided to you and all other participants during this hotel stay have a normal nicotine content. The nicotine content is about the same as what would be available in a typical cigarette purchased on the market today.” In the VLNC condition, participants were told “All of the cigarettes provided to you and all other participants during this hotel stay have a very low nicotine content. The nicotine content is about 97% less than what would be available in a typical cigarette purchased on the market today.”
Participants
Daily smokers were recruited from the Charleston, South Carolina area. Participants were screened on the phone or via a REDCap survey for initial eligibility before being invited to an in-person lab session. Participants provided informed consent and were screened for eligibility. Inclusion criteria included: a) at least 18 years old; b) smoking at least five cigarettes daily for the past month (CO>8; if CO<8 ppm, then urine cotinine concentration > 2000 ng/mL (NicAlert test≥6)); c) smoking ≤30 cigarettes daily for the past month (to ensure participants had the potential to increase their smoking while in the hotel, d) willingness to stay in a local hotel for two four-night stays during the prearranged dates; e) no use of illegal drugs excluding cannabis. Exclusion criteria included: a) interest in quitting smoking in the next two months; b) unwillingness to use research cigarettes as part of the trial; c) use of non-cigarette tobacco products, binge drinking, or self-reported illicit drug use > 9 days in the past month; d) pregnant, trying to become pregnant, or breastfeeding; e) current use of nicotine replacement therapies or other pharmacotherapies for the purpose of stopping smoking; and f) any medical or psychiatric condition that rendered the participant unable to fully participate in the study or which posed a safety issue.
Hotel Phase Procedures
Prior to each hotel stay, participants arrived at the research clinic for intake at 11AM on Monday morning (Day 1) to complete baseline procedures. Participants provided a fresh urine sample to verify no use of illicit drugs (except cannabis) and non-pregnancy. Participants were instructed to not bring any tobacco products with them to the hotel and their belongings were searched for contraband by study staff (i.e., non-study tobacco, alcohol, illicit drugs, unmarked medications, weapons). Participants were then transported to the hotel via shuttle service. Participants arrived at the hotel at 2:30 PM Monday (Day 1) and remained at the hotel until 12:30 PM on Friday (Day 5). Each participant was provided with their own smoking-friendly hotel room. Departing the hotel for any reason resulted in study withdrawal. Participants were required to stay at the hotel and could not have visitors. During the day, participants were allowed to use hotel amenities as they wished (e.g., hotel pool). At night, a curfew was enacted requiring participants to stay in their rooms (9:00PM-7:00AM). Lunch and dinner were provided by a catering company, and breakfast was served by the hotel. Alcohol or illicit drug use was not allowed. Some entertainment was provided to mitigate boredom (e.g., puzzles, coloring, games), but participants were encouraged to bring activities (e.g., books, computers) to keep themselves occupied. Participants were asked to not fraternize with non-study hotel guests. Study staff were on the hotel premises at all times and regularly patrolled the hotel.
Each hotel stay comprised approximately four 24-hour periods. Participants reported four times per day—8AM, 12PM, 4PM, and 8PM for study check-ins. Participants were required to collect all of their urine in 24-hr urine containers (returned at 12PM check in each day), and to collect their first void urine separately (returned at 8AM check in each day). At each check-in participants provided an expired breath CO sample (CoVita Miro+pro Smokerlyzer Monitor) and had the opportunity to purchase cigarettes within the “cigarette store”. At select check-in visits, participants completed self-report surveys via REDCap. Participants collected all of their used cigarette butts (returned at 12PM on Days 2–5, 4 collections/week). Participants who completed all aspects of the study could earn $885.
30 days after participants’ second hotel stay, participants completed a follow-up call. The primary purpose of these calls was to ask participants whether they used any non-study tobacco products while at the hotel. Participants had already received payment and staff emphasized that participant responses would not impact their compensation or participation in any way.
