Abstract
As the primary indication for corneal transplantation, the pathogenesis of keratoconus remains elusive. Aiming to identify whether any mutation from extracellular-matrix (ECM)-related genes contributes to the patients with sporadic cases of keratoconus (KC) from Chinese Han population, one hundred and fifty-three participants in total were enrolled in our study, including fifty-three KC patients and one hundred healthy controls. Mutational analysis of three ECM-related genes (LOX, COL5A1 and TIMP3) with next-generation sequencing and Sanger sequencing was performed. To further confirm the function of three ECM-related genes in the pathogenesis of keratoconus, we performed Real-time Quantitative PCR in vitro. Results showed that three new sequence variants (c.95 G > A in LOX, c.1372 C > T in COL5A1 and c.476 C > T in TIMP3) were identified in aforementioned ECM-related genes in KC patients without being detected among the healthy controls. According to the results of QPCR, we found that the expression levels of LOX and TIMP3 were decreased in the KC patients, while COL5A1 showed no significant difference of expression. This is the first time to screen so many ECM-related genes in Chinese keratoconus patients using next-generation sequencing. We find numerous underlying causal variants, enlarging lots of mutation spectrums and thus providing new sites for other investigators to replicate and for further research.
Subject terms: Gene expression, Next-generation sequencing
Introduction
Keratoconus (KC) is a progressive disorder characterized by central cornea thinning and ectasia in a cone-shape fashion, leading to myopia, irregular astigmatism and even vision loss1,2. KC usually occurs from the second decade to the fourth decade of life, and affects both genders. The prevalence of KC ranges from 900 to 3300 per 100,000 in recent population-based studies2. Clinically, corneal tomography is most frequently used for diagnosis of KC, while mild or subclinical KC can be diagnosed by posterior corneal elevation abnormalities3. The treatments of KC often begin with verbal guidance such as not rubbing eyes and wearing contact lenses, with 10–20% of patients finally turning to corneal transplantation3,4. Collagen cross-linking (CXL) is a novel intervention effective in the therapy of KC.
However, the exact etiology and pathogenesis of KC remains unclear, in which genetic, environmental, biomechanical and biochemical factors may involve3. Eye rubbing5, atopy6,7 and sun exposure2 are important environmental factors indicating a high risk of KC. In addition, there are increasing evidences suggesting a genetic predisposition in the pathogenesis of keratoconus, with lots of genomic loci and genes identified, including visual system homeobox 1 (VSX1)8–10, superoxide dismutase 1 (SOD1)10,11, transforming growth factor beta-induced (TGFβI)12 and microRNA 184 (MIR184)13,14. Nevertheless, it is to be further explored whether and how these genomic loci and genes participate in the progression of KC.
As a major component of the cornea, the corneal stroma rich in extracellular matrix (ECM) plays an important role in cornea diseases, thinning of which cannot resist normal intraocular pressure, causing cornea protruding and finally developing KC. Over years, studies on the relationship between ECM and KC have been more and more conducted, and many ECM-related genes and corresponding proteins have been found to be potentially involved in the pathogenesis of KC, such as glycoprotein fibronectin (FN1)15, integrin15, metalloproteinase (MMP9)15,16, tissue inhibitor of metalloproteinase (TIMP1, TIMP2)15,16, thrombospondin1 (THBS1)15,17, transforming growth factor beta-induced gene (TGFBI)15,18,19, et al. LOX, COL5A1 and TIMP3 were three ECM-related genes identified in this study. The LOX gene is located on the 5q23.2 chromosomal region, including seven exons and six introns20. The inactive 50 kDa pro-enzyme is first produced until processed by pro-collagen C-proteinases—mammalian Tolloids and bone morphogenetic protein-1 (BMP-1) to become active enough to cross link collagens and elastin by catalyzing oxidative deamination of peptidyl lysines20–23. The COL5A1 gene, located on 9q34.2, encodes the α1 chain of type V collagen, which regulates collagen fibrillogenesis24,25. Collagen V is a quantitatively minor component in most tissues, and often functions in a heterotypic form with collagen I26,27. Collagen V has different isoforms, of which the most abundant and ubiquitous form is the heterotrimer [α1(V)]2α2(V), then α1(V)α2(V)α3(V) and [α1(V)]2α4(V), with [α1(V)]3 homotrimer the least common form showing a more restricted expression pattern28. The high proportion of type V collagen in the cornea leads to the large number of nucleation sites, which may account for the great number and small diameter of fibril necessary for transparency29,30. The TIMP3 gene is on 22q12.3, containing 5 exons. The gene products TIMP3 is a tissue specific, endogenous inhibitor of metalloproteinase (MMP), thus playing an important role in extracellular matrix remodeling and potentially KC progression31,32.
