Abstract
Objective
Increased myelopoiesis has been linked to risk of atherosclerotic cardiovascular disease (ACD). Excessive myelopoiesis can be driven by dyslipidemia and cholesterol accumulation in hematopoietic stem and progenitor cells (HSPC) and may involve increased signaling via Janus kinase 2 (JAK2). Constitutively activating JAK2 mutants drive biased myelopoiesis and promote development of myeloproliferative neoplasms (MPN) or clonal hematopoiesis, conditions associated with increased risk of ACD. JAK2 inhibitors have been developed as a therapy for MPNs. The potential for JAK2 inhibitors to protect against atherosclerosis has not been tested. We therefore assessed the impact of JAK2 inhibition on atherogenesis.
Methods
A selective JAK2 inhibitor TG101348 (fedratinib) or vehicle was given to high-fat high-cholesterol Western diet (WD)–fed wild-type (WT) or Apoe−/− mice. Hematopoietic cell profiles, cell proliferation, and atherosclerosis in WT or Apoe−/− mice were assessed.
Results
TG101348 selectively reversed neutrophilia, monocytosis, HSPC, and granulocyte-macrophage progenitor (GMP) expansion in Apoe−/− mice with decreased cellular phosphorylated STAT5 and ERK1/2 and reduced cell cycling and BrdU incorporation in HSPCs, indicating inhibition of JAK/STAT signaling and cell proliferation. Ten-week WD feeding allowed the development of marked aortic atherosclerosis in Apoe−/− mice which was substantially reduced by TG101348.
Conclusions
Selective JAK2 inhibition reduces atherogenesis by suppressing excessive myelopoiesis in hypercholesterolemic Apoe−/− mice. These findings suggest selective JAK2 inhibition as a potential therapeutic approach to decrease ACD risk in patients with increased myelopoiesis and leukocytosis.
Electronic supplementary material
The online version of this article (10.1007/s10557-020-06943-9) contains supplementary material, which is available to authorized users.
Keywords: JAK2 inhibitor, TG101348 (Fedratinib), Atherosclerosis, Myelopoiesis
Introduction
Atherosclerosis is a lipoprotein-driven chronic inflammatory disease of the arterial wall [1]. Myeloid cells including monocytes, macrophages, neutrophils, and dendritic cells are critical players in innate and acquired immunity that are also responsible for the initiation and progression of atherosclerosis. It is generally accepted that a major proportion of myeloid cells in atherosclerotic plaques are recruited from circulating blood cells. Leukocytosis is associated with increased all-cause mortality [2, 3], due in large part to the increased morbidity and mortality of ischemic atherosclerotic cardiovascular diseases (ACD) [3, 4]. While leukocytosis could be a marker of other processes such as infection, there is considerable evidence that leukocytosis directly promotes the entry of monocytes and neutrophils into the arterial wall, increasing atherosclerosis and thrombosis [4].
Dyslipidemia and accumulation of cholesterol in hematopoietic stem and progenitor cells (HSPC) may link increased HSPC proliferation with myeloid bias to atherogenesis. Mice deficient in the adenosine triphosphate-binding cassette (ABC) transporters A1 and G1 (ABCA1 and ABCG1), which promote cholesterol efflux from myeloid cells, developed monocytosis, neutrophilia, and expansion of HSPC [5]. A similar observation was made in Western diet (WD)–fed hypercholesterolemic Ldlr−/− and Apoe−/− mice [6], where monocytosis and neutrophilia were associated with HSPC expansion. Competitive BM transplantation experiments suggested a cell intrinsic proliferative advantage and biased myelopoiesis of Apoe−/− or Abca1−/−Abcg1−/− HSPCs relative to WT HSPCs giving rise to increased numbers of myeloid progenitors, monocytes, and neutrophils [5, 6]. In these studies, the cell intrinsic proliferative advantage of HSPCs was associated with increased cell surface levels of the common β subunit of the GM-CSF/IL-3 receptor (CBS) and increased GM-CSF/IL-3 signaling [5, 6]. Importantly, CBS deficiency in WD-fed Apoe−/− mice reduced HSPC expansion, monocytosis, and neutrophilia and decreased atherogenesis, causally linking aberrant cell proliferation signaling in hematopoietic cells to excess myelopoiesis, monocytosis, neutrophilia, and atherogenesis [7].
Janus kinase 2 (JAK2) is a non-receptor tyrosine kinase and a critical node in multiple growth factor and cytokine-mediated signaling pathways regulating hematopoiesis, including the GM-CSF/IL-3 signaling cascade [8]. Loss of JAK2 is embryonically lethal in mice [9], due to defective hematopoiesis. Conditional knockout of Jak2 primarily in hematopoietic cells in adult mice results in a marked defect in hematopoiesis and hematopoietic stem cell function [10, 11]. The critical role of JAK2 in myelopoiesis in humans is illustrated by JAK2 mutations. Acquired activating mutations of JAK2 in hematopoietic tissues, with JAK2V617F as the most common, drive development of myeloproliferative neoplasms (MPNs) [12]. JAK2V617F is also one of several genetic variants that drive clonal hematopoiesis of indeterminate potential (CHIP) which is common in the elderly [13, 14]. Both JAK2V617F-driven MPN and CHIP are associated with increased risk of CVD [15, 16].