Cigarette Store
Spectrum research cigarettes were provided by the National Institute on Drug Abuse4. Each week, participants were provided with a $72.00 account balance for purchasing cigarettes, which could be purchased one pack at a time at any check in. Each pack “cost” $6.00, representing a price similar to the national average19. Thus, the starting account balance provided enough funds for participants to purchase up to three packs of cigarettes per day, and participants could purchase no more than three packs in 24-hours. Participants were instructed not to share their cigarettes with anyone else. At the end of each week, participants returned any unused cigarettes for $0.30/cigarette, and any remaining account balance was provided to the participants as additional compensation.
Measures
The primary outcomes were: 1) CPD; and 2) expired CO. The primary CPD assessment utilized the returned cigarette butts from each participant, but a secondary analysis utilized the total number of cigarettes purchased by each participant after subtracting those that were returned at the end of the week (divided by 4 to standardize ‘per day’). Secondary outcomes include urinary smoke exposure biomarkers, nicotine exposure biomarkers (urinary total nicotine equivalents [TNEs]), the 15-item Minnesota Nicotine Withdrawal Scale (MNWS), a modified Questionnaire of Smoking Urges20 which assessed craving to smoke the study cigarettes (QSU) (both assessed on Hotel Days 2–5 at 8AM), and a modified version of the Cigarette Evaluation Scale (CES)21, assessed at the first check in after participants received the study cigarettes (Hotel Day 1, 8PM) and on the final day (Hotel Day 5, 8AM). TNEs were calculated as the molar sum of nicotine, cotinine, hydroxycotinine, and nicotine-1’N-oxide. Both QSU factors were analyzed (Factor 1: desire and intention to smoke; Factor 2: relief from negative affect or withdrawal)22. The CES was analyzed using five empirically-derived subscales as previously described21. Urinary metabolites, including free and glucuronide conjugated forms of nicotine metabolites as well as volatile organic compounds, were measured by an isotope dilution high performance chromatography/tandem mass spectrometric method (HPLC- or UPLC-MS/MS)23,24. The main text includes analysis of two urinary smoke exposure biomarkers: acrolein metabolite N-acetyl-S-(3-hydroxypropyl)-L-cysteine (hydroxypropyl-MA), and acrylonitrile metabolite N-acetyl-S-(cyanoethyl)-L-cysteine (cyanoethyl-MA). Data for other urinary biomarkers of smoke exposure are included in eTable1. Urinary analyses combined the 24-hr sample and first void sample to report the total biomarker excreted during each 24-hr period (μmol/day, 4 samples/condition). Urinary analyses were completed at the Centers for Disease Control and Prevention. Adverse events were assessed each morning or when spontaneously reported. Adverse events were monitored at subsequent check-ins until resolved, or participants were encouraged to follow-up with a primary care physician after the study. A licensed medical professional was available for consult regarding adverse events when needed.
Statistical Analyses
An intended sample size of 20 smokers was chosen based on the expectation that it would provide 80% power to detect an effect size of 0.65, corresponding to a 20% increase in smoke and toxicant exposure, which would be consistent with compensatory increases in smoking observed when smokers switch to ventilated cigarettes with reduced nicotine yield (i.e., light cigarettes)10,25,26. Data were analyzed using R (version 3.4.2)27. A linear mixed-model was used. The model included terms for cigarette nicotine content (VLNC and NNC), time, cohort, a random effect for subject, and a nicotine content x time interaction. A significant nicotine content x time interaction indicates that the impact of using VLNC cigarettes changed across the hotel week. If the nicotine content x time interaction was significant at the α=0.05 level, the pairwise VLNC versus NNC contrast was reported separately for each timepoint. Otherwise, the interaction term was dropped from the model and a single main effect for nicotine content was reported. For most outcomes, the time variable is synonymous with day (CPD, urinary biomarkers, MNWS), but in some cases there are more assessments (CO, 16 check-ins/week) or fewer assessments (CES, 2 assessments/week). Urinary biomarkers were log transformed prior to analyses and the effect of nicotine content was summarized by the ratio of geometric means (RGM=VLNC/NNC). Otherwise, the effect of nicotine content was reported as mean difference (MD) in the outcome between conditions (VLNC–NNC). Because the purpose of this research was to examine the potential for an unintended negative consequence of nicotine reduction, we considered it more important to avoid Type II error than Type 1 error. Thus, we did not correct for multiple comparisons (α=0.05).