In this study, we aimed to make further explorations on the biomechanical nature of KC, and sequenced several ECM-related genes in a Chinese Han population by next-generation sequencing. According to the results of sequencing, we found that three variants in three genes respectively (c.95 G > A in LOX, c.1372 C > T in COL5A1 and c.476 C > T in TIMP3) might play a role in the pathogenesis of keratoconus. Further QPCR conduction showed that the expression levels of LOX and TIMP3 were decreased in the KC patients, while COL5A1 showed no significant difference of expression between KC and healthy controls.
Results
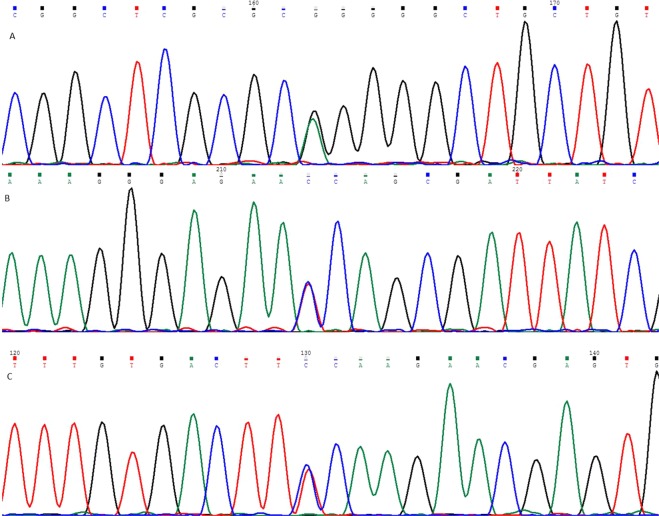
A total of 53 keratoconus (KC) patients and 100 healthy controls were included in this study. Demographic characteristics of all KC patients and three patients carrying target variants were shown in Table 1 and Table 2 respectively. According to the results of next-generation sequencing in KC patients, three single nucleotide variants were separately identified in three extracellular-matrix related genes (c.95 G > A in LOX, c.1372 C > T in COL5A1 and c.476 C > T in TIMP3). Sanger sequencing were then conducted in 100 healthy controls to rule out the possibility of false positives. Sequencing chromatograms of the three mutations were shown in Fig. 1, and all were located in the exon regions of the corresponding genes. None of the three mutations were classified as tolerated according to SIFT (Table 3).
Table 1.
The general demographic characteristics of the 53 keratoconus patients in this study.
| Demographic characteristics | ||
|---|---|---|
| Sex | Males | 41 (77.36%) |
| Females | 12 (22.64%) | |
| Age | 27.04 ± 6.35 | |
| Age at diagnosis (year) | 20.06 ± 4.18 | |
| Disease laterality | OD | 4 (7.55%) |
| OS | 6 (11.32%) | |
| OU | 43 (81.13%) | |
| Diopter (OD) | −6.78 ± 4.14 | |
| Diopter (OS) | −6.14 ± 3.4 | |
| Corneal transplantation history | Positive | 1 (1.89%) |
| Negative | 52 (98.11%) | |
| Corneal thickness (OD, at the thinnest point) | 461.45 ± 56.64 | |
| Corneal thickness (OS, at the thinnest point) | 471.47 ± 50.7 | |
Table 2.
The demographic characteristics of the 3 keratoconus patients carrying target mutations in this study.
| Mutation | Sex | Age | Age at diagnosis | Disease laterality | Diopter (OD) | Diopter (OS) | Corneal transplantation history | Corneal thickness (OD) | Corneal thickness (OS) |
|---|---|---|---|---|---|---|---|---|---|
| c.95 G > A in LOX | Male | 28 | 21 | OU | −6.5 | −5.25 | negative | 467 | 489 |
| c.1372 C > T in COL5A1 | Male | 32 | 25 | OU | −5.75 | −11.5 | negative | 501 | 468 |
| c.476 C > T in TIMP3 | Male | 20 | 15 | OU | −4.75 | −9.5 | negative | 472 | 444 |
Figure 1.
Sequence chromatograms of LOX (A), COL5A1 (B) and TIMP3 (C).
Table 3.