Subsequent to the discovery of JAK2V617F as the major mutation that drives MPN, JAK2 inhibitors were developed for MPN therapy. Ruxolitinib was the first JAK1/2 inhibitor that has been approved for the treatment of intermediate or high-risk myelofibrosis [17], including primary myelofibrosis, post-polycythemia vera myelofibrosis, and post-essential thrombocythemia myelofibrosis. While JAK2 inhibition ameliorates MPN phenotypes driven by JAK2 or related gene mutations in both mouse models and humans, its impact on excessive myelopoiesis and atherogenesis caused by hypercholesterolemia and defective cholesterol efflux from HSPCs and myeloid progenitors is unknown. In this study, we have assessed the impact of treatment with TG101348 (fedratinib), a selective inhibitor of JAK2 [18, 19], on myelopoiesis and atherosclerosis in WD-fed Apoe−/− mice.
Materials and Methods
Mice
WT (C57BL/6) and Apoe−/− (B6.129P2-APOE<tm1 Unc>/J) female mice were purchased from the Jackson Laboratory. At 8 weeks of age, all mice were fed a WD (21% milk fat, 0.2% cholesterol; catalog no. TD88137; Harlan Teklad) for the specified period of time. One week after WD feeding, Apoe−/− mice and WT mice received TG101348 in phosphate-buffered saline (PBS) containing 0.5% methyl-cellulose (Sigma) or vehicle (PBS containing 0.5% methyl-cellulose) via oral gavage, at a dose of 120 mg/kg per day in the first week, and 240 mg/kg per day in the following days. Where appropriate, mice will be euthanized by CO2 asphyxiation followed by cervical dislocation, a method consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. All animal experiments and procedures were designed according to NIH guidelines and approved by Columbia University IACUC.
Complete Blood Count Analysis
Complete blood count analysis was performed using freshly drawn blood via angular vein and the FORCYTE Veterinary Analyzer (Oxford Science Inc.).
Flow Cytometry
For identification of monocytes and neutrophils from whole blood, we used the following strategy. Blood was drawn via angular vein collected into EDTA tubes, which were immediately placed on ice. RBCs were lysed (BD pharm Lyse; BD Biosciences), and leukocytes were pelleted by centrifugation, and resuspended in HBSS (0.1% BSA w/v, 5 mM EDTA). Cells were stained with a cocktail of antibodies against CD45-pacific blue, Ly6-C/G-PerCP-Cy5.5 (BD Biosciences), CD115-APC, and CD11b-PEcy7(eBioscience). Samples were analyzed on an LSR-II (BD Biosciences). Neutrophil were defined as CD45hi Ly6-C/Ghi CD115lo. Monocytes were identified as CD45hiCD115hi and further subdivided into Ly6-Chi and Ly6-Clo [20, 21].
For BM hematopoietic cell profiling, BM cells were stained and analyzed as previously described [22]. Briefly, BM cells from mouse femurs and tibias were stained with a cocktail of antibodies to lineage-committed cells (CD45R, CD19, CD11b, CD3e, TER-119, CD2, CD8, CD4, and Ly-6C/G, all FITC conjugated; Bioscience), Sca 1-pacific blue and c-Kit-BV605 to identify HSPC (Lin− Sca1+ c-Kit+) cells and HSPCs (Lin− Sca1− c-Kit+) together with antibodies against CD16/CD32 (FcγRII/III)-BV510 and CD34-APC to separate CMP (Lin− Sca1− c-Kit+, CD34int, FcγRII/IIIint), granulocyte-macrophage progenitor (GMP) (Lin− Sca1− c-Kit+, CD34int, FcγRII/IIIhi), and MEP (Lin− Sca1− c-Kit+, CD34low, FcγRII/IIIlow). Where further identification of MEP population was required, ERP was defined as Lin− Sca1− c-Kit+, CD34low, FcγRII/IIIlow, CD71+ CD41−, and MKP as Lin− Sca1− c-Kit+, CD34low, FcγRII/IIIlow, and CD71− CD41+. Cell cycle was quantified using 7-AAD. Phospho-flow was performed using antibodies to PE conjugated p-ERK1/2 or p-STAT5 [22, 23]. Briefly, BM cells were stained with antibodies described above and then fixed by 2% PFA in the room temperature for 10 min. The cells were then resuspended by Perm Buffer III (BD Biosciences) 30 min in the ice. After washing and resuspending by staining buffer, the PE Anti-ERK1/2(BD Phosflow) and PE Anti-STAT5(BD Phosflow) were added and incubated in room temperature for 30 min and then analyzed by flow cytometry.