Results
Participants
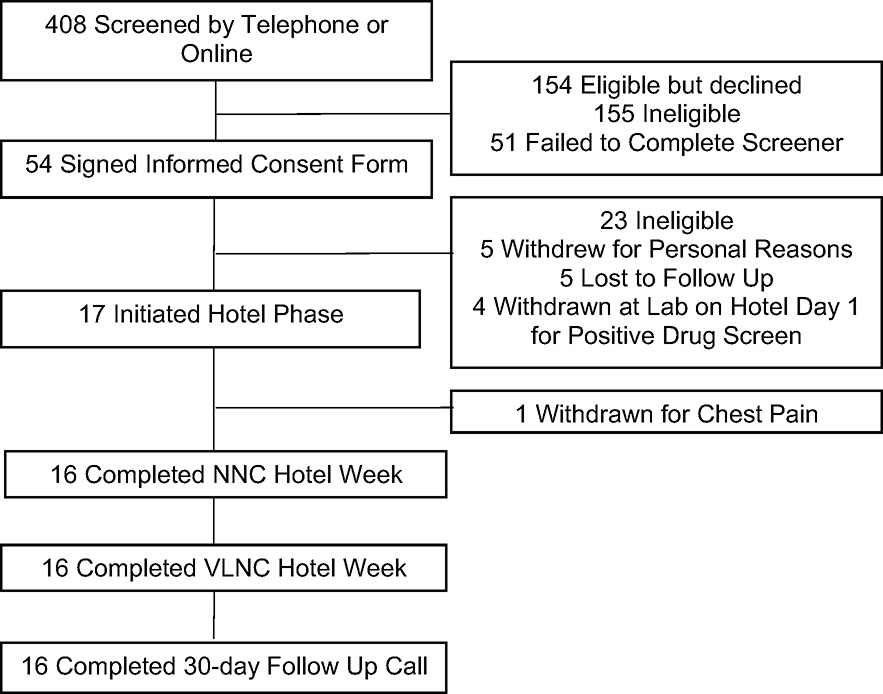
Figure 1 shows a CONSORT diagram of participant flow; Table 1 shows relevant demographic and baseline smoking characteristics of the final sample. One participant experienced chest pain during the NNC week. The participant was transferred to an emergency room for evaluation but was given a diagnosis of indigestion and discharged home. Because the participant left the hotel, they were withdrawn, and their data were excluded from analyses. The final sample includes 16 participants.
Figure 1.
CONSORT diagram. One participant was withdrawn during the NNC week because they left the hotel to be evaluated for chest pain. Analyzed sample included 16 participants.
Table 1.