Three novel mutations respectively of LOX, COL5A1 and TIMP3 identified in KC patients.
| Gene | Nucleotide change | Amino acid change | Position | Gene region | Mutation effect | SIFT score |
|---|---|---|---|---|---|---|
| LOX | c.95 G > A | P32L | 121413586 | exonic | nonsynonymous SNV | 0.01 |
| COL5A1 | c.1372 C > T | S159F | 33255204 | exonic | nonsynonymous SNV | 0 |
| TIMP3 | c.476 C > T | P458S | 137623956 | exonic | nonsynonymous SNV | 0.01 |
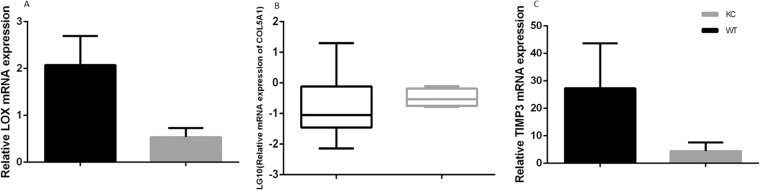
Based on the results of sequencing, QPCR was further conducted to explore the molecular manifestations of aforementioned three genes in 6 KC patients and 4 healthy controls. There were of no significant difference on demographic characteristics between KC and control groups (Table 4). Results of QPCR (Fig. 2) showed that mRNA expression of LOX and TIMP3 were significantly higher in KC corneas compared to controls (P < 0.01 and P = 0.0297 respectively), while no significant differences were observed on COL5A1 expression (P = 0.6252).
Table 4.
Demographic characteristics of 6 keratoconus patients and 4 healthy controls for QPCR experiment.
| Group | Sex (%) | Age (year) | |
|---|---|---|---|
| Keratoconus patients | Males | 66.7% | 44.67 ± 18.02 |
| Females | 33.3% | ||
| Healthy controls | Males | 75% | 53.75 ± 13.7 |
| Females | 25% | ||
| P value | 0.807 | 0.419 | |
Figure 2.
Relative mRNA expressions of LOX (left, A), COL5A1 (middle, B) and TIMP3 (right, C) in the KC patients (grey panels) and healthy controls (black panel).
Discussion
In this study, three novel mutations (c.95 G > A in LOX, c.1372 C > T in COL5A1 and c.476 C > T in TIMP3) leading to the following amino acid substitutions P32L, P458S and S159F, were recognized in a Chinese Han population of 53 keratoconus (KC) patients. All mutations were discovered in sporadic cases by next generation sequencing and validated in 100 healthy controls by Sanger sequencing and identified as damaging according to the results of SIFT. SIFT is an online tool distinguishing damaging amino acid substitutions from tolerant ones, which is based on sequence homology and the severity of the corresponding amino acid change18,19. The outstanding advantage of SIFT is not requiring structure but having similar power to those that use structure20,21. Further QPCR performance showed a decreased mRNA expression of LOX and TIMP3 in KC corneas, with no significant discrepancy found in COL5A1 expression.
KC is a multifactorial disease with the exact etiology remaining unclear to date. Stromal thinning is an important hallmark of KC, with more studies focusing on the mechanisms of biomechanical factors in the pathogenesis of KC33–35. In our study, LOX, COL5A1 and TIMP3 were three ECM-related genes playing different roles in the pathogenesis of KC. First, COL5A1 is a component of ECM regulating collagen fibrillogenesis24,25. Then, LOX catalyzes the formation of covalent bonds between elastin and collagens which promotes the maturation of ECM36–38. Finally, TIMP3 works to resist the function of MMP or facilitate the apoptosis of stromal cells, thus playing a unique role in ECM remodeling38. These all implicated that instability of ECM may be a crucial mechanism underlying the pathogenesis of KC.
