Abstract
Background:
Diet is associated with colorectal cancer (CRC) survival. Yet, adherence to nutrition guidelines is low among CRC survivors.
Methods:
We conducted a pilot trial among CRC survivors to evaluate a 12-week remote dietary intervention. Participants received print materials and were randomized (1:1) to intervention (website, text messages) or wait-list control. Primary outcomes included feasibility and acceptability. We also explored change in diet from 0 to 12 and 24 weeks and change from 0 to 12 weeks in anthropometry and circulating biomarkers. Trial Registration: NCT02965521.
Results:
We randomized 50 CRC survivors (25 intervention, 25 control). Retention was 90% at 12 weeks and 84% at 24 weeks. Participants had a median age of 55 years and were 66% female, 70% non-Hispanic white, and 96% had a college degree. The intervention arm responded to a median 15 (71%) of 21 text messages that asked for a reply (IQR: 8, 19) and visited the website a median of 13 (15%) days [interquartile range (IQR): 1, 33] out of the 84 study days.
Conclusions:
In conclusion, we developed a web-based dietary intervention for CRC survivors. Our pilot results suggest CRC survivors may engage more with text messages than a study website. Research to improve tailoring of text messages, while maintaining scalability, is needed.
Impact:
Remote dietary interventions using text messages may be feasible for CRC survivors.
Keywords: behavior, nutrition, digital health, technology, diet record
INTRODUCTION
Over 1.2 million Americans currently live with colorectal cancer (CRC) in the United States (US) (1). While these individuals are living longer, CRC remains the second-leading cause of cancer death in the US (2).
Diet after CRC may impact survival (3). In particular, adherence to the American Cancer Society (ACS) guidelines, including a diet rich in fruits, vegetables, and whole grains and low in red and processed meats (4), after CRC diagnosis is associated with lower risk of CRC-specific and all-cause mortality (5,6). Unfortunately, adherence to these recommendations is low among individuals with CRC (5,6). Thus, interventions are needed to help people with CRC adopt dietary recommendations.
Growing data suggest that behavioral interventions using web and mobile technology are acceptable and may modify dietary behavior (7–12). Because these interventions can be largely automated, they may be more scalable in cancer survivorship care compared to in-person or telephone counseling (13,14). However, the feasibility of a remotely delivered technology-based dietary intervention among CRC survivors is not known.
Identifying a feasible and acceptable dietary intervention that is also scalable is a critical step toward the long-term goal of determining the effect of dietary change on CRC survival. Thus, we developed a website with a simple diet tracking tool and a 12-week text messaging program to help individuals with CRC make recommended dietary changes. We conducted a 2-arm randomized controlled pilot study to determine the feasibility and acceptability of the intervention. To inform the design of a larger definitive study, we also explored change in diet, anthropometry, and circulating biomarkers of cardiometabolic health.
MATERIALS AND METHODS
We conducted a pilot study of a remotely delivered dietary intervention among 50 individuals with colon or rectal cancer (Survivor Choices for Eating and Drinking; SUCCEED). At enrollment, participants were randomized 1:1 to receive the intervention for 12 weeks or wait-list control. We included a wait list control to facilitate enrollment and maximize feedback on the intervention. All participants were asked to complete 4-day diet records and surveys at 0, 12, and 24 weeks. Written informed consent was obtained from all participants and the study was conducted in accordance with recognized ethical guidelines (e.g., Declaration of Helsinki, CIOMS, Belmont Report, U.S. Common Rule) and approved by the institutional review board of the University of California, San Francisco (UCSF).
Study Population
To be eligible, individuals must have been diagnosed with colon or rectal adenocarcinoma; not be actively undergoing chemotherapy; be considered disease-free or have stable disease; be able to speak and read English; have access to a mobile phone with Internet and text messaging capabilities; and able to navigate websites, fill out forms on the web, communicate by email, and have regular access to the Internet. We excluded individuals who were already meeting four or more of the six target dietary behaviors at enrollment (based on self-reported intake of vegetables, whole grains, fish, processed meat, sugar-sweetened beverages, alcohol; Table 1).
Table 1.
Target dietary factors in a web-based dietary intervention with text messages for people with colorectal cancer.
| Foods to Increase | |
|---|---|
| Vegetables | Eat 5 or more servings of vegetables every day. Choose a wide variety of fresh or frozen produce. |
| Whole Grains | Eat 3 or more servings of whole grains per day. Choose fiber-rich, whole grain foods over refined grain foods. |
| Fish | Eat 2 or more servings of fish per week. Choose varieties rich in omega-3 fatty acids, such as salmon, trout, and herring. |
| Foods to Limit | |
| Processed Meat | Limit intake of processed meats. Choose lean protein sources over red and processed meats, such as fish, skinless chicken or turkey, beans, lentils, nuts, tofu, and nonfat plain yogurt. |
| Sweetened Beverages | Avoid sugar-sweetened beverages. Drink water or coffee with no sugar. |
| Alcohol | If you drink alcohol, do so in moderation. Men, limit intake to 2 servings per day; women, limit intake to 1 serving per day. |
Recruitment, Screening, and Informed Consent
Participants were identified through the gastrointestinal oncology clinic at UCSF between April 2017 and May 2018. We also advertised at local clinics, support groups, and a national conference. If interested, individuals were asked to complete a screening survey online and have their provider complete a form to verify clinical information (e.g., diagnosis, stage, treatment). The screening survey asked participants how many servings of vegetables, whole grains, processed meat, fish, sugar-sweetened beverages, and alcohol they usually consume. A definition of the food group as well as serving size was provided. For vegetables, participants were asked to report how many servings they consume each day, with nine options ranging from zero to nine or more servings/day. For the other food groups, participants were asked “on average, how many servings of [food group] do you eat?” and were provided with nine options, ranging from never to four or more servings/day.
After an individual was determined to be eligible, they were sent a consent form via mail, e-mail, or fax. A research coordinator scheduled a time to review the consent form over the phone. At the conclusion of the call, the research coordinator asked the participant to confirm that they understood the consent form, and if so, asked them to sign a paper or electronic consent form.
Clinical Procedures and Data Collection
Following consent, participants were asked to complete 4-day diet records using the National Cancer Institute’s Automated Self-administered Dietary Assessment Tool (ASA24) and surveys using REDCap electronic data capture tools hosted at UCSF (15–18). Participants repeated the 4-day diet records and surveys at 12 and 24 weeks. Participants were also asked to go to a LabCorp for a fasting blood draw and anthropometric assessment at enrollment and 12 weeks.