Demographic and Baseline Smoking Characteristics n=16
| Variable | Level/Unit | |
|---|---|---|
| Age | Years, M (range) | 38.87 (26–63) |
| Gender | Male, n (%) | 8 (50%) |
| Race | White, n (%) | 14 (88%) |
| Black, n (%) | 1 (6%) | |
| Ethnicity | Hispanic, n (%) | 2 (12.5%) |
| Education | Some college or more, n (%) | 8 (50%) |
| Menthol Status | Menthol, n (%) | 7 (44%) |
| CPD | M (range) | 14.75 (7–30) |
| Smoking Initiation | Daily smoking age, M (range) | 16.50 (10–19) |
| TNE | nmol/mL, Geometric M (range) | 63.10 (29.5–169.8) |
| CO | ppm, M (range) | 28.66 (12–63) |
| FTND | M (range) | 5.06 (2–10) |
CPD
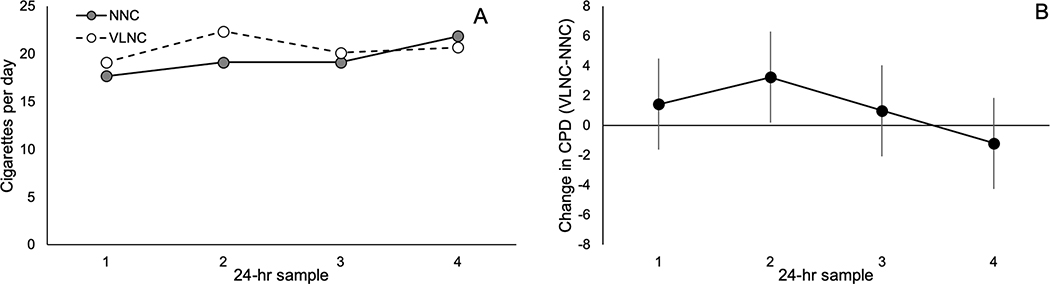
There was no significant effect of nicotine content (MD=1.13, 95% CI=−0.41, 2.66, p=0.15), and no nicotine content x time interaction on cigarettes smoked per day (p=0.23, Figure 2). A secondary analysis compared the NNC and VLNC conditions for average CPD as measured by total cigarettes purchased, and also did not indicate a significant effect of nicotine content (MD=1.3, 95% CI=−2.05, 4.64, p=0.42).
Figure 2.
Cigarettes smoked per day during both conditions of hotel phase
A) Average cigarettes smoked per day during both conditions of the hotel phase. Each day was calculated as the total number of cigarettes butts returned by the participants at the 12PM check in on Days 2–5. B) Mean change in average cigarettes per day during VLNC week (VLNC-NNC, ± 95% confidence interval)
Smoke and Toxicant Exposure
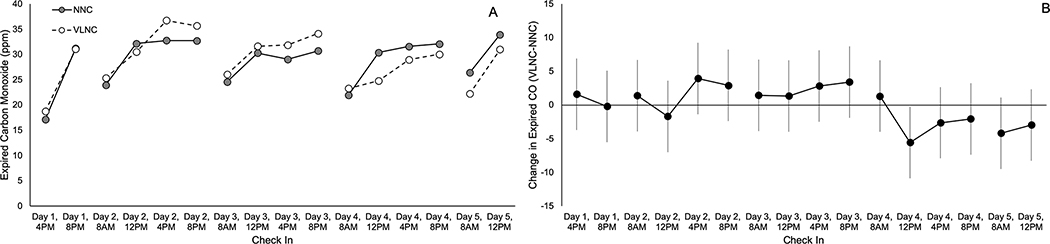
There was no significant effect of nicotine content (MD=0.07, 95% CI=−1.27, 1.4, p=0.92), and no nicotine content x time interaction on expired CO (p=0.27, Figure 3). There was a significant nicotine x time interaction on hydroxypropyl-MA (p=0.02). Follow up tests revealed that hydroxypropyl-MA was significantly increased during the VLNC condition for the first 24-hr sample (RGM=1.53, 95% CI=1.01, 2.33, p=0.046), but not 24-hr samples 2–4 (RGM: 0.88, 95% CI=0.58,1.33, p=0.54; 1.35, 95% CI=0.90, 2.02, p=0.15; 0.68, 95% CI=0.46,1.03, p=0.07). There was no significant effect of cigarette nicotine content or nicotine x time interaction on the 24-hr sample for cyanoethyl-MA (RGM=1.10, 95% CI=0.87, 1.39, p=0.45, interaction p=0.25). Data from other smoke exposure biomarkers are reported in eTable1.
Figure 3.
Expired carbon monoxide for each check in during both conditions of hotel phase
A) Mean of expired carbon monoxide at each check in during both conditions of the hotel phase. B) Mean change in average expired carbon monoxide during the VLNC week (VLNC-NNC, ± 95% confidence interval). Breaks in the data indicate separate hotel days.