The LOX gene encodes a copper-dependent amine oxidase which is crucial for cross-linking of ECM, the stability of which is thus guaranteed39. Recently, more and more studies have shown LOX as a candidate gene for KC38–46. Multiple LOX mutations have been detected in sporadic (rs1800449 in the Iranian population40, rs2956540 in the Chinese44 and European45 population) and familial (rs2956540, rs10519694 rs1800449 and rs2288393 in the American population46) cases. Molecular evidences further showed a positive correlation between decreased expression and activity of LOX and the severity of KC41,42. In our study, we found a new mutation (c.95 G > A) in LOX, accompanied by a reduction in LOX mRNA expression in KC patients. It can be well explained that destruction of normal LOX expression destroys ECM maturation by reduced cross-linking of collagen fibers in the cornea stroma, leading to cornea biomechanical instability and thinning31,47, which is a remarkable characteristic of KC. It can be further proved by observing reduced crosslinks between collagen and elastic fibers in LOX-null mice48,49. So it is a reasonable guess that normal LOX expression in the cornea is important in stabilizing the structure and function of the cornea; mutations in LOX may affect expression of corresponding proteins by alternative splicing42. Collagen cross linking (CXL) is a relatively safe and well-tolerated treatment to KC50, the principle of which is similar to the function mechanism of LOX. The finding that LOX was higher expressed in the high-response-to-CXL group compared to the low response group51 further confirmed the function of LOX and its potential role in KC. However, some SNPs (rs295654044–46, rs1051969446, rs180044940,46 and rs228839346) confirmed in previous studies was undetected in this study, which may be due to the limited number of patients and different populations. Otherwise, a study in 2012 even did not find any pathogenic variant in KC38, and another gene expression microarray study showed a oppositely increasing trend in the KC patients compared to the normal controls52. These all indicated the elusive pathogenic mechanisms of KC in which LOX may include various mutants and play multiple roles by different signal pathways.
COL5A1 is a kind of central-cornea-thickness (CCT)-related gene that codes for an alpha chain of Collagen V53. Many population studies have found that COL5A1 was associated with corneal thinning54–56, which is characteristic of KC. SNPs rs7044529 and rs1536482 of COL5A1 were also indicated to be related with KC in several population studies53,54,57. However, some subsequent analyses of KC population showed no significant difference of COL5A1 minor allele frequency (MAF) between KC patients and controls40,44, making the function of COL5A1 in KC confusing. In our study, a new mutation in COL5A1 (c.1372 C > T) was detected. However, the result of QPCR that the mRNA expression was of no significant difference between KC patients and healthy controls (P = 0.6264) was beyond expectation. We speculate that COL5A1 may be a potentially pathogenic locus for KC as verified in many previous studies53,58, but not all KC patients carry the mutation of this gene, perhaps related with race, region and so on. In addition, although the expression level of COL5A1 in KC was comparable with that in the control, the structure or function of that protein might have been damaged in the KC group, thus also contributing the development of KC. As for the discrete mechanisms, it is well expected that the different compositions of Collagen V may play a role in the pathogenesis of KC as α1 homotrimer being the predominant form is not able to be well incorporated into ECM, finally destroying the integrity and stability of ECM59. The construction of conditional-col5α2-knock-out mice model verified this opinion from the other perspective29. The targeted deletion of col5α2 caused the homotrimer [α1(V)]3 the major form which is unable to be absorbed into the heterotypic collagen fibrils, thus impairing skin matrix organization59. Another murine model also showed that the cornea was thinner and had fewer collagen fibrils in heterozygous col5α1 null mice than in wild type mice30,60.
The protein product of gene TIMP3 is a type of tissue inhibitor of metalloproteinase (TIMP), which functions against matrix metalloproteinase (MMP) to protect tissues from irreversible destruction32,61. The capacity of TIMP3 on ECM remodeling makes it a candidate for KC progression. However, few studies focused on the relationship between TIMP3 and KC, and no pathogenic variants have been found so far32. As for molecular findings, different studies showed contradictory results38,62. Ji-Eun Lee et al.62 found that TIMP3 was underexpressed in KC patients compared to controls, while Matthews et al. represented a high expression of TIMP3 and active apoptosis in KC corneas38. In our study, we discovered a new mutation (c.476 C > T) in TIMP3, and found a decreased expression of TIMP3 in KC patients. It was expected that TIMP3 functioned as an inhibitor of MMP in the healthy cornea, and disruption of its normal structure or function caused itself unable to protect tissues from irreversible destruction of extracellular matrix62, finally turning to KC. However, previous studies have shown that TIMP3 could easily trigger apoptosis of neighboring cells when in a matrix-bound and high-concentration form. Therefore, concurrent detections of apoptosis markers and TIMP3 expression might further distinguish the comprehensive functions of TIMP3 in KC.