Randomization
Eligible, consented participants who completed enrollment assessments were randomly assigned in blocks of 2 and 4 to intervention or control with allocation weight of 1:1. A study biostatistician (LZ) generated the randomization scheme prior to the start of the study and this was uploaded into REDCap. The Randomization module in REDCap was then used to determine participants’ intervention assignment.
Intervention Development and Description
We designed a 12-week web-based dietary intervention using human centered design methodology with the UCSF School of Medicine Technology (SOM Tech) (19); a patient advisory board (PAB) of five CRC survivors; and a registered dietician at the UCSF Helen Diller Family Comprehensive Cancer Center. The PAB participated in intervention development and tested a prototype for one week. We then conducted semi-structured interviews with PAB members and revised the intervention.
The goal of the intervention was to increase vegetables, whole grains, and fish and decrease processed meats, sugar-sweetened beverages, and alcohol. These goals were based on the ACS guidelines plus literature on diet and CRC survival (4,20–23). The intervention included print materials, a personalized report, one 15–30 minute session with a study coordinator in person (22%) or by phone (78%), a study website, and text messages for 12 weeks. The personalized report indicated whether the participant currently met, almost met, or did not meet the six target dietary recommendations at enrollment (Table 1). The orientation session focused on how to use the study website; no dietary counseling or health coaching was performed. The intervention incorporated theoretical constructs from the Theory of Planned Behavior and Social Cognitive Theory and addressed outcome expectations, self-efficacy, goal setting, self-monitoring, and social support (24–26).
Website features:
The website was designed to be used on a computer, tablet, or phone and built on the UCSF Drupal 8 infrastructure. We collected and stored data from participants securely with a username and password. Data collected through the website was encrypted and transmitted to a HIPAA-compliant secure server. The website included goal setting, daily tracking of target food groups, visual summaries of tracked dietary intake and progress toward goals, recommendations, recipes, meal planning, frequently asked questions, and a profile page. Participants could contact the study team with questions through the website. In the profile page, participants could turn tracking on or off. For example, if an individual abstained from alcohol, they could turn it “off” and alcoholic drinks would not show in the goals, tracking, or progress pages. Prompts to visit the website were included in 13 text messages, and up to an additional 5 messages depending on participants’ responses. No reminders were sent to participants based on their engagement with the website. Data on website use by participant was obtained through logins, tracking data in the study website (which recorded responses by study ID, date and time), as well as google analytics. See Supplemental Figure 1a–1d for screenshots of the website.
Text message program:
We sent text messages to participants via Twilio (www.twilio.com). The messages included educational content (e.g., how to estimate serving size, what is a whole grain); prompts to set goals, track, meal plan, try recipes; motivational messages; quizzes; and challenges. Participants could set the time of day (i.e., morning, afternoon, evening) that they received messages and turn the messages on/off through the website. Twenty-one of the 84 text messages (25%) asked the participants for a reply. See Supplemental Table 1 for the first 14 text messages.
Wait-list Control
Participants randomized to the control arm received print materials on diet after CRC at enrollment. After completion of the 12-week assessments, controls had the option to receive the intervention from 12 to 24 weeks; 21 of the 25 control participants received the intervention. These individuals received access to the study website, had a brief orientation session on how to use the website, and 12-weeks of daily text messages.
Outcome Measures
Feasibility and Acceptability:
We assessed the intervention’s feasibility and acceptability at 12 weeks in the intervention arm and 24 weeks in the control arm (among individuals who received the intervention from 12 to 24 weeks). To assess the intervention’s feasibility, we evaluated adherence (frequency of using the study website and response rates to text messages that asked for a reply) and attrition (proportion of participants who completed the 12-week diet records). A priori, we stated that the intervention would be considered feasible if we achieved ≥70% adherence and ≤20% attrition in the intervention arm (27,28).
We explored the acceptability of the intervention through a self-administered investigator-developed online questionnaire. Participants were asked to what degree they agreed (strongly agree, agree, undecided, disagree, strongly disagree) with nine statements about the text messages. For the study website, participants were asked to rate the quality of the website, overall and each page, from poor (0) to excellent (100). Participants were asked how satisfied they were overall and could provide open-ended feedback.
Dietary assessment:
Participants were asked to complete 4-day diet records using ASA24 at 0, 12, and 24 weeks (29). Participants received instructions with screenshots to guide them through completing a diet record on ASA24. Participants who had trouble using the online system were allowed to submit paper diaries, which were entered into ASA24 by one trained researcher blinded to the participants’ assigned study arm. If a participant submitted a paper diary at enrollment, they completed paper diaries at subsequent time points. In total, 11 (22%) enrollment diaries, 16 (36%) 12-week diaries, and 14 (35%) 24-week diaries were entered by the researcher. All of the days recorded had acceptable calorie intakes, defined as between the 5th and 95th percentile of calorie intakes based on ASA24 users in the National Health and Nutrition Examination Survey (600 – 4400 kcal/d for women; 650 – 5700 kcal/d for men) (30).
Dietary outcomes of interest included change in vegetables, whole grains, fish, processed meat, sugar-sweetened beverages, and alcohol from enrollment to 12 and 24 weeks. We also examined change in refined grains, to explore if participants replaced refined grains with whole grains or added the recommended servings of whole grains without decreasing refined grain intake. We measured diet at 24 weeks in both arms to explore whether changes that occurred in the intervention arm from 0 to 12 weeks were maintained at 24 weeks, and whether individuals in the control arm who received the intervention from 12 to 24 weeks made any changes.
Body Size, Blood Pressure, and Blood Values:
Participants were asked to go LabCorp, an accredited clinical laboratory, at 0 and 12 weeks. A trained technician measured participants’ height, weight, waist circumference and blood pressure and took a fasting blood sample. The technician was an employee of LabCorp and blinded to the participants’ assigned group. LabCorp visits were optional, and two participants chose not to complete them at enrollment. Assessments were done according to the LabCorp standard operating procedures. From the fasting blood sample, LabCorp measured C-reactive protein (CRP), hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Power and sample size
We powered the study based on the proportion of participants who adhered to the intervention, defined as using the website and/or engaging with text messages. Using a 1-sided 1-sample binomial test with α=0.1, 20 participants who complete the trial in the intervention arm, and a null hypothesis that 70% would adhere to the intervention, we estimated that we would have 80% power to reject the null hypothesis if the observed adherence was 47% or less. We randomized 50 participants to account for up to 20% drop-out.
