Abstract
Bacterial biofilms are formed by the complex but ordered regulation of intra- or inter-cellular communication, environmentally responsive gene expression, and secretion of extracellular polymeric substances. Given the robust nature of biofilms due to the non-growing nature of biofilm bacteria and the physical barrier provided by the extracellular matrix, eradicating biofilms is a very difficult task to accomplish with conventional antibiotic or disinfectant treatments. Synthetic biology holds substantial promise for controlling biofilms by improving and expanding existing biological tools, introducing novel functions to the system, and re-conceptualizing gene regulation. This review summarizes synthetic biology approaches used to eradicate biofilms via protein engineering of biofilm-related enzymes, utilization of synthetic genetic circuits, and the development of functional living agents. Synthetic biology also enables beneficial applications of biofilms through the production of biomaterials and patterning biofilms with specific temporal and spatial structures. Advances in synthetic biology will add novel biofilm functionalities for future therapeutic, biomanufacturing, and environmental applications.
Keywords: biofilm control, synthetic biology, genetic circuit, protein engineering, quorum sensing, quorum quenching
Graphical Abstract
Synthetic biology can enable the eradication of harmful biofilms and the development of beneficial biofilms.
1. Introduction
Biofilms are sessile microbial aggregates resulted from cooperation and competition between microbes (Dang and Lovell, 2005; Elias and Banin, 2012; Nadell et al., 2016) within a self-produced matrix of extracellular polymeric substances (EPS) composed of polysaccharides, proteins, lipids and nucleic acids that enhance surface adherence and microbial aggregation (Costa et al., 2018; Flemming and Wingender, 2010). Typically, biofilm formation causes detrimental effects in various areas including industrial manufacturing (Xu et al., 2017), the environment (Beech and Sunner, 2004; Scheerer et al., 2009), food safety (Zhao et al., 2017), and health (Miquel et al., 2016). Many chronic infections are closely related to the biofilm state (Costerton et al., 1999; Lebeaux et al., 2014), and bacterial colonization of medical devices and implants such as catheters, contact lenses, mechanical cardiac valves, and dental implants can lead to device-related infections (Costerton et al., 2005; Stoodley et al., 2013). Biofilms formed on industrial production lines, heat exchangers, and working surfaces lead to corrosion and damage to machinery, as well as contamination of raw materials and products (Jia et al., 2019; Y. Li et al., 2018). In addition, biofilms formed in food processing facilities can contaminate food products (Brooks and Flint, 2008; Galié et al., 2018), contributing to foodborne outbreaks (Srey et al., 2013).
Biofilms serve to protect bacteria from antimicrobial agents by forming physical barriers composed of EPS that reduce the diffusion of toxic compounds and by slowing bacterial growth inside the biofilms, which mitigates the efficacy of antimicrobial agents (Mah and O’Toole, 2001). Although mechanical brushing and cleaning can effectively remove biofilms from accessible surfaces (Berger et al., 2018; González-Rivas et al., 2018), it is difficult or impossible to access biofilm-colonized surfaces in many cases. For example, biofilms on indwelling medical devices (Khatoon et al., 2018), industrial pipes (Liu et al., 2014), and food processing equipment (González-Rivas et al., 2018) are not easily accessible and require advanced physical, chemical, and biological methods for eradication. Advanced physical methods, such as pulsed electric (del Pozo et al., 2009; Khan et al., 2016), pulsed light (Garvey et al., 2015), magnetic (Geilich et al., 2017; H. Park et al., 2011), sonication (Baumann et al., 2009; Bjerkan et al., 2009), and cold plasma (Abramzon et al., 2006; Gilmore et al., 2018) approaches, have been used to remove or destroy surface biofilms. Chemical treatments, including the use of surfactants (Percival et al., 2017; Simões et al., 2005; Splendiani et al., 2006), disinfectants [e.g., chlorine (Kim et al., 2008; Lee et al., 2011) and hydrogen peroxide (Lin et al., 2011; Lineback et al., 2018)], and antibiotics (Ciofu et al., 2017), have also been applied to control biofilms. Biological approaches for biofilm control (Roy et al., 2018) include interfering with signaling pathways via quorum sensing (QS) (e.g., autoinducers) (Boles and Horswill, 2008; Brackman and Coenye, 2014; Hammer and Bassler, 2003; Herzberg et al., 2006; McNab et al., 2003) or secondary messenger molecules [e.g., cyclic di-guanosine monophosphate (c-di-GMP)] (Arora et al., 2015; Barraud et al., 2015; Valentini and Filloux, 2016), inhibiting stringent responses [e.g., alarmone (p)ppGpp] (Chávez de Paz et al., 2012; de la Fuente-Núñez et al., 2014), dispersing extracellular polymeric components by enzymatic disruption (Powell et al., 2018; Xavier et al., 2005), cleaving peptidoglycan [e.g., transglycosylase (Stapleton et al., 2007) and endolysin (Shen et al., 2013)], and altering the membrane potential or permeabilization [e.g., lantibiotics (Mathur et al., 2018) and polymyxins (Lima et al., 2019; S. C. Park et al., 2011)]. Furthermore, surface materials with anti-biofilm coatings (Cattò and Cappitelli, 2019) and smart antibacterial surfaces (X. Li et al., 2018) have been developed for anti-biofilm strategies. Various anti-biofilm compounds, including natural products [essential oils (Jafri et al., 2019) or fatty acids (Marques et al., 2015; Thibane et al., 2010)] and synthesized nanoparticles (Allaker, 2010; Mi et al., 2018), have been investigated for the inhibition or dispersion of biofilms.
Synthetic biology is the intersection of biology and engineering and has been harnessed to engineer commensal and probiotic bacteria as genetically programmable sensors and drug delivery devices (Bradley et al., 2016; Duan et al., 2015; Maxmen, 2017) and incorporate synthetic metabolic pathways to produce useful chemicals ranging from biofuels, foods, and pharmaceuticals in the form of microbial consortia (Carocho and Ferreira, 2013; Jia et al., 2016; Volke and Nikel, 2018). Tools employing synthetic biology approaches are also used to investigate the organization of biofilms, uncover the mechanisms of actions of anti-biofilm agents and design strategies to combat biofilms (Brenner and Arnold, 2011; Hong et al., 2012; Hwang et al., 2017). By understanding the formation of microbial consortia, we can design and engineer microbial ecosystems for biomedical, industrial and biotechnological purposes. Recent seminal reviews have summarized the synthetic biology tools used to engineer microbial communities (Bittihn et al., 2018; Jia et al., 2016; Kong et al., 2018). Here, we specifically focus on reviewing synthetic biology tools and strategies to eradicate and engineer biofilms.
2. Biofilms and Signaling Molecules
2.1. Biofilm development and persistence
Biofilms develop through the cellular processes of initial reversible and irreversible attachment, microcolony formation, and maturation. When biofilms become sufficiently mature, single planktonic cells are dispersed from the biofilms (Costerton et al., 1999) (Fig. 1A). Biofilm development involves the regulation of hundreds of biofilm-specific genes including those related to stress responses, QS, motility, cell-surface appendages, metabolism, and transport (Domka et al., 2007). Biofilm communities release diverse inter- and intra-cellular signaling molecules that directly affect the population and dynamic structure of biofilms (Giaouris et al., 2015; Karatan and Watnick, 2009). These bioactive compounds range from small signaling molecules known as autoinducers, D-amino acids, and metabolites, to higher-order proteins that mediate bacterial interactions (Karatan and Watnick, 2009; Kostakioti et al., 2013). As a result, biofilms are quite robust and frequently require costly, repetitive physical and chemical treatment applications for removal. Biofilms are typically treated through the external addition of disinfectants or antimicrobials, unless physical debridement, such as mechanical brushing, is used (Berger et al., 2018; González-Rivas et al., 2018). However, external biocide addition shows very limited efficacy, mainly because of the mass transfer limitation in complex biofilms mixed with EPS, as well as the nonmetabolizing nature of the cells inside biofilms (Anderson and O’Toole, 2008), which survive under high concentrations of antibiotics. Compared with planktonic counterparts, biofilms are 10- to 1,000-fold more resistant to various antimicrobials (Davies, 2003). Therefore, novel approaches are required to eradicate biofilm bacteria.
Figure 1. Biofilm formation and signaling.
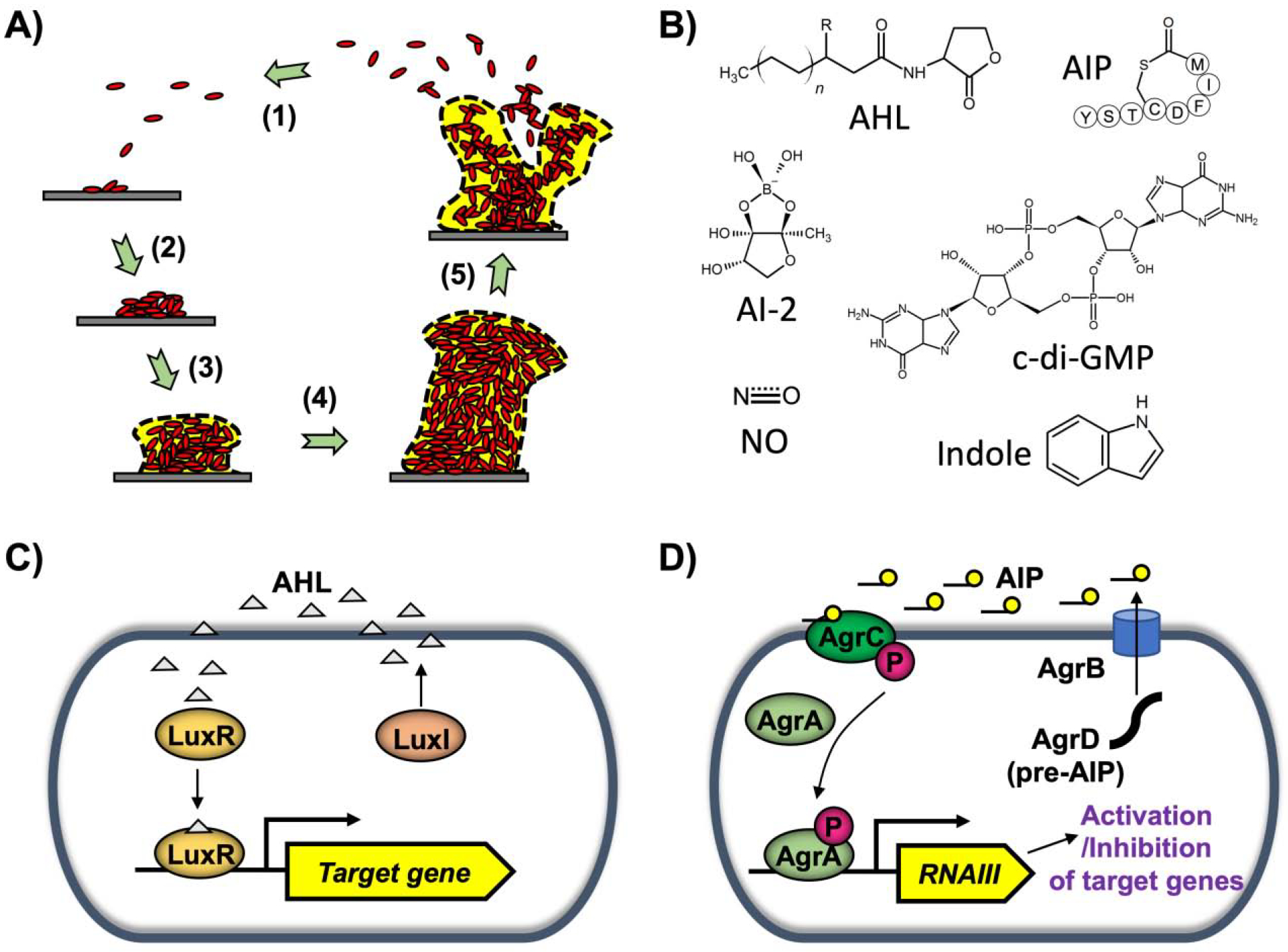
A) Biofilm developmental stages: 1) attachment, 2) cell-to-cell adhesion, 3) proliferation, 4) maturation, and 5) dispersal. B) Signaling molecules involved in biofilm formation: acylhomoserine lactone (AHL), autoinducing peptide (AIP), autoinducer-2 (AI-2), cyclic di-guanosine monophosphate (c-di-GMP), indole, and nitric oxide (NO). C) Gram-negative quorum sensing (QS). In V. fischeri, LuxI synthesizes 3oC6HSL (AHL). LuxR forms a complex with AHL, and the complex activates target gene expression. D) Gram-positive QS. In S. aureus, AgrD is processed to form AIP. Upon sensing AIP, AgrC phosphorylates AgrA, which in turn induces RNAIII production. RNAIII activates or inhibits target gene expression.
