Abstract
Seagrass meadows are considered important natural carbon sinks due to their capacity to store organic carbon (Corg) in sediments. However, the spatial heterogeneity of carbon storage in seagrass sediments needs to be better understood to improve accuracy of Blue Carbon assessments, particularly when strong gradients are present. We performed an intensive coring study within a sub-tropical estuary to assess the spatial variability in sedimentary Corg associated with seagrasses, and to identify the key factors promoting this variability. We found a strong spatial pattern within the estuary, from 52.