Statistical Analyses
Enrollment characteristics were summarized by arm and overall. Frequency distributions and percentages were used to summarize categorical measures and medians (interquartile range; IQR) were used to describe continuous measures. Point estimates of adherence (frequency of using the study website, response rate to text messages that asked for a reply) and attrition (completion of diet records) were calculated by arm.
To estimate the effect of the intervention on diet, we calculated each participant’s average servings per day for the target food groups based on the 4-day diet records at enrollment, 12, and 24 weeks. We then calculated the median (IQR) intake of each target food group at each time point by arm. Because alcohol has different recommendations by sex, and to explore the effect of the intervention separately in men and women, we reported each food group by sex. We described the absolute change in intake from 0 to 12 and 0 to 24 weeks using medians and IQR. We also described the difference in changes from enrollment to 12 weeks between the two groups using means and 95% confidence interval (CI).
We adhered to the CONSORT guidelines for reporting pilot trials and did not perform statistical tests (31). Analyses were conducted using SAS v. 9.4.
RESULTS
As shown in Figure 1, we screened 94 individuals for eligibility. Of these, 19 (20%) were ineligible; 14 (15%) met 4 or more of the 6 target dietary behaviors, 4 were receiving chemotherapy, and 1 did not have CRC. Among the remaining individuals, 11 decided not to consent after completing the screening survey and 14 did not complete enrollment procedures. After screening, consent, and completion of enrollment procedures, 50 individuals were randomized 1:1 to intervention (n=25) or wait-list control (n=25). All participants received their assigned intervention. Follow-up based on completion of the diet record was 90% at 12-weeks and 84% at 24-weeks. By arm, these values were 22 (88%) in the intervention arm and 23 (92%) in the control arm at 12-weeks and 22 (88%) in the intervention arm and 20 (80%) in the control arm at 24-weeks.
Figure 1. CONSORT Flow Diagram.

Figure 1 shows the flow of participants from screening to end of study. Follow-up at 12 and 24 weeks was defined by completion of the 4-day diet record.
Characteristics of the study sample are presented in Table 2. The intervention vs. control group was slightly older (median, 57 vs. 54 years), had a higher BMI (median, 26.9 vs. 25.5 kg/m2), and were more likely to be female, have rectal cancer, and have elevated cholesterol.
Table 2.
Characteristics of 50 individuals with colorectal cancer participating in a pilot trial of a remotely delivered, web-based dietary intervention with text messages.
| Characteristic | Total | Intervention | Control |
|---|---|---|---|
| N | 50 | 25 | 25 |
| Age at enrollment, years, median (IQR) | 55 [50, 62] | 57 [51, 64] | 54 [50, 57] |
| BMI, kg/m2, median (IQR) | 26.8 [23.7, 31.1] | 26.9 [24.4, 29.5] | 25.5 [22.8, 32.0] |
| Male, N (%) | 17 (34) | 7 (28) | 10 (40) |
| Non-Hispanic white, N (%) | 35 (70) | 18 (72) | 17 (68) |
| College degree, N (%) | 48 (96) | 25 (100) | 23 (92) |
| Works full-time, N (%) | 28 (56) | 13 (52) | 15 (60) |
| Married, N (%) | 34 (69) | 17 (71) | 17 (68) |
| Cancer Type | |||
| Colon cancer, N (%) | 33 (66) | 14 (56) | 19 (76) |
| Rectal cancer, N (%) | 17 (34) | 11 (44) | 6 (24) |
| Months since diagnosis, median (IQR) | 22 [12, 39] | 22 [8, 49] | 22 [14, 25] |
| Tumor Stage, N (%) | |||
| I | 6 (12) | 4 (16) | 2 (8) |
| II | 6 (12) | 2 (8) | 4 (16) |
| III | 35 (70) | 18 (72) | 17 (68) |
| IV | 3 (6) | 1 (4) | 2 (8) |
| Current ostomy, N (%) | 11 (22) | 6 (25) | 5 (20) |
| Smoking status, N (%) | |||
| Never | 38 (76) | 22 (88) | 16 (64) |
| Past | 10 (20) | 3 (12) | 7 (28) |
| Current | 2 (4) | 0 | 2 (8) |
| History of diabetes mellitus, N (%) | 1 (2) | 0 | 1 (4) |
| History of elevated cholesterol, N (%) | 11 (22) | 8 (32) | 3 (12) |
| History of high blood pressure, N (%) | 15 (30) | 6 (24) | 9 (36) |
| Regular aspirin use, N (%) | 26 (52) | 14 (58) | 12 (48) |
IQR, interquartile range; BMI, body mass index
Feasibility of the intervention (adherence)
Responses to text messages were relatively stable over the 12 weeks in the intervention arm (Figure 2a). For any given text message, the median number of responses was 15 (60%) (IQR: 15, 16). When examining text message responses by participant, those in the intervention arm responded to a median of 15 (71%) of the 21 text messages that asked for a reply (IQR: 8, 19).
Figure 2. Text message response rate and study website use during the 12-week intervention among the 25 people with colorectal cancer randomized to the intervention arm.