2.2. Regulation of biofilm formation via signaling molecules
Diverse signaling molecules are involved during bacterial biofilm formation (Fig. 1B). QS is a cell-cell communication process in bacteria mediated by the production and detection of extracellular chemicals known as autoinducers (Popat et al., 2015; Waters and Bassler, 2005). QS allows bacteria to coordinate their gene expression in a population-driven manner. Acyl-homoserine lactones (AHLs) are a major autoinducer signal mediating QS in Gram-negative bacteria (Papenfort and Bassler, 2016). The LuxI/LuxR system of Vibrio fischeri is known as a classical AHL QS system (Fuqua et al., 1994) (Fig. 1C). LuxI synthesizes the autoinducer N-(3-oxo-hexanoyl)-L-homoserine lactone (3oC6HSL), and LuxR forms a complex with 3oC6HSL, resulting in broad gene expression activation (Fuqua et al., 2001; Kumar and Rajput, 2018). In contrast, Gram-positive bacteria use modified oligopeptides as autoinducers, which are detected by membrane-bound two-component signaling proteins that transduce information via a series of phosphorylation events (Kleerebezem et al., 1997). The agr (accessory gene regulator) system of Staphylcoccus aureus is an example of a QS system in Gram-positive bacteria (Queck et al., 2008) (Fig. 1D). agrD encodes a propeptide possessing the autoinducing peptide (AIP) signal sequence (Zhang et al., 2002). The propeptide is processed by cleavage of the N-terminal signal peptide by S. aureus signal peptidase B (SpsB) and C-terminal tail by AgrB, and the mature AIP is then secreted into the extracellular environment (Kavanaugh et al., 2007). Sensor transmembrane histidine kinase AgrC and its cognate response regulator AgrA constitute a classical bacterial two-component signal transduction system. Once AIP binds to AgrC, AgrA is phosphorylated and subsequently binds to P2 and P3 promoter regions. This enables RNAII production that further triggers AIP synthesis, along with induction of RNAIII that regulates genes related to virulence, biofilm formation, and other processes (Koenig et al., 2004; Queck et al., 2008). Such QS signaling plays an important role in biofilm formation (Boles and Horswill, 2008). In V. cholerae and S. aureus, increased cell density inhibits biofilm formation (Boles and Horswill, 2008; Hammer and Bassler, 2003), while activation of QS circuits (two LuxI/R-type QS circuits, LasI/R and RhlI/R) in Pseudomonas aeruginosa stimulates biofilm formation (Duan and Surette, 2007). Autoinducer-2 (AI-2) is a species-nonspecific autoinducer produced by both Gram-negative and Gram-positive bacteria (Schauder and Bassler, 2001). It is synthesized by S-ribosylhomocysteine lyase (LuxS), which converts S-ribosylhomocysteine to homocysteine and (S)-4,5-dihydroxy-2,3-pentanedione (DPD). DPD is then processed into AI-2 molecules (Xavier et al., 2007). AI-2 was studied in regulations of intra- and inter- species biofilms. In the environment of dental plaque, hundreds of bacterial species constitute mixed-species biofilms, and Streptococcus gordonii is a main colonizer among them as AI-2 production from S. gordonii on teeth induces the consecutive colonization by other bacteria such as Porphyromonas gingivalis (McNab et al., 2003). The addition of AI-2 leads to an increase in biofilm formation in E. coli (Herzberg et al., 2006). In addition, AI-2 from Klebsiella pneumoniae could promote its early biofilm formation (Balestrino et al., 2005).
Another common signaling molecule is c-di-GMP, a ubiquitous second messenger present in almost all bacteria. c-di-GMP is the central regulator of biofilm formation, as it mediates the switch between the motile and sessile forms of bacteria (Valentini and Filloux, 2016). c-di-GMP is synthesized from two guanosine-5’-triphosphate molecules by diguanylate cyclases (DGCs), and is degraded into 5’-phosphoguanylyl-(3’−5’)-guanosine and guanosine monophosphate by phosphodiesterases (PDEs). Various microorganisms are reported to express multiple DGC and PDE enzymes (Hengge, 2009; Römling et al., 2013; Sondermann et al., 2012). This enzymatic redundancy might be beneficial to bacteria through each enzyme’s specific activation and inactivation in response to different environmental conditions.
Indole is an intercellular signaling molecule produced from tryptophan by the enzyme tryptophanase TnaA (Lee and Lee, 2010). Indole has diverse roles including in spore formation, plasmid stability, drug resistance, biofilm formation, and virulence in indole-producing bacteria. The effect of indole on biofilm formation is controversial. Indole was initially reported to enhance biofilm formation in E. coli S17–1. However, indole inhibits biofilm formation in nine nonpathogenic E. coli as well as the pathogenic E. coli O157: H7 strain. Indole was recently reported to repress persister cells, which are metabolically dormant cell populations (J. H. Lee et al., 2016).
2.3. Regulation of biofilm dispersal via signaling molecules
Nitric oxide (NO) is a simple gas and a biological signaling molecule found to induce biofilm dispersal across a wide range of bacterial species (Arora et al., 2015; Barraud et al., 2015). Because of the broad-spectrum anti-biofilm effects of NO, NO-releasing materials and prodrugs have also been explored (Barraud et al., 2012; Hetrick et al., 2009). Increased understanding of the role of NO in biofilm formation through its regulation of intracellular c-di-GMP concentrations, QS, and cellular nitrogen metabolism has helped reveal the action mechanism of known drugs and identify novel targets for drug development (Rinaldo et al., 2018). NO sensors such as H-NOX (heme-nitric oxide/oxygen binding) or NosP (nitric oxide sensing protein) affect biofilm formation by regulating c-di-GMP concentrations and QS (Hossain and Boon, 2017; Rinaldo et al., 2018). Understanding H-NOX and NosP mechanisms in bacteria could lead to better control of bacterial biofilms and biofilm-related infections (Williams et al., 2018).
Natural amino acids predominantly participating in protein synthesis are in the L-form, while D-amino acids are found in the cell walls of bacteria. Recently, D-amino acids have been demonstrated to act as regulatory signals for cell wall remodeling and biofilm disassembly (Cava et al., 2011; Kolodkin-Gal et al., 2010). In living organisms, D-amino acids are synthesized by the action of racemases that convert amino acids from L-form to D-form (Tanner, 2002). D-amino acids disperse biofilms by interfering with the anchoring of amyloid fibers that link biofilm cells together (Kolodkin-Gal et al., 2010; Oppenheimer-Shaanan et al., 2013) and prevent biofilm formation by altering the cell wall composition (Bucher et al., 2015). Furthermore, mixtures of D-amino acids have been shown to promote biocide treatments against biofilm communities in a water-cooling tower (Jia et al., 2017) and to reduce biofilms in dental unit waterlines (Ampornaramveth et al., 2018). Due to the distinctive mechanisms and biological roles of D-amino acids (Aliashkevich et al., 2018), the application of D-amino acids is an appealing anti-biofilm approach, either alone or in combination with established antimicrobials.
It should be noted that during biofilm formation, the synthesis and degradation of inter- and intracellular signaling molecules are regulated in response to key environmental factors such as temperature (Lee et al., 2008; Lee and Lee, 2010; Townsley and Yildiz, 2015), pH (Chopp et al., 2003; Lee and Lee, 2010), osmotic pressure (Hengge, 2008; Valverde and Haas, 2008), and nutrient conditions (Stanley and Lazazzera, 2004). Hence, signaling molecules are excellent candidates for controlling biofilm formation and eradication.
3. Synthetic Biology Approaches
With an enhanced understanding of biofilms (Flemming et al., 2016) and a growing synthetic biology toolkit (Bittihn et al., 2018; Brenner et al., 2008; Jia et al., 2016), the ability to control biofilms (Wood et al., 2011) continues to expand. An important strategy in controlling biofilms is based on the ability of molecules produced inside biofilms to bypass the mass transport barriers created by extracellular polymeric substances, thereby reaching concentrations sufficiently high to regulate target biofilms. This approach may address numerous biofilm-associated challenges in environmental, agricultural, industrial, and medical areas. The biofilm eradication strategies using protein engineering and synthetic biology are summarized below.
3.1. Protein engineering of biofilm-controlling enzymes
Protein engineering is a potential strategy to enhance the activity of global regulator proteins related to biofilm formation (Fig. 2A). H-NS (histone-like nucleoid structuring protein) represses transcription by recognizing curved DNA sequences and was the first engineered regulator used to control biofilm formation without signaling molecules (Hong et al., 2010b). The variant H-NS K57N was found to reduce biofilm formation, showing an opposite function compared to the biofilm-promoting activity of wild-type H-NS (Hong et al., 2010b). Another global regulator Hha (high hemolysin activity) was engineered to promote biofilm dispersal, resulting in nearly complete biofilm dispersal (Hong et al., 2010a). Proteins with the ability to bind signaling molecules have been engineered for controlled biofilm formation and enhanced dispersal. E. coli does not produce AHLs because it lacks an AHL synthase, but it senses AHL signals through the AHL receptor SdiA, a homologue of LuxR (Dyszel et al., 2010). SdiA was engineered via random and site-directed mutagenesis to regulate biofilm formation in the presence of AHLs or indole (Lee et al., 2009). Like E. coli, the foodborne pathogen Salmonella enterica does not produce AHL signals but does contain the receptor SdiA for AHL, which regulates S. enterica adhesion as well as resistance to host immune responses (Bai and Rai, 2016). BdcA was identified as a c-di-GMP-binding protein and engineered to increase biofilm dispersal through a single amino acid replacement at E50Q (Ma et al., 2011a). In addition, BdcA of E. coli was found to control biofilm dispersal in P. aeruginosa and Rhizobium meliloti (Ma et al., 2011b). Therefore, protein engineering of global regulators or signaling molecule-binding proteins enables enhanced biofilm eradication or can be used to modulate the microbial activity of biofilm formation.
Figure 2. Biofilm cell killing and eradication.
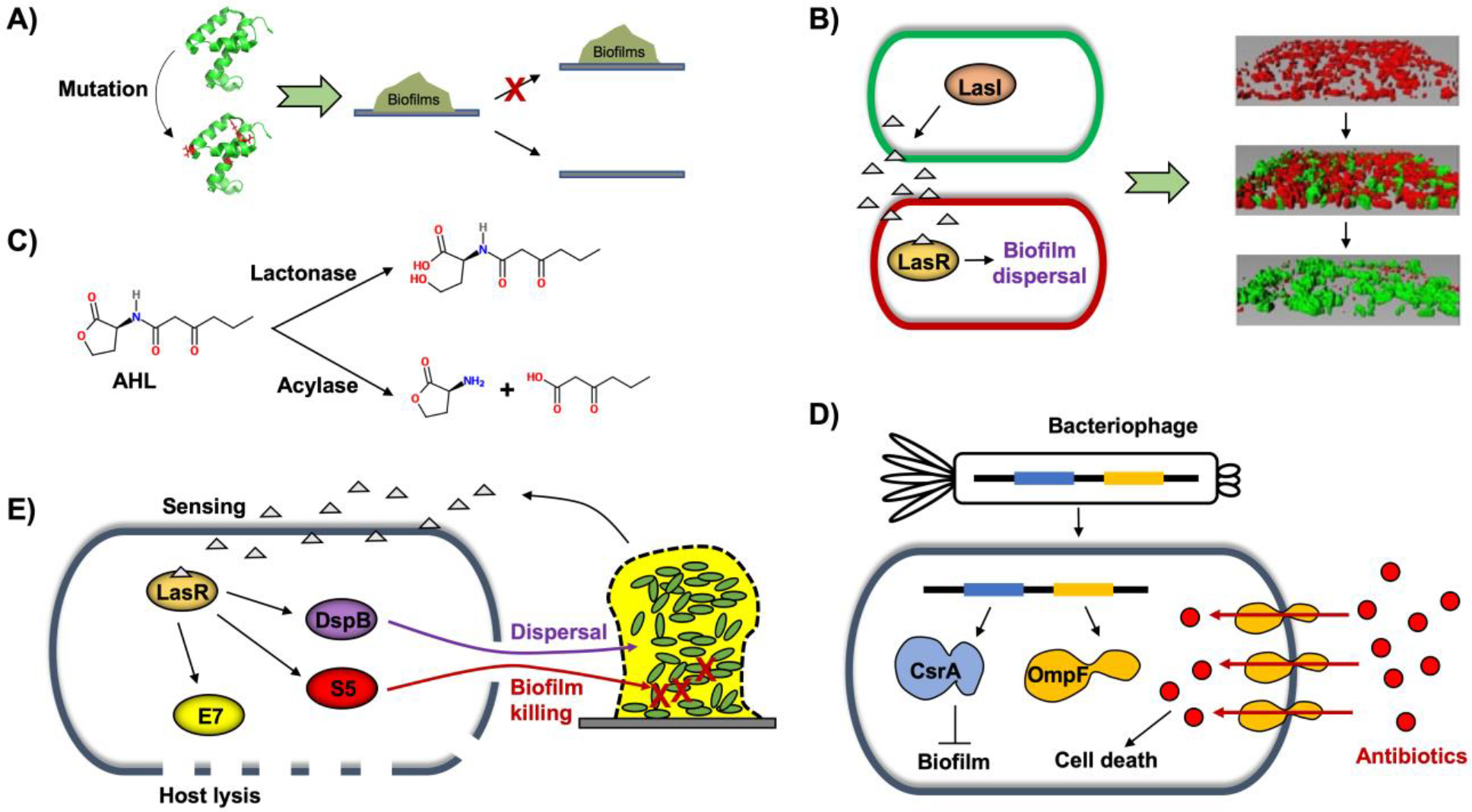
A) Protein engineering via random or site-directed mutagenesis to induce biofilm dispersal. B) Synthetic QS genetic circuit to enable biofilm displacement. LasI in the green cell produces AHL, and the LasR/AHL complex in the red cell induces biofilm dispersal [biofilm images from (Hong et al., 2012)]. C) Quorum quenching to disrupt AHL. Lactonase hydrolyzes lactone rings, and acylase cleaves acyl groups, which inhibits biofilms. D) Engineered bacteriophage for biofilm cell killing via enhanced antibiotic penetration along with biofilm inhibition via induction of the biofilm-inhibiting enzyme CsrA. E) Engineered probiotic strain to sense and kill pathogen biofilms. Colin E7 lysin (E7) disrupts the probiotic host cells, pyocin S5 (S5) kills P. aeruginosa in biofilms, and dispersin B (DspB) degrades the biofilm matrix.