Nicotine Exposure and Compliance
The VLNC condition was associated with the expected decrease in nicotine exposure. There was a significant nicotine content x time interaction on urinary TNEs (p<0.001). In follow-up analyses, there was a significant decrease in the VLNC condition compared to the NNC condition for 24-hr samples 2–4 (RGM: 0.34, 95% CI=0.25, 0.46; 0.18, 95% CI=0.13, 0.24; 0.08, 95% CI=0.06, 0.11, ps<0.001).
All of the participants self-reported that they were 100% compliant with the study cigarettes while staying in the hotel. In a previously published paper, cutoffs were established for classifying study participants as completely compliant with VLNC cigarettes using estimates of the 95th percentile for the distribution of urinary TNEs and urinary cotinine for compliant individuals28. For two of the 16 participants, the final 24-hr sample in the VLNC week exceeded both of these cutoffs (TNEs and cotinine). A sensitivity analysis for all outcomes was conducted after excluding these two participants, and the pattern of results is the same. Results for the primary outcomes from this sensitivity analysis are shown in eTable3.
Withdrawal, Craving, and Cigarette Subjective Effects
There was a significant increase in total MNWS scores associated with the VLNC condition (MD=3.19, 95% CI=1.77, 4.60, p<0.001), but the interaction with time was not significant (p=0.95). However, there was no significant effect of nicotine content, or nicotine content x time interaction on the QSU total score (MD=0.02, 95% CI=−0.2, 0.25, p=0.84, interaction p=0.94), QSU Factor 1 (MD=−0.07, 95% CI=−0.36, 0.23, p=0.65, interaction p=0.75), or QSU Factor 2 (MD=0.12, 95% CI=−0.07, 0.30, p=0.22, interaction p=0.75).
For the CES, the VLNC cigarettes were rated significantly lower than the NNC cigarettes on the Satisfaction (MD=−1.8, 95% CI=−2.31, −1.3, p<0.001), Enjoyment of the Sensations in the Respiratory Tract (MD=−1.59, 95% CI=−2.18, −1.01, p<0.001), and Craving Relief subscales (MD=−1.87, 95% CI=−2.66, −1.09, p<0.001). For the Psychological Reward subscale, there was a significant nicotine content x time interaction (p=0.02), and the VLNC cigarettes were rated significantly lower than the NNC cigarettes on Day 1 (MD=−1.31, 95% CI=−1.85, −0.78), but not Day 5. The interaction between nicotine content and time was not significant for any of the other subscales. There was no effect of nicotine content or significant interaction for the Aversion subscale.
Adverse Events
There were no serious adverse events reported by any of the participants. There were seven adverse events reported during the NNC condition, and 18 reported during the VLNC condition. The most commonly reported adverse events were also frequently reported events in other nicotine reduction clinical trials5 (e.g., cough, sore throat). There were no adverse events rated severe by study staff. See eTable3 for a frequency table of all AEs reported.
Discussion
The data from this trial provide critical information about the impact of nicotine reduction on compensation in the context of complete compliance with VLNC cigarettes (i.e., when no other cigarettes are available). Prior studies examining the effects of nicotine reduction have relied on self-determined participant compliance with use of reduced nicotine cigarettes and have shown non-compliance with instructions4–6,8,16,29. The present study, using an unblinded within-subjects design, sequestered smokers in a local smoking-friendly hotel and allowed exclusive access to study cigarettes. There was no significant compensatory smoking as measured by CPD or expired CO. Similarly, the majority of smoke exposure biomarkers did not significantly increase during VLNC smoking. Across most outcomes, treatment effects were in the direction of a slight increase during the VLNC condition (e.g., 1.13 CPD increase in VLNC condition), and in Figures 2-4, it is clear that these small effect sizes are driven by increases in smoking behavior and smoke exposure early in the hotel phase (CPD, 2nd 24-hr collection; CO, Hotel Days 2–3). However, across all measures, by the end of the hotel phase there was no evidence of compensatory smoking across any measure of smoking or toxicant exposure. In sum, these data indicate that any compensatory increases in smoking behavior are temporary, and within 96 hours, there is no evidence of compensatory smoking.