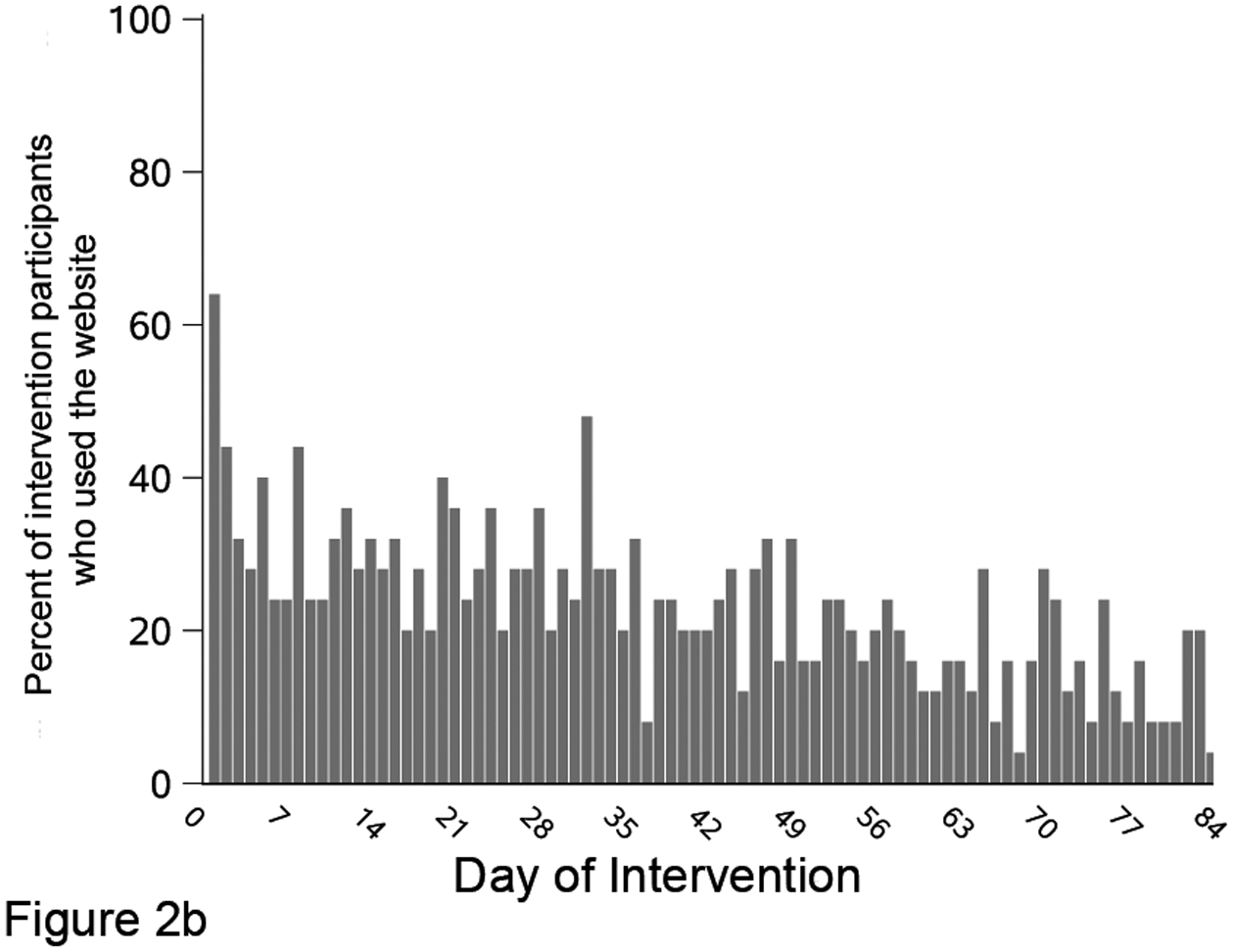
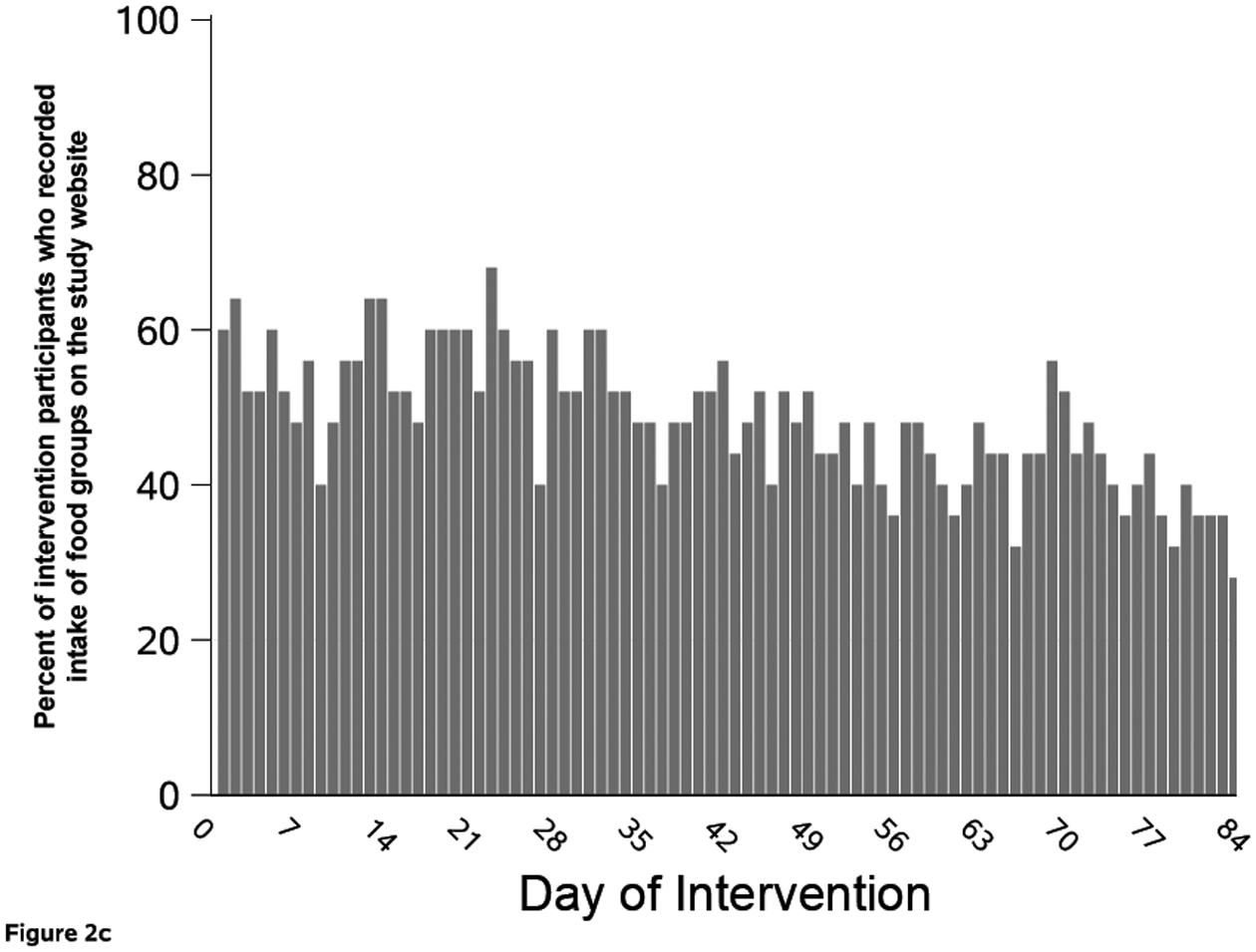
Figure 2 shows the percent of intervention arm (n=25) who responded to each text message that asked for a reply (A); visited the study website by study day (B); and had recorded food group intake on the study website, by study day (C). Participants could track intake of food groups for multiple days at one visit.