Proliferation Assays
Bone marrow cells were isolated from WD-fed WT or Apoe−/− mice treated with vehicle or TG101348 (240 mg/day) for 6 weeks and then were cultured in IMDM (Invitrogen) with 10% FBS and 1 μM BrdU (Sigma). After 12-h incubation, cells were stained as described above (lineage changed to APC-conjugated and CD34 changed to PE-conjugated antibodies) for progenitor cells together with FITC-anti BrdU (Biolegend). Proliferation was quantified as percentage of BrdU + cells by flow cytometry.
Atherosclerosis Study
The thoracic and abdominal descending aortas were collected from WD-fed Apoe−/− mice following systemic perfusion with PBS by cardiac puncture of mice anesthetized with isoflurane vaporizer (inhale, ~ 5% isoflurane) and stained with Oil Red O, following fixation with 10% buffered formalin. Aortas were pinned in silicon dishes, and Oil Red O–positive areas were quantified using ImageJ software and expressed as the percentage of the total aorta area.
Plasma Cholesterol Level Measurement
Plasma was collected from blood sample by centrifugation for 10 min at 10,000×g using a refrigerated centrifuge. Total cholesterol levels were measured using the Cholesterol E kit (Wako Diagnostics) as per the manufacturer’s instructions.
Statistics
The number of mice used was estimated by power analysis based on the data from our previous studies. Normality assumption of the data distribution was assessed using Kolmogorov-Smirnov test. Data were analyzed by unpaired t test if data were normally distributed and two groups were involved. One-way ANOVA was used for more than two groups. Two-tailed analysis was performed in all statistical analyses. p value less than 0.05 was considered a significant difference.
Results
TG101348 Reverses Monocytosis and Neutrophilia in WD-Fed Apoe−/− Mice
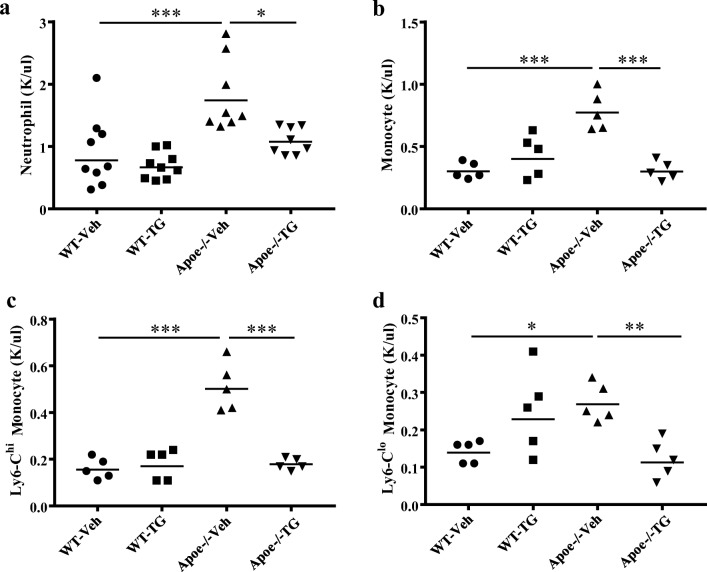
To assess the impact of TG101348 on hematopoiesis, we administered vehicle or TG101348 to WD-fed WT or Apoe−/− mice at a dose that sustained the plasma concentration above the cellular IC50 and effectively reduced myelopoiesis, hematocrit, and leukocytosis in a murine model of MPN induced by hematopoietic Jak2V617F expression [18]. Consistent with the previous report, TG101348 did not cause apparent abnormalities or toxicities in either WT or Apoe−/− mice, with no difference in gain of body weight (Supplemental Figure 1A, B). WD feeding in combination with APOE deficiency markedly increased plasma total cholesterol levels, as expected. TG101348 did not affect plasma cholesterol or HDL levels (Supplemental Figure 1C, D). Apoe−/− mice WD-fed for one week showed monocytosis and neutrophilia relative to the WD-fed WT mice (supplemental Figure 2A, B) and continued WD-feeding made this leukocytosis even more pronounced (Fig. 1), as reported [6, 24, 25]. WD feeding, relative to chow diet, moderately increases plasma cholesterol levels and has no effect on white blood cell counts including monocyte and neutrophil counts in WT mice [24]. At the time point just before vehicle or TG101348 treatment, the baseline levels of monocytes, neutrophils, platelets, and hemoglobin concentration showed no difference between vehicle and TG101348 groups (Supplemental Figure 2A, B; Supplemental Figure 3A, B). However, after 30 days of TG101348 treatment, the neutrophilia and monocytosis in Apoe−/− mice was reduced to the level of the WT mice (Fig. 1a, b). Ly6Chi monocytes preferentially enter atherosclerotic lesions where they differentiate to macrophages that are actively involved in progression of atherosclerosis [24, 25]. Apoe−/− mice showed a marked increase in Ly6Chi monocyte which was reversed by TG101348 (Fig. 1c, Supplemental Figure 5). TG101348 also reduced Ly6Clo monocytes in Apoe−/− mice (Fig. 1d). In contrast, TG101348 showed no effects on neutrophil and monocyte counts in the WT mice (Fig. 1a, b) nor did it affect platelet counts in either group (Supplemental Figure 3D). TG101348 slightly decreased hemoglobin levels and red blood cell counts in WT and Apoe−/− mice (Supplemental Figure 3C and Supplemental Figure 4). These findings indicate that TG101348 selectively reverses monocytosis and neutrophilia in WD-fed Apoe−/− mice.