There was a significant increase in withdrawal symptoms associated with the VLNC condition, consistent with some5,30, but not all4,16,31, clinical trials investigating nicotine reduction. The study design would be expected to generate higher levels of withdrawal than observed in clinical trials, and potentially greater withdrawal than would be seen following implementation of a mandated policy because smokers in this study were unable to use non-cigarette nicotine products (e.g., nicotine replacement, e-cigarettes). Importantly, no participants left the hotel during the VLNC week due to withdrawal (or for any reason), and the increase in total withdrawal symptoms was small (3.19-point increase on 0–60 scale), indicating that withdrawal was mild. Participants also rated VLNC cigarettes as significantly less satisfying, an effect which has been reported elsewhere32–34.
A strength of our study is that we captured multiple measures of smoke and toxicant exposure. Additional strengths were the high degree of experimental control, naturalistic setting provided by the hotel, use of a study bank for purchasing cigarettes, and the unblinded design which mimics the real-world conditions following a mandated nicotine reduction policy. However, there were several weaknesses that deserve attention. First, the sample size was smaller than originally planned (n=20) because of the challenges associated with recruiting for an inpatient study. However, the sample size is consistent with other lab-based and within-subject design studies of smoking behavior10,13. Furthermore, the treatment estimates consistently show no effect by Day 4 across multiple measures of smoking behavior and smoke exposure, and the confidence intervals are relatively small, suggesting that a larger sample size is unnecessary (eFigure1). For example, by Day 4, there was a 1.19 cigarette decrease in CPD in the VLNC condition (95% confidence interval: 4.24 cigarette decrease, 1.87 cigarettes increase). Second, while the hotel setting provides a more naturalistic research setting than a residential facility or other in-patient environment, it is still somewhat artificial in comparison to the real-world setting of clinical trials that utilize field trial methodology. Finally, we recognize that our study sample may not generalize to a wider smoking population (e.g., limited racial diversity). In order to participate, participants needed to be able to be sequestered from family, friends, and work for two four-night hotel stays. This may have limited the representativeness of the final sample as many smokers would have other obligations that made inclusion impossible.
In this trial, there was no evidence of compensatory smoking after four days of exclusive VLNC cigarette use. These data are consistent with data from one previous trial showing that smokers who exclusively received VLNC cigarettes over 11 days did not compensate31. There are a variety of differences between this trial and the previous one (between-subjects design, double-blind, no study cigarette bank, setting)31. However, both utilized a high degree of experimental control and failed to find evidence of sustained compensation, which provides reassuring replication across designs. These data are also consistent with those from a variety of clinical trials which have not shown increases in CPD or smoke/toxicant exposure associated with VLNC cigarettes4–7,16,29. Together, this trial and the body of work surrounding nicotine reduction suggest that increased smoke and toxicant exposure as a result of compensatory increases in smoking behavior is unlikely following a reduction in nicotine to very low levels.
Supplementary Material
Acknowledgements
The authors would like to thank the following individuals for their work in data collection: Lisa Coles, Noelle Natale, Amy Boatright, David Braak, Nathan Silvestri, and Mason Deinema. Thank you to Dr. Kevin Gray for serving as the licensed medical provider. The research reported in this manuscript was supported by the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products (R03DA045197 to TTS). Salary support during the preparation of the manuscript was provided by the National Institute on Drug Abuse (K01DA047433 to TTS; K01DA043413 to LRP). The content is solely the responsibility of the authors and does not necessarily represent the official position of the National Institutes of Health, Food and Drug Administration, or Centers for Disease Control and Prevention. Dr. Koopmeiners completed all statistical analyses. Dr. Smith had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Support: The research reported in this manuscript was supported by the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products (R03DA045197 to TTS). Salary support during the preparation of the manuscript was provided by the National Institute on Drug Abuse (K01DA047433 to TTS; K01DA043413 to LRP).
Footnotes
Conflict of Interest: Dr. Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies. Dr. Carpenter has received consultant honoraria from Pfizer. All other authors have no conflicts to report.