The response rate to text messages among people initially assigned to the control arm was lower and declined over time (Supplemental Figure 2a). For any given text message that asked for a reply, the median number of responders was 12 (57%; IQR: 10, 13). When examining text message response by participant, those in the control arm responded to a median of 9 (43%) out of the 21 text messages that asked for a reply (IQR: 7, 17).
Intervention arm participants went to the website throughout the 12-week study, although website use declined over time (Figure 2b). On any given study day, the median number of participants who visited the study website was 6 (24%; IQR: 4, 7). When examining website use by participant, those randomized to the intervention arm went to the website a median of 13 (15%) out of 84 days (IQR: 1, 33 days). Five participants did not visit the website and three visited once during the 12 weeks. In contrast, five participants went to the study website on more than half of the 84 study days; the most frequent user visited the website on 63 days. Twenty (80%) intervention participants tracked a food on the website at least once (Figure 2c). Among trackers, the median number of days with at least one food group tracked was 48 days (57%; IQR: 25, 82 days). This value was higher than website visits because participants often recorded multiple days of intake at each visit. When an individual tracked, they entered data for all six target food groups 53% of the time.
Website use among those initially assigned to the control group was lower compared to the intervention arm (Supplemental Figure 2b). On any given study day between week 12–24, the median number of control participants who visited the study website was 3 (14%; IQR: 2, 5). When examining website use by participant, those randomized to the control group went to the study website a median of 4 (5%) out of 84 days (IQR: 0, 21 days). Seven control participants did not visit the website and two went on one day. In contrast, three control participants went to the study website on more than half of the 84 days; the most frequent control user went to the website on 72 days. Seventeen (81%) control participants tracked a food group on the website at least once (Supplemental Figure 2c). Among trackers, the median number of days with at least one food group tracked was 33 days (39%; IQR: 22, 78 days). As with the intervention arm, this value was higher than website visits because participants recorded multiple days of intake per visit.
Based on self-report, participants accessed the website from an iPhone (24, 57%), computer (23, 55%), android phone (9, 21%), iPad (7, 17%), and/or android tablet (2, 5%). The most used page was Tracking (69% reported they used this page frequently) followed by Recipes (40%), View Progress (37%), Set Goals (23%), Recommendations (17%), Meal Planning (14%), and FAQ (6%).
Acceptability of the intervention
The feedback questionnaire was completed by 22 of the 25 intervention participants at 12-weeks and 20 of the 21 control participants who received the intervention at 24-weeks. Of these, 31 (74%) were satisfied or very satisfied with the text messages, 27 (64%) said the content of the messages was interesting, and 33 (79%) said the frequency of the messages (1 per day) was ideal. Feedback on the text messages was generally positive. However, some participants commented that they would have liked additional personalization. For example, “…it would be great to tailor some of the messages if I select what I am already good at” and “the language was impersonal” (see Supplemental Table 2 for participant quotes).
Overall, 28 (64%) participants were satisfied or very satisfied, 11 (26%) were neutral, and three (10%) were dissatisfied or very dissatisfied with the intervention. The one participant who responded very dissatisfied was in the intervention arm and stated that the website’s simplified tracking “cancelled out a lot of things on my diet (ex. [I didn’t know where to add things such as miso soup).” The two participants who responded “dissatisfied” were both in the control arm; one of whom experienced a recurrence during the first 12 weeks of the study prior to receiving the intervention.
Change in diet
Intake at enrollment and absolute change from enrollment of vegetables, whole and refined grains, fish, processed meats, sugar-sweetened beverages, and alcoholic drinks at 0, 12, and 24-weeks are presented in Table 3. The intervention arm had higher intake of whole grains at 12 weeks compared to controls (difference in means comparing the intervention to control group at 12 weeks: 0.9 servings/d; 95% CI: 0.1, 1.6). This difference appeared to be driven by women. Additionally, the intervention arm’s increase in whole grains appeared to be maintained at 24 weeks. Controls had no clear improvements in diet from 0 to 12 or 24 weeks.
Table 3.
Dietary intake based on 4-day diet records at baseline, 12, and 24 weeks among 50 individuals with colorectal cancer participating in a pilot randomized controlled trial of a 12-week web-based dietary intervention with text messages. The intervention arm received the intervention from 0 to 12 weeks; controls received the intervention from 12 to 24 weeks.
