Abstract
Psychological distress is a public health issue as it contributes to the development of human diseases including neuropathologies. Parkinsońs disease (PD), a chronic, progressive neurodegenerative disorder, is caused by multiple factors like aging, mitochondrial dysfunction and/or stressors. In PD, a substantial loss of substantia nigra (SN) neurons leads to rigid tremors, bradykinesia and chronic fatigue. Several studies have reported that the hypothalamic-pituitary-adrenal (HPA) axis is altered in PD patients, leading to an increase level of cortisol which contributes to neurodegeneration and oxidative stress. We hypothesized that chronic psychological distress induces PD-like symptoms and promotes neurodegeneration in WT rats and exacerbates PD pathology PINK1 knockout (KO) rats, a well validated animal model of PD. We measured the bioenergetics profile (oxidative phosphorylation and glycolysis) in the brain by employing an XF24e Seahorse Extracellular Flux analysis in young rats subjected to predator-induced psychological distress. In addition, we analyzed anxiety-like behavior, motor function, expression of antioxidant enzymes, mitochondrial content and neurotrophic factors (BDNF) in the brain. Overall, we observed that psychological distress diminished up to 50 % of mitochondrial respiration and glycolysis in the prefrontal cortex (PFC) derived from both WT and PINK1-KO rats. Mechanistically, the level of antioxidant proteins, mitochondrial content, and BDNF were significantly altered. Finally, psychological distress robustly induced anxiety and Parkinsonian symptoms in WT rats and accelerated certain symptoms of PD in PINK1-KO rats. For the first time, our collective data suggest that psychological distress can phenocopy aspects of PD neuropathology, disrupt brain energy production as well as induce ataxia-like behavior.
Keywords: PARK7, SOD, CAT, Corticosterone, TOM20, BDNF
Introduction
Parkinson’s disease (PD) is a multifactorial, progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra (SN) and the accumulation of protein aggregates named Lewy bodies. Beyond the dopaminergic system, the cholinergic, catecholaminergic and serotonergic neurons are also affected leading to locomotor dysfunction, hypokinesia, muscular rigidity, bradykinesia, akinesia, loss of gait and balance [1, 2]. Besides damaging the midbrain, there is growing evidence that suggests that PD also affects the cerebral cortex. Indeed, significant atrophy in the cortex, due to loss of dendrites and accumulation of Lewy bodies, has been observed in individuals with late stage PD [3]. In addition, a substantial loss of neurons of the prefrontal cortex (PFC) and associated motor areas can impair cognitive processes [4] and motor function in PD [5]. PD is a multifactorial disease caused by aging (> 65 years), exposure to intoxicants/toxins, suboptimal diet, mitochondrial dysfunction and protein aggregation. However, while most PD cases are sporadic (unknown cause), approximately 10 % of PD cases are caused by mutations in over more than 18 different genes, including mutations in phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) [6, 7]. Mutations in the PINK1 gene is associated with autosomal recessive, early onset forms of PD. PINK1 is a serine/threonine (ser/thr) kinase localized in mitochondrial and cytosolic compartments involved in the regulation of mitochondrial structure, function and turnover [8, 9]. This protein is critical for neuronal homeostasis, as loss of endogenous PINK1 is associated with enhanced Drp1-dependent mitochondrial fission, increased oxidative stress, altered levels of antioxidant enzymes including catalase (CAT) and superoxide dismutase 1 and 2 (SOD1 and SOD2), overt macroautophagy, lysosomal expansion, reduced neuronal development of cortical neurons, progressive loss of dendrites, and onset of symptoms in PD [8, 10]. Several genetic murine models of PD (as PINK1, Parkin or DJ-1 knockouts rats and mice), that are widely used for the study of neurodegeneration and PD-associated symptoms, develop motor and non-motor symptoms of PD, loss of dopaminergic neurons and mitochondrial dysfunction, starting at early ages [11–13]. Indeed, at 2-months old, PINK1-KO rats manifest progressive vocalization and oromotor deficits [14], impairment of motor function [15], increase of the neurochemical metabolite glutamine (which is cytotoxic in high concentrations) in striatum and larger sizes of ventricles in males [16]. However, little/insufficient data has been collected regarding the dopaminergic system in the brain or of brain mitochondria state at this young age in PINK1-KO rats. For example, lack of Parkin, an ubiquitin-protein ligase that is phosphorylated by PINK1, induces alteration in dopaminergic neurotransmission at 2-months old PINK1-KO rats [17]. At 3 months of age, PINK1-KO rats exhibit bioenergetics and proteomic alterations in isolated striatal synaptic mitochondria. [18]. Moreover, 3-months old PINK1-KO rats show changes in the level of antioxidant in the brain including a decrease in the level of glutathione and SOD and an increase in glial fibrillary activity protein, and a disruption in oxidative phosphorylation with a concomitant decrease cytochrome C oxidase, NADH and ATP level, in various brain regions [19]. However, several studies did not observed any motor dysfunction (tapered beam balance and rotarod test) or cognitive decline at this age [19]. At the age of 4 months, PINK1-KO rats develop progressive PD motor and non-motor symptoms caused by progressive neurodegeneration especially in SN and striatum and oxidative brain damage [12, 14, 16, 20, 21]. Also, depletion of PINK1 causes diminished basal oxygen consumption rates (OCRs), coupling efficiency and ATP production in striatal slices from old mice [22].
It is worth noting that over more than 50 % of PD cases are comorbid with major clinical depression [23] and characterized by a significant decrease in the serum level of neurotrophic factors including brain-derived neurotrophic factor (BDNF) [24, 25]. Therefore, the observation that PD can be comorbid with different psychiatric conditions (e.g. major clinical depression) raises the possibility that major adverse life events and highly stressful experiences can trigger the development and manifestation of clinical symptoms and contribute to neurodegeneration in PD [26, 27]. We hypothesized that stressful events in life can contribute to the pathology of PD or exacerbate it. The premise for formulating this hypothesis is that previous literature has shown that exposure to stress induces neuroinflammation and neuronal damage in different brain regions in WT animals, and intensifies motor and no-motor symptoms of PD and the degeneration of dopaminergic neurons in non-genetic models [26, 28–31].
To date, psychological stress (psychological distress), as induced by single traumatic events or chronic stress, can contribute to an increase in oxidative stress in the brain and promote neurodegeneration [32]. While a temporary increase in cortisol may be beneficial, the exposure to chronic stress can elicit a detrimental increase in the level of cortisol and contribute to chronic inflammation in the whole body including the immune, brain and peripheral tissues. Indeed, psychological distress can elicit oxidative stress and causes myriad of pathological effects in cells including: promotes mitochondrial dysfunction as a consequence of reactive oxygen species (ROS) generated via electron slippage between complex I and II, induces the misfolding proteins, deregulates the transcriptional machinery, reduces level of antioxidant enzymes (CAT, SOD1 and SOD2), and alters the expression and activity of the oxidation sensor (DJ-1). Collectively, these biochemical changes adversely affect the nervous and immune systems [33–35]. The chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis includes the release of corticosterone/cortisol and can adversely affect the neuronal circuitry by inducing neurodegeneration (loss of dendrites). Indeed, several studies have reported that the HPA axis is altered and unbalanced in PD patients and lead to a significant increase in the level of cortisol [36, 37]. Given that a detrimental increase in cortisol can contribute to neurodegeneration and oxidative stress, it is conceivable that chronic psychological stress can exacerbate PD pathology [30, 38].
In this study, we surmised that psychological distress can significantly alter energy production in the brain and elicit oxidative stress in PINK1 knockout (KO) rats, a bone fide in vivo rodent model of PD that faithfully recapitulates mitochondrial dysfunction, progressive loss of midbrain dopamine neurons, and the onset of motor symptoms of PD [12].
Our data suggest that psychological distress equally affects brain energy production in young WT and PINK1-KO rats, pathological effects that coincide with alterations in plasma level of corticosterone, mitochondrial content, and of antioxidants such as DJ-1 and SOD and BDNF level in the SN and the PFC. Overall, our data show that psychological distress can promote alterations in brain bioenergetics, anxiety and elicit motor deficits (ataxia-like behavior) in a similar manner as in PD in the absence of neurodegeneration of midbrain dopamine neurons.
Materials and Methods
Study design
To investigate how psychological distress and/or a lack of endogenous PINK1 affects the energy production in the brain, the bioenergetics profile (oxidative phosphorylation and glycolysis) was assessed in the PFC and the SN by employing an XF24e Seahorse Extracellular Flux Analyzer in young, wild type (WT) and in Parkinsonian rats (PINK1-KO) subjected to predator-induced psychological distress. Also, to correlate energy production to alterations in mitochondria turnover and the level of key molecular players implicated in oxidative stress and neurotrophic signaling, we employed Western blot (WB) to analyze the protein level of DJ-1, mitochondrial content (TOM20), brain-derived neurotrophic factor (BDNF), and antioxidants enzymes like cytosolic superoxide dismutase (SOD1), mitochondrial superoxide dismutase (SOD2) and catalase (CAT).
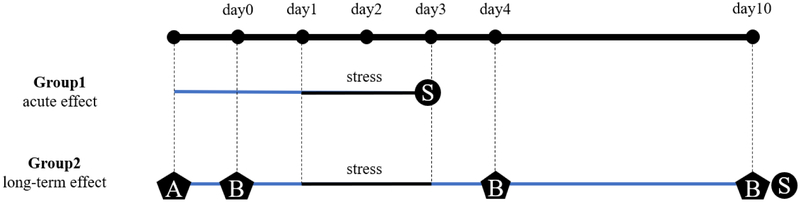
The overall experimental design of this study is shown in Fig. 1. Briefly, two cohorts of animals were used to analyze the brain energy metabolism under acute effect (short term) of emotional distress induced by exposure to cat odor: 20 males (10 WT and 10 PINK1-KO rats, 5 stressed and 5 unstressed rats for each genotype) and 20 females (10 WT and 10 PINK1-KO rats, 5 stressed and 5 unstressed rats for each genotype). The rats were stressed or left untreated for three days and sacrificed on the third day via euthanasia to harvest brain tissue (Fig. 1).
Fig 1.
Experimental design. Two animal cohorts were used: cohort 1 were females (10 WT, 10 PINK1-KO) and males (10 WT, 10 PINK1-KO), distributed in four conditions (WT S-, WT S+, PINK1-KO S-, PINK1-KO S+) of 5 animals per group. Cohort 2 were males (8 WT, 10 PINK1-KO). Cohort 1 (for the study of acute effect of stress) was stressed for three days and the tissue collection was made on the third day. Cohort 2 (for the study of long-term effect of stress) was trained and tested for behavior and motor function, then stressed for three days (day1-day3), tested again for behavior and motor function in day 4, rest for 7 days, and finally tested for the third time for behavior and motor function and tissue collected in day 10. A: Training for rotarod test and beam balance test; B: Testing for rotarod test, grip strength test, beam balance test and cat-odor avoidance test; S: Sacrifice, tissue collection, Seahorse XF24 metabolic assay.