3.2. Synthetic biology for eradicating biofilms
3.2.1. Quorum sensing genetic circuits
Bacterial QS systems have been important components of a wide variety of engineered biological devices. Autoinducers are useful as input signals because they diffuse freely in liquid media and penetrate cells easily (Choudhary and Schmidt-Dannert, 2010). Because the engineered cells synthesize their own QS signals, they are able to self-monitor cell density and modulate their activities without oversight (Hong et al., 2012; Ryan and Dow, 2008). Synthetic QS circuit systems have great potential in that population-driven QS switches may be utilized to develop synthetic genetic networks for a variety of applications such as to engineer bidirectional communication, construct a predator-prey ecosystem, and create a synthetic symbiotic ecosystem (Wood et al., 2011). The LasI/R and RhlI/R pairs, the two best-characterized QS systems of P. aeruginosa, have been widely used for synthetic genetic circuits. LasI produces the autoinducer molecule, N-(3-oxo-dodecanoyl)-L-homoserine lactone (3oC12HSL), which is sensed by LasR. Likewise, RhlI produces N-butyryl-L-homoserine lactone (C4HSL) that is sensed by RhlR (Pesci et al., 1997). For biofilm formation, the RhlI/R QS system was utilized to demonstrate important roles for self-organization and aggregation in a synthetic biofilm consortium. The LasI/R system in combination with the engineered biofilm-dispersal enzymes Hha and BdcA showed excellent biofilm displacement upon sensing QS signals (Hong et al., 2012). In this system, the second biofilm (disperser) is grown in the existing biofilm (colonizer), and QS signaling molecules are produced by LasI and accumulate inside the dual-species biofilm. The QS molecules form a complex with LasR, which triggers dispersal of the colonizer biofilm through increased c-di-GMP levels mediated by the BdcA variant (Fig. 2B). Then, the disperser biofilm can be disrupted by inducing the Hha variant with a chemical switch, resulting in cell death in the biofilm. The synthetic QS circuit was applied to prevent membrane biofouling and/or to degrade environmental pollutants (Wood et al., 2016). This beneficial biofilm was able to limit its own thickness on wastewater treatment membrane by secreting and sensing the signaling molecule controlling c-di-GMP levels mediated by the BdcA variant. In addition, the engineered biofilm also prevented biofilm formation by deleterious bacteria through NO generation and was able to degrade the environmental pollutant epichlorohydrin via epoxide hydrolase. Thus, the use of this beneficial biofilm enabled the development of a living biofouling-resistant membrane system. The QS circuit systems for controlling biofilms can provide insights into how beneficial biofilms can be developed to prevent or eradicate deleterious biofilms for various applications.
3.2.2. Quorum quenching enzymes
Finding ways to subvert microbes by interfering with their communication signals is important for combating antibiotic resistance and other biofilm-related situations (Marx, 2014). Quorum quenching (QQ) is the mechanism by which QS is inhibited or interrupted. One strategy here is to process, modify or degrade the signaling molecules that are required for cellular communication, thereby preventing the buildup of biofilms (Grandclément et al., 2016). The majority of QQ studies have focused on hydrolysis of N-acyl homoserine lactones using lactonases that break down lactone rings in AHLs along with acylases that cleave acyl groups (Oh and Lee, 2018) (Fig. 2C). Bacterial or enzymatic QQ has been applied for antifouling strategies in membrane bioreactors (MBRs) for wastewater treatment (Oh and Lee, 2018). For example, AHL-producing bacteria on the surface of membrane were decreased by recombinant E. coli producing lactonase AiiA from Bacillus thuringiensis (Oh et al., 2012) and AiiO from Agrobacterium tumefaciens (Oh et al., 2017). Production of EPS and expression of genes related to microbial attachment and agglomeration were found to be reduced with enzymatic QQ treatment (Kim et al., 2013). Rhodococcus erythropolis W2 was used to degrade AHLs via both its oxido-reductase and AHL-acylase activities (Uroz et al., 2005). Because AI-2 signaling molecules are secreted by both Gram-negative and Gram-positive bacteria, targeting AI-2 for QQ is another useful strategy. LsrK (luxS-regulated kinase) that phosphorylates AI-2 is considered to be a QQ enzyme, as purified LsrK with added ATP significantly decreased the AI-2 signaling of S. typhimurium, E. coli, and V. harveyi (Roy et al., 2010). Farnesol, a chemical compound secreted from Candida albicans, is effective in repressing AI-2 synthesis and mitigating biofouling in MBRs (K. Lee et al., 2016). Metagenomic approaches have been applied to find a system for modifying AI-2 (Weiland-Bräuer et al., 2016). An indigenous bacterium Acinetobacter sp. DKY-1 was found to inactivate AI-2 by secreting a hydrophilic AI-2 QQ compound with a molecular weight of less than 400 Da, but the mechanistic details remain to be determined (Lee et al., 2018). QQ enzymes or chemical compound production systems integrated into synthetic genetic circuits would enable elaborate control of biofilm prevention and eradication.
3.2.3. Bacteriophages
Bacteriophages can penetrate the inner layers of biofilms because phage depolymerases can degrade EPS components (Azeredo and Sutherland, 2008). Single-type phages (Curtin and Donlan, 2006; Pires et al., 2011) as well as multi-phage cocktails (Fu et al., 2010; Sillankorva et al., 2010) have been applied for biofilm destruction or inhibition. Bacteriophages have great potential for engineering as antimicrobial agents, vehicles for drug delivery and vaccines, and the assembly of new materials (Pires et al., 2016). Synthetic biology has been used to develop reinforced bacteriophages that can efficiently kill deleterious biofilm cells by introducing biofilm-degrading or -inhibiting enzymes or enhancing antibiotic penetration. For example, a T7 phage was engineered to produce the biofilm-degrading enzyme dispersin B (DspB) during phage infection (Lu and Collins, 2007). dspB from Actinobacillus actinomycetemcomitans was integrated into the phage genome under the T7 φ10 promoter, leading to dspB transcription by the T7 RNA polymerase upon phage infection of E. coli TG1 biofilms. Along with cell killing by the phages, DspB simultaneously attacked the biofilm matrix by hydrolyzing the biofilm-promoting adhesin β−1,6-N-acetyl-D-glucosamine of E. coli. The engineered enzymatic phage reduced the E. coli biofilm by 2 orders of magnitude compared to the wild-type non-enzymatic phage treatment. A bacteriophage was also designed to increase the antibiotic susceptibility of biofilm cells (Lu and Collins, 2009). The M13mp18 phage, a modified non-lytic filamentous M13 phage, was engineered to contain csrA that encodes a biofilm repressor CsrA with or without ompF that encodes a porin for quinolone penetration (Fig. 2D). Infection with the engineered phage enhanced the antibiotic ofloxacin’s bactericidal effect, resulting in more effective killing of the biofilm as well as planktonic cells compared to unmodified phage treatment (Lu and Collins, 2009). In order to overcome the narrow substrate specificity of biofilm-degrading enzymes (e.g., DspB), a QQ enzyme was integrated into bacteriophage that was more effective in inhibiting mixed species biofilms by disrupting AHL signals (Pei and Lamas-Samanamud, 2014). Lactonase AiiA from Bacillus sp. cleaves the lactone rings of diverse AHLs (Wang et al., 2004). A T7 bacteriophage was engineered to express aiiA controlled by the T7 φ10 promoter, and this QQ phage treatment was effective in inhibiting P. aeruginosa and E. coli dual-species biofilm formation via both cell lysis and AHL degradation (Pei and Lamas-Samanamud, 2014). Genetically engineered phages will be further developed by integrating novel biofilm inhibitory functions.
3.2.4. Probiotics
Probiotics are beneficial microbes that enhance host immunity (Hill et al., 2014) and inhibit pathogens (Ohland and MacNaughton, 2010). Probiotic bacteria also have the ability to inhibit biofilm formation (Fang et al., 2018; Shao et al., 2019; Woo and Ahn, 2013). Due to their beneficial health effects, probiotics have been considered as an engineering host for human therapeutic application. E. coli Nissle 1917 strain (EcN) is one of the best characterized probiotics and has been used for the clinical treatment of intestinal disorders (Heselmans et al., 2005; Schultz, 2008; Sonnenborn and Schulze, 2009) and engineered for enhancing live biotherapeutics such as tumor detection (Ozdemir et al., 2018), hyperammonemia treatment (Kurtz et al., 2019), and as a drug delivery vehicle (Mckay et al., 2018). For biofilms, wild-type EcN has the ability to inhibit biofilm formation of pathogenic and non-pathogenic E. coli (Fang et al., 2018; Hancock et al., 2010) as well as the Gram-positive pathogens Staphylococcus aureus and S. epidermidis in co-cultures (Fang et al., 2018). EcN was engineered to sense, kill, and inhibit pathogenic biofilms for preventing P. aeruginosa gut infection in Caenorhabditis elegans and mouse models (Hwang et al., 2017) (Fig. 2E). The alr and dadX genes in the EcN genome were knocked out to enable the mutant EcN strain to become a D-alanine auxotroph, which stabilized retention of the plasmid expressing alr. The engineered EcN contained a synthetic genetic circuit. In response to the QS molecule 3oC12HSL from P. aeruginosa, the engineered EcN produced E7 lysis protein to open the host cell, S5 pyocin to kill P. aeruginosa, and DspB to degrade the biofilm matrix. The engineered EcN with antimicrobial and anti-biofilm enzymes disrupted the existing biofilm and prevented biofilm formation of P. aeruginosa (Hwang et al., 2017). Taken together, synthetic genetic circuits can enhance the prophylactic and therapeutic activities of probiotics against biofilm-forming pathogens.
3.3. Synthetic biology for engineering biofilms
Although the elimination of deleterious biofilm cells is crucial, biofilms may have beneficial potential if their pattern, thickness, composition, and metabolism can be controlled in a tunable, spatial, and temporal manner. Engineered biofilms can be applied for bioremediation (Brune and Bayer, 2012; Mangwani et al., 2016), wastewater treatment (Karadag et al., 2015; Lewandowski and Boltz, 2011), biocorrosion control (Jia et al., 2019; Morikawa, 2006; Narenkumar et al., 2016; Zuo, 2007), biofuel production (Heimann, 2016; Hoh et al., 2016), specialty and bulk chemical biorefinery (Rosche et al., 2009; Wang et al., 2017), biomedical microelectromechanical systems (bioMEMS) devices (Fernandes et al., 2010), and pharmaceutical testing (Stewart, 2015). Synthetic genetic circuits and signaling can facilitate the design and development of such biofilm control systems.