Fig. 1.
TG101348 selectively reverses monocytosis and neutrophilia in WD-fed Apoe−/− mice. After 1-week WD feeding, female mice were treated with vehicle or TG101348 for 30 days and peripheral blood cell profiles were assessed as described in “Materials and methods.” a Neutrophil counts assessed by automated blood cell analyzer. b Total monocyte, c Ly6Chi, and d Ly6Clo monocyte counts were determined with 5 randomly selected samples by flow cytometry in combination with automated blood cell analyzer. One-way ANOVA. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
TG101348 Reverses HSPC Expansion in WD-Fed Apoe−/− Mice
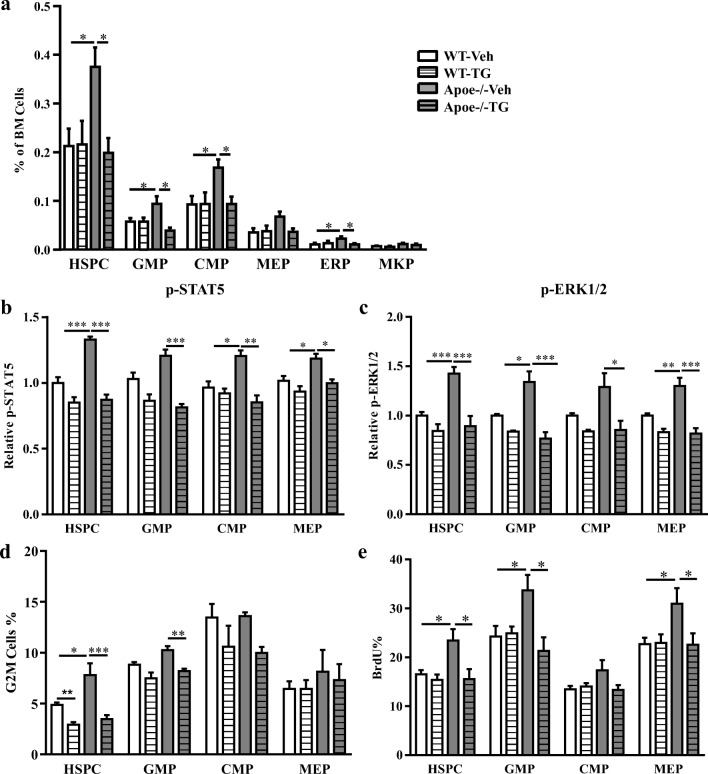
Previous studies indicate that neutrophilia and monocytosis in Apoe−/− mice are associated with increased proliferation and expansion of multipotential progenitor cells (HSPCs) [6]. This HSPC expansion appears to be critical for aberrant myelopoiesis and leukocytosis in Apoe−/− mice [6, 7]. To assess the impact of TG101348 on HSPC expansion and myelopoiesis, we analyzed bone marrow hematopoietic cell profiles. Consistent with previous findings [6], the HSPC population was expanded in Apoe−/− mice fed WD for 10 weeks relative to the WT control (Fig. 2a) and so were common myeloid progenitors (CMP) and GMPs. TG101348 treatment in the last 9 weeks of WD feeding reversed the expansion of HSPCs and myeloid progenitors in Apoe−/− mice but showed no effects on these cell populations in WT mice (Fig. 2a). WD-fed Apoe−/− mice also showed splenomegaly (Supplemental Figure 6A, B), likely as a result of increased extramedullary hematopoiesis as reported [6]. TG101348 selectively reversed the splenomegaly in Apoe−/− mice and showed no effect on spleen weight in WT mice (Supplemental Figure 6A, B).
Fig. 2.