To analyze the long-term effect of emotional distress on brain energy metabolism, a second cohort of 18 male rats was used: 8 WT and 10 PINK1-KO rats, stressed and unstressed rats for each genotype. The experiments were conducted over a period of ten days. Prior to exposure to psychological stress, the animals were trained and tested for motor function by using the rotarod test, grip strength test, beam balance test and the cat-odor avoidance test. The animals were stressed or left untreated for three days; the day following exposure to psychological stress (day 4) and seven days following exposure to cat odor (day 10), the rats were tested again for motor function and anxiety behavior. Between day 4 and day 10, the animals rested in their home cages without undergoing additional experimentation or treatments. On day 10, after being exposed to a battery of behavioral tests, the rats were sacrificed, and the tissues were collected and processed for analyzing the bioenergetics profiles in midbrains/cortical slices and for downstream analysis of protein levels of the aforementioned markers as described in Figure 1.
The behavioral tests were conducted after various training trails in both WT and PINK1-KO rats and a specific order was established in a manner that causes less stress as further described. In the day 0, day 4 and day 10 (around 7 am), the animal was allowed to rest and acclimate in the experimental room for 40 min followed by analyzing for the muscular force of the forelimbs and hindlimbs by employing a grip strength test. The decision to first conduct the grip strength analysis is based on a series of training trials that showed that this behavioral test was the least stressful of the three (e.g. more compliance by the animal to perform this test). After approximately 30 min of rest, the animal was tested in the rotarod apparatus to assess motor coordination and allowed the rat to rest another 30 min before the performing the cat-odor avoidance test. After the 20 min of free exploration in the light-dark box, the animal rested for another 30 min in the home cage, and was finally tested for the equilibrium and fine motor coordination in the beam balance apparatus. Is important to mention that all the animals were treated the same manner per the order of the behavioral tests. The behavioral tests were conducted in the non-active period (light cycle) from 7 am to 11 am for all the experimental groups.
Animals
After receiving approval from the Institutional Animal Care and Use Committees (IACUC) at University of Nevada in Reno (protocol #0572), males and females Long-Evans rats (WT and PINK1-KO rats, Horizon Discovery), of 9 to 11 weeks age and weighting from 250 – 350 g were employed for this study and were humanly treated per Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines [39]. The animals were maintained under controlled temperature 25–26 °C, 12/12 h light/dark cycle, with food and water ad libitum and individually caged three days before the behavioral assays.
Stress and behavior: cat-odor avoidance test
To induce psychological distress, rats were exposed to cat urine for three consecutive days for one hour (between 9 and 11 am) as previously published with minor modifications [34]. In brief, prior to stress exposure, rats were habituated for 20 min in the test cage, returned for other 20 min in the housing cage, then transferred into the test cage to stress the rats with cat urine impregnated on a piece of cloth. It is worth noting that cat urine was obtained from the bladder by performing sterile punctures from three different cats, pooled and 0.5 mL was dispensed in cotton fabric and stored at −20 °C until used [34]. It is worth nothing that the major urinary protein of the cat is a non-volatile component in urine that is predominantly responsible for inducing psychological distress in rats [40].
To quantify the anxiety induced by the psychological stress, the animals′ behavior in the experimental cage was recorded for 20 min before being exposed to the stressor (day 0) and following several stress episodes (days 4 and 10). The videos were analyzed for the average time that the animal spent hiding, exploring, and approaching, either smelling or playing with the piece of cloth (see Fig 1), by employing JWatcher™ software (version 0.9).
Motor function tests
The rotarod test is an experimental procedure that measures the capacity of the animal to maintain the equilibrium on a rod in constant or progressive velocity rotation. This method is often used to assess for motor deficits in response to pharmacological treatments [41, 42]. In the present study, an accelerating rotarod apparatus (Panlab Harvard Apparatus, 76–0772) was employed. On the first day, the animal was trained and placed on a rotating rod (20 rpm) for 2 min. After 10 min of resting in the home cage, the animal was tested again at gradually increasing speeds from 4 to 40 rpm. On the second day, the same procedure was made and the latency time to fall off was recorded. The same assay (without training) was repeated a day after the exposure to stress (day 4), and after 7 days following stress (day 10), see Fig. 1.
The grip strength test is a behavioral test used to measure the muscle strength in rodents [43, 44]. In the present study, muscle strength was measured three times in the hind limbs and front limbs. The grip strength score was calculated as the maximum strength (g) divided by the animal weight (g). The highest strength value for each rat was recorded and considered as the maximum muscular force. This test was conducted before stress exposure, a day after the stress exposure (day 4), and after 7 days following stress (day 10), as seen in Fig. 1.
The beam balance test is another behavioral test used to measure the equilibrium and fine motor coordination by allowing rats to cross through beams of sequentially decreasing widths [42]. In the present study, a beam balance apparatus (Panlab Harvard Apparatus) composed of a goal black box and two ledge tapered beams of 2 cm and 6 cm were mounted on two tripods at an angle of 15° from the floor, with the highest point (90 cm) set at the level of the goal box. It is worth noting that a safety ledge was mounted below the beam and a light source was positioned at the other end of the beam in order to motivate the rat to climb the beam up to the goal box. Animals were trained once on both beams for decreasing widths, followed by three trials per beam with 1–2 min of rest between trials upon reaching the goal box. The cross time of each beam (s) and the number of foot slips were assessed by employing the JWatcher™ software (version 0.9). Finally, to study the long-term effect of distress on motor coordination, the beam balance test was conducted before stress exposure (day 0), a day after the stress exposure (day 4) and after 7 days following stress (day 10).
Brain samples
Rats were anesthetized with 5 % isoflurane via inhalation and transcardially perfused with 100 mL (10 mL/min) of artificial cerebrospinal fluid (aCSF) solution (120 mM NaCl, 3.5 mM KCl, 1.3 mM CaCl2, 1 mM MgCl2, 0.4 mM KH2PO4, 5 mM HEPES, 10 mM Glucose, pH 7.4) at 37 °C. The brains were microdissected and immersed in oxygenated aCSF at 37 °C. The right hemispheres were coronally sliced by employing a vibratome (VT 1200S, Leica Microsystems, Germany) at 150 μm. The PFC and the SN were localized by using the stereotaxic atlas of Paxinos and Watson [45]. The brain slices were maintained in the same oxygenated aCSF at 37 °C while mounting in the islet capture microplate (XF24 islet capture microplate, Seahorse Bioscience) as previously described [46].
Agilent Seahorse XF24 metabolic assays
To measure the effects of psychological distress on brain energy production, a XF24® Extracellular Flux Analyzer was employed to measure the real-time oxygen consumption rates (OCRs) and extracellular acidification rates (ECARs) in the brain slices as proxies of mitochondrial function and glycolysis respectively. In the present study, we analyzed the bioenergetics profiles from five brain slices derived from each animal that were placed into the wells of the islet capture microplate and OCRs and ECARs were measured as previously published, with minor adjustments [46]. In brief, biopsy punches of 1.2 mm diameter from each brain region were carefully isolated and mounted on the bottom of each well from the plate. Mesh capture screens, previously submerged in XF Base Medium, were carefully mounted immediately over the tissue and 700 μL of Agilent Seahorse XF Base Medium (supplemented with 2 mM L-glutamine, 1 mM Na pyruvate, 10 mM glucose and 4 mg/ml BSA, pH 7.4) was added into each well. Three dilutions of pharmacological reagents/toxins were prepared in XF Medium as follows: 25 μM oligomycin from Streptomyces diastatochromogenes (1404-19-9, Sigma Aldrich), 15 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 370-86-86, Sigma Aldrich) and a mixture of 20 μM antimycin-A from Streptomyces sp. (1397– 94-0, Sigma Aldrich) with 20 μM rotenone (R8875, Sigma Aldrich). 75 μL of each drug were injected successively in a final volume of 925 μL in each well. Four wells, one per row, were employed as blank wells for each microplate. The following parameters of the mitochondrial stress assay was set up as previously published [46] but with the following modifications. Each measuring cycle was programmed to consist 3 min of mixing, 3 min of waiting and OCRs and ECARs were measured for 2 min. In addition, five cycles were employed to analyze the baseline OCRs, seven cycles were used to evaluate ATP-dependent OCRs (oligomycin), five measurements were used to measure maximal OCRs (FCCP), and six cycles to evaluate mitochondrial-dependent OCRs.
The following bioenergetics parameters were evaluated in brain slices: the non-mitochondrial OCRs (calculated as the minimum OCRs after injection of rotenone and antimycin-A), basal respiration (calculated as the last OCRs measurement prior to the first injection), the maximal respiration (considered as the maximum rate measurement following FCCP injection), the proton (H+) leak (calculated as the minimum OCRs measurement following oligomycin injection), the ATP production (calculated as the last rate measurement before oligomycin injection), and the spare respiratory capacity (assessed by calculating maximal respiration minus the basal OCRs). In addition, the following parameters of ECARs were analyzed: the basal glycolysis (calculated as the last rate measured before oligomycin injection), and the glycolytic capacity (calculated as the difference between maximum ECARs following FCCP injection and the last rate measured before oligomycin injection).
Western Blot assays
Brain tissue was homogenized in cold RIPA buffer (150 mM NaCl, 1 % Triton X-100, 0.5 % Sodium Deoxycholate, 0.1 % SDS, 50 mM Tris Base, 2 mM PMSF, pH 8) and tissue debris was removed by centrifuging the lysate at 19,000 g for 20 min at 4 °C. Approximately 20 μg of total protein of the resulting supernatant was electrophoresed on a 12 % SDS-PAGE gels at 100 V by using a Biorad minigel system. The proteins were subsequently transferred from the agarose gel to PVDF membranes in a semi-dry condition by using a semi-dry transfer apparatus (Biorad). The PDVF membranes were then blocked in 4 % milk diluted in 1X TBST (15 mM Tris HCl, 4.6 mM Tris Base, 6.6 M NaCl, 2 % Tween-20, pH 7.6), washed extensively in 1X TBST, and incubated for 12 h with the primary antibody: DJ-1 (Abcam, Rabbit anti-PARK7/DJ1 antibody [EP2815Y], ab76008, 1:5000, 23 kDa), SOD1 (Abcam, Rabbit anti-Superoxide Dismutase 1 antibody, ab16831, 1:2000, 17 kDa), SOD2 (Abcam, Rabbit anti-SOD2/MnSOD antibody, ab13533, 1:5000, 25 kDa), CAT (Abcam, Rabbit anti-Catalase antibody, ab16731, 1:2000, 55 kDa), BDNF (Abcam, Rabbit anti-BDNF antibody [EPR1292], ab108319, 1:500, 14 and 28 kDa), TOM20 (Santa Cruz Biotechnology, Rabbit anti-Tom20 antibody (FL-145), sc11415, 1:500, 17 kDa). As internal control was used β-tubulin (Abcam, Mouse Anti-beta Tubulin antibody [1E1-E8-H4], ab131205, 1:5000, 50 kDa). Following incubation with primary antibodies, the PDVF membranes were incubated with the respective horse radish peroxidase (HRP)-conjugated secondary antibody (Invitrogen, Goat anti-Rabbit IgG, 65–6120, and Invitrogen Goat anti-Mouse IgG, 62–6520) for 2 h at room temperature (25 °C), and finally analyzed by chemiluminescence detection (ChemiDoc™ MP Imaging System (170–8280), Bio-Rad).