3.3.1. Biofilm patterning
Biofilm formation requires complex gene regulation processes (Domka et al., 2007) that are difficult to manipulate when attempting to generate a desired structure or pattern. An optogenetic module was developed for microprinting biofilms (Huang et al., 2018; Ryu et al., 2017). Light-activated diguanylate (BphS) that synthesizes c-di-GMP under near-infrared light (Ryu and Gomelsky, 2014) and phosphodiesterase (BlrP1) that hydrolyzes c-di-GMP under blue light (Barends et al., 2009) were used to bidirectionally regulate c-di-GMP levels. Near-infrared light (632 nm) illumination increased the level of c-di-GMP, resulting in attachment of the cells to a cover glass surface, while blue light (434 nm) decreased the level of c-di-GMP to allow detachment. Dual-color illumination enabled biofilm patterning with a high spatial resolution (Huang et al., 2018) (Fig. 3A). Another biofilm patterning utilized the expression of membrane adhesion proteins in response to blue light (Jin and Riedel-Kruse, 2018). E. coli was engineered to contain a light-activated transcriptional promoter (pDawn) that optically controls the expression of an adhesin gene (Ag43). Upon blue light illumination, biofilm formation was increased and optically patterned with a 25 μm spatial resolution. Furthermore, a photoswitchable interaction between nMag and pMag proteins (Kawano et al., 2015) was also developed to control bacterial adhesion (Chen and Wegner, 2017) (Fig. 3B). pMag protein was produced on the surface of E. coli in the presence of blue light to allow the engineered strain to adhere to the immobilized nMag protein on the material surface. This adhesion was reversible. The binding was released in the dark, allowing tunable and biorthogonal control (Chen and Wegner, 2017). The ability to maintain biofilm levels at a desired thickness is important for bioremediation and bioproduction (Zhang and Poh, 2018). The CRISPRi/dCas9 system was applied to control the expression of the wcaF gene involved in the synthesis of colanic acid, a key EPS component in E. coli biofilm formation. Depending on the level of the guide RNA (gRNA) controlled by a chemical inducer, wcaF gene expression was regulated by gRNA-dCas9 binding to the chromosomal wcaF locus. Temporal induction resulted in different levels of biofilm thickness. When the circuit was combined with the blue light-mediated expression system, biofilm thickness could be controlled by switching the light. Furthermore, production of the antimicrobial peptide nisin was utilized to achieve robust and tunable spatial structures (Kong et al., 2017). The external nisin gradient resulted in no fluorescence or cell death at a low nisin concentration, fluorescence induction without killing the cells at medium nisin level, and cell death without fluorescence at a high level of nisin, which created band-pass patterns. Mixed nisin producer and responder species generated dynamic spatial structures consistent with the computational model (Kong et al., 2017).
Figure 3. Biofilm utilization for patterning and biomaterial production.
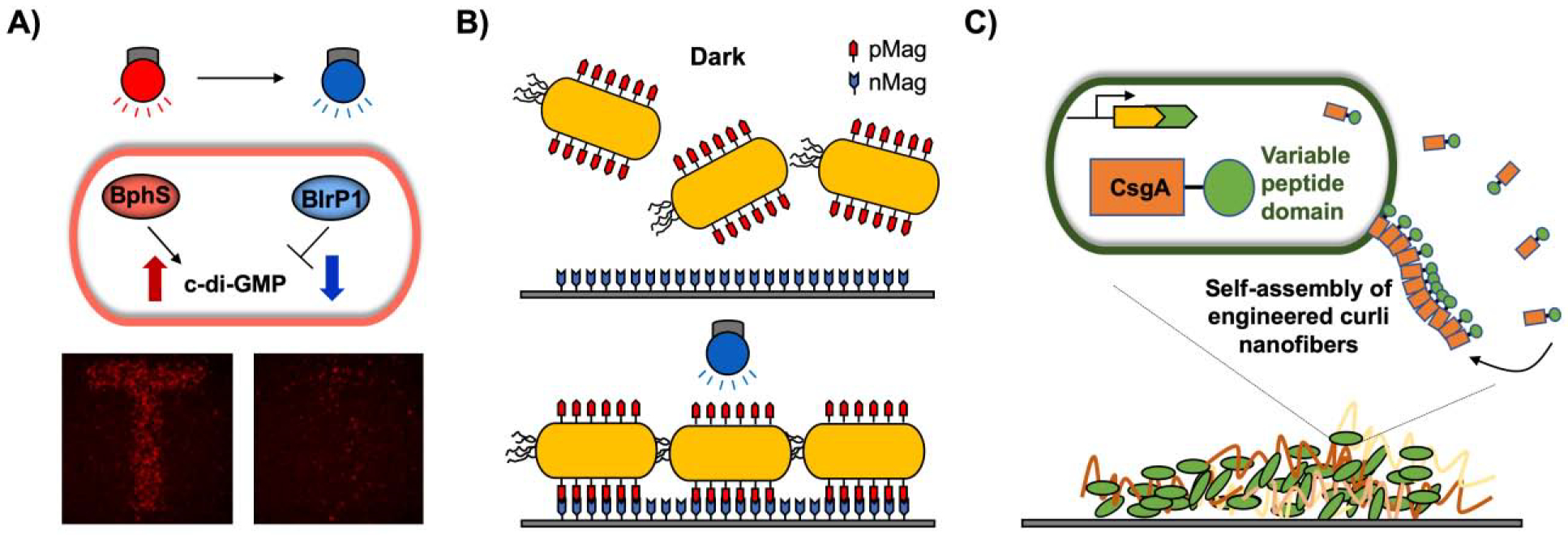
A) Optogenetic biofilm patterning using light-switchable c-di-GMP regulation. BphS activated by near-infrared light synthesizes c-di-GMP, while BlrP1 activated by blue light degrades c-di-GMP, resulting in biofilm formation and dispersal, respectively [biofilm images from (Huang et al., 2018)]. B) pMag on the microbial surface and nMag on the material surface form heterodimers with blue light. C) Engineered microbe produces the self-assembled curli nanofiber CsgA with a variable peptide domain, which confers new functions.
3.3.2. Biomaterial production
Biofilms can be developed as a biological platform for producing self-assembling functional materials (Nguyen et al., 2014) (Fig. 3C). Biofilm-Integrated Nanofiber Display (BIND) was developed to produce engineered amyloid protein CsgA, a major component of the curli fibrils of E. coli biofilms. The engineered CsgA containing functional peptide domains was self-assembled upon secretion and facilitated nanoparticle biotemplating, substrate adhesion, and site-specific protein immobilization on the BIND system (Botyanszki et al., 2015; Nguyen et al., 2014). The same amyloid protein was applied to create environmentally switchable conductive biofilms by using an inducible synthetic riboregulator circuit and interfacing the self-assembled curli fibrils with inorganic materials such as gold nanoparticles to introduce an electro-conductive property (Chen et al., 2014). 3D printing of bacteria was used to create biofilm-based functional materials for bioremediation and biomedical applications (Schaffner et al., 2017). Patterned biofilms were demonstrated by engineered curli production on the 3D-printed E. coli (Schmieden et al., 2018). Synthetic biology will guide the engineering of self-assembled polymer production and direct the assembly of patterned biofilms (Majerle et al., 2019).
4. Perspective
Intra- or inter-species phenomena occur in mixed-species biofilms, which exhibit dynamic interactions among bacteria (Giaouris et al., 2015). The cooperative interactions between biofilm bacterial species are achieved through cell-cell communication, metabolic cooperation, or spatial organization (Elias and Banin, 2012). However, there are also competitive interactions regarding nutrient uptake, occupation of spatial resources, or with the production of anti-biofilm agents (Giaouris et al., 2015). Synthetic biology approaches can help understand and engineer such cooperative and competitive behaviors among different bacterial species in biofilms. Studies on the beneficial characteristics of probiotic bacteria in inhibiting deleterious biofilms are growing (Fang et al., 2018; Hager et al., 2019; Wasfi et al., 2018). Ribosomally-synthesized antimicrobial proteins such as pyocins (Oluyombo et al., 2019; Smith et al., 2011) or colicins (Brown et al., 2012; Jin et al., 2019, 2018; Rendueles et al., 2014) that exhibit target-specific bacterial killing could be used with probiotics to eradicate harmful biofilms without affecting the overall beneficial or commensal microbial consortia.
Biofilms with higher productivity and tolerance to toxic inhibitors can serve as microbial cell factories (Berlanga and Guerrero, 2016) for producing chemicals such as ethanol (Todhanakasem et al., 2014), acetone, butanol (Förberg and Häggström, 1985), and succinyl acid (Urbance et al., 2004). Coordinating synthetic biofilm communities is becoming more important in industrial biochemical production (Berlanga and Guerrero, 2016). The morphology and spatial organization of catalytic biofilms must be programmed along with engineering of their metabolic pathways for biochemical production (Volke and Nikel, 2018). A 3D printing approach combined with synthetic genetic controls will enhance the design and assembly of synthetic biofilm catalysts.
In addition to bacterial biofilms, fungal biofilms on implanted devices and on epithelial and endothelial surfaces can cause recurrent infections with increased drug resistance (Desai et al., 2014; Kernien et al., 2018). Candida, Aspergillus, and Cryptococcus are the most prominent clinically relevant fungi involved in the resilience of fungal biofilms to host immunity (Kernien et al., 2018). Antimicrobial peptides naturally found in living organisms can effectively treat fungal biofilms without eliciting an immune response. For example, histatin-5 (Hst-5) from human saliva is an antifungal peptide that can inhibit the growth of Candida albicans (Baev et al., 2002) but has limited antifungal activity due to its rapid degradation at the site of action (Moffa et al., 2015a). Recently, liposome encapsulation has enabled the prolonged delivery of Hst-5 (Zambom et al., 2019), and the design of proteolysis-resistant peptides has been shown to stabilize Hst-5, resulting in enhanced antifungal activity (Ikonomova et al., 2019, 2018), which may be applied for the control of fungal biofilms (Moffa et al., 2015b). In contrast to harmful fungal biofilms, some fungal biofilms are beneficial. For example, the formation of fungal–bacterial biofilms on the plant root promotes plant growth by supplying essential nutrients and providing plant growth-promoting substances (Gentili and Jumpponen, 2006; Herath et al., 2015). Such symbiotic relationships between plants and microbes, including fungi and bacteria (Goh et al., 2013; Hassani et al., 2018), have resulted the development of biofilmed biofertilizers, presenting a viable alternative for chemical fertilizers in agriculture (Zakeel and Safeena, 2019). Despite the need to control fungal biofilms in medical, industrial, and agricultural applications, synthetic biology techniques for fungal cells are still in the early developmental stages (Hennig et al., 2015). Fungal QS (Albuquerque and Casadevall, 2012) and pheromone communication (Hennig et al., 2015) may be attractive targets for modulating fungal biofilms.
Signaling molecules exhibit some drawbacks in the control of biofilms and thus require further improvement. As mentioned above, QS molecules have been widely utilized in synthetic biology (Choudhary and Schmidt-Dannert, 2010; Hong et al., 2012; Ryan and Dow, 2008), as signals produced in the host cell can bind to receptors of the target cell, resulting in population-driven responses (Popat et al., 2015; Waters and Bassler, 2005). However, QS signal production and detection are strain-specific (Hawver et al., 2016); therefore, it is difficult to apply QS circuits to target non-model strains or species that have different QS systems or that lack QS signal recognition, which commonly arise in real-world situations. In contrast, c-di-GMP is a nearly ubiquitous bacterial signal (Hengge, 2009; Römling et al., 2013; Sondermann et al., 2012) that regulates biofilm formation, but it acts intracellularly (Valentini and Filloux, 2016). This lack of signal diffusion to other cells limits the development of a c-di-GMP genetic circuit and the corresponding control strategy to the host cells. Nitric oxide (NO) signaling in nitrogen metabolism is involved in c-di-GMP metabolism (Rinaldo et al., 2018), and NO production can be triggered by the external addition of chemicals (Barraud et al., 2012; Hetrick et al., 2009) to modulate c-di-GMP production in a broad range of bacteria. Therefore, combining ubiquitous c-di-GMP regulation and strain-specific QS systems will enable the development of a broad spectrum of synthetic genetic circuits for the control of complex biofilms. Additionally, bioactive phytochemicals found in natural products, such as green tea leaves (Qais et al., 2019) and medicinal plant extracts (Shukla and Bhathena, 2016), that exhibit broad-spectrum QS and biofilm inhibition may be integrated in the development of biofilm-controlling genetic circuits. Furthermore, models of the effects of signaling molecules in biofilm communities (Abisado et al., 2018; Emerenini et al., 2015; Frederick et al., 2011) can aid in the design and validation of synthetic biological circuits for effective biofilm control.