References
- 1.US Congress. Family Smoking Prevention and Tobacco Control Act. H.R. 1256 (111th congress)2009. [Google Scholar]
- 2.Apelberg BJ, Feirman SP, Salazar E, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration (FDA). FDA accounces comprehensive regulatory place to shift trajectory of tobacco-related disease, death. 2017.
- 4.Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatsukami DK, Luo X, Jensen JA, et al. Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TT, Koopmeiners JS, Tessier K, et al. Randomized trial of low nicotine cigarettes and transdermal nicotine. Am J Prev Med. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiffman S, Kurland BF, Scholl SM, Mao JM. Nondaily Smokers’ Changes in Cigarette Consumption With Very Low-Nicotine-Content Cigarettes: A Randomized Double-blind Clinical Trial. JAMA Psychiatry. 2018;75(10):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates C The tobacco endgame - a critical review of the policy ideas. The counterfacual 2015; https://www.clivebates.com/the-tobacco-endgame-a-critical-review-of-the-policy-ideas/#3.3 Accessed Decembver 14, 2015.
- 10.Benowitz NL, Jacob P 3rd, Bernert JT, et al. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1376–1383. [DOI] [PubMed] [Google Scholar]
- 11.Kozlowski LT, O’Connor RJ. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002;11 Suppl 1:I40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henningfield JE, Griffiths RR. Effects of ventilated cigarette holders on cigarette smoking by humans. Psychopharmacology (Berl). 1980;68(2):115–119. [DOI] [PubMed] [Google Scholar]
- 13.Sutton SR, Feyerabend C, Cole PV, Russell MA. Adjustment of smokers to dilution of tobacco smoke by ventilated cigarette holders. Clin Pharmacol Ther. 1978;24(4):395–405. [DOI] [PubMed] [Google Scholar]
- 14.Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86(2–3):294–300. [DOI] [PubMed] [Google Scholar]
- 15.Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl). 2012;223(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boatman JA, Vock DM, Koopmeiners JS, Donny EC. Estimating causal effects from a randomized clinical trial when noncompliance is measured with error. Biostatistics. 2018;19(1):103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardone N, Donny EC, Hatsukami DK, et al. Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016;111(12):2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drope J, Schluger N, Cahn Z, et al. The Tobacco atlas. Atlanta: American Cancer Society and Vital Strategies.; 2018. [Google Scholar]
- 20.Toll BA, McKee SA, Krishnan-Sarin S, O’Malley S S. Revisiting the factor structure of the questionnaire on smoking urges. Psychol Assess. 2004;16(4):391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 22.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–1476. [DOI] [PubMed] [Google Scholar]
- 23.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta. 2012;750:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei B, Feng J, Rehmani IJ, et al. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell MA, Sutton SR, Iyer R, Feyerabend C, Vesey CJ. Long-term switching to low-tar low-nicotine cigarettes. Br J Addict. 1982;77(2):145–158. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt AR, Kirkham AJ, Mariner DC, Baldry AG, Cumming G. Long-term effects of switching to cigarettes with lower tar and nicotine yields. Psychopharmacology (Berl). 1989;99(1):80–86. [DOI] [PubMed] [Google Scholar]
- 27.Team RC. R: A language and environment for statistical computing. Vienna, Austria: 2018. [Google Scholar]
- 28.Denlinger RL, Smith TT, Murphy SE, et al. Nicotine and Anatabine Exposure from Very Low Nicotine Content Cigarettes. Tob Regul Sci. 2016;2(2):186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatsukami DK, Hertsgaard LA, Vogel RI, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P 3rd. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–2485. [DOI] [PubMed] [Google Scholar]
- 31.Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. [DOI] [PubMed] [Google Scholar]
- 32.Higgins ST, Heil SH, Sigmon SC, et al. Addiction Potential of Cigarettes With Reduced Nicotine Content in Populations With Psychiatric Disorders and Other Vulnerabilities to Tobacco Addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arger CA, Heil SH, Sigmon SC, et al. Preliminary validity of the modified Cigarette Evaluation Questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp Clin Psychopharmacol. 2017;25(6):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.