TG101348 reverses excessive proliferation and expansion of HSPCs in WD-fed Apoe−/− mice. Female mice were treated with vehicle or TG101348 for 9 weeks. a Hematopoietic cell profile in bone marrow. Progenitor cells were defined as HSPC (Lin− Sca1+ c-Kit+), CMP (Lin− Sca1− c-Kit+ CD34intFcγRII/IIIint), GMP (Lin− Sca1− c-Kit+ CD34intFcγRII/IIIhi), MEP (Lin− Sca1− c-Kit+ CD34lowFcγRII/IIIlow), ERP(Lin− Sca1− c-Kit+ CD34lowFcγRII/IIIlowCD71+ CD41−), and MKP (Lin− Sca1− c-Kit+ CD34lowFcγRII/IIIlowCD71− CD41+) by flow cytometry. b Phospho-flow of p-STAT5 and c p-ERK1/2 relative MFI in HSPCs. d Percentage G2M phase positive cells of HSPCs. e HSPC proliferation was determined by BrdU incorporation. Four to six randomly selected sample per group were used for each of the assays. One-way ANOVA. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. n = 4 (e) to 6 (a–d) mice per group
Increased HSPC proliferation and expansion in Apoe−/− mice reflect enhanced signaling in pathways involved in cell survival and proliferation, including IL-3/GM-CSF classical signaling pathways [6]. Indeed, levels of pSTAT5 and pERK1/2, the downstream signaling molecules of IL-3/GM-CSF pathways, in HSPCs from Apoe−/− mice were increased (Fig. 2b, c) as assessed by phospho-flow cytometry, as reported [6], indicating increased proliferation signaling. pSTAT5 and pERK1/2 were also increased in GMPs and CMPs (Fig. 2b, c). Consistent with increased proliferation signaling, cell cycling, as assessed by G2M phase positive cells, and cell proliferation, as assessed by BrdU incorporation, in HSPCs from Apoe−/− mice also was increased (Fig. 2d, e), as reported [6]. TG101348 reversed the increase in pSTAT5, pERK1/2, cell cycling, and BrdU incorporation in HSPCs and GMPs (Fig. 2b–e, Supplemental Figure 7A, B; Supplemental Figure 8). Together, these data suggest that TG101348 reverses HSPC expansion by inhibiting cell proliferation signaling that involves JAK2, STAT5, and ERK1/2.
TG101348 Decreases Atherosclerosis in Apoe−/− Mice
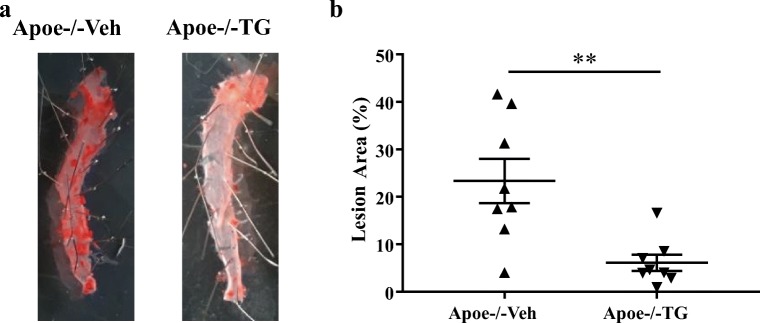
We next assessed the impact of TG101348 on atherogenesis in WD-fed Apoe−/− mice. Apoe−/− mice were fed WD for 10 weeks and treated with TG101348 or vehicle in the last 9 weeks as detailed in “Materials and methods.” TG101348 did not affect plasma total cholesterol levels following the treatment for 9 weeks (not shown), consistent with no change of HDL or non-HDL cholesterol levels following TG101348 treatment for 30 days (Supplemental Figure 1B). Atherogenesis was assessed by en face Oil Red O staining of the descending aorta. WD feeding induced marked Oil Red O stained and neutral lipid-rich aortic atherosclerosis in Apoe−/− mice, and TG101348 substantially reduced atherosclerosis lesion area by ~ 74% (Fig. 3a, b). Together, these data indicate that TG101348 reduces atherogenesis in Apoe−/− mice at least in part by selective suppression of HSPC expansion, excessive myelopoiesis, and leukocytosis.
Fig. 3.
TG101348 suppressed atherosclerosis progression in Apoe−/− mice. Female Apoe−/− mice were fed WD for 1 week following by 9-week WD feeding with vehicle or TG101348 treatment. a Oil red O staining of aortas. b Plaque area as a percentage of total area. Unpaired t test. Data are presented as mean ± SEM. **p < 0.01
Discussion
Our studies demonstrate that TG101348 decreases atherosclerosis in Apoe−/− mice, likely by selective inhibition of hematopoietic JAK2 that results in suppression of excessive myelopoiesis driven by enhanced cell proliferation signaling in HSPCs and myeloid progenitors and reversal of HSPC expansion and leukocytosis. This suggests the possible translation of TG101348 therapy in reducing the risk of ACD in association with moderate myeloproliferation with or without JAK2 mutations.