5. Conclusion
Control of biofilms, including their eradication and utilization, has been hampered due to insufficient knowledge of biofilm development and the limitations of biological toolkits. Recent investigations of biofilm physiology and synthetic biology advancements can facilitate fine control of biofilms, resulting in the efficient eradication of deleterious biofilms without the use of antibiotics and beneficial utilization of engineered biofilms. However, such synthetic biology approaches for controlling biofilms remain in the early stages. Rather than a single gene or signaling molecule, multiple factors contribute simultaneously or in series at the different stages of biofilm development. Hence, multi-stage and multi-target strategies may be required to achieve the desired level of biofilm control, which will be enabled by mimicking native biofilm formation and dispersal processes. Growing sets of synthetic biology tools as well as continued investigations into biofilm regulation will provide insights for biofilm-controlling strategies and their application in medical, food-processing, agricultural, industrial, and environmental fields.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R15AI130988), the National Science Foundation (CBET 1917130), and the Interdisciplinary Seed Funding Grants program of Wanger Institute for Sustainable Energy Research at Illinois Institute of Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare no competing financial interests.
References
- Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR, 2018. Bacterial quorum sensing and microbial community interactions. MBio 9, e02331–17. 10.1128/mBio.02331-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramzon N, Joaquin JC, Bray J, Brelles-Mariño G, 2006. Biofilm destruction by RF high-pressure cold plasma jet. IEEE Trans. Plasma Sci 34, 1304–1309. 10.1109/TPS.2006.877515 [DOI] [Google Scholar]
- Albuquerque P, Casadevall A, 2012. Quorum sensing in fungi-a review. Med. Mycol 50, 337–345. 10.3109/13693786.2011.652201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliashkevich A, Alvarez L, Cava F, 2018. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front. Microbiol 9, 683 10.3389/fmicb.2018.00683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaker RP, 2010. Critical review in oral biology & medicine: The use of nanoparticles to control oral biofilm formation. J. Dent. Res 89, 1175–1186. 10.1177/0022034510377794 [DOI] [PubMed] [Google Scholar]
- Ampornaramveth RS, Akeatichod N, Lertnukkhid J, Songsang N, 2018. Application of D-amino acids as biofilm dispersing agent in dental unit waterlines. Int. J. Dent 2018, 9413925 10.1155/2018/9413925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GG, O’Toole GA, 2008. Innate and induced resistance mechanisms of bacterial biofilms, in: Romeo T (Ed.), Bacterial Biofilms. Springer, Berlin, Heidelberg, pp. 85–105. 10.1007/978-3-540-75418-3_5 [DOI] [PubMed] [Google Scholar]
- Arora DP, Hossain S, Xu Y, Boon EM, 2015. Nitric oxide regulation of bacterial biofilms. Biochemistry 54, 3717–3728. 10.1021/bi501476n [DOI] [PubMed] [Google Scholar]
- Azeredo J, Sutherland IW, 2008. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol 9, 261–266. 10.2174/138920108785161604 [DOI] [PubMed] [Google Scholar]
- Baev D, Li XS, Dong J, Keng P, Edgerton M, 2002. Human salivary histatin 5 causes disordered volume regulation and cell cycle arrest in Candida albicans. Infect. Immun 70, 4777–4784. 10.1128/IAI.70.9.4777-4784.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai AJ, Rai RV, 2016. Effect of small chain N acyl homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J. Food Sci. Technol 53, 3609–3614. 10.1007/s13197-016-2346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrino D, Haagensen JAJ, Rich C, Forestier C, 2005. Characterization of type 2 quorum sensing in Klebsiella pneumoniae and relationship with biofilm formation. J. Bacteriol 187, 2870–2880. 10.1128/JB.187.8.2870-2880.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barends TRM, Hartmann E, Griese JJ, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I, 2009. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459, 1015–1018. 10.1038/nature07966 [DOI] [PubMed] [Google Scholar]
- Barraud N, Kardak BG, Yepuri NR, Howlin RP, Webb JS, Faust SN, Kjelleberg S, Rice SA, Kelso MJ, 2012. Cephalosporin-3’-diazeniumdiolates: targeted NO-donor prodrugs for dispersing bacterial biofilms. Angew. Chemie - Int. Ed 51, 9057–9060. 10.1002/anie.201202414 [DOI] [PubMed] [Google Scholar]
- Barraud N, Kelso MJ, Rice SA, Kjelleberg S, 2015. Nitric oxide : a key mediator of biofilm dispersal with applications in infectious diseases. Curr. Pharm. Des 21, 31–42. 10.2174/1381612820666140905112822 [DOI] [PubMed] [Google Scholar]
- Baumann AR, Martin SE, Feng H, 2009. Removal of Listeria monocytogenes biofilms from stainless steel by use of ultrasound and ozone. J. Food Prot 72, 1306–1309. 10.4315/0362-028x-72.6.1306 [DOI] [PubMed] [Google Scholar]
- Beech IB, Sunner J, 2004. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol 15, 181–186. 10.1016/j.copbio.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Berger D, Rakhamimova A, Pollack A, Loewy Z, 2018. Oral biofilms: development, control, and analysis. High-Throughput 7, 24 10.3390/HT7030024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga M, Guerrero R, 2016. Living together in biofilms: the microbial cell factory and its biotechnological implications. Microb. Cell Fact 15, 165 10.1186/s12934-016-0569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittihn P, Din MO, Tsimring LS, Hasty J, 2018. Rational engineering of synthetic microbial systems: from single cells to consortia. Curr. Opin. Microbiol 45, 92–99. 10.1016/j.mib.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerkan G, Witso E, Bergh K, 2009. Sonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitro. Acta Orthop. 80, 245–250. 10.3109/17453670902947457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Horswill AR, 2008. agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4, e1000052 10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botyanszki Z, Tay PKR, Nguyen PQ, Nussbaumer MG, Joshi NS, 2015. Engineered catalytic biofilms: Site-specific enzyme immobilization onto E. coli curli nanofibers. Biotechnol. Bioeng 112, 2016–2024. 10.1002/bit.25638 [DOI] [PubMed] [Google Scholar]
- Brackman G, Coenye T, 2014. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des 21, 5–11. 10.2174/1381612820666140905114627 [DOI] [PubMed] [Google Scholar]
- Bradley RW, Buck M, Wang B, 2016. Tools and principles for microbial gene circuit engineering. J. Mol. Biol 428, 862–888. 10.1016/j.jmb.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Brenner K, Arnold FH, 2011. Self-organization, layered structure, and aggregation enhance persistence of a synthetic biofilm consortium. PLoS One 6, e16791 10.1371/journal.pone.0016791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner K, You L, Arnold FH, 2008. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26, 483–489. 10.1016/j.tibtech.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Brooks JD, Flint SH, 2008. Biofilms in the food industry: problems and potential solutions. Int. J. Food Sci. Technol 43, 2163–2176. 10.1111/j.1365-2621.2008.01839.x [DOI] [Google Scholar]
- Brown CL, Smith K, McCaughey L, Walker D, 2012. Colicin-like bacteriocins as novel therapeutic agents for the treatment of chronic biofilm-mediated infection. Biochem. Soc. Trans 40, 1549–1552. 10.1042/bst20120241 [DOI] [PubMed] [Google Scholar]
- Brune KD, Bayer TS, 2012. Engineering microbial consortia to enhance biomining and bioremediation. Front. Microbiol 3, 203 10.3389/fmicb.2012.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher T, Oppenheimer-Shaanan Y, Savidor A, Bloom-Ackermann Z, Kolodkin-Gal I, 2015. Disturbance of the bacterial cell wall specifically interferes with biofilm formation. Environ. Microbiol. Rep 7, 990–1004. 10.1111/1758-2229.12346 [DOI] [PubMed] [Google Scholar]
- Carocho M, Ferreira ICFR, 2013. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol 51, 15–25. 10.1016/j.fct.2012.09.021 [DOI] [PubMed] [Google Scholar]
- Cattò C, Cappitelli F, 2019. Testing anti-biofilm polymeric surfaces: Where to start? Int. J. Mol. Sci 20, 3794 10.3390/ijms20153794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava F, Lam H, de Pedro MA, Waldor MK, 2011. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell. Mol. Life Sci 68, 817–831. 10.1007/s00018-010-0571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez de Paz LE, Lemos JA, Wickström C, Sedgley CM, 2012. Role of (p)ppGpp in biofilm formation by Enterococcus faecalis. Appl. Environ. Microbiol 78, 1627–1630. 10.1128/AEM.07036-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AY, Deng Z, Billings AN, Seker UOS, Lu MY, Citorik RJ, Zakeri B, Lu TK, 2014. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat. Mater 13, 515–523. 10.1038/nmat3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wegner SV, 2017. Blue light switchable bacterial adhesion as a key step toward the design of biofilms. ACS Synth. Biol 6, 2170–2174. 10.1021/acssynbio.7b00197 [DOI] [PubMed] [Google Scholar]
- Chopp DL, Kirisits MJ, Moran B, Parsek MR, 2003. The dependence of quorum sensing on the depth of a growing biofilm. Bull. Math. Biol 65, 1053–1079. 10.1016/S0092-8240(03)00057-0 [DOI] [PubMed] [Google Scholar]
- Choudhary S, Schmidt-Dannert C, 2010. Applications of quorum sensing in biotechnology. Appl. Microbiol. Biotechnol 86, 1267–1279. 10.1007/s00253-010-2521-7 [DOI] [PubMed] [Google Scholar]
- Ciofu O, Rojo-Molinero E, Macià MD, Oliver A, 2017. Antibiotic treatment of biofilm infections. Apmis 125, 304–319. 10.1111/apm.12673 [DOI] [PubMed] [Google Scholar]
- Costa OYA, Raaijmakers JM, Kuramae EE, 2018. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol 9, 1636 10.3389/fmicb.2018.01636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Montanaro L, Arciola CR, 2005. Biofilm in implant infections: its production and regulation. Int. J. Artif. Organs 28, 1062–1068. 10.1177/039139880502801103 [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP, 1999. Bacterial bofilms: a common cause of persistent infections. Science 284, 1318–1322. 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Donlan RM, 2006. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother 50, 1268–1275. 10.1128/AAC.50.4.1268-1275.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H, Lovell CR, 2005. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev 80, 91–138. 10.1128/MMBR.00037-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D, 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2, 114–122. 10.1038/nrd1008 [DOI] [PubMed] [Google Scholar]
- de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, Hancock REW, 2014. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10, e1004152 10.1371/journal.ppat.1004152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R, 2009. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother 53, 35–40. 10.1128/AAC.00237-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai JV, Mitchell AP, Andes DR, 2014. Fungal biofilms, drug resistance, and recurrent Infection. Cold Spring Harb. Perspect. Med 4, a019729 10.1101/cshperspect.a019729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domka J, Lee J, Bansal T, Wood TK, 2007. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol 9, 332–346. 10.1111/j.1462-2920.2006.01143.x [DOI] [PubMed] [Google Scholar]
- Duan FF, Liu JH, March JC, 2015. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 64, 1794–1803. 10.2337/db14-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K, Surette MG, 2007. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol 189, 4827–4836. 10.1128/JB.00043-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyszel JL, Soares JA, Swearingen MC, Lindsay A, Smith JN, Ahmer BMM, 2010. E. coli K-12 and EHEC genes regulated by SdiA. PLoS One 5, e8946 10.1371/journal.pone.0008946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S, Banin E, 2012. Multi-species biofilms: living with friendly neighbors. FEMS Microbiol. Rev 36, 990–1004. [DOI] [PubMed] [Google Scholar]