Densitometric analysis of immunoreactive bands for technical replicates of each protein marker of interest was performed by using NIH ImageJ 1.50i software (Bethesda, MD) as previously published [8]. The integrated density for each immunoreactive band of interest was normalized to β-tubulin and corrected for background by using rolling ball radius of 25 pixels.
Immunofluorescence assay
Indirect immunolabeling of brain slices was performed as previously published [8] with the following minor modifications. In brief, the left cerebral hemispheres from psychologically distressed and unstressed rats were fixed with 4 % paraformaldehyde (PFA, w/v), dehydrated by using 30 % sucrose solution, embedded in a mixture of OCT containing with 30 % sucrose solution, frozen at −20 °C and then sliced by employing a cryostat (Leica CM1510-S). The 12 μm brain slices of the SN (stereotaxic coordinates [45] were: 2.88 mm to 4.32 mm from interaural line and −6.12 mm to −4.64 mm from bregma) were collected on pre-coated slides (Superfrost Plus Gold Microscope Slides, Fisher Scientific) and stored at −20 °C until used. After blocking with 5 % bovine serum albumin (BSA) for 30 min, the brain tissue was incubated for 2 h with tyrosine hydroxylase (TH) antibody (Thermo Fisher Scientific P21962, 1:200). Next, the brain slices were washed extensively in 1X PBST and then incubated with the secondary antibody (Alexa Fluor 647, goat anti-rabbit IgG, 1:1000). Finally, nuclei were visualized by exposing the brain slices to nucleic acid stain 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) mixed in a solution of 70 % glycerol at a final concentration of 1.25μg/mL to visualize nuclei. The brain slices were then coverslipped and imaged by using an EVOS-FL Cell Imaging System (Life Technologies) with a 4X objective. The captured images were analyzed using ImageJ 1.50i software. In order to assess for neurodegeneration of SN dopamine neurons in response to an absence of endogenous PINK1 or exposure to psychological distress, the integrated TH fluorescence intensity in the SN was quantified by using NIH ImageJ (Bethesda, MD, version 1.50i) for each brain slice. The integrated density was then normalized to the total number of cells, as identified by counting the number of nuclei that stained with DAPI, for each brain slice analyzed for the SN. The ratio of the integrated density of TH fluorescence to the number of DAPI- stained nuclei is an accurate and well accepted metric to assess for neurodegeneration of midbrain dopamine neurons as previously published [47–49]. Also, it is important to note that the complete SN was analyzed including the SN pars compacta, SN pars reticulata and SN pars lateralis, a well-validated method to assess for neurodegeneration of midbrain dopamine neurons in PD models as previously published [12, 50–52].
ELISA assay
The levels of corticosterone were measured in blood plasma obtained from rats in the day of sacrifice via intracardial puncture and collected in vacutainer tubes containing 7.2 mg of potassium-EDTA (K2EDTA). In brief, blood was centrifuged at 1,000 × g for 10 min at room temperature and the plasma was stored at −80 °C until needed. The level of corticosterone in plasma samples was measured by employing the Cayman Corticosterone ELISA Kit (Cayman chemical, Cat No. 501320; Ann Arbor, MI, USA) per manufacturer’s instructions. The amount of corticosterone was measured in plasma by analyzing the optical density at 412 nm by employing a microplate reader (SpectraMax M3, Molecular Devices). It is worth noting that the assay range was 8.2 – 5000 pg/mL and sensitivity of 30 pg/mL.
Statistical analyses
Unless otherwise indicated, results are expressed as mean ± S.E.M. from at least three independent experiments. Behavioral data in the same rats prior and after exposure to psychological distress were analyzed by employing a Student’s t test (two-tailed) for pairwise comparisons. Multiple group comparisons were done by performing two-way ANOVA followed by Bonferroni-corrected Tukey’s test by using the GraphPad Prism software (version 6.0). p values less than 0.05 were considered statistically significant.
Results
Given that a detrimental increase in glucocorticoids and cortisol can contribute to neurodegeneration and oxidative stress, we surmised that chronic psychological stress can exacerbate clinical symptoms and alter brain energy metabolism in the SN and the PFC, two brain regions that degenerate in PD. Psychological distress was induced by exposing WT and PINK1-KO rats to cat urine as previously published [34, 40]. To certify that our methodology can induce psychological distress, plasma corticosterone level was measured (Fig. 2D) following exposure of rats to cat urine, a stressor that causes an imbalance in the HPA axis [36, 38, 53]. We also evaluated the effect of distress on motor performance, anxiety behavior, number of dopaminergic neurons in the SN, energy production in slices of the PFC and the SN, as well as the protein level of several antioxidant enzymes, neurotrophic factor, and mitochondria by WB as further described below.
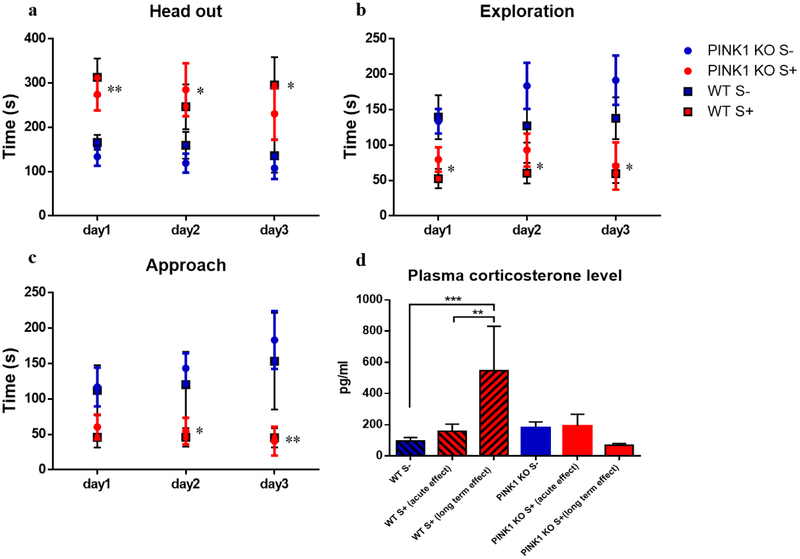
Fig 2.
Psychological distress induces anxiety-like behavior in WT and PINK1-KO rats. Corticosterone response is uncoupled in PINK1-KO rats. (a) time spent in head out position (alert behavior), for day1: **p ≤ 0.01, WT S+ compared with WT S- and PINK1-KO S+ compared with PINK1-KO S-; for day2: *p ≤ 0.05, PINK1-KO S+ compared with PINK1-KO S-; for day3: *p ≤ 0.05, WT S+ compared with WT S-; (b) time of exploration in the open part of the light-dark box (exploration), *p ≤ 0.05, WT S+ compared with WT S- and PINK1-KO S+ compared with PINK1-KO S-; and (c) time of examination of the piece of cotton impregnated (or not) with cat urine (approach), *p ≤ 0.05, **p ≤ 0.01, PINK1-KO S+ compared with PINK1-KO S-. n = 10 rats (5 females and 5 males) per group. Data are expressed as mean ± SEM, unpaired t test; (d) plasma corticosterone level in male and female rats, n = 4–10 per group. Data are expressed as mean ± SEM. **p ≤ 0.01, ***p ≤ 0.001.
Psychological distress can induce Parkinsonian-like motor symptoms and anxiety-like behavior in vivo
Consistent with previous published studies in Sprague-Dawley rats [34], psychological distress induced by cat urine leads to anxiety-like behavior in Long Evans WT rats. Indeed, stressed WT and PINK1-KO rats significantly explored less (exploration time), spent more time with the head out in a defensive position (head out time) and they approached the cloth impregnated with cat urine significantly less (approach) compared to unstressed WT rats (Fig. 2a, Fig. 2b and Fig. 2c). We observed no significant sex-related differences in behavioral test performance in response to stress exposure (day 1- day 3). The anxiety-like behavior lasted at least one week after exposure to stress, as rats spent almost twice the time in a defensive position (Fig. 3a), implying that this stress model faithfully reproduces long-term psychological distress as previously published [34]. However, the lack of endogenous PINK1 did not change the performance in the cat-odor avoidance test (Fig. 2, 3a). This data is contrary to a previous study that showed that PINK1-KO rats exhibited anxious-like behavior in open-field test at 4 months of age [12].
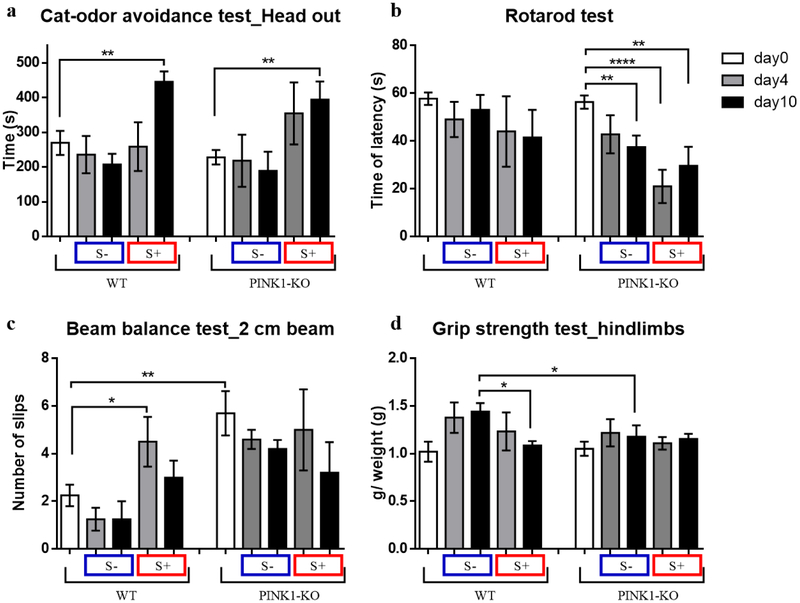
Fig 3.