In conclusion, this study discovered three novel variants in three ECM-related genes respectively in the Chinese Han population (c.95 G > A in LOX, c.1372 C > T in COL5A1, and c.476 C > T in TIMP3), and the results of QPCR indicated that the abnormally low expression of LOX and TIMP3 might contribute to the development of KC, all these highlighting the importance of ECM in the pathogenesis of KC. The result that the expression of COL5A1 was of no significant difference between the control and KC group, did not negate the potential role of COL5A1 in the pathogenesis of KC, but indicated the complex mechanisms underlying KC among different races, regions, and so on. Besides, change of function is as important as change of the expression level in the pathogenesis of diseases, so the additional detection of COL5A1 function by mutation screening and the SIFT score or other methods may better translate the result of QPCR. This study enlarges KC-related mutation spectrums and the novel mutations found here can be used for further validation and research, making a deep understanding of KC and thus contributing to the development of KC therapy. However, Because of the limited samples obtained in this study, further larger and multi-center population studies need to be taken to confirm the danger of these variants as well as functional experiments to deep dig into the nature of KC.
Methods
The study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the ethics committee of Second Affiliated Hospital, Medical College of Zhejiang University, Hangzhou, China. Written informed consent was obtained from all participating individuals or their guardians after explanation of possible consequences of the study.
Study participants
Totally, fifty-three clinically affected isolated keratoconus patients of Chinese Han ethnicity and one hundred unrelated population-matched healthy controls without any ocular or systemic disorders were recruited from Eye Center of Second Affiliated Hospital, Medical College of Zhejiang University, during the period of 2013 to 2015. Following thorough inquiry, negative family histories taken, each participate underwent a comprehensive ocular and systemic evaluation. Any keratoconus cases with co-existing allergy/atopy or secondary to causes such as trauma, Laser-Assisted in situ Keratomileusis (LASIK) or other refractive surgeries, Ehlers Danlos syndrome, Down syndrome, Osteogenesis Imperfecta and pellucid marginal degeneration were excluded from the study.
The diagnosis of keratoconus was carried out by an experienced ophthalmologist based on key features exhibited through slit-lamp biomicroscopy, cycloplegic retinoscopy, and corneal topography63. Slit-lamp biomicroscopy was used to identify well-established clinical signs of keratoconus including stromal corneal thinning, Vogt’s striae and Fleischer rings in participants. The oil droplet sign and scissoring of the red reflex were assessed by retinoscopy performed with a fully dilated pupil. Patients were considered keratoconus if they had at least one clinical sign accompanied with a confirmatory videokeratography map63. The detailed criterion selected in this article was posterior corneal elevation ≥ +20 um within the central 5 mm and inferior-superior dioptric asymmetry (I-S value)> 1.2 diopters (D), with the steepest keratometry > 47D13.
Mutation screening
Peripheral blood samples of all above-mentioned participants were collected in Vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ, USA) containing ethylene diamine tetraacetic acid (EDTA) and genomic DNA was isolated using the Simgen Blood DNA mini kit (Simgen, Hangzhou, China)64. Mutation screening was performed using genomic DNA samples from affected participants as well as healthy controls. For patients, several extracellular-matrix (ECM)-related genes suggested involved in keratoconus were screened by next-generation sequencing and confirmed by directly sequencing. Subsequently, probable pathogenic variants were analyzed in a healthy control population using Sanger sequencing analysis. All coding regions comprised of all exons, intron-exon junctions and promoter regions of the candidate genes were amplified by polymerase chain reaction (PCR) using specific primer sequences. The PCR products were isolated by electrophoresis on 1.0% agarose gels and sequenced with the BigDye Terminator Cycle sequencing kit V3.1 (Applied Biosystems, Foster City, CA) on an Applied Biosystems ABI3730 Sequence Analyzer. The sequencing results were analyzed using Polyphred and compared with the sequences in the NCBI GenBank database1.
Bioinformatics analysis
To predict the effect of this amino acid substitution on the protein, we used the online tools SIFT (Sorting Intolerant Form Tolerant, http://sift.jcvi.org/) programs. Using structural and comparative evolutionary considerations, the prediction result of SIFT ranges from 0 to 1 based on evolutionary conservation. The amino acid substitution is predicted damaging if the score is ≤0.05, and tolerated if the score is >0.05.
Isolation of RNA, cDNA synthesis, and real-time QPCR
Total corneas were collected from 6 keratoconus patients and 4 healthy controls who were met with the aforementioned criteria listed in the Study Participant part. Debrided cells were immediately transferred to −80 °C for storage until processing for RNA extraction.