| Median [IQR], servings/day | Between group difference in means (95% CI)1 at 12 weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Absolute change | Absolute change | |||||||
| Enrollment | 12 weeks | 24 weeks1 | Enrollment1 | 12 weeks | 24 weeks2 | |||
| Total (n=50) | 25 | 22 | 22 | 25 | 23 | 20 | ||
| Vegetables | 1.9 [1.4, 2.4] | −0.4 [−0.7, 0] | 0.3 [−0.7, 0.9] | 2.0 [1.6, 2.5] | −0.2 [−0.8, 0] | −0.2 [−0.9, 0.3] | −0.3 (−0.9, 0.2) | |
| Whole grains | 0.7 [0.3, 1.3] | 0.4 [0, 1.7] | 0.3 [−0.3, 1.2] | 0.7 [0.1, 1.2] | 0 [−0.5, 0.5] | 0.2 [−0.1, 1.6] | 0.9 (0.1, 1.6) | |
| Refined grains | 3.7 [1.9, 6.2] | −0.7 [−1.9, 0.8] | −0.6 [−2.6, 2.5] | 3.3 [1.4, 4.7] | 0.2 [−1.1, 1.2] | −0.7 [−1.6, 2.2] | −0.5 (−1.7, 0.7) | |
| Fish | 0.5 [0, 1.2] | 0 [−0.4, 1.5] | 0 [−0.1, 0.9] | 1.1 [0, 1.6] | 0 [−1.2, 0.2] | 0.3 [−1.0, 1.1] | 0.2 (−0.6, 0.9) | |
| Processed meats | 0.1 [0, 0.6] | 0 [−0.4, 0.2] | 0 [0, 0.2] | 0.1 [0, 0.8] | 0 [−0.1, 0.4] | 0 [−0.2, 0.2] | −0.2 (−0.7, 0.4) | |
| Sugar-sweetened beverages | 0.1 [0, 0.6] | 0 [−0.5, 0] | −0.1 [−0.5, 0] | 0 [0, 0.3] | 0 [−0.4, 0] | 0 [−0.1, 0.2] | 0 (−0.1, 0.2) | |
| Women (n=33) | 18 | 15 | 15 | 15 | 14 | 11 | ||
| Vegetables | 2.2 [1.8, 3.0] | −0.4 [−1.8, 0] | 0.1 [−0.9, 0.9] | 1.9 [1.5, 2.4] | −0.2 [−0.8, 0] | −0.1 [−0.5, 0.1] | −0.3 (−1.0, 0.5) | |
| Whole grains | 0.9 [0.5, 1.9] | 0.4 [0, 2.1] | 0.2 [−0.4, 1.2] | 0.3 [0, 1.0] | 0.2 [−0.3, 0.6] | 0.1 [0, 1.9] | 1.2 (0.2, 2.2) | |
| Refined grains | 3.1 [1.5, 6.2] | −0.4 [−1.2, 1.6] | −0.3 [−2.6, 3.0] | 3.3 [1.4, 4.7] | 0.2 [−1.1, 1.2] | −0.7 [−1.6, 2.2] | 0.1 (−1.2, 1.4) | |
| Fish | 0.1 [0, 1.1] | 0.9 [0, 1.9] | 0 [0, 0.9] | 1.1 [0, 2.6] | −0.2 [−1.5, 0.2] | 0 [−1.1, 1.1] | 0.1 (−1.0, 1.2) | |
| Processed meats | 0.1 [0, 0.5] | 0 [−0.2, 0.3] | 0 [0, 0.3] | 0.1 [0, 0.5] | 0 [−0.1, 0.3] | 0 [0, 0.4] | −0.3 (−1.1, 0.5) | |
| Sugar-sweetened beverages | 0.2 [0, 0.7] | 0 [−0.6, 0] | −0.2 [−0.6, 0] | 0 [0, 0.3] | 0 [0, 0.1] | 0 [0, 0.3] | 0.1 (−0.1, 0.2) | |
| Alcohol | 0 [0, 0.3] | 0 [−0.2, 0] | 0 [−0.2, 0] | 0.3 [0, 1.8] | 0 [−0.3, 0.3] | −0.3 [−0.8, 0.3] | −0.3 (−0.8, 0.3) | |
| Men (n=17) | 7 | 7 | 7 | 10 | 9 | 9 | ||
| Vegetables | 1.5 [1.3, 2.2] | −0.3 [−0.6, 2.0] | 0.5 [−0.5, 1.1] | 2.2 [1.7, 2.7] | −0.4 [−0.6, −0.2] | −0.5 [−1.5, 0.6] | −0.1 (−1.4, 1.1) | |
| Whole grains | 0.3 [0, 0.8] | 0.2 [−0.1, 0.8] | 0.3 [−0.1, 2.6] | 1.0 [0.4, 1.3] | −0.2 [−0.6, 0] | 0.4 [−0.2, 1.3] | 0.8 (−0.8, 2.5) | |
| Refined grains | 4.3 [3.7, 7.5] | −1.2 [−5.8, 0.5] | −2.0 [−3.9, 1.9] | 3.2 [1.4, 4.7] | 0.2 [−1.1, 3.2] | 0.4 [−0.9, 2.0] | −1.3 (−3.8, 1.2) | |
| Fish | 1.2 [0.6, 3.1] | −1.2 [−2.7, 0] | 0 [−1.2, 3.1] | 1.0 [0, 1.3] | 0 [−0.3, 0.2] | 0.9 [0, 1.1] | −0.4 (−1.7, 0.9) | |
| Processed meats | 0.4 [0, 1.8] | 0 [−1.1, 0.2] | 0 [−1.8, 0.2] | 0.2 [0, 1.0] | 0.1 [0, 0.4] | 0 [−0.3, 0] | 0.1 (−0.5, 0.7) | |
| Sugar-sweetened beverages | 0.1 [0, 0.3] | 0 [−0.3, 0] | 0 [−0.1, 0.1] | 0.2 [0, 0.7] | −0.1 [−0.4, 0] | 0 [−0.7, 0] | 0 (−0.2, 0.2) | |
| Alcohol | 1.2 [0, 4.4] | 0 [−0.4, 0.6] | −0.7 [−1.6, 0] | 0.7 [0, 1.7] | 0 [−0.3, 0.9] | 0.2 [0, 0.6] | 0 (−2.1, 2.0) | |
Difference in means is intervention group minus the control group.
21 control participants opted to receive the intervention from 12 to 24 weeks; 20 of whom had diet record data available at 24 weeks.
We calculated the percentage of participants who met our target recommendations at each time point by arm (Figure 3). Overall, adherence to the recommendations was low. The recommendation to avoid or limit alcohol to moderate levels had the highest adherence rates at 80% and 76% in the intervention and control arms, respectively, at enrollment. Only one participant in the intervention arm met the recommendation to consume 5 servings/d of vegetables at enrollment; no participant achieved this target at 12 or 24 weeks. We observed modest improvements in the percentages of people meeting recommendations for whole grains (0, 12, and 24 week percentages, intervention: 0%, 16%, 12%; control: 4%, 0%, 16%); fish in the intervention arm (56% at enrollment and 68% at 12 weeks); and sugar-sweetened beverages in the intervention arm (36%, 48%, and 52% at 0, 12 and 24 weeks).
Figure 3. Adherence to the target dietary recommendations among 50 people with colorectal cancer, by study arm and time point.