The clearest evidence for a role of excessive myelopoiesis in neutrophilia and monocytosis comes from studies of animal models with hypercholesterolemia and genetic deficiency in genes involved in cholesterol efflux from hematopoietic cells such as Abca1−/−/Abcg1−/− or Apoe−/− mice [5, 6]. However, excessive myelopoiesis and leukocytosis also occur in hypercholesterolemic mice such as long-term WD-fed Ldlr−/− without deficiencies in cholesterol efflux genes [6]. Ldlr−/− mice with loss of one copy of Apoa1 also showed HSPC expansion, monocytosis, and neutrophilia [26]. Importantly, HDL-C levels in children with familial hypercholesterolemia showed an inverse correlation with monocyte counts [26]. Cell membrane cholesterol accumulation in HSPCs and myeloid progenitors increases surface CBS levels, leading to enhanced IL3- or GM-CSF-mediated cell proliferation signaling [5–7]. Although the precise mechanism responsible for the increased CBS expression at the cell surface remains elusive, hypercholesterolemia appears to disrupt a negative feedback desensitization response that downregulates CBS in response to IL3 or GM-CSF stimulation [27, 28]. Hypercholesterolemia-triggered neutrophilia and mobilization of Ly6Chi monocytes from the spleen as a result of extramedullary hematopoiesis in WD-fed Apoe−/− mice have been shown to promote atherogenesis [29, 30] and reversal of neutrophilia and reduction of Ly6Chi monocytes by TG101348 likely contributes to reduced atherogenesis. Our studies demonstrate for the first time that selective inhibition of JAK2 reverses excessive myelopoiesis and reduces atherosclerosis in hypercholesterolemic mice.
Subsequent to the discovery of JAK2VF as a major mutation that drives development of MPN, JAK inhibitors were developed as a therapy for MPN. Following the approval of the JAK1/2 inhibitor ruxolitinib, TG101348 (fedratinib) was developed as a selective JAK2 inhibitor; clinical trials met the primary endpoint (the proportion of patients with a ≥ 35% reduction in spleen volume) with TG101348 for patients who had MPN and showed no response or intolerance to ruxolitinib [19]. Recently, the FDA has approved fedratinib for treatment of myelofibrosis [31]. TG101348 slightly decreased red blood cell counts and hemoglobin content in both WT and Apoe−/− mice, consistent with anemia as a detected adverse effect of fedratinib in MPN therapy in clinical trials [32]. Other potential adverse effects of JAK inhibitors could include increases in LDL cholesterol and in body weight. However, since they may reflect amelioration of the underlying MPN [33, 34], they do not necessarily indicate an adverse effect on metabolic health.
Like many other tyrosine kinase inhibitors, inhibition of JAK2 by TG101348 is selective and the specificity is not absolute. TG101348 has been reported to inhibit FLT3 and RET, although with much higher IC50 relative to that of JAK2 [18]. FLT3 has a critical role in hematopoietic progenitor development and function [35]. In contrast to Jak2−/−, targeted disruption of Flt3 results in normal, healthy, and fertile adult mice, in association with no morphological and quantitative changes of peripheral blood cells [36]. The only detectable impact of Flt3−/− on mature blood cells in bone marrow was on lymphocytes, particularly reduction of B cells [36]. We did not detect any effect of TG101348 on lymphocytes in our models. Thus, we conclude that the primary impact of TG101348 on myelopoiesis in WD-fed Apoe−/− mice is mediated by selective inhibition of JAK2, although minor effect mediated by FLT3 inhibition cannot be excluded.
In addition to MPNs, JAK inhibitors have been developed for therapy of other immune and inflammatory disorders such as rheumatoid arthritis and inflammatory bowel disease [37] reflecting the role of JAKs in regulation of type I and type II cytokine receptor–mediated signaling [37]. Atherosclerosis has long been recognized as a chronic inflammatory disease [1], and innate and adaptive immunities in the setting of hypercholesterolemia are a major contributor to development and progression of atherosclerosis [38]. Prominent examples include enhanced inflammasome activation and IL-1β production in atherosclerotic macrophages [39, 40]. The translational significance was demonstrated by the CANTOS trial showing that IL-1β antagonism decreases the risk of ACD [41, 42]. Enhanced inflammatory responses with increased production of pro-inflammatory cytokines and transcriptional and epigenetic reprogramming of HSPCs and myeloid progenitors in hypercholesterolemia or other settings of chronic systemic inflammation such as rheumatoid arthritis could lead to biased myelopoiesis and leukocytosis, increasing atherogenesis [43, 44]. This could form a vicious cycle with biased myelopoiesis/leukocytosis and chronic inflammatory responses strengthening and exacerbating each other. While not specifically tested in this study, it is possible that TG101348 reduces atherogenesis by suppressing type I and type II cytokine receptor–mediated pro-inflammatory responses in the setting of hypercholesterolemia in Apoe−/− mice, in addition to reversal of leukocytosis, including acting directly on macrophages [45].