Total RNA was extracted using TRIzol reagent (Invitrogen), and reverse transcription was performed with ReverTra Ace (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Real-time QPCR was performed on a Light-Cycler Roche480 (Roche Molecular Systems) using the SYBR Green Master Kit (Bimake). The mRNA levels were calculated using the ΔΔCt method. All qPCR primers and their sequences were as follows: LOX (5-CTTGCACGTTTCCAATCGCA-3, 5-ATGCCAAGGGTGGGATTCAG-3), COL5A1 (5-ACGGGAATGGCGAGAACTAC-3, 5-GAGCAGTTTCCCACGCTTGA-3), TIMP3 (5-ACCGAGGCTTCACCAAGATG-3, 5-CAGGGGTCTGTGGCATTGAT-3), and GADPH (5-GAATGGGCAGCCGTTAGGAA-3, 5-AAAAGCATCACCCGGAGGAG-3).
Statistical analysis
All results were expressed as the mean ± S.D. The p value was calculated using the GraphPad Prism version 5 statistical program and determined by two-tailed Student’s t test (LOX, lg10 (COL5A1) and TIMP3). A value of p < 0.05 was considered statistically significant.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 81670834); the National Natural Science Foundation of China (Grant no. 81970781); the Natural Science Foundation of Zhejiang Province (no. LY17H090004); the National Natural Science Foundation of China (Grant no. 81800807); and the National Natural Science Foundation of China (Grant no. 81800869).
Author contributions
Shentu X.C. designed and reviewed the study. Xu X.Y. designed the study, performed the experimental work, analyzed the results, interpreted the results, and drafted the manuscript. Zhang X. designed the study and performed the experimental work. Cui Y.L., Yang H., Ping X.Y., Wu J., Jin X.M. and Huang X.D. performed the experimental work. Yu X.N. reviewed this article.
Data availability
Readers are welcome to comment on the online version of the paper. All data included in this study are available upon request by contact with the corresponding author Xingchao Shentu (stxc@zju.edu.cn).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu X, Chen B, Zhang X, Shentu X. Identification of seven novel ZNF469 mutations in keratoconus patients in a Han Chinese population. Mol. Vis. 2017;23:296–305. [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon-shaag A, Millodt M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed. Res. Int. 2016;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes JA, et al. Global consensus on keratoconus and ectatic Diseases. Cornea. 2015;34:359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 4.Brown SE, Simmasalam R, Antonova N, Gadaria N, Asbell PA. Progression in keratoconus and the effect of corneal cross-linking on progression. Eye Contact Lens. 2014;40:331–338. doi: 10.1097/ICL.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 5.Gordon-Shaag A, Millodot M, Shneor E. The epidemiology and etiology of keratoconus. Int. J. Keratoconus Ectatic Corneal Dis. 2012;1:7–15. doi: 10.5005/jp-journals-10025-1002. [DOI] [Google Scholar]
- 6.Kaya V, et al. Evaluation of the corneal topographic characteristics of keratoconus with orbscan II in patients with and without atopy. Cornea. 2007;26:945–948. doi: 10.1097/ICO.0b013e3180de1e04. [DOI] [PubMed] [Google Scholar]
- 7.Nemet AY, Vinker S, Bahar I, Kaiserman I. The association of keratoconus with immune disorders. Cornea. 2010;29:1261–1264. doi: 10.1097/ICO.0b013e3181cb410b. [DOI] [PubMed] [Google Scholar]
- 8.Saee-rad S, et al. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol. Vis. 2011;17:3128–3136. [PMC free article] [PubMed] [Google Scholar]
- 9.Jeoung JW, et al. VSX1 gene and keratoconus: genetic analysis in Korean patients. Cornea. 2012;31:746–750. doi: 10.1097/ICO.0b013e3181e16dd0. [DOI] [PubMed] [Google Scholar]
- 10.Moschos MM, et al. Polymorphism analysis of VSX1 and SOD1 genes in Greek patients with keratoconus. Ophthalmic Genet. 2015;36:213–217. doi: 10.3109/13816810.2013.843712. [DOI] [PubMed] [Google Scholar]
- 11.Udar N, et al. SOD1: A candidate gene for keratoconus. Invest. Ophthalmol. Vis. Sci. 2006;47:3345–3351. doi: 10.1167/iovs.05-1500. [DOI] [PubMed] [Google Scholar]
- 12.Guan T, Liu C, Ma Z, Ding S. The point mutation and polymorphism in keratoconus candidate gene TGFBI in Chinese population. Gene. 2012;503:137–139. doi: 10.1016/j.gene.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Amero KK, et al. Screening of the seed region of MIR184 in keratoconus patients from Saudi Arabia. Biomed. Res. Int. 2015;2015:604508. doi: 10.1155/2015/604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechner J, et al. Mutational analysis of MIR184 in sporadic keratoconus and myopia. Invest. Ophthalmol. Vis. Sci. 2013;54:5266–5272. doi: 10.1167/iovs.13-12035. [DOI] [PubMed] [Google Scholar]