- Emerenini BO, Hense BA, Kuttler C, Eberl HJ, 2015. A mathematical model of quorum sensing induced biofilm detachment. PLoS One 10, e0132385 10.1371/journal.pone.0132385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K, Jin X, Hong SH, 2018. Probiotic Escherichia coli inhibits biofilm formation of pathogenic E. coli via extracellular activity of DegP. Sci. Rep 8, 4939 10.1038/s41598-018-23180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R, Luo X, Tsao CY, Payne GF, Ghodssi R, Rubloff GW, Bentley WE, 2010. Biological nanofactories facilitate spatially selective capture and manipulation of quorum sensing bacteria in a bioMEMS device. Lab Chip 10, 1128–1134. 10.1039/b926846d [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J, 2010. The biofilm matrix. Nat. Rev. Microbiol 8, 623–633. 10.1080/0892701031000072190 [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S, 2016. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol 14, 563–575. 10.1038/nrmicro.2016.94 [DOI] [PubMed] [Google Scholar]
- Förberg C, Häggström L, 1985. Control of cell adhesion and activity during continuous production of acetone and butanol with adsorbed cells. Enzyme Microb. Technol 7, 230–234. 10.1016/0141-0229(85)90073-0 [DOI] [Google Scholar]
- Frederick MR, Kuttler C, Hense BA, Eberl HJ, 2011. A mathematical model of quorum sensing regulated EPS production in biofilm communities. Theor. Biol. Med. Model 8, 8 10.1186/1742-4682-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM, 2010. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother 54, 397–404. 10.1128/AAC.00669-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C, Parsek MR, Greenberg EP, 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet 35, 439–468. 10.1146/annurev.genet.35.102401.090913 [DOI] [PubMed] [Google Scholar]
- Fuqua WC, Winans SC, Greenberg EP, 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol 176, 269–275. 10.1128/jb.176.2.269-275.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galié S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F, 2018. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol 9, 898 10.3389/fmicb.2018.00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey M, Rabbitt D, Stocca A, Rowan N, 2015. Pulsed ultraviolet light inactivation of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Water Environ. J 29, 36–42. 10.1111/wej.12088 [DOI] [Google Scholar]
- Geilich BM, Gelfat I, Sridhar S, van de Ven AL, Webster TJ, 2017. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials 119, 78–85. 10.1016/j.biomaterials.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Gentili F, Jumpponen A, 2006. Potential and possible uses of bacterial and fungal biofertilizers, in: Rai M (Ed.), Handbook of Microbial Biofertilizers. The Haworth Press Incorporated, pp. 1–18. 10.1201/9781482277760 [DOI] [Google Scholar]
- Giaouris E, Heir E, Desvaux M, Hébraud M, Møretrø T, Langsrud S, Doulgeraki A, Nychas G-J, Kačániová M, Czaczyk K, Ölmez H, Simões M, 2015. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol 6, 841 10.3389/fmicb.2015.00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore BF, Flynn PB, O’Brien S, Hickok N, Freeman T, Bourke P, 2018. Cold plasmas for biofilm control: opportunities and challenges. Trends Biotechnol. 36, 627–638. 10.1016/j.tibtech.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CH, Veliz Vallejos DF, Nicotra AB, Mathesius U, 2013. The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J. Chem. Ecol 39, 826–839. 10.1007/s10886-013-0326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rivas F, Ripolles-Avila C, Fontecha-Umaña F, Ríos-Castillo AG, Rodríguez-Jerez JJ, 2018. Biofilms in the spotlight: Detection, quantification, and removal methods. Compr. Rev. Food Sci. Food Saf 17, 1261–1276. 10.1111/1541-4337.12378 [DOI] [PubMed] [Google Scholar]
- Grandclément C, Tannières M, Moréra S, Dessaux Y, Faure D, 2016. Quorum quenching: role in nature and applied developments. FEMS Microbiol. Rev 40, 86–116. 10.1093/femsre/fuv038 [DOI] [PubMed] [Google Scholar]
- Hager CL, Isham N, Schrom KP, Chandra J, McCormick T, Miyagi M, Ghannoum MA, 2019. Effects of a novel probiotic combination on pathogenic bacterial-fungal polymicrobial biofilms. MBio 10, e00338–19. 10.1128/mbio.00338-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL, 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol 50, 101–114. 10.1046/j.1365-2958.2003.03688.x [DOI] [PubMed] [Google Scholar]
- Hancock V, Dahl M, Klemm P, 2010. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J. Med. Microbiol 59, 392–399. 10.1099/jmm.0.008672-0 [DOI] [PubMed] [Google Scholar]
- Hassani MA, Durán P, Hacquard S, 2018. Microbial interactions within the plant holobiont. Microbiome 6, 58 10.1186/s40168-018-0445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawver LA, Jung SA, Ng WL, 2016. Specificity and complexity in bacterial quorum-sensing systemsa. FEMS Microbiol. Rev 40, 738–752. 10.1093/femsre/fuw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann K, 2016. Novel approaches to microalgal and cyanobacterial cultivation for bioenergy and biofuel production. Curr. Opin. Biotechnol 38, 183–189. 10.1016/j.copbio.2016.02.024 [DOI] [PubMed] [Google Scholar]
- Hengge R, 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol 7, 263–273. 10.1038/nrmicro2109 [DOI] [PubMed] [Google Scholar]
- Hengge R, 2008. The two-component network and the general stress sigma factor RpoS (σS) in Escherichia coli, in: Utsumi R (Ed.), Bacterial Signal Transduction: Networks and Drug Targets. Springer, New York, pp. 40–53. 10.1007/978-0-387-78885-2_4 [DOI] [PubMed] [Google Scholar]
- Hennig S, Rödel G, Ostermann K, 2015. Artificial cell-cell communication as an emerging tool in synthetic biology applications. J. Biol. Eng 9, 13 10.1186/s13036-015-0011-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath HMLI, Menikdiwela KR, Igalavithana AD, Seneviratne G, 2015. Developed fungal-bacterial biofilms having nitrogen fixers: Universal biofertilizers for legumes and non-legumes, in: de Bruijn FJ (Ed.), Biological Nitrogen Fixation. John Wiley & Sons, Inc, pp. 1041–1046. 10.1002/9781119053095.ch102 [DOI] [Google Scholar]
- Herzberg M, Kaye IK, Peti W, Thomas K, Wood TK, 2006. YdgG (TqsA) controls biofilm formation in Escherichia coli K-12 through autoinducer 2 transport. J. Bacteriol 188, 587–598. 10.1128/JB.188.2.587-598.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heselmans M, Reid G, Akkermans LMA, Savelkoul H, Timmerman H, Rombouts FM, 2005. Gut flora in health and disease: potential role of probiotics. Curr. Issues Intest. Microbiol 6, 1–7. [PubMed] [Google Scholar]
- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH, 2009. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 30, 2782–2789. 10.1016/j.biomaterials.2009.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME, 2014. Expert consensus document: The International scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol 11, 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Hoh D, Watson S, Kan E, 2016. Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chem. Eng. J 287, 466–473. 10.1016/j.cej.2015.11.062 [DOI] [Google Scholar]
- Hong SH, Hegde M, Kim J, Wang X, Jayaraman A, Wood TK, 2012. Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device. Nat. Commun 3, 613 10.1038/ncomms1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH, Lee J, Wood TK, 2010a. Engineering global regulator Hha of Escherichia coli to control biofilm dispersal. Microb. Biotechnol 3, 717–728. 10.1111/j.1751-7915.2010.00220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH, Wang X, Wood TK, 2010b. Controlling biofilm formation, prophage excision and cell death by rewiring global regulator H-NS of Escherichia coli. Microb. Biotechnol 3, 344–356. 10.1111/j.1751-7915.2010.00164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain S, Boon EM, 2017. Discovery of a novel nitric oxide binding protein and nitric-oxide-responsive signaling pathway in Pseudomonas aeruginosa. ACS Infect. Dis 3, 454–461. 10.1021/acsinfecdis.7b00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xia A, Yang G, Jin F, 2018. Bioprinting living biofilms through optogenetic manipulation. ACS Synth. Biol 7, 1195–1200. 10.1021/acssynbio.8b00003 [DOI] [PubMed] [Google Scholar]
- Hwang IY, Koh E, Wong A, March JC, Bentley WE, Lee YS, Chang MW, 2017. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat. Commun 8, 15028 10.1038/ncomms15028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomova SP, Moghaddam-Taaheri P, Jabra-Rizk MA, Wang Y, Karlsson AJ, 2018. Engineering improved variants of the antifungal peptide histatin 5 with reduced susceptibility to Candida albicans secreted aspartic proteases and enhanced antimicrobial potency. FEBS J. 285, 146–159. 10.1111/febs.14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomova SP, Moghaddam-Taaheri P, Wang Y, Doolin MT, Stroka KM, Hube B, Karlsson AJ, 2019. Effects of histatin 5 modifications on antifungal activity and kinetics of proteolysis. Protein Sci. 1–14 10.1002/pro.3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri H, Ansari FA, Ahmad I, 2019. Prospects of essential oils in controlling pathogenic biofilm, New Look to Phytomedicine. Elsevier Inc. 10.1016/b978-0-12-814619-4.00009-4 [DOI] [Google Scholar]
- Jia R, Li Y, Al-Mahamedh HH, Gu T, 2017. Enhanced biocide treatments with D-amino acid mixtures against a biofilm consortium from a water cooling tower. Front. Microbiol 8, 1538 10.3389/fmicb.2017.01538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia R, Unsal T, Xu D, Lekbach Y, Gu T, 2019. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad 137, 42–58. 10.1016/j.ibiod.2018.11.007 [DOI] [Google Scholar]
- Jia X, Liu C, Song H, Ding M, Du J, Ma Q, Yuan Y, 2016. Design, analysis and application of synthetic microbial consortia. Synth. Syst. Biotechnol 1, 109–117. 10.1016/j.synbio.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Kightlinger W, Hong SH, 2019. Optimizing cell-free protein synthesis for increased yield and activity of colicins. Methods Protoc. 2, 28 10.3390/mps2020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Kightlinger W, Kwon Y-C, Hong SH, 2018. Rapid production and characterization of antimicrobial colicins using Escherichia coli-based cell-free protein synthesis. Synth. Biol 3, ysy004 10.1093/synbio/ysy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Riedel-Kruse IH, 2018. Biofilm Lithography enables high-resolution cell patterning via optogenetic adhesin expression. Proc. Natl. Acad. Sci 115, 3698–3703. 10.1073/pnas.1720676115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadag D, Köroğlu OE, Ozkaya B, Cakmakci M, 2015. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem 50, 262–271. 10.1016/j.procbio.2014.11.005 [DOI] [Google Scholar]
- Karatan E, Watnick P, 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev 73, 310–347. 10.1128/mmbr.00041-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh JS, Thoendel M, Horswill AR, 2007. A role for type I signal peptidase in Staphylococcus aureus quorum sensing. Mol. Microbiol 65, 780–798. 10.1111/j.1365-2958.2007.05830.x [DOI] [PubMed] [Google Scholar]
- Kawano F, Suzuki H, Furuya A, Sato M, 2015. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun 6, 6256 10.1038/ncomms7256 [DOI] [PubMed] [Google Scholar]
- Kernien JF, Snarr BD, Sheppard DC, Nett JE, 2018. The interface between fungal biofilms and innate immunity. Front. Immunol 8, 1968 10.3389/fimmu.2017.01968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SI, Blumrosen G, Vecchio D, Golberg A, Mccormack MC, Yarmush ML, Hamblin MR, Austen WG, 2016. Eradication of multidrug-resistant Pseudomonas biofilm with pulsed electric fields. Biotechnol. Bioeng 113, 643–650. 10.1002/bit.25818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI, 2018. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 10.1016/j.heliyon.2018.e01067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-W, Oh H-S, Kim S-R, Lee K-B, Yeon K-M, Lee C-H, Kim S, Lee J-K, 2013. Microbial population dynamics and proteomics in membrane bioreactors with enzymatic quorum quenching. Appl. Microbiol. Biotechnol 97, 4665–4675. 10.1007/s00253-012-4272-0 [DOI] [PubMed] [Google Scholar]
- Kim J, Pitts B, Stewart PS, Camper A, Yoon J, 2008. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob. Agents Chemother 52, 1446–1453. 10.1128/AAC.00054-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem M, Quadri LEN, Kuipers OP, de Vos WM, 1997. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol 24, 895–904. 10.1046/j.1365-2958.1997.4251782.x [DOI] [PubMed] [Google Scholar]
- Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK, 2004. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol 186, 7549–7555. 10.1128/jb.186.22.7549-7555.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R, 2010. D-amino acids trigger biofilm disassembly. Science 328, 627–629. 10.1126/science.1188628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Blanchard AE, Liao C, Lu T, 2017. Engineering robust and tunable spatial structures with synthetic gene circuits. Nucleic Acids Res. 45, 1005–1014. 10.1093/nar/gkw1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Meldgin DR, Collins JJ, Lu T, 2018. Designing microbial consortia with defined social interactions. Nat. Chem. Biol 14, 821–829. 10.1038/s41589-018-0091-7 [DOI] [PubMed] [Google Scholar]
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ, 2013. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med 3, a010306 10.1101/cshperspect.a010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Rajput A, 2018. Phylogenomics and evolutionary perspective of quorum sensing regulators (LuxI/LuxR) in prokaryotes, in: Kalia VC (Ed.), Quorum Sensing and Its Biotechnological Applications. Springer, Singapore, pp. 61–70. 10.1007/978-981-13-0848-2_4 [DOI] [Google Scholar]
- Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, Kotula JW, Antipov E, Dagon Y, Denney WS, Wagner DA, West KA, Degar AJ, Brennan AM, Miller PF, 2019. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med 11, eaau7975 10.1126/scitranslmed.aau7975 [DOI] [PubMed] [Google Scholar]
- Lebeaux D, Ghigo J-M, Beloin C, 2014. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev 78, 510–543. 10.1128/mmbr.00013-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Maeda T, Hong SH, Wood TK, 2009. Reconfiguring the quorum-sensing regulator SdiA of Escherichia coli to control biofilm formation via indole and N-acylhomoserine lactones. Appl. Environ. Microbiol 75, 1703–1716. 10.1128/AEM.02081-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhang X-S, Hegde M, Bentley WE, Jayaraman A, Wood TK, 2008. Indole cell signaling occurs primarily at low temperatures in Escherichia coli. ISME J 2, 1007–1023. 10.1038/ismej.2008.54 [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim YG, Gwon G, Wood TK, Lee J, 2016. Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express 6, 123 10.1186/s13568-016-0297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee J, 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev 34, 426–444. 10.1111/j.1574-6976.2009.00204.x [DOI] [PubMed] [Google Scholar]
- Lee K, Kim Y-W, Lee S, Lee SH, Nahm CH, Kwon H, Park P-K, Choo K-H, Koyuncu I, Drews A, Lee C-H, Lee J-K, 2018. Stopping autoinducer-2 chatter by means of an indigenous bacterium (Acinetobacter sp. DKY-1): a new antibiofouling strategy in a membrane bioreactor for wastewater treatment. Environ. Sci. Technol 52, 6237–6245. 10.1021/acs.est.7b05824 [DOI] [PubMed] [Google Scholar]
- Lee K, Lee S, Lee SH, Kim S-R, Oh H-S, Park P-K, Choo K-H, Kim Y-W, Lee J-K, Lee C-H, 2016. Fungal quorum quenching: a paradigm shift for energy savings in membrane bioreactor (MBR) for wastewater treatment. Environ. Sci. Technol 50, 10914–10922. 10.1021/acs.est.6b00313 [DOI] [PubMed] [Google Scholar]
- Lee WH, Wahman DG, Bishop PL, Pressman JG, 2011. Free chlorine and monochloramine application to nitrifying biofilm: Comparison of biofilm penetration, activity, and viability. Environ. Sci. Technol 45, 1412–1419. 10.1021/es1035305 [DOI] [PubMed] [Google Scholar]
- Lewandowski Z, Boltz JP, 2011. Biofilms in water and wastewater treatment, in: Wilderer P (Ed.), Treatise on Water Science. Elsevier Science, pp. 529–570. 10.1016/B978-0-444-53199-5.00095-6 [DOI] [Google Scholar]
- Li X, Wu B, Chen H, Nan K, Jin Y, Sun L, Wang B, 2018. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J. Mater. Chem. B 6, 4274–4292. 10.1039/C8TB01245H [DOI] [PubMed] [Google Scholar]
- Li Y, Xu D, Chen C, Li X, Jia R, Zhang D, Sand W, Wang F, Gu T, 2018. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol 34, 1713–1718. 10.1016/j.jmst.2018.02.023 [DOI] [Google Scholar]
- Lima MR, Ferreira GF, Neto WRN, de Melo Monteiro J, Santos ÁRC, Tavares PB, Denadai ÂML, Bomfim MRQ, dos Santos VL, Marques SG, de Souza Monteiro A, 2019. Evaluation of the interaction between polymyxin B and Pseudomonas aeruginosa biofilm and planktonic cells: Reactive oxygen species induction and zeta potential. BMC Microbiol. 19, 1–9. 10.1186/s12866-019-1485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-M, Svoboda KKH, Giletto A, Seibert J, Puttaiah R, 2011. Effects of hydrogen peroxide on dental unit biofilms and treatment water contamination. Eur. J. Dent 5, 47–59. [PMC free article] [PubMed] [Google Scholar]
- Lineback CB, Nkemngong CA, Wu ST, Li X, Teska PJ, Oliver HF, 2018. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 7, 154 10.1186/s13756-018-0447-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tang B, Gu Q, Yu X, 2014. Elimination of the formation of biofilm in industrial pipes using enzyme cleaning technique. MethodsX 1, 130–136. 10.1016/j.mex.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TK, Collins JJ, 2009. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci 106, 4629–4634. 10.1073/pnas.0800442106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TK, Collins JJ, 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci 104, 11197–11202. 10.1073/pnas.0704624104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Yang Z, Pu M, Peti W, Wood TK, 2011a. Engineering a novel c-di-GMP-binding protein for biofilm dispersal. Environ. Microbiol 13, 631–642. 10.1111/j.1462-2920.2010.02368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Zhang G, Wood TK, 2011b. Escherichia coli BdcA controls biofilm dispersal in Pseudomonas aeruginosa and Rhizobium meliloti. BMC Res. Notes 4, 447 10.1186/1756-0500/4/447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah T-FC, O’Toole GA, 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39. 10.1016/S0966-842X(00)01913-2 [DOI] [PubMed] [Google Scholar]
- Majerle A, Schmieden DT, Jerala R, Meyer AS, 2019. Synthetic biology for multiscale designed biomimetic assemblies: from designed self-assembling biopolymers to bacterial bioprinting. Biochemistry 58, 2095–2104. 10.1021/acs.biochem.8b00922 [DOI] [PubMed] [Google Scholar]
- Mangwani N, Kumari S, Das S, 2016. Bacterial biofilms and quorum sensing: fidelity in bioremediation technology. Biotechnol. Genet. Eng. Rev 32, 43–73. 10.1080/02648725.2016.1196554 [DOI] [PubMed] [Google Scholar]
- Marques CNH, Davies DG, Sauer K, 2015. Control of biofilms with the fatty acid signaling molecule cis-2-decenoic acid. Pharmaceuticals 8, 816–835. 10.3390/ph8040816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx V, 2014. Stop the microbial chatter. Nature 511, 493–497. 10.1038/511493a [DOI] [PubMed] [Google Scholar]
- Mathur H, Field D, Rea MC, Cotter PD, Hill C, Ross RP, 2018. Fighting biofilms with lantibiotics and other groups of bacteriocins. npj Biofilms Microbiomes 4, 9 10.1038/s41522-018-0053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxmen A, 2017. Living therapeutics: Scientists genetically modify bacteria to deliver drugs. Nat. Med 23, 5–7. 10.1038/nm0117-5 [DOI] [PubMed] [Google Scholar]
- Mckay R, Ghodasra M, Schardt J, Quan D, Pottash AE, Shang W, Jay SM, Gregory FP, Chang MW, March JC, William EB, 2018. A platform of genetically engineered bacteria as vehicles for localized delivery of therapeutics: toward applications for Crohn’s disease. Bioeng. Transl. Med 3, 209–221. 10.1002/btm2.10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ, 2003. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol 185, 274–284. 10.1128/JB.185.1.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi G, Shi D, Wang M, Webster TJ, 2018. Reducing bacterial infections and biofilm formation using nanoparticles and nanostructured antibacterial surfaces. Adv. Healthc. Mater 7, 1800103 10.1002/adhm.201800103 [DOI] [PubMed] [Google Scholar]
- Miquel S, Lagrafeuille R, Souweine B, Forestier C, 2016. Anti-biofilm activity as a health issue. Front. Microbiol 7, 592 10.3389/fmicb.2016.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffa EB, Machado MAAM, Mussi MCM, Xiao Y, Garrido SS, Giampaolo ET, Siqueira WL, 2015a. In vitro identification of histatin 5 salivary complexes. PLoS One 10, e0142517 10.1371/journal.pone.0142517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffa EB, Mussi MCM, Xiao Y, Garrido SS, Machado MAAM, Giampaolo ET, Siqueira WL, 2015b. Histatin 5 inhibits adhesion of C. albicans to reconstructed human oral epithelium. Front. Microbiol 6, 885 10.3389/fmicb.2015.00885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M, 2006. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J. Biosci. Bioeng 101, 1–8. 10.1263/jbb.101.1 [DOI] [PubMed] [Google Scholar]
- Nadell CD, Drescher K, Foster KR, 2016. Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol 14, 589–600. 10.1038/nrmicro.2016.84 [DOI] [PubMed] [Google Scholar]
- Narenkumar J, Madhavan J, Nicoletti M, Benelli G, Murugan K, Rajasekar A, 2016. Role of bacterial plasmid on biofilm formation and its influence on corrosion of engineering materials. J. Bio- Tribo-Corrosion 2, 24 10.1007/s40735-016-0054-z [DOI] [Google Scholar]
- Nguyen PQ, Botyanszki Z, Tay PKR, Joshi NS, 2014. Programmable biofilm-based materials from engineered curli nanofibres. Nat. Commun 5, 4945 10.1038/ncomms5945 [DOI] [PubMed] [Google Scholar]
- Oh H-S, Lee C-H, 2018. Origin and evolution of quorum quenching technology for biofouling control in MBRs for wastewater treatment. J. Memb. Sci 554, 331–345. 10.1016/J.MEMSCI.2018.03.019 [DOI] [Google Scholar]
- Oh H-S, Tan CH, Low JH, Rzechowicz M, Siddiqui MF, Winters H, Kjelleberg S, Fane AG, Rice SA, 2017. Quorum quenching bacteria can be used to inhibit the biofouling of reverse osmosis membranes. Water Res 112, 29–37. 10.1016/j.watres.2017.01.028 [DOI] [PubMed] [Google Scholar]
- Oh HS, Yeon KM, Yang CS, Kim SR, Lee CH, Park SY, Han JY, Lee JK, 2012. Control of membrane biofouling in MBR for wastewater treatment by quorum quenching bacteria encapsulated in microporous membrane. Environ. Sci. Technol 46, 4877–4884. 10.1021/es204312u [DOI] [PubMed] [Google Scholar]
- Ohland CL, MacNaughton WK, 2010. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Liver Physiol 298, G807–G819. 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- Oluyombo O, Penfold CN, Diggle SP, 2019. Competition in biofilms between cystic fibrosis isolates of Pseudomonas aeruginosa is shaped by R-pyocins. MBio 10, e01828–18. 10.1128/mbio.01828-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Shaanan Y, Steinberg N, Kolodkin-Gal I, 2013. Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol 21, 594–601. 10.1016/j.tim.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Ozdemir T, Fedorec AJH, Danino T, Barnes CP, 2018. Synthetic biology and engineered live biotherapeutics: toward increasing system complexity. Cell Syst 7, 5–16. 10.1016/j.cels.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Papenfort K, Bassler BL, 2016. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol 14, 576–588. 10.1038/nrmicro.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Park HJ, Kim JA, Lee SH, Kim JH, Yoon J, Park TH, 2011. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 84, 41–45. 10.1016/j.mimet.2010.10.010 [DOI] [PubMed] [Google Scholar]
- Park SC, Park Y, Hahm KS, 2011. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci 12, 5971–5992. 10.3390/ijms12095971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R, Lamas-Samanamud GR, 2014. Inhibition of biofilm formation by T7 bacteriophages producing quorum-quenching enzymes. Appl. Environ. Microbiol 80, 5340–5348. 10.1128/aem.01434-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival SL, Mayer D, Malone M, Swanson T, Gibson D, Schultz G, 2017. Surfactants and their role in wound cleansing and biofilm management. J. Wound Care 26, 680–690. 10.12968/jowc.2017.26.11.680 [DOI] [PubMed] [Google Scholar]