Psychological distress induces motor deficit and anxiety-like behavior. (a) time spent in alert behavior (head out time), (b) time of latency to fall in accelerating rotarod apparatus, (c) number of hindlimbs slips in the 2 cm beam balance, (d) grip strength of hindlimbs, in males before stress exposure (day 0), after stress exposure (day 4) and after 7 days of rest (day 10), n = 4–5 male rats per group. Data are mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, unpaired t-test.
Interestingly, in this Parkinsonian model, unstressed PINK1-KO showed motor deficits starting at an age of 2 and a half months (Fig. 3c). Indeed, we observed a significant reduction in fine motor coordination and balance (Fig. 3c), in a similar manner as previously reported for 7-month old PINK1-KO rats (Supplementary Fig. 1). In addition, unstressed PINK1-KO rats exhibited a significant reduction (p ≤ 0.01) on the rotarod test performance in day 10 and this physical fatigue was intensified by the exposure to cat urine immediately (day 4) and 7 days following stress exposure (day 10), suggesting that stress exacerbates motor symptoms of PD. On the other hand, WT rats showed the same rotarod performance in the absence or following exposure to stress (Fig. 3b). However, psychological distress reduced the WT rats’ strength gain (p ≤ 0.05) on the 10th day (Fig. 3d), and reduced fine motor coordination and equilibrium as evident by a significant increase in the number of foot slips on the 2 cm beam (Fig. 3c).
Moreover, exposure to cat urine elevated plasma corticosterone level in WT rats at 7 days but not at 4 days following stress exposure (Fig. 2d). Unexpectedly, unstressed and stressed PINK1-KO rats showed no significant changes in plasma corticosterone concentration (Fig. 2d), presumably because they have an uncoupled HPA axis as shown by previous studies [21, 54]. Also, we observed no significant sex differences in plasma corticosterone level in both WT and PINK1-KO rats.
Energy metabolism (OCRs and ECARs) in the brain is altered by psychological distress
By using an XF24e Extracellular Flux Analyzer to measure neuronal metabolism ex vivo, we observed that exposing both WT and PINK1-KO rats to three episodes of cat urine-mediated psychological distress is sufficient to significantly reduce energy metabolism (decrease mitochondrial respiration and glycolysis) in brain slices of the PFC and SN (Fig. 4 and Fig. 5).
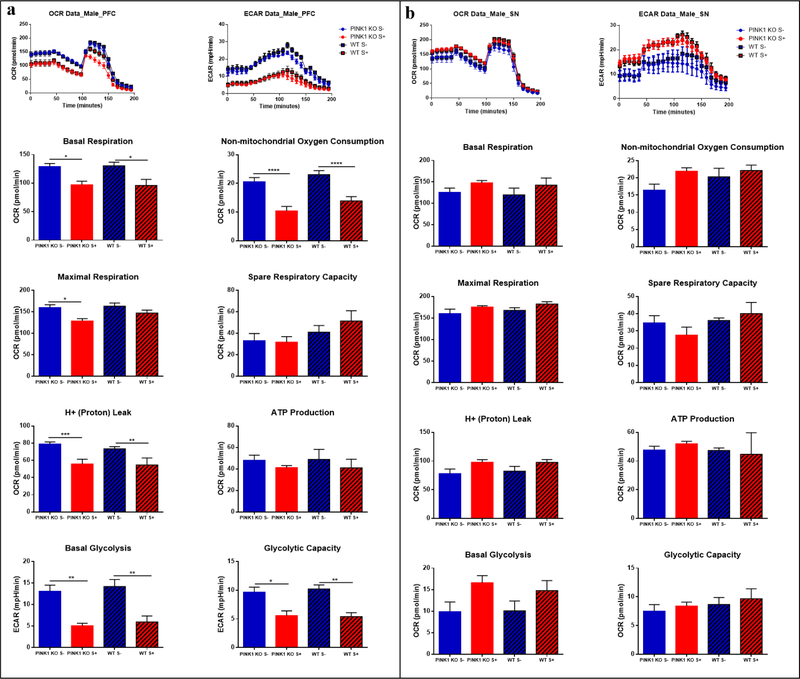
Fig 4.
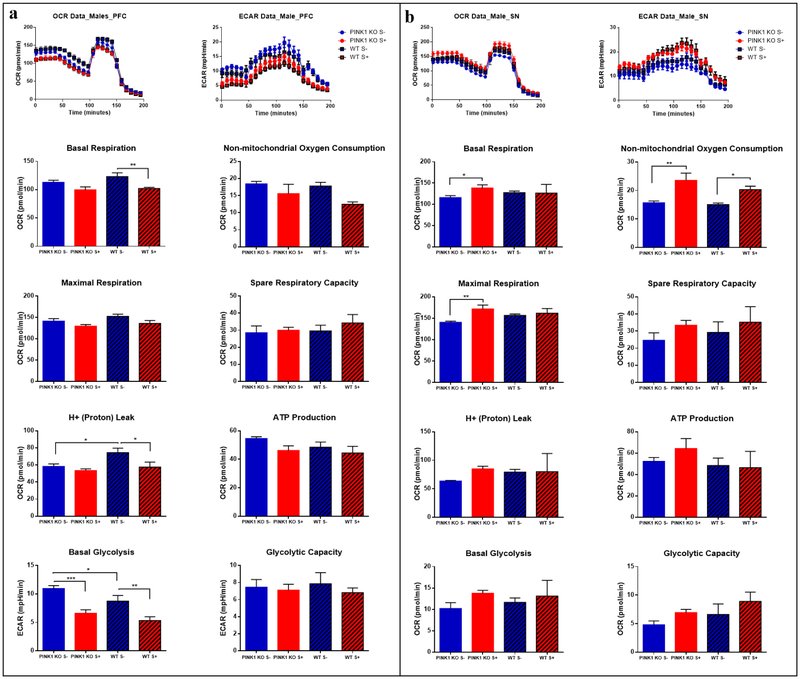
Acute effect of psychological distress on males: energy reduction in prefrontal cortex (PFC) but without changes in substantia nigra (SN). (a) Oxygen Consumption Rate (OCR) and Extracellular acidification rate (ECAR) in the PFC; (b) and in the SN. The oxygraphs of compiled data obtain per group (n = 5 rats) are shown on the top for OCRs (left oxygraph) and the ECARs (right oxygraph) for (a) and (b). The means ± SEM of the bioenergetics parameters that were graphed include: Basal Respiration, Non-mitochondrial Oxygen Consumption, Spare Respiratory Capacity, Maximal Respiration, H+ (Proton) Leak, ATP Production, Basal Glycolysis, Glycolytic Capacity. For the assay it was used: 25 μM Oligomycin, 15 μM FCCP, 20 μM Antimycin, 20 μM Rotenone; n = 5 per group. Data are expressed as mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, two-way ANOVA with Bonferronís multiple comparisons test.
Fig 5.
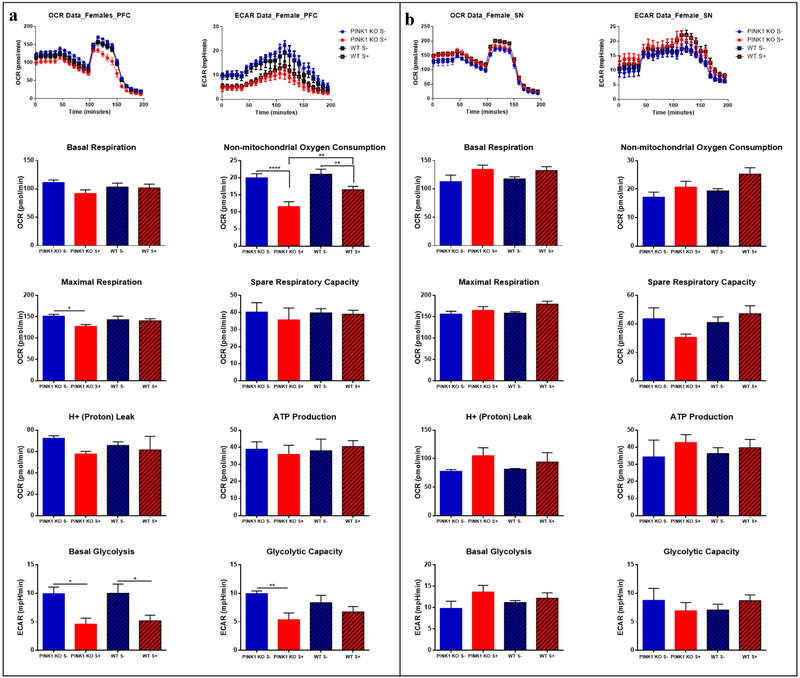
Acute effect of psychological distress on females: energy reduction in prefrontal cortex (PFC) without any effect on substantia nigra (SN). (a) Parameters of oxygen consumption rates (OCRs) and Extracellular acidification rates (ECARs) in the PFC in (b) and in the SN. The oxygraphs of compiled data obtain per group (n = 5 rats) are shown on the top for OCRs (left oxygraph) and the ECARs (right oxygraph) in (a) and (b). The means ± SEM of the bioenergetics parameters that were graphed include: Basal Respiration, Non-mitochondrial Oxygen Consumption, Spare Respiratory Capacity, Maximal Respiration, H+ (Proton) Leak, ATP Production, Basal Glycolysis, Glycolytic Capacity. For each mitochondrial stress assay the following concentrations of reagents were used: 25 μM Oligomycin, 15 μM FCCP, 20 μM Antimycin, 20 μM Rotenone; n = 5 per group. Data are expressed as mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, two-way ANOVA with Bonferronís multiple comparisons test.
In the PFC, psychological distress caused a significant reduction in non-mitochondrial OCRs and basal OCRs (p ≤ 0.05), and a significant decrease in proton leak (p ≤ 0.001) in both WT and PINK1-KO rats. Also, we observed a significant decrease in maximal OCRs in the PFC of both male and female PINK1-KO rats. In addition, basal glycolysis and glycolytic capacity significantly diminished in both males and females (Fig. 4a and Fig. 5a). Interestingly, the alterations in brain bioenergetics induced by psychological distress was more prominent in the PFC of males compared to females (Fig. 4a and Fig. 5a), an effect that persisted up to seven days following stress exposure (Fig. 6a). Overall, these data suggest that psychological distress can have negative long-term effects in this brain region (Fig. 6a).
Fig 6.