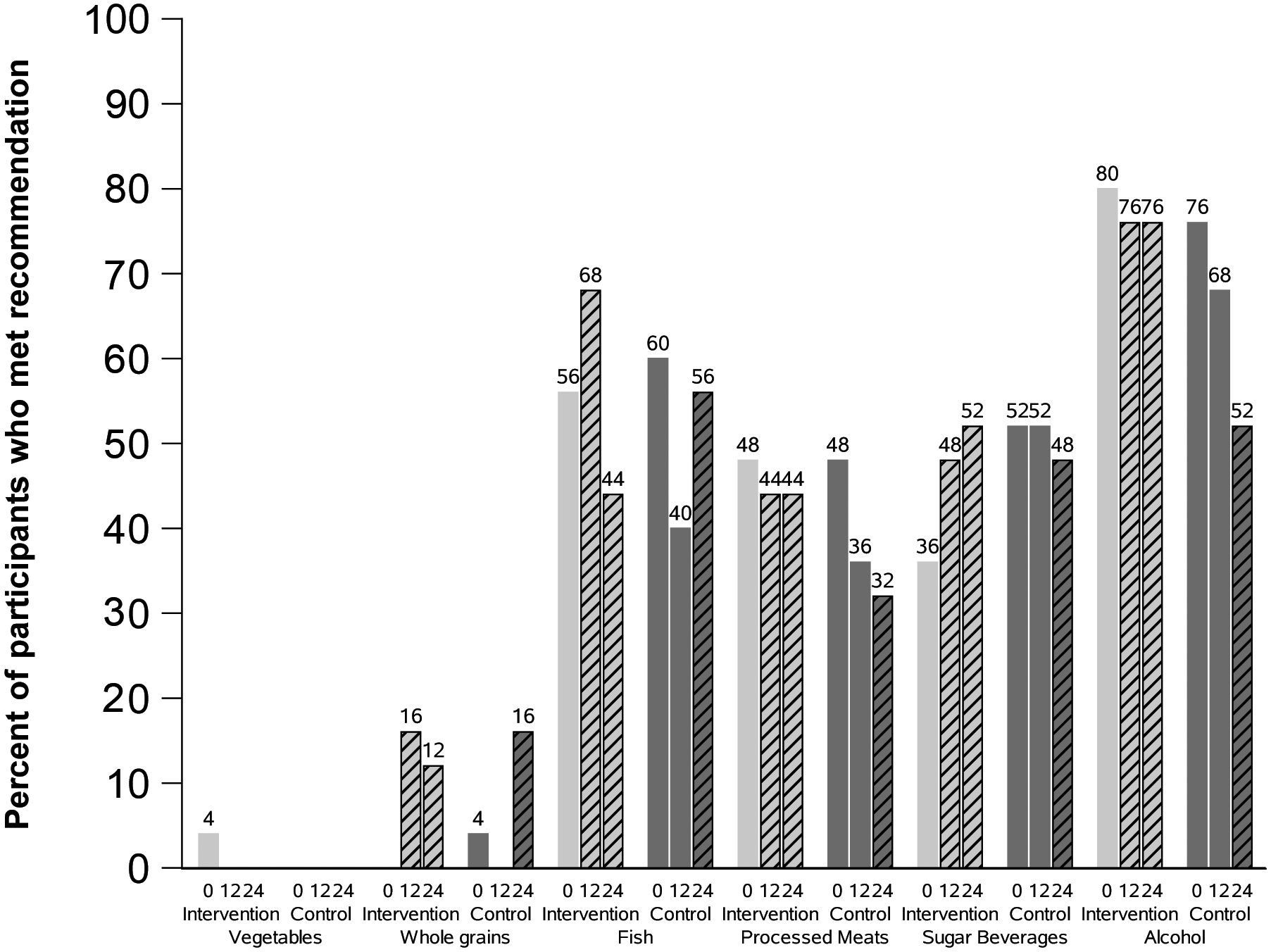
Figure 3 shows the percentage of participants who met the target intake of each of the six food groups at enrollment, 12, and 24 weeks. Light gray bars are for the intervention group and dark gray bars are for the control group. Slashed bars indicate post-intervention time points (12 and 24 weeks for the intervention arm and 24 weeks for the wait-list control arm).
Change in anthropometric and blood values
Anthropometric and blood values at 0 and 12 weeks are listed in Supplemental Table 3. On average, participants did not lose weight or waist circumference during the study. Compared to controls, which had no improvements, the intervention arm appeared to have lower systolic blood pressure at 12 weeks (difference in means: −7 mmHg; 95% CI: −14, 1).
DISCUSSION
We developed a remotely delivered web-based dietary intervention for CRC survivors. The text message program met the pre-specified criteria for feasibility (>70% adherence), but the study website with a simple diet tracking tool did not. While use of the website was less than intended, intervention participants appeared to increase whole grain consumption.
Our study had a number of strengths. We targeted our intervention for CRC survivors, because diet may impact CRC outcomes and this cancer site has been under-represented in past dietary interventions (3,26). Additionally, we enrolled a fairly diverse study population including 30% non-white and 34% men. Most of the research on lifestyle interventions for cancer survivors has been conducted in white women with breast cancer (26). However, women were still over-represented in our study population compared to all people diagnosed with CRC. Our study population was also younger and had a high level of education, which may limit the generalizability of the findings. Future studies are needed to determine the intervention’s feasibility and acceptability in populations with more men, older individuals, and/or less education.
The ACS recommends five servings/day of fruits and vegetables. We focused on vegetables only, due to literature suggesting that carbohydrates may be associated with an increased risk of CRC recurrence and death (32). However, given that only one participant met the goal of 5 serving/d of vegetables at enrollment and no participants met the goal at 12 or 24 weeks, a lower target for vegetable intake may be more reasonable for this population. A pilot trial of 17 overweight/obese adults suggest that a 12-week digital health intervention targeting only vegetable intake was successful for increasing vegetable consumption (33). It is possible sequential diet goals, where participants are only asked to change one thing at a time, would be more successful for increasing overall healthy diet in the long term.
We did not include recommendations on food quantity or calorie intake in our intervention. Data on body weight and survival after CRC are mixed, and it is not known if calorie restriction is beneficial in CRC survivors (34). Accordingly, we did not see a change in body weight in the intervention arm from 0 to 12 weeks, suggesting participants did not change their calorie intake while participating in the study.
While the intervention appeared feasible and acceptable, a number of aspects could be improved. The optimal frequency of website use/diet tracking to achieve dietary change is not known (35,36). It is possible that less frequent tracking is needed to change intake of food groups, such as whole grains, compared to managing calorie intake for weight loss. Additionally, a key area for improvement is personalization of text messages. Advances in technology, including artificial intelligence, hold promise for tailoring messages while maintaining scalability (37). In addition, it would be of interest to test the independent effect of text messages in a future study using a multi-arm or factorial design.