- Pesci EC, Pearson JP, Seed PC, Iglewski BH, 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol 179, 3127–3132. 10.1128/jb.179.10.3127-3132.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires D, Sillankorva S, Faustino A, Azeredo J, 2011. Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res. Microbiol 162, 798–806. 10.1016/j.resmic.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Pires DP, Cleto S, Sillankorva S, Azeredo J, Lu TK, 2016. Genetically engineered phages: a review of advances over the last decade. Microbiol. Mol. Biol. Rev 80, 523–543. 10.1128/MMBR.00069-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat R, Cornforth DM, McNally L, Brown SP, 2015. Collective sensing and collective responses in quorum-sensing bacteria. J. R. Soc. Interface 12, 20140882 10.1098/rsif.2014.0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LC, Pritchard MF, Ferguson EL, Powell KA, Patel SU, Rye PD, Sakellakou SM, Buurma NJ, Brilliant CD, Copping JM, Menzies GE, Lewis PD, Hill KE, Thomas DW, 2018. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. npj Biofilms Microbiomes 4, 13 10.1038/s41522-018-0056-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qais FA, Khan MS, Ahmad I, 2019. Broad-spectrum quorum sensing and biofilm inhibition by green tea against gram-negative pathogenic bacteria: Deciphering the role of phytocompounds through molecular modelling. Microb. Pathog 126, 379–392. 10.1016/j.micpath.2018.11.030 [DOI] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach THL, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M, 2008. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32, 150–158. 10.1016/j.molcel.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendueles O, Beloin C, Latour-Lambert P, Ghigo J-M, 2014. A new biofilm-associated colicin with increased efficiency against biofilm bacteria. ISME J 8, 1275–1288. 10.1038/ismej.2013.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo S, Giardina G, Mantoni F, Paone A, Cutruzzolà F, 2018. Beyond nitrogen metabolism: nitric oxide, cyclic-di-GMP and bacterial biofilms. FEMS Microbiol. Lett 365, fny029 10.1093/femsle/fny029 [DOI] [PubMed] [Google Scholar]
- Römling U, Galperin MY, Gomelsky M, 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev 77, 1–52. 10.1128/mmbr.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosche B, Li XZ, Hauer B, Schmid A, Buehler K, 2009. Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol. 27, 636–643. 10.1016/j.tibtech.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Roy R, Tiwari M, Donelli G, Tiwari V, 2018. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9, 522–554. 10.1080/21505594.2017.1313372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V, Fernandes R, Tsao C-Y, Bentley WE, 2010. Cross species quorum quenching using a native AI-2 processing enzyme. ACS Chem. Biol 5, 223–232. 10.1021/cb9002738 [DOI] [PubMed] [Google Scholar]
- Ryan RP, Dow JM, 2008. Diffusible signals and interspecies communication in bacteria. Microbiology 154, 1845–1858. 10.1099/mic.0.2008/017871-0 [DOI] [PubMed] [Google Scholar]
- Ryu M-H, Fomicheva A, Moskvin OV, Gomelsky M, 2017. Optogenetic module for dichromatic control of c-di-GMP signaling. J. Bacteriol 199, e00014–17. 10.1128/jb.00014-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MH, Gomelsky M, 2014. Near-infrared light responsive synthetic c-di-GMP module for optogenetic applications. ACS Synth. Biol 3, 802–810. 10.1021/sb400182x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner M, Rühs PA, Coulter F, Kilcher S, Studart AR, 2017. 3D printing of bacteria into functional complex materials. Sci. Adv 3, eaao6804 10.1126/sciadv.aao6804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder S, Bassler BL, 2001. The languages of bacteria. Genes Dev. 15, 1468–1480. 10.1101/GAD.899601 [DOI] [PubMed] [Google Scholar]
- Scheerer S, Ortega-Morales O, Gaylarde C, 2009. Microbial deterioration of stone monuments-an updated overview, in: Laskin AI, Sariaslani S, Gadd GM (Eds.), Advances in Applied Microbiology. Elsevier, pp. 97–139. 10.1016/S0065-2164(08)00805-8 [DOI] [PubMed] [Google Scholar]
- Schmieden DT, Basalo Vázquez SJ, Sangüesa H, Van Der Does M, Idema T, Meyer AS, 2018. Printing of patterned, engineered E. coli biofilms with a low-cost 3D printer. ACS Synth. Biol 7, 1328–1337. 10.1021/acssynbio.7b00424 [DOI] [PubMed] [Google Scholar]
- Schultz M, 2008. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm. Bowel Dis 14, 1012–1018. 10.1002/ibd.20377 [DOI] [PubMed] [Google Scholar]
- Shao X, Fang K, Medina D, Wan J, Lee JL, Hong SH, 2019. The probiotic, Leuconostoc mesenteroides, inhibits Listeria monocytogenes biofilm formation. J. Food Saf e12750 10.1111/jfs.12750 [DOI] [Google Scholar]
- Shen Y, Köller T, Kreikemeyer B, Nelson DC, 2013. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother 68, 1818–1824. 10.1093/jac/dkt104 [DOI] [PubMed] [Google Scholar]
- Shukla V, Bhathena Z, 2016. Broad spectrum anti-quorum sensing activity of tannin-rich crude extracts of Indian medicinal plants. Scientifica. 2016, 5823013 10.1155/2016/5823013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillankorva S, Neubauer P, Azeredo J, 2010. Phage control of dual species biofilms of Pseudomonas fluorescens and Staphylococcus lentus. Biofouling 26, 567–575. 10.1080/08927014.2010.494251 [DOI] [PubMed] [Google Scholar]
- Simões M, Pereira MO, Vieira MJ, 2005. Action of a cationic surfactant on the activity and removal of bacterial biofilms formed under different flow regimes. Water Res. 39, 478–486. 10.1016/j.watres.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Smith K, Martin L, Rinaldi A, Rajendran R, Ramage G, Walker D, 2011. Activity of pyocin S2 against Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother 56, 1599–1601. 10.1128/aac.05714-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Shikuma NJ, Yildiz FH, 2012. You’ve come a long way: c-di-GMP signaling. Curr. Opin. Microbiol 15, 140–146. 10.1016/j.mib.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenborn U, Schulze J, 2009. The non-pathogenic Escherichia coli strain Nissle 1917-features of a versatile probiotic. Microb. Ecol. Health Dis 21, 122–158. [Google Scholar]
- Splendiani A, Livingston AG, Nicolella C, 2006. Control of membrane-attached biofilms using surfactants. J. Anat 94, 15–23. 10.1002/bit [DOI] [PubMed] [Google Scholar]
- Srey S, Jahid IK, Ha S. Do, 2013. Biofilm formation in food industries: a food safety concern. Food Control 31, 572–585. 10.1016/j.foodcont.2012.12.001 [DOI] [Google Scholar]
- Stanley NR, Lazazzera BA, 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol 52, 917–924. 10.1111/j.1365-2958.2004.04036.x [DOI] [PubMed] [Google Scholar]
- Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ, 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J. Bacteriol 189, 7316–7325. 10.1128/JB.00734-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, 2015. Prospects for anti-biofilm pharmaceuticals. Pharmaceuticals 8, 504–511. 10.3390/ph8030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Hall-Stoodley L, Costerton B, DeMeo P, Shirtliff M, Gawalt E, Kathju S, 2013. Biofilms, biomaterials, and device-related infections, in: Modjarrad K, Ebnesajjad S (Eds.), Handbook of Polymer Applications in Medicine and Medical Devices. William Andrew Publishing, Oxford, pp. 77–101. 10.1016/B978-0-323-22805-3.00005-0 [DOI] [Google Scholar]
- Tanner ME, 2002. Understanding nature’s strategies for enzyme-catalyzed racemization and epimerization. Acc. Chem. Res 35, 237–246. 10.1021/ar000056y [DOI] [PubMed] [Google Scholar]
- Thibane VS, Kock JLF, Ells R, van Wyk PWJ, Pohl CH, 2010. Effect of marine polyunsaturated fatty acids on biofilm formation of Candida albicans and Candida dubliniensis. Mar. Drugs 8, 2597–2604. 10.3390/md8102597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todhanakasem T, Sangsutthiseree A, Areerat K, Young GM, Thanonkeo P, 2014. Biofilm production by Zymomonas mobilis enhances ethanol production and tolerance to toxic inhibitors from rice bran hydrolysate. N. Biotechnol 31, 451–459. 10.1016/j.nbt.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Townsley L, Yildiz FH, 2015. Temperature affects c-di-GMP signalling and biofilm formation in Vibrio cholerae. Environ. Microbiol 17, 4290–4305. 10.1111/1462-2920.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbance SE, Pometto AL, DiSpirito AA, Denli Y, 2004. Evaluation of succinic acid continuous and repeat-batch biofilm fermentation by Actinobacillus succinogenes using plastic composite support bioreactors. Appl. Microbiol. Biotechnol 65, 664–670. 10.1007/s00253-004-1634-2 [DOI] [PubMed] [Google Scholar]
- Uroz S, Chhabra SR, Cámara M, Williams P, Oger P, Dessaux Y, 2005. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 151, 3313–3322. 10.1099/mic.0.27961-0 [DOI] [PubMed] [Google Scholar]
- Valentini M, Filloux A, 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem 291, 12547–12555. 10.1074/jbc.R115.711507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde C, Haas D, 2008. Small RNAs controlled by two-component systems, in: Utsumi R (Ed.), Bacterial Signal Transduction: Networks and Drug Targets. Springer, New York, pp. 54–79. 10.1007/978-0-387-78885-2_5 [DOI] [Google Scholar]
- Volke DC, Nikel PI, 2018. Getting bacteria in shape: synthetic morphology approaches for the design of efficient microbial cell factories. Adv. Biosyst 2, 1800111 10.1002/adbi.201800111 [DOI] [Google Scholar]
- Wang J, Liu W, Liu T, 2017. Biofilm based attached cultivation technology for microalgal biorefineries—A review. Bioresour. Technol 244, 1245–1253. 10.1016/j.biortech.2017.05.136 [DOI] [PubMed] [Google Scholar]
- Wang LH, Weng LX, Dong YH, Zhang LH, 2004. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL-lactonase). J. Biol. Chem 279, 13645–13651. 10.1074/jbc.M311194200 [DOI] [PubMed] [Google Scholar]
- Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM, 2018. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med 22, 1972–1983. 10.1111/jcmm.13496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL, 2005. Qurorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol 21, 319–346. 10.1146/annurev.cellbio.21.012704.131001 [DOI] [PubMed] [Google Scholar]
- Weiland-Bräuer N, Kisch MJ, Pinnow N, Liese A, Schmitz RA, 2016. Highly effective inhibition of biofilm formation by the first metagenome-derived AI-2 quenching enzyme. Front. Microbiol 7, 1098 10.3389/fmicb.2016.01098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Nisbett L-M, Bacon B, Boon E, 2018. Bacterial heme-based sensors of nitric oxide. Antioxid. Redox Signal 29, 1872–1887. 10.1089/ars.2017.7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Ahn J, 2013. Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. Lett. Appl. Microbiol 56, 307–313. 10.1111/lam.12051 [DOI] [PubMed] [Google Scholar]
- Wood TK, Hong SH, Ma Q, 2011. Engineering biofilm formation and dispersal. Trends Biotechnol. 29, 87–94. 10.1016/j.tibtech.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TL, Guha R, Tang L, Geitner M, Kumar M, Wood TK, 2016. Living biofouling-resistant membranes as a model for the beneficial use of engineered biofilms. Proc. Natl. Acad. Sci 113, 2802–2811. 10.1073/pnas.1521731113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier JB, Picioreanu C, Abdul Rani S, van Loosdrecht MCM, Stewart PS, 2005. Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix - A modelling study. Microbiology 151, 3817–3832. 10.1099/mic.0.28165-0 [DOI] [PubMed] [Google Scholar]
- Xavier KB, Miller ST, Lu W, Kim JH, Rabinowitz J, Pelczer I, Semmelhack MF, Bassler BL, 2007. Phosphorylation and processing of the quorum-sensing molecule autoinducer-2 in enteric bacteria. ACS Chem. Biol 2, 128–136. 10.1021/cb600444h [DOI] [PubMed] [Google Scholar]
- Xu D, Jia R, Li Y, Gu T, 2017. Advances in the treatment of problematic industrial biofilms. World J. Microbiol. Biotechnol 33, 97 10.1007/s11274-016-2203-4 [DOI] [PubMed] [Google Scholar]
- Zakeel MCM, Safeena MIS, 2019. Biofilmed biofertilizer for sustainable agriculture, in: Ansari RA, Mahmood I (Eds.), Plant Health Under Biotic Stress. Springer, Singapore, pp. 65–82. 10.1007/978-981-13-6040-4_3 [DOI] [Google Scholar]
- Zambom CR, da Fonseca FH, Crusca E, da Silva PB, Pavan FR, Chorilli M, Garrido SS, 2019. A novel antifungal system with potential for prolonged delivery of histatin 5 to limit growth of Candida albicans. Front. Microbiol 10, 1667 10.3389/fmicb.2019.01667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Poh CL, 2018. Regulating exopolysaccharide gene wcaF allows control of Escherichia coli biofilm formation. Sci. Rep 8, 13127 10.1038/s41598-018-31161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gray L, Novick RP, Ji G, 2002. Transmembrane topology of AgrB, the protein involved in the post-translational modification of AgrD in Staphylococcus aureus. J. Biol. Chem 277, 34736–34742. 10.1074/jbc.M205367200 [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhao F, Wang J, Zhong N, 2017. Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. RSC Adv 7, 36670–36683. 10.1039/c7ra02497e [DOI] [Google Scholar]
- Zuo R, 2007. Biofilms: Strategies for metal corrosion inhibition employing microorganisms. Appl. Microbiol. Biotechnol 76, 1245–1253. 10.1007/s00253-007-1130-6 [DOI] [PubMed] [Google Scholar]