Long-lasting effects of psychological distress on males: energy reduction in prefrontal cortex (PFC) but significant increase in substantia nigra (SN). Psychological distress induced long-lasting alterations on energy metabolism in the PFC and in the SN. (a) Parameters of oxygen consumption rate (OCR) and extracellular acidification rates (ECARs) in the PFC; (b) and in the SN. The oxygraphs of compiled data obtain per group (n = 5 rats) shown in (a) and (b) are shown on the top for OCRs (left oxygraph) and the ECARs (right oxygraph). The means ± SEM of the bioenergetics parameters that were graphed include: Basal Respiration, Non-mitochondrial Oxygen Consumption, Spare Respiratory Capacity, Maximal Respiration, H+ (Proton) Leak, ATP Production, Basal Glycolysis, Glycolytic Capacity. For each mitochondrial stress assay the following reagents and concentrations were used: 25 μM Oligomycin, 15 μM FCCP, 20 μM Antimycin, 20 μM Rotenone; n = 4–5 per group. Data are expressed as mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, two-way ANOVA with Bonferronís multiple comparisons test.
However, unlike the PFC, the SN showed an increase in energy demand immediately following psychological distress. Indeed, OCRs and ECARs tended to increase in both male and female WT or PINK1-KO rats exposed to psychological distress (Fig. 4b, Fig. 5b). However, a significant increase in non-mitochondrial OCR in the SN of both WT and PINK1-KO rats were observed seven days following psychological distress (Fig. 6b). Interestingly, psychological distress induces long-term effects on the brain as we observed increased basal and maximal respiration in PINK1-KO rats (p ≤ 0.01). Thus, our compiled bioenergetics data suggest that the metabolism in the SN of PINK1-KO rats is enhanced seven days after the rats were exposed to cat urine (Fig. 6b).
Psychological distress induces changes in the abundance of antioxidant enzymes and neurotrophic proteins in the brain
Given that psychological distress altered brain metabolism, which can be partly due to an increase in oxidative stress, we then surmised that psychological distress might alter the antioxidant response pathways and neurotrophic factors. By performing WB analysis in brain tissue, we observed that psychological distress robustly altered the level of various antioxidants enzymes (SOD1, SOD2, CAT), the expression of BDNF and DJ-1 and level of mitochondria content (TOM20) in the PFC, midbrain, hippocampus and striatum in male and female WT rats (Table 1). In psychologically stressed male WT rats, we observed an increase in SOD2 and a decrease in DJ1 and TOM20 in the PFC, a decrease in CAT, TOM20, mature or cleaved BDNF (mBDNF) and in the ratio of cleaved to uncleaved BDNF (mBDNF/proBDNF) expression in midbrain, and a significant decrease in TOM20 expression in striatum. In addition, psychological distress induced modest alterations in the levels of the aforementioned biomarkers in the hippocampus in both male and female WT rats. The female WT rats were less affected by stress as we observed a decrease in the level of SOD2 and an increase in the level of BDNF in the PFC, and a significant decrease in the level of SOD1 and SOD2 in the striatum. This data is consistent with our bioenergetics data (Fig. 4 and Fig. 5), where females rats, either WT or PINK1-KO, were more resilient to changes in response to distress compared to male rats. Furthermore, the midbrain of male PINK1-KO rats showed reduction in CAT, TOM20 and BDNF expression, in a similar manner as in psychologically distressed male WT rats (p ≤ 0.05). Moreover, in the male PINK1-KO rats hippocampus, we observed significant increases in the endogenous levels of CAT, DJ1 and BDNF (p ≤ 0.05). Also, in female PINK1-KO animals, we observed a decrease in the level of SOD2 and CAT in the PFC, a decrease in DJ1 expression in the hippocampus and a decrease in the level of SOD1, SOD2 and BDNF in the striatum (p ≤ 0.05). Unexpectedly, WB data show that psychological distress did not significantly affect the aforementioned biomarkers in the brains of PINK1-KO rats, as we only observed a significant increase in the level of CAT in the PFC of males and a decrease in the level of the same enzyme in the midbrain of female rats (Table 1).
Table 1.
Psychological distress alters antioxidant proteins and neurotrophic factor expression in prefrontal cortex, midbrain, hippocampus and striatum in male and female WT and PINK1-KO rats (acute effect). Females seem to be less affected by stress than males. It was analyzed: cytosolic superoxide dismutase (SOD1), mitochondrial superoxide dismutase (SOD2), catalase (CAT), DJ-1, mitochondria content (TOM20), brain-derived neurotrophic factor (BDNF) in mature form (mBDNF) and the ratio between mBDNF and the precursor of BDNF (proBDNF), mBDNF/proBDNF. S- represents the unstressed group and S+ represents the stressed group. Data were obtained by Western blot and normalized using WT group without stress (WT S-) as a unit. n = 5 per group. Data are expressed as mean ± SD.
| MALES | FEMALES | ||||||
|---|---|---|---|---|---|---|---|
| WT | PINK 1 KO | WT | PINK 1 KO | ||||
| S− | S+ | S− | S+ | S− | S+ | S− | S+ |
| 1 | 1.06 ± 0.26 | 0.98 ± 0.13 | 1.06 ± 0.11 | 1 | 0.95 ± 0.19 | 0.97 ± 0.33 | |
| 1 | 1.16 ± 0.18 * | 1.02 ± 0.12 | 1.07 ± 0.11 | 1 | 0.72 ± 0.1 *** | 0.65 ± 0.15 **** | |
| 1 | 1.16 ± 0.12 | 1.14 ± 0.2 | 1.38 ± 0.18 † | 1 | 0.8 ± 0.26 | 0.53 ± 0.14 ** | |
| 1 | 0.81 ± 0.17 * | 0.87 ± 0.16 | 0.91 ± 0.09 | 1 | 0.98 ± 0.16 | 0.95 ± 0.23 | |
| 1 | 0.79 ± 0.17 * | 0.96 ± 0.15 | 0.99 ± 0.07 | 1 | 0.8 ± 0.21 | 0.88 ± 0.19 | |
| 1 | 0.8 ± 0.39 | 1.23 ± 0.31 | 1.2 ± 0.7 | 1 | 1.46 ± 0.54 * | 0.85 ± 0.2 | |
| 1 | 0.75 ± 0.37 | 1.15 ± 0.34 | 1.48 ± 0.9 | 1 | 1.27 ± 0.55 | 0.75 ± 0.13 | |
| 1 | 0.85 ± 0.29 | 0.77 ± 0.22 | 0_72 ± 0.13 | 1 | 0.87 ± 0.19 | 1.08 ± 0.36 | |
| 1 | 0.92 ± 0.23 | 0.95 ± 0.15 | 0.93 ± 0.08 | 1 | 0.95 ± 0.14 | 0.98 ± 0.43 | |
| 1 | 0.43 ± 0.15 **** | 0.48 ± 0.21 **** | 0.68 ± 0.17 | 1 | 1.23 ± 0.24 | 0.89 ± 0.26 | |
| 1 | 0.9 ± 0.14 | 0.99 ± 0.25 | 1.05 ± 0.31 | 1 | 1.09 ± 0.18 | 1.33 ± 0.53 | |
| 1 | 0.69 ± 0.06 *** | 0.76 ± 0.16 ** | 0.71 ± 0.13 | 1 | 0.83 ± 0.24 | 0.91 ± 0.28 | |
| 1 | 0.68 ± 0.22 ** | 0.79 ± 0.17 * | 0.7 ± 0.15 | 1 | 0.9 ± 0.25 | 0.93 ± 0.45 | |
| 1 | 0.62 ± 0.18 *** | 0.44 ± 0.1 **** | 0.49 ± 0.12 | 1 | 0.82 ± 0.16 | 0.73 ± 0.35 | |
| 1 | 0.91 ± 0.21 | 0.99 ± 0.11 | 0.86 ± 0.24 | 1 | 0.92 ± 0.42 | 0.88 ± 0.37 | |
| 1 | 0.98 ± 0.13 | 1.09 ± 0.06 | 0.999 ± 0.19 | 1 | 0.96 ± 0.24 | 0.94 ± 0.18 | |
| 1 | 1.37 ± 0.21 | 1.66 ± 0.46 ** | 1.5 ± 0.27 | 1 | 0.79 ± 0.18 | 1.03 ± 0.26 | |
| 1 | 1.88 ± 0.69 | 2.49 ± 1.2 * | 2.37 ± 1.16 | 1 | 0.9 ± 0.23 | 0.81 ± 0.09 * | |
| 1 | 1.29 ± 0.33 | 1.63 ± 0.33 | 1.76 ± 0.85 | 1 | 0.93 ± 0.34 | 0.94 ± 0.16 | |
| 1 | 1.21 ± 0.17 | 1.39 ± 0.29 * | 1.31 ± 0.4 | 1 | 1.09 ± 0.46 | 0.97 ± 0.5 | |
| 1 | 1.12 ± 0.13 | 1.33 ± 0.2 * | 1.37 ± 0.41 | 1 | 0.75 ± 0.45 | 0.71 ± 0.18 | |
| 1 | 0.95 ± 0.09 | 0.97 ± 0.09 | 0.97 ± 0.08 | 1 | 0.77 ± 0.18 ** | 0.73 ± 0.09 ** | |
| 1 | 0.94 ± 0.09 | 0.95 ± 0.13 | 1.04 ± 0.08 | 1 | 0.81 ± 0.07 * | 0.78 ± 0.12 * | |
| 1 | 0.91 ± 0.07 | 1.12 ± 0.22 | 1.13 ± 0.28 | 1 | 0.74 ± 0.23 | 0.75 ± 0.23 | |
| 1 | 0.999 ± 0.06 | 1.03 ± 0.15 | 1.02 ± 0.08 | 1 | 1.03 ± 0.09 | 0.95 ± 0.09 | |
| 1 | 0.79 ± 0.18 * | 0.91 ± 0.07 | 0.88 ± 0.22 | 1 | 0.8 ± 0.18 | 0.83 ± 0.27 | |
| 1 | 0.99 ± 0.44 | 1.01 ± 0.09 | 1.16 ± 0.35 | 1 | 0.85 ± 0.25 | 0.78 ± 0.28 | |
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001,
p ≤ 0.0001 compared with WT S-,
p ≤ 0.05 compared with PINK1-KO S-, one-way ANOVA with Fisheŕs LSD test.