- 15.Bykhovskaya Y, Gromova A, Makarenkova HP, Rabinowitz YS. Abnormal regulation of extracellular matrix and adhesion molecules in corneas of patients with keratoconus. Int. J. Keratoconus Ectatic Corneal Dis. 2016;5:63–70. doi: 10.5005/jp-journals-10025-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier SA. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin. Exp. Ophthalmol. 2001;29:340–344. doi: 10.1046/j.1442-9071.2001.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 17.Macé M, et al. Comparative transcriptome and network biology analyses demonstrate antiproliferative and hyperapoptotic phenotypes in human keratoconus corneas. Invest. Ophthalmol. Vis. Sci. 2011;52:6181–6191. doi: 10.1167/iovs.10-70981. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz YS, Dong L, Wistow G. Gene expression profile studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest. Ophthalmol. Vis. Sci. 2005;46:1239–1246. doi: 10.1167/iovs.04-1148. [DOI] [PubMed] [Google Scholar]
- 19.Tai TY, et al. Keratoconus associated with corneal stromal amyloid deposition containing TGFBIp. Cornea. 2009;28:589–593. doi: 10.1097/ICO.0b013e31818c9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hämäläinen ER, et al. Molecular cloning of human lysyl oxidase and assignment of the gene to chromosome 5q23.3–31.2. Genomics. 1991;11:508–516. doi: 10.1016/0888-7543(91)90057-L. [DOI] [PubMed] [Google Scholar]
- 21.Trackman PC, Bedell-Hogan D, Tang J, Kagan HM. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J. Biol. Chem. 1992;267:8666–8671. [PubMed] [Google Scholar]
- 22.Uzel MI, et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J. Biol. Chem. 2001;276:22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Pischon N, Palamakumbura AH, Trackman PC. Intracellular distribution of the lysyl oxidase propeptide in osteoblastic cells. Am. J. Physiol. Cell Physiol. 2007;292:C2095–C2102. doi: 10.1152/ajpcell.00613.2006. [DOI] [PubMed] [Google Scholar]
- 24.Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 1990;95:649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 25.Linsenmayer TF, et al. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J. Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/S0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 27.Birk, D. E. & Brückner, P. Collagens, suprastructures, and collagen fibril assembly. The Extracellular Matrix: an Overview (2010).
- 28.Roulet M, Ruggiero F, Karsenty G, LeGuellec D. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: a clue for understanding collagen V function in developing connective tissues. Cell Tissue Res. 2007;327:323–332. doi: 10.1007/s00441-006-0294-1. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J. Cell Sci. 2011;124:4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segev F, et al. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest. Ophthalmol. Vis. Sci. 2006;47:565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- 31.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee JE, Oum BS, Choi HY, Lee SU, Lee JS. Evaluation of differentially expressed genes identified in keratoconus. Mol. Vis. 2009;15:2480–2487. [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes S, Boote C, Tuft SJ, Quantock AJ, Meek KM. A study of corneal thickness, shape and collagen organisation in keratoconus using videokeratography and X-ray scattering techniques. Exp. Eye Res. 2007;84:423–434. doi: 10.1016/j.exer.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Meek KM, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest. Ophthalmol. Vis. Sci. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 35.Hayes S, et al. The effect of riboflavin/UVA collagen cross-linking therapy on the structure and hydrodynamic behaviour of the ungulate and rabbit corneal stroma. PLoS One. 2013;8:e52860. doi: 10.1371/journal.pone.0052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 1991;5:206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 37.Finney J, Moon HJ, Ronnebaum T, Lantz M, Mure M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014;546:19–32. doi: 10.1016/j.abb.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De BP, et al. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol. Vis. 2011;17:2482–2494. [PMC free article] [PubMed] [Google Scholar]
- 39.Dudakova L, Jirsova K. The impairment of lysyl oxidase in keratoconus and in keratoconus-associated disorders. J. Neural Transm. 2013;120:977–982. doi: 10.1007/s00702-013-0993-1. [DOI] [PubMed] [Google Scholar]
- 40.Hasanian-Langroudi F, Saravani R, Validad MH, Bahari G, Yari D. Association of lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet. 2015;36:309–314. doi: 10.3109/13816810.2014.881507. [DOI] [PubMed] [Google Scholar]
- 41.Shetty R, et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol. Vis. 2015;21:12–25. [PMC free article] [PubMed] [Google Scholar]