In summary, our studies suggest JAK2 inhibition as a novel therapeutic approach to decrease ACD risk associated with excessive myelopoiesis and leukocytosis.
Electronic Supplementary Material
Supplemental Figure 1. (A-B) Body weight after and following 9 weeks of vehicle or TG101348 (8-10 mice/group). (C) plasma total cholesterol levels and (D) HDL-cholesterol following 30 days of vehicle or TG101348 treatment (8-10 mice/group). One-way ANOVA. Data are mean ± SEM. **p<0.01, ***p<0.001. Supplemental Figure 2. Baseline levels of (A) neutrophil or (B) monocyte counts in peripheral blood (at day 0 before vehicle or TG101348 treatment) were shown. One-way ANOVA. Data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Supplemental Figure 3. WT and Apoe-/- mice hemoglobin and platelet counts. (A) The baseline hemoglobin concentration and (B) platelet counts in peripheral blood (at day 0 before vehicle or TG101348 treatment). (C) hemoglobin concentration and (D) platelet counts in peripheral blood following vehicle or TG101348 treatment for 30 days were shown. One-way ANOVA. Data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Supplemental Figure 4. Red blood cell counts following treatment with vehicle or TG101348 for 30 days. One-way ANOVA. Data are mean ± SEM. ***p<0.001. Supplemental Figure 5. Flow cytometry gating strategy of blood neutrophil and monocyte. Supplemental Figure 6. Decreased spleen weight in TG101348 treated Apoe-/- mice. Mice were fed WD for one week following by 9 weeks WD with vehicle or TG101348 treatment. (A) Absolute spleen weight and (B) spleen/body weight ratios. One-way ANOVA. Data are mean ± SEM. ***p<0.001. Supplemental Figure 7. Representative flow cytometric histograms of (A) p-STAT5 and (B) p-ERK1/2 in HSPC. Supplemental Figure 8. Flow cytometry gating strategy of hematopoietic progenitor cells. (PDF 414 kb)
Acknowledgments
Y. Tang, W. Liu, W. Wang, T. Fidler, and B. Woods performed research and analyzed data. N. Wang, A.R. Tall, and RL. Levine designed research, analyzed data, or wrote the article.
Funding Information
This study was supported by the National Institutes of Health Grant RO1 HL137663 (to A.R. Tall) and RO1 HL118567 (to N. Wang) and by Leducq Foundation Transatlantic Network. The Columbia University CCTI and Diabetes Research Center Flow Cytometry Cores, supported in part by the Office of the Director, National Institutes of Health, under awards S10RR027050, S10OD020056, and 5P30DK063608, were used for this study.
Compliance with Ethical Standards
Conflict of Interest
Alan R. Tall is a scientific advisory board member for Amgen, Staten Biotech, and Fortico Biotech and a consultant for Janssen, CSL, and the Medicines Company. Ross L. Levine is on the supervisory board of Qiagen and is a scientific advisor to Loxo, Imago, C4 Therapeutics, and Isoplexis which include equity interest. He receives research support from and consulted for Celgene and Roche and has consulted for Lilly, Janssen, Astellas, Morphosys, and Novartis. He has received honoraria from Roche, Lilly, and Amgen for invited lectures and from Gilead for grant reviews.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Yang Tang and Wenli Liu are co-first author.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alan R. Tall and Nan Wang contributed equally to this work.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.de Labry LO, Campion EW, Glynn RJ, Vokonas PS. White blood cell count as a predictor of mortality: results over 18 years from the Normative Aging Study. J Clin Epidemiol. 1990;43(2):153–157. doi: 10.1016/0895-4356(90)90178-r. [DOI] [PubMed] [Google Scholar]
- 3.Asadollahi K, Beeching NJ, Gill GV. Leukocytosis as a predictor for non-infective mortality and morbidity. QJM. 2010;103(5):285–292. doi: 10.1093/qjmed/hcp182. [DOI] [PubMed] [Google Scholar]
- 4.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25(4):658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 5.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328(5986):1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121(10):4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Subramanian M, Abramowicz S, et al. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler Thromb Vasc Biol. 2014;34(5):976–984. doi: 10.1161/ATVBAHA.113.303097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7(9):673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 10.Akada H, Akada S, Hutchison RE, Sakamoto K, Wagner KU, Mohi G. Critical role of Jak2 in the maintenance and function of adult hematopoietic stem cells. Stem Cells. 2014;32(7):1878–1889. doi: 10.1002/stem.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SO, Wamsley HL, Bae K, Hu Z, Li X, Choe SW, Slayton WB, Oh SP, Wagner KU, Sayeski PP. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8(3):e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Fröhling S, Döhner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Levine RL, Belisle C, Wadleigh M, Zahrieh D, Lee S, Chagnon P, Gilliland DG, Busque L. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood. 2006;107(10):4139–4141. doi: 10.1182/blood-2005-09-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy M, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, Barbui T, European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114–124. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 17.