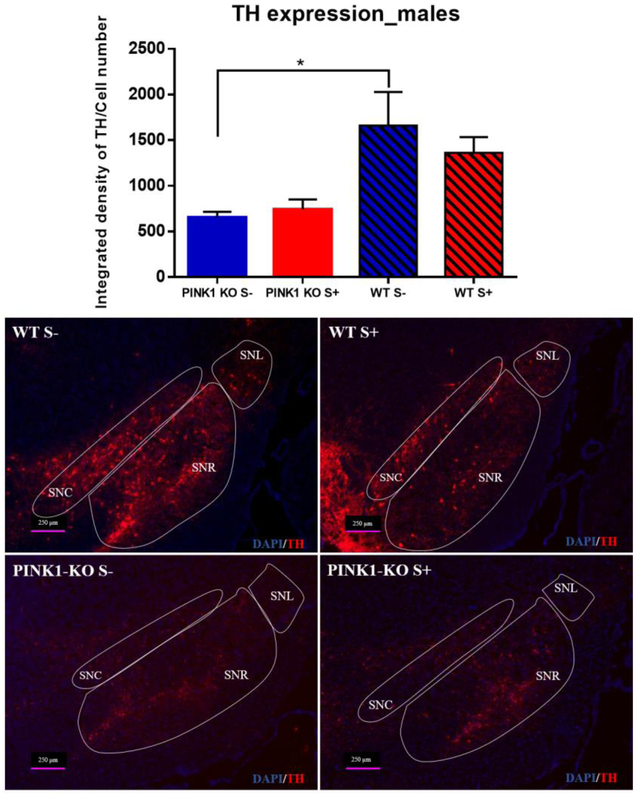
Given that psychological distress altered the level of antioxidant enzymes, BDNF, and mitochondrial content, we then surmised that psychological distress might contribute to degeneration of SN neurons. However, we observed that the integrated fluorescence density of TH normalized to cell number in the SN was not significantly altered in response to psychological distress in WT rats as evaluated by IHC (Fig. 7), suggesting that the psychological distress does not induce neurodegeneration in this model. On the other hand, PINK1-KO rats showed a significant decrease in the integrated density of TH in the SN compared to WT rats (p ≤ 0.05), which is consistent with the motor deficits observed in unstressed PINK1 KO rats (Fig. 3c). IHC data suggest that PINK1-KO rats show early loss of dopaminergic neurons (Fig. 7), consistent with the fact that humans harboring homozygous PINK1 mutations develop juvenile onset forms of monogenic PD [55].
Fig 7.
Loss of dopaminergic neurons in SN from PINK1-KO rats, without changes in WT rats. Immunofluorescence assay for detection of tyrosine hydroxylase (TH) expression in all areas of the SN from male rats (acute effect). n = 5 per group. Data are expressed as mean of the ratio of the integrated density to the total cell number in the SN ± SEM. * p ≤ 0.05, one-way ANOVA with Fisheŕs LSD test. SNC: substantia nigra pars compacta, SNR: substantia nigra pars reticulata, SNL: substantia nigra pars lateralis, abbreviations after [45].
Discussion
It is highly recognized that acute psychological distress can have myriad of pathological effects on the body and mental health [32, 56]. However, the detrimental effects of long-term psychological distress in the brain are beginning to be elucidated within the last decade. Indeed, there is increasing evidence that psychological distress can induce a chronic release of corticosteroids and brain inflammation [28], promote the retraction of dendrites and synaptic connections [29], induce neurodegeneration (hippocampal atrophy), depression-like behavior and memory deficit in both rodents and humans [53, 57]. As expected, the present study showed that psychological distress increases plasma corticosterone in WT rats. Unexpectedly, corticosterone level was constant in any condition in PINK1-KO rats (Fig. 2d). However, psychological distress induced an increase in anxiety-like behavior, motor dysfunction (Fig. 3), metabolic impairments in the PFC and SN (Fig. 4–6) and altered expression of BDNF in the brain (Table 1) in both WT and PINK1-KO rats alike. It is conceivable that an increase in oxidative stress - caused by exposure to environmental stressors - can adversely promote mitochondrial dysfunction, elicits protein aggregation and alters mitochondrial trafficking in neurons, cellular pathology that is associated with aging and neurodegenerative diseases [6]. Indeed, the present study suggests that psychological distress can be a risk factor that can significantly drive neuropathology and clinical symptoms of PD, detrimental effects that can manifest at an early age. We offer the following pathological mechanisms by which psychological distress can induce ataxia-Parkinsonian like symptoms and alterations in brain neurochemistry as further elaborated below.
To this date, it is not clear whether high levels of cortisol or corticosterone, as caused by psychological distress, can influence the progression of PD. While high level of those hormones was associated with the onset of motor dysfunction [31] and cognitive impairment [58], several conflicting reports suggested that PD patients can exhibit either an increase [59] or a decrease [60] of plasma cortisol level, and an increase in salivary cortisol [61] compared to healthy individuals. Other reports showed no correlation between the level of cortisol and PD pathology [62]. Consistent with the fact that psychological distress can alter the HPA axis, our in vivo data show that plasma corticosterone level is enhanced in stressed WT rats and is maintain one week following stress exposure (Fig. 2d). However, no significant changes were observed in corticosterone level in Parkinsonian rats suggesting that the absence of endogenous PINK1 could have detrimental effects on the HPA axis regulation, consistent with the behavioral phenotype previously described [12, 14]. A recent study showed that the loss of endogenous PINK1 in mice disrupts the HPA axis integrity, as stressed PINK1-KO mice exhibit no changes in serum corticosterone level. However, hippocampal glucocorticoids receptors were significantly diminished two weeks following stress exposure [54]. Moreover, PINK1-KO rats showed altered dopamine, glutamate and acetylcholine level in striatum, after 4 months of age [21].
Furthermore, depletion of endogenous PINK1 caused no bioenergetic alterations in the PFC and SN (Fig. 4–6), two brain regions strongly affected in PD [1, 3–6]. This data is in stark contrast to previous studies that showed disruption in mitochondrial electron transport chain activity and in the level of proteins that modulate mitochondrial respiration in the striatum of 3-month old PINK1-KO rats [18] and, also, diminished the level of mitochondrial complex proteins (NADH and cytochrome C oxidase) and ATP level in the PFC, SN, striatum and deep cerebellar nuclei [19]. Moreover, a recent study found diminished coupling efficiency but no changes in basal OCR or ATP production in striatal slices derived from young PINK1-KO mice [22]. However, an alteration in brain energy metabolism was observed following psychological distress in both WT and PINK1-KO animals. Hence, our data suggest that highly stressful events can adversely affect energy production in the brain. Indeed, distress induced a 50 % decline of bioenergetics pathways (oxidative phosphorylation and glycolysis) in the PFC of male rats (Fig. 4a). Like psychological distress, aging has been shown to also contribute to a decrease in brain energy production. Indeed, a decrease of oxidative phosphorylation and glycolysis might be caused to aging due to a reduction of glucose utilization (decreased activity of glycolytic or TCA cycle enzymes) or a decrease in glucose uptake in the brain, pathological events that may contribute to age-related neurodegeneration [63]. Hence, both psychological distress and aging can affect brain energy metabolism and antioxidant response pathways. For the first time, our ex vivo bioenergetic data suggest that psychological stress can have long-term detrimental effects on brain energy production (Fig. 6a), which can be a risk factor that contributes to neuropathology in PD.
In addition, we observed that psychological distress induces a mild increase of OCRs and ECARs in the SN (Fig. 4b, Fig. 5b and Fig. 6b), presumably in response to oxidative stress and inflammation [64–66]. A previous study reported that exposing rats to five days of cat odor induced robust neuroinflammation as evident by an accumulation of microglia and recruitment of leukocytes in area postrema [28], a phenomenon that contributes to neurodegeneration [66]. Unlike the PFC, we observed an increase, albeit not significant, in basal glycolysis and oxidative phosphorylation and ATP production in SN (Fig. 4b, Fig. 5b and Fig. 6b). In PINK1-KO rats, an increase in metabolism was observed after seven days following stress exposure (Fig.6 b). Those changes in the PFC and SN bioenergetics may occur as a response to the altered antioxidant-response pathway and neurotrophic signaling (BDNF) level found in different brain regions (Table 1), which can hasten the neurodegeneration process and thereby induce PD-like motor symptoms (Fig. 3). Perhaps, a more prolonged period of stress or exposing rats to additional episodes of psychological distress would have significantly changed the energy production in the SN.
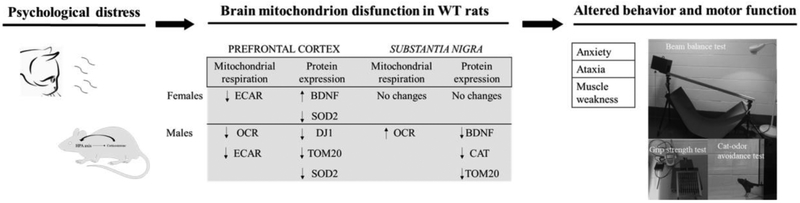
Mitochondrial dysfunction underlies the etiology of a myriad of brain-degenerative diseases including PD. Psychological distress caused alterations in mitochondrial content in WT rats (Table 1), induces impairment of motor function and anxiety-like behavior (Fig. 3) which coincides with the bioenergetic alterations observed in the brain (Fig. 4 and Fig. 5). Indeed, a reduction in brain energy metabolism (Fig. 4), a decline in mitochondrial content, a low level of BDNF and of DJ-1 in the PFC and midbrain (Table 1) following exposure to psychological distress is correlated with the development of PD-like motor symptoms (Fig. 8). BDNF is a neurotrophin implicated in synaptic development and plasticity and has been shown to regulate brain bioenergetics. A decrease in the level of BDNF in the brain and plasma in humans is highly comorbid with psychiatric disorders as previously described [67]. Moreover, DJ-1 is a ROS-sensing protein whose antioxidant and esterase activities are enhanced via the transition of the oxidation state of the catalytic cysteine (Cys106) to modulate oxidative stress in cells. A decrease level and function of DJ-1 in the brain leads to motor deficiency and other symptoms of PD [68, 69]. In a like manner, a depletion of PINK1 alters antioxidant pathways (Table 1) and disrupts motor coordination, as assessed by the beam balance test (Fig. 3c) compared with WT rats performance, data that coincides with a previous study [15]. Interestingly, psychological distress induced a significant decrease of strength and coordination in hind limbs in WT rats (Fig. 3c and 3d) and only the physical performance (Fig. 3b) was affected in PINK1-KO rats. Although psychological distress can elicit motor dysfunction in WT rats as in untreated Parkinsonian rats (Fig, 3c), our data does not imply that psychological stress causes Parkinsonian symptoms given the lack of neurodegeneration of midbrain neurons (Fig. 7). Hence our data warrants future studies to identify pathological factors that contribute to motor impairment in response to psychological distress. The impairment of motor function is associated with neurodegeneration in the frontal cortex as observed in brain-degenerative diseases including PD [4, 5] or Huntington disease [70]. Our WB data showed no significant changes in the level of mitochondria, neurotrophic factors, antioxidants and of other proteins of interest (Table 1) in stressed Parkinsonian rats brain even though they showed an impairment in energy metabolism (Fig. 4 and Fig. 5), motor dysfunction (Fig. 3b) and increased anxiety (Fig. 3a). It is conceivable that a lack of endogenous PINK1 activates a myriad of signaling pathways in other brain regions that promotes neurodegeneration in the SN. The hippocampus, known to harbor critical stress-related hormones receptors [71], showed an increase in the brain levels of CAT, DJ-1 and BDNF (Table 1), presumably as a response to oxidative stress induced by lack of PINK1 [8, 19, 72], and which can leads to changes in neuronal morphology including the retraction and eventual loss of dendritic networks [73].