- 42.Dudakova L, et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp. Eye Res. 2012;104:74–81. doi: 10.1016/j.exer.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Pahuja N, et al. Differential molecular expression of extracellular matrix and iInflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest. Opthalmol Vis. Sci. 2016;57:5372–5382. doi: 10.1167/iovs.16-19677. [DOI] [PubMed] [Google Scholar]
- 44.Sahebjada S, et al. Evaluating the association between keratoconus and the corneal thickness genes in an independent Australian population. Invest. Opthalmol Vis. Sci. 2013;54:8224–8228. doi: 10.1167/iovs.13-12982. [DOI] [PubMed] [Google Scholar]
- 45.Dudakova L, et al. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur. J. Hum. Genet. 2015;23:1581–1583. doi: 10.1038/ejhg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bykhovskaya Y, et al. Variation in the lysyl Oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest. Ophthalmol. Vis. Sci. 2012;53:4152–4157. doi: 10.1167/iovs.11-9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bykhovskaya Y, Margines B, Rabinowitz YS. Genetics in keratoconus: where are we? Eye Vis. 2016;3:16. doi: 10.1186/s40662-016-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 49.Xiao Q, Ge G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron. 2012;5:261–273. doi: 10.1007/s12307-012-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzotta C, et al. In vivo confocal microscopy after corneal collagen cross-linking. Ocul. Surf. 2015;13:298–314. doi: 10.1016/j.jtos.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Shetty R, et al. Outcomes of corneal cross-linking correlate with cone-specific lysyl oxidase expression in patients with keratoconus. Cornea. 2017;37:369–374. doi: 10.1097/ICO.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen K, Birkenkamp-Demtröder K, Ehlers N, Orntoft TF. Identification of differentially expressed genes in keratoconus epithelium analyzed on microarrays. Invest. Opthalmol Vis. Sci. 2003;44:2466–2476. doi: 10.1167/iovs.02-0671. [DOI] [PubMed] [Google Scholar]
- 53.Li X, et al. Genetic association of COL5A1 variants in keratoconus patients suggests a complex connection between corneal thinning and keratoconus. Invest. Ophthalmol. Vis. Sci. 2013;54:2696–2704. doi: 10.1167/iovs.13-11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitart V, et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum. Mol. Genet. 2010;19:4304–4311. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- 55.Vithana EN, et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum. Mol. Genet. 2011;20:649–658. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 Influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010;6:e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoehn R, et al. Population-based meta-analysis in Caucasians confirms association with COL5A1 and ZNF469 but not COL8A2 with central corneal thickness. Hum. Genet. 2012;131:1783–1793. doi: 10.1007/s00439-012-1201-3. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genet. 2013;45:155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chanut-Delalande H, et al. Development of a functional skin matrix requires deposition of collagen V heterotrimers. Mol. Cell Biol. 2004;24:6049–6057. doi: 10.1128/MCB.24.13.6049-6057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wenstrup RJ, et al. Murine model of the Ehlers-Danlos syndrome: col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J. Biol. Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 61.Johnson MD, et al. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J. Cell Physiol. 1994;160:194–202. doi: 10.1002/jcp.1041600122. [DOI] [PubMed] [Google Scholar]
- 62.Matthews FJ, Cook SD, Majid MA, Dick AD, Smith VA. Changes in the balance of the tissue inhibitor of matrix metalloproteinases (TIMPs)-1 and -3 may promote keratocyte apoptosis in keratoconus. Exp. Eye Res. 2007;84:1125–1134. doi: 10.1016/j.exer.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Lechner J, et al. Enrichment of pathogenic alleles in the brittle cornea gene, ZNF469, in keratoconus. Hum. Mol. Genet. 2014;23:5527–5535. doi: 10.1093/hmg/ddu253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shentu, X., Miao, Q., Tang, X., Yin, H. & Zhao, Y. Identification and functional analysis of a novel MIP gene mutation associated with congenital cataract in a Chinese family. Plos one10, e0126679 (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Readers are welcome to comment on the online version of the paper. All data included in this study are available upon request by contact with the corresponding author Xingchao Shentu (stxc@zju.edu.cn).