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, Gozo M, McDowell E, Levine RL, Doukas J, Mak CC, Noronha G, Martin M, Ko YD, Lee BH, Soll RM, Tefferi A, Hood JD, Gilliland DG. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13(4):311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P, Jourdan E, Winton E, Silver RT, Schouten HC, Passamonti F, Zweegman S, Talpaz M, Lager J, Shun Z, Mesa RA. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4(7):e317–ee24. doi: 10.1016/S2352-3026(17)30088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28(11):2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 21.Gower RM, Wu H, Foster GA, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31(1):160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Oh S, Koester M, Abramowicz S, Wang N, Tall AR, Welch CL. Enhanced megakaryopoiesis and platelet activity in hypercholesterolemic, B6-Ldlr−/−, Cdkn2a-deficient mice. Circ Cardiovasc Genet. 2016;9(3):213–222. doi: 10.1161/CIRCGENETICS.115.001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med. 2013;19(5):586–594. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolani S, Pagler TA, Murphy AJ, Bochem AE, Abramowicz S, Welch C, Nagareddy PR, Holleran S, Hovingh GK, Kuivenhoven JA, Tall AR. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis. 2013;229(1):79–85. doi: 10.1016/j.atherosclerosis.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Moczygemba M, Huston DP. Proteasomal regulation of betac signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization. J Clin Invest. 2001;108(12):1797–1806. doi: 10.1172/JCI13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Tang Y, Wang Y, Tascau L, Balcerek J, Tong W, Levine RL, Welch C, Tall AR, Wang N. LNK/SH2B3 loss of function promotes atherosclerosis and thrombosis. Circ Res. 2016;119(6):e91–e103. doi: 10.1161/CIRCRESAHA.116.308955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 30.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125(2):364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedratinib Becomes New Option in Myelofibrosis. Cancer Discov, 2019. [DOI] [PubMed]
- 32.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tefferi A, Nicolosi M, Penna D, Mudireddy M, Szuber N, Lasho TL, Hanson CA, Ketterling RP, Gangat N, Pardanani AD. Development of a prognostically relevant cachexia index in primary myelofibrosis using serum albumin and cholesterol levels. Blood Adv. 2018;2(15):1980–1984. doi: 10.1182/bloodadvances.2018018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugliese L, Bernardini I, Pacifico N, Peverini M, Damaskopoulou E, Cataldi S, Albi E. Severe hypocholesterolaemia is often neglected in haematological malignancies. Eur J Cancer. 2010;46(9):1735–1743. doi: 10.1016/j.ejca.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 36.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3(1):147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17(1):78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerterp M, Fotakis P, Ouimet M, et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation. 2018;138(9):898–912. doi: 10.1161/CIRCULATIONAHA.117.032636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, CANTOS Trial Group Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 43.Murphy AJ, Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J. 2016;37(14):1113–1121. doi: 10.1093/eurheartj/ehv718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun Ü, Hajishengallis G, Netea MG, Chavakis T. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172(1–2):147–161. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, Wei L, Pfeffer LM, Berliner JA. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282(43):31460–31468. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A-B) Body weight after and following 9 weeks of vehicle or TG101348 (8-10 mice/group). (C) plasma total cholesterol levels and (D) HDL-cholesterol following 30 days of vehicle or TG101348 treatment (8-10 mice/group). One-way ANOVA. Data are mean ± SEM. **p<0.01, ***p<0.001. Supplemental Figure 2. Baseline levels of (A) neutrophil or (B) monocyte counts in peripheral blood (at day 0 before vehicle or TG101348 treatment) were shown. One-way ANOVA. Data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Supplemental Figure 3. WT and Apoe-/- mice hemoglobin and platelet counts. (A) The baseline hemoglobin concentration and (B) platelet counts in peripheral blood (at day 0 before vehicle or TG101348 treatment). (C) hemoglobin concentration and (D) platelet counts in peripheral blood following vehicle or TG101348 treatment for 30 days were shown. One-way ANOVA. Data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Supplemental Figure 4. Red blood cell counts following treatment with vehicle or TG101348 for 30 days. One-way ANOVA. Data are mean ± SEM. ***p<0.001. Supplemental Figure 5. Flow cytometry gating strategy of blood neutrophil and monocyte. Supplemental Figure 6. Decreased spleen weight in TG101348 treated Apoe-/- mice. Mice were fed WD for one week following by 9 weeks WD with vehicle or TG101348 treatment. (A) Absolute spleen weight and (B) spleen/body weight ratios. One-way ANOVA. Data are mean ± SEM. ***p<0.001. Supplemental Figure 7. Representative flow cytometric histograms of (A) p-STAT5 and (B) p-ERK1/2 in HSPC. Supplemental Figure 8. Flow cytometry gating strategy of hematopoietic progenitor cells. (PDF 414 kb)