In conclusion, we developed a web-based dietary intervention for CRC survivors. Our pilot results suggest CRC survivors may engage more with text messages than a study website. Research to improve tailoring of text messages, while maintaining scalability, is needed.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patient advocates; Greta Macaire, RD; and the team at UCSF School of Medicine (SOM) Tech and Y3TI for their help developing the SUCCEED intervention. Research reported in this publication was supported by the National Cancer Institute of the NIH under Award Number K07CA197077 to E Van Blarigan. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians 2014;64(4):252–71 doi 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.SEER. 2016. February 24 SEER Stat Fact Sheets: Prostate Cancer. National Cancer Institute; <seer.cancer.gov/statfacts/html/prost.html>. Accessed 2016 February 24. [Google Scholar]
- 3.Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol 2015;33(16):1825–34 doi 10.1200/JCO.2014.59.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62(4):243–74 doi 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 5.Van Blarigan EL, Fuchs CS, Niedzwiecki D, Zhang S, Saltz LB, Mayer RJ, et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol 2018;4(6):783–90 doi 10.1001/jamaoncol.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinter MA, McCullough ML, Gapstur SM, Campbell PT. Associations of Pre- and Postdiagnosis Diet Quality With Risk of Mortality Among Men and Women With Colorectal Cancer. J Clin Oncol 2018:JCO1800714 doi 10.1200/JCO.18.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg DM, Levine EL, Askew S, Foley P, Bennett GG. Daily text messaging for weight control among racial and ethnic minority women: randomized controlled pilot study. J Med Internet Res 2013;15(11):e244 doi 10.2196/jmir.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw RJ, Bosworth HB, Hess JC, Silva SG, Lipkus IM, Davis LL, et al. Development of a Theoretically Driven mHealth Text Messaging Application for Sustaining Recent Weight Loss. JMIR Mhealth Uhealth 2013;1(1):e5 doi 10.2196/mhealth.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadmus-Bertram L, Wang JB, Patterson RE, Newman VA, Parker BA, Pierce JP. Web-based self-monitoring for weight loss among overweight/obese women at increased risk for breast cancer: the HELP pilot study. Psychooncology 2013;22(8):1821–8 doi 10.1002/pon.3219. [DOI] [PubMed] [Google Scholar]
- 10.Spring B, Duncan JM, Janke EA, Kozak AT, McFadden HG, DeMott A, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med 2013;173(2):105–11 doi 10.1001/jamainternmed.2013.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, et al. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Archives of internal medicine 2012;172(10):789–96 doi 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyles H, McLean R, Neal B, Doughty RN, Jiang Y, Mhurchu CN. Using mobile technology to support lower-salt food choices for people with cardiovascular disease: protocol for the SaltSwitch randomized controlled trial. BMC Public Health 2014;14:950 doi 10.1186/1471-2458-14-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumpkins CY, Mabachi N, Lee J, Pacheco C, Greiner KA, Geana M. A Prescription for Internet Access: Appealing to Middle-Aged and Older Racial and Ethnic Minorities Through Social Network Sites to Combat Colorectal Cancer. Health Commun 2017;32(7):916–20 doi 10.1080/10410236.2016.1195679. [DOI] [PubMed] [Google Scholar]
- 14.Selsky C, Luta G, Noone AM, Huerta EE, Mandelblatt JS. Internet access and online cancer information seeking among Latino immigrants from safety net clinics. J Health Commun 2013;18(1):58–70 doi 10.1080/10810730.2012.688248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81 doi 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute; 2014 Automated Self-Administered 24-Hour Recall (ASA24)-2014. [Google Scholar]
- 17.Kirkpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE, Kahle LL, et al. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. The American journal of clinical nutrition 2014;100(1):233–40 doi 10.3945/ajcn.114.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park Y, Dodd KW, Kipnis V, Thompson FE, Potischman N, Schoeller DA, et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am J Clin Nutr 2018;107(1):80–93 doi 10.1093/ajcn/nqx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman M, Huang TTK, Breland JY. Design Thinking in Health Care. Prev Chronic Dis 2018;15:E117 doi 10.5888/pcd15.180128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Agriculture and U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2010. Washington, D.C.2010. December 2010. [Google Scholar]
- 21.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Archives of internal medicine 2012;172(7):555–63 doi 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs MA, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS ONE 2014;9(6):e99816 doi 10.1371/journal.pone.0099816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 2007;298(7):754–64 doi 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 24.Pinto BM, Floyd A. Theories underlying health promotion interventions among cancer survivors. Semin Oncol Nurs 2008;24(3):153–63 doi 10.1016/j.soncn.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Bandura A Human agency in social cognitive theory. Am Psychol 1989;44(9):1175–84. [DOI] [PubMed] [Google Scholar]
- 26.Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv 2015;9(2):305–38 doi 10.1007/s11764-014-0413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol 2013;31(18):2313–21 doi 10.1200/jco.2012.45.5873. [DOI] [PubMed] [Google Scholar]
- 28.Van Blarigan EL, Chan H, Van Loon K, Kenfield SA, Chan JM, Mitchell E, et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): a pilot randomized controlled trial. BMC Cancer 2019;19(1):218 doi 10.1186/s12885-019-5427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett WC. Nutritional Epidemiology. Oxford University Press; 1998. [Google Scholar]
- 30.ASA24 2018. Reviewing and cleaning ASA24 data. <https://epi.grants.cancer.gov/asa24/resources/asa24-data-cleaning.pdf>.
- 31.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239 doi 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyerhardt JA, Sato K, Niedzwiecki D, Ye C, Saltz LB, Mayer RJ, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst 2012;104(22):1702–11 doi 10.1093/jnci/djs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mummah SA, Mathur M, King AC, Gardner CD, Sutton S. Mobile Technology for Vegetable Consumption: A Randomized Controlled Pilot Study in Overweight Adults. JMIR Mhealth Uhealth 2016;4(2):e51 doi 10.2196/mhealth.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol 2008;26(25):4109–15 doi 10.1200/jco.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc 2011;111(1):92–102 doi 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner-McGrievy GM, Dunn CG, Wilcox S, Boutte AK, Hutto B, Hoover A, et al. Defining Adherence to Mobile Dietary Self-Monitoring and Assessing Tracking Over Time: Tracking at Least Two Eating Occasions per Day Is Best Marker of Adherence within Two Different Mobile Health Randomized Weight Loss Interventions. J Acad Nutr Diet 2019. doi 10.1016/j.jand.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oka R, Nomura A, Yasugi A, Kometani M, Gondoh Y, Yoshimura K, et al. Study Protocol for the Effects of Artificial Intelligence (AI)-Supported Automated Nutritional Intervention on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Diabetes Ther 2019;10(3):1151–61 doi 10.1007/s13300-019-0595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