Fig 8.
Distress can induce mitochondrial failure driving Parkinsońs disease phenocopy in WT rats. Psychological distress induces brain mitochondrion disfunction characterized by decrease OCRs and ECARs in PFC, increase OCR in SN and change antioxidants and BDNF expression, and diminish mitochondrial content in both regions. This brain injury leads to motor disfunction and anxiety-like behavior.
In neurons, it is conceivable that the lack of endogenous PINK1 affects mitochondrial structure, function, elicits oxidative stress and reduces the trafficking of mitochondria to dendrites neurons [8], which reduces the provision of energy to critical areas of the neuron. Although the cause of selective neurodegeneration in the SN remains unknown, it is believed that circadian rhythms, as regulated by L-type channels, can make SN neurons highly susceptible to neurodegeneration induced by oxidative stress [74]. The present study highlights the importance of PINK1 in the survival and function of dopaminergic neurons. Given that the absence of PINK1 is implicated with deregulation of calcium signaling in the cytosol and mitochondria [75, 76], our IHC data shows that the absence of PINK1 function is sufficient to induce neurodegeneration in PINK1-KO rats at an early age (Fig. 7) which is consistent with the presence of motor deficits at this age (Fig 3c), albeit one and a half month younger rats than what previous studies have shown [12, 14, 20]. In these other studies, it was observed that PINK1-KO rats exhibited a progressive loss of dopaminergic neurons at 4 months (25 %) and up to 50 % at 8 months age [12].
Interestingly, important gender-differences in brain bioenergetics were found as stressed female WT and PINK1-KO rats showed less bioenergetic alterations in the PFC and in the SN compared with the males (Fig. 4 and 5). In addition, our WB data in brain suggest that male WT rats are more sensitive to psychological distress (Table 1). Hence, it is conceivable that females have alternative pathways for modulating stress and are less likely to be adversely affected by psychological distress, presumably due to the strong neuroprotective effects of estrogen and its immunomodulatory effects on immune system cells (e.g. migration through blood-brain barrier) [77]. A recent study described important gender-related differences in PINK1-KO rats in terms of cumulative oxidative damage in total brain, ventricle sizes and neurochemical metabolites concentration in striatum [12], which may explain why males are more likely to develop PD than females, data that is consistent with our results.
Conclusions
Overall, our data suggest that psychological distress can elicit bioenergetics alterations in the PFC and SN, induces fatigue, anxiety and accelerates motor deficits in a similar manner as in PD (refer to conceptual model in Fig. 8) without inducing SN degeneration. In addition, our data suggest that environmental stressors such as psychological stress and mitochondrial dysfunction, induced by loss of PINK1 function, modestly interact to exacerbate some PD symptoms (rotarod performance). This neuropathology is associated with a decrease in antioxidant response and neurotrophic signaling. Finally, our collective behavioral and metabolic data warrant future studies to analyze the potential role of PINK1 in modulating the proprioception system.
Supplementary Material
Acknowledgements
We thank Drs. Robert Renden, Ángel G. Diaz-Sanchez, and Gilberto Mercado Mercado for their valuable technical support and constructive criticisms. This study was funded by the Pennington Foundation (Nevada), by National Institutes of Health (NIH) grant R01 NS105783 and by Consejo Nacional de Ciencia y Tecnología (CONACYT) 254483, 2833 and MG-fellowship by CONACYT.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical Approval and Consent to participate
All animal experiments were performed according the ARRIVE guidelines and by using an animal protocol (#572) that was approved by the IACUC at the University of Nevada, Reno, US.
Competing interests
The authors declare that they have no competing interests.
Availability of supporting data
All data of this study are available from the corresponding authors.
REFERENCES
- 1.Sulzer D, Surmeier DJ (2014) Neuronal vulnerability, pathogenesis and Parkinson’s disease. Mov Disord 28:41–50. 10.1002/mds.25095.Neuronal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willard AM, Bouchard RS, Gittis AH (2015) Differential degradation of motor deficits during gradual dopamine depletion with 6-hydroxydopamine in mice. Neuroscience 301:254–267. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurtig HI, Trojanowski JQ, Galvin J, et al. (2000) Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 54:1916–1921. 10.1212/wnl.54.10.1916 [DOI] [PubMed] [Google Scholar]
- 4.Narayanan NS, Rodnitzky RL, Uc E (2013) Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s Disease. Rev Neurosci 24:1–17. 10.1515/revneuro-2013-0004.Prefrontal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herz DM, Siebner HR, Hulme OJ, et al. (2014) Levodopa reinstates connectivity from prefrontal to premotor cortex during externally paced movement in Parkinson’ s disease. Neuroimage 90:15–23. 10.1016/j.neuroimage.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 6.Poewe W, Seppi K, Tanner CM, et al. (2017) Parkinson disease. Nature 3:1–21. 10.1038/nrdp.2017.13 [DOI] [Google Scholar]
- 7.Valente EM, Salvi S, Ialongo T, et al. (2004) PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol 56:336–341. 10.1002/ana.20256 [DOI] [PubMed] [Google Scholar]
- 8.Das Banerjee T, Dagda RY, Dagda M, et al. (2017) PINK1 regulates mitochondrial trafficking in dendrites of cortical neurons through mitochondrial PKA. J Neurochem 142:545–559. 10.1111/jnc.14083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine S, Youle RJ (2018) PINK1 import regulation ; a fine system to convey mitochondrial stress to the cytosol. BMC Biol 16:1–12. 10.1186/s12915-017-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodson MW, Guo M (2007) Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Curr Opin Neurobiol 17:331–337. 10.1016/j.conb.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 11.Creed RB, Goldberg MS (2018) New developments in genetic rat models of Parkinson’s disease. Mov Disord 33:717–729. 10.1002/mds.27296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dave KD, De Silva S, Sheth NP, et al. (2014) Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis 70:190–203. 10.1016/j.nbd.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 13.Kyser TL, Dourson AJ, McGuire JL, et al. (2019) Characterization of motor and non-motor behavioral alterations in the Dj-1 (PARK7) knockout rat. J Mol Neurosci 1:298–311. 10.1007/s12031-019-01358-0 [DOI] [PubMed] [Google Scholar]
- 14.Grant LM, Kelm-Nelson CA, Hilby BL, et al. (2015) Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 gene knockout rat model of Parkinson’s disease. J Neurosci Res 93:1713–1727. 10.1002/jnr.23625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquis JM, Lettenberger SE, Kelm-Nelsona CA (2020) Early-onset Parkinsonian behaviors in female Pink1−/− rats. Behav Brain Res 377:1–15. 10.1016/j.bbr.2019.112175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren X, Hinchie A, Swomley A, et al. (2019) Profiles of brain oxidative damage, ventricular alterations, and neurochemical metabolites in the striatum of PINK1 knockout rats as functions of age and gender: Relevance to Parkinson disease. Free Radic Biol Med 143:146–152. 10.1016/j.freeradbiomed.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gemechu JM, Sharma A, Yu D, et al. (2018) Characterization of Dopaminergic System in the Striatum of Young Adult Park2 −/− Knockout Rats. Sci Rep 8:1–19. 10.1038/s41598-017-18526-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stauch KL, Villeneuve LM, Purnell PR, et al. (2016) Loss of Pink1 modulates synaptic mitochondrial bioenergetics in the rat striatum prior to motor symptoms: concomitant complex I respiratory defects and increased complex II-mediated respiration. Proteomics - Clin Appl 10:1205–1217. 10.1002/prca.201600005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris CF, Morrison TR, Iriah S, et al. (2018) Evidence of neurobiological changes in the presymptomatic PINK1 knockout rat. J Parkinsons Dis 8:281–301. 10.3233/JPD-171273 [DOI] [PubMed] [Google Scholar]
- 20.Villeneuve LM, Purnell PR, Boska MD, Fox HS (2016) Early expression of Parkinson’s disease-related mitochondrial abnormalities in PINK1 knockout rats. Mol Neurobiol 53:171–186. 10.1007/s12035-014-8927-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creed RB, Menalled L, Casey B, et al. (2019) Basal and evoked neurotransmitter levels in Parkin, DJ-1, PINK1 and LRRK2 knockout rat striatum. Neuroscience 409:169–179. 10.1016/j.neuroscience.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhi L, Qin Q, Muqeem T, et al. (2018) Loss of PINK1 causes age-dependent decrease of dopamine release and mitochondrial dysfunction. Neurobiol Aging 75:1–10. 10.1016/j.neurobiolaging.2018.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgonovo J, Allende-Castro C, Laliena A, et al. (2017) Changes in neural circuitry associated with depression at pre-clinical, pre-motor and early motor phases of Parkinson’s disease. Park Relat Disord 35:17–24. 10.1016/j.parkreldis.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Liu H, Du X, et al. (2017) Association of low serum BDNF with depression in patients with Parkinson’s disease. Park Relat Disord 41:73–78. 10.1016/j.parkreldis.2017.05.012.This [DOI] [PubMed] [Google Scholar]
- 25.Scalzo P, Kummer A, Bretas TL, et al. (2010) Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’ s disease. J Neurol 257:540–545. 10.1007/s00415-009-5357-2 [DOI] [PubMed] [Google Scholar]
- 26.Smith AD, Castro SL, Zigmond MJ (2002) Stress-induced Parkinson’ s disease: a working hypothesis. Physiol Behav 77:527–531. 10.1016/S0031-9384(02)00939-3 [DOI] [PubMed] [Google Scholar]
- 27.Fontoura JL, Baptista C, Pedroso FDB, et al. (2017) Depression in Parkinson’s disease: the contribution from animal studies. Parkinsons Dis 2017:1–8. 10.1155/2017/9124160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas-Caraveo A, Perez-Ishiwara DG, Martinez-Martinez A (2015) Chronic Psychological Distress as an Inducer of Microglial Activation and Leukocyte Recruitment into the Area Postrema. Neuroimmunomodulation 1–11. 10.1159/000369350 [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmerle AM, Dickerson JW, Herman JP, Seroogy KB (2014) Stress exacerbates experimental Parkinson’s disease. Mol Psychiatry 19:638–640. 10.1038/mp.2013.108 [DOI] [PubMed] [Google Scholar]
- 31.Smith LK, Jadavji NM, Colwell KL, et al. (2008) Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. Eur J Neurosci 27:2133–2146. 10.1111/j.1460-9568.2008.06177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sierra-Fonseca JA, Gosselink KL (2018) Tauopathy and neurodegeneration: A role for stress. Neurobiol Stress 9:105–112. 10.1016/j.ynstr.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mejia-Carmona GE, Gosselink KL, Pérez-Ishiwara G, Martínez-Martínez A (2015) Oxidant/antioxidant effects of chronic exposure to predator odor in prefrontal cortex, amygdala, and hypothalamus. Mol Cell Biochem 406:121–129. 10.1007/s11010-015-2430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigoruţă M, Vargas-Caraveo A, Vázquez-Mayorga E, et al. (2018) Blood mononuclear cells as speculum of emotional stress analyzed by synchrotron infrared spectroscopy and a nootropic drug. Spectrochim Acta - Part A Mol Biomol Spectrosc 204:475–483. 10.1016/j.saa.2018.06.075 [DOI] [PubMed] [Google Scholar]
- 35.Mejia-Carmona GE, Gosselink KL, de la Rosa L a., et al. (2014) Evaluation of antioxidant enzymes in response to predator odor stress in prefrontal cortex and amygdala. Neurochem J 8:125–128. 10.1134/S181971241402007X [DOI] [Google Scholar]
- 36.Höschl C, Hajek T (2001) Hippocampal damage mediated by corticosteroids — a neuropsychiatric research challenge. Eur Arch Psychiatry Clin Neurosci 251:81–88. 10.1007/bf03035134 [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Zhang Y, Wu W, et al. (2017) Chronic glucocorticoid exposure activates BK-NLRP1 signal involving in hippocampal neuron damage. J Neuroinflammation 14:1–13. 10.1186/s12974-017-0911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrero M-T, Estrada C, Maatouk L, Vyas S (2015) Inflammation in Parkinsońs disease: role of glucocorticoids. Front Neuroanat 9:1–12. 10.3389/fnana.2015.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilkenny C, Browne WJ, Cuthill IC, et al. (2010) Animal research: reporting in vivo experiments: The ARRIVE guidelines. PLoS Biol 8:1–5. https://doi.org/e1000412. doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dielenberg RA, McGregor IS (2001) Defensive behavior in rats towards predatory odors: A review. Neurosci Biobehav Rev 25:597–609. 10.1016/S0149-7634(01)00044-6 [DOI] [PubMed] [Google Scholar]
- 41.Rozas G, Labandeira García JL (1997) Drug-free evaluation of rat models of parkinsonism and nigral grafts using a new automated rotarod test. Brain Res 749:188–199. 10.1016/S0006-8993(96)01162-6 [DOI] [PubMed] [Google Scholar]
- 42.Carter RJ, Morton JA, Dunnett SB (2001) Motor coordination and balance in rodents. Curr Protoc Neurosci 1–14. 10.1002/0471142301.ns0812s15. [DOI] [PubMed] [Google Scholar]
- 43.Maurissen JPJ, Marable BR, Andrus AK, Stebbins KE (2003) Factors affecting grip strength testing. Neurotoxicol Teratol 25:543–553. 10.1016/S0892-0362(03)00073-4 [DOI] [PubMed] [Google Scholar]
- 44.Cabe PA, Tilson HA, Mitchell CL, Dennis R (1978) A simple recording grip strength device. Pharmacol Biochem Behav 8:101–102. 10.1016/0091-3057(78)90131-4 [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C (2007) The Rat Brain in stereotaxic coordinates, 6th ed [DOI] [PubMed] [Google Scholar]
- 46.Fried NT, Moffat C, Seifert EL, Oshinsky ML (2014) Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am J Physiol Cell Physiol 307:1017–1030. 10.1152/ajpcell.00332.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giguère N, Delignat-Lavaud B, Herborg F, et al. (2019) Increased vulnerability of nigral dopamine neurons after expansion of their axonal arborization size through D2 dopamine receptor conditional knockout. PLOS Genet 15:1–26. 10.1371/journal.pgen.1008352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callio J, Oury TD, Chu CT (2005) Manganese superoxide dismutase protects against 6- hydroxydopamine injury in mouse brains. J Biol Chem 280:18536–18542. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xavier LL, Viola GG, Ferraz AC, et al. (2005) A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res Protoc 16:58–64. 10.1016/j.brainresprot.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 50.Salvatore MF, McInnis TR, Cantu MA, et al. (2019) Tyrosine hydroxylase inhibition in substantia nigra decreases movement frequency. Mol Neurobiol 56:2728–2740. 10.1007/s12035-018-1256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozina EA, Khakimova GR, Khaindrava VG, et al. (2014) Tyrosine hydroxylase expression and activity in nigrostriatal dopaminergic neurons of MPTP-treated mice at the presymptomatic and symptomatic stages of parkinsonism. J Neurol Sci 340:198–207. 10.1016/j.jns.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 52.Mulcahy P, O’Doherty A, Paucard A, et al. (2012) Development and characterisation of a novel rat model of Parkinsońs disease induced by sequential intranigral administration of AAV-α-synuclein and the pesticide, rotenone. Neuroscience 203:170–179. 10.1016/j.neuroscience.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Zhao Y, Wang Z (2015) Chronic corticosterone exposure reduces hippocampal astrocyte structural plasticity and induces hippocampal atrophy in mice. Neurosci Lett 592:76–81. 10.1016/j.neulet.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 54.Agnihotri SK, Sun L, Yee BK, et al. (2019) PINK1 deficiency is associated with increased deficits of adult hippocampal neurogenesis and lowers the threshold for stress-induced depression in mice. Behav Brain Res 363:161–172. 10.1016/j.bbr.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Deng H, Le W, Shahed J, et al. (2008) Mutation analysis of the parkin and PINK1 genes in American Caucasian early-onset Parkinson disease families. Neurosci Lett 430:18–22. 10.1016/j.neulet.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 56.Pfau ML, Russo SJ (2015) Peripheral and central mechanisms of stress resilience. Neurobiol Stress 1:66–79. 10.1016/j.ynstr.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lupien SJ, De Leon M, De Santi S, et al. (1998) Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1:69–73. 10.1038/271 [DOI] [PubMed] [Google Scholar]
- 58.Lara VP, Caramelli P, Teixeira AL, et al. (2013) High cortisol levels are associated with cognitive impairment no-dementia (CIND) and dementia. Clin Chim Acta 423:18–22. 10.1016/j.cca.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 59.Hartmann A, Veldhuis JD, Deuschle M, et al. (1997) Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol Aging 18:285–289. 10.1016/S0197-4580(97)80309-0 [DOI] [PubMed] [Google Scholar]
- 60.Bellomo G, Santambrogio L, Fiacconi M, et al. (1991) Plasma profiles of adrenocorticotropic hormone, cortisol, growth hormone and prolactin in patients with untreated parkinson’s disease. J Neurol 238:19–22. 10.1007/BF00319704 [DOI] [PubMed] [Google Scholar]
- 61.Djamshidian A, O’Sullivan SS, Papadopoulos A, et al. (2011) Salivary cortisol levels in Parkinson’s disease and its correlation to risk behaviour. J Neurol Neurosurg Psychiatry 82:1107–1111. 10.1136/jnnp.2011.245746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volpi R, Caffarra P, Boni S, et al. (1997) ACTH/Cortisol involvement in the serotonergic disorder affecting the parkinsonian brain. Neuropsychobiology 35:73–78. 10.1159/000119394 [DOI] [PubMed] [Google Scholar]
- 63.Hoyer S (1982) The young-adult and normally aged brain. Its blood flow and oxidative metabolism. A review - part I. Geriatr, Arch Gerontol 1:101–116. 10.1016/0167-4943(82)90021-8 [DOI] [PubMed] [Google Scholar]
- 64.Kayser E, Sedensky MM, Morgan PG (2016) Region-specific defects of respiratory capacities in the Ndufs4 (KO) Mouse Brain. PLoS One 4:1–18. 10.1371/journal.pone.0148219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin F, Sancheti H, Patil I, Cadenas E (2017) Energy metabolism and inflammation in brain aging and Alzheimer’s Disease. Free Radic Biol Med 100:108–122. 10.1016/j.freeradbiomed.2016.04.200.Energy [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J-K, Tran T, Tansey MG (2009) Neuroinflammation and Parkinson’s Disease. J Neuroimmune Pharmacol 4:419–429. 10.1007/978-1-4614-5836-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cattaneo A, Cattane N, Begni V, et al. (2016) The human BDNF gene : peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl Psychiatry 6:1–10. 10.1038/tp.2016.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma N, Rao SP, Kalivendi SV, et al. (2019) The deglycase activity of DJ-1 mitigates α-synuclein glycation and aggregation in dopaminergic cells: Role of oxidative stress mediated downregulation of DJ-1 in Parkinson’s disease. Free Radic Biol Med 135:28–37. 10.1016/j.freeradbiomed.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Chandran JS, Lin X, Zapata A, et al. (2008) Progressive behavioral deficits in DJ-1 -deficient mice are associated with normal nigrostriatal function. Neurobiol Dis 29:505–514. 10.1016/j.nbd.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf RC, Vasic N, Schönfeldt-Lecuona C, et al. (2007) Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington’s disease: Evidence from event-related fMRI. Brain 130:2845–2857. 10.1093/brain/awm210 [DOI] [PubMed] [Google Scholar]
- 71.McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dagda RK, Cherra SJ, Kulich SM, et al. (2009) Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 284:13843–13855. 10.1074/jbc.M808515200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lakshminarasimhan H, Chattarji S (2012) Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One 7:1–6. 10.1371/journal.pone.0030481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA (2010) What causes the death of dopaminergic neurons in Parkinson’s disease? Prog Brain Res 183:59–77. 10.1016/S0079-6123(10)83004-3 [DOI] [PubMed] [Google Scholar]
- 75.Heeman B, Van Den Haute C, Aelvoet S, et al. (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci 124:1115–1125. 10.1242/jcs.078303 [DOI] [PubMed] [Google Scholar]
- 76.Gandhi S, Wood-Kaczmar A, Yao Z, et al. (2009) PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33:627–638. 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maggioli E, Mcarthur S, Mauro C, et al. (2016) Estrogen protects the blood – brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun 51:212–222. 10.1016/j.bbi.2015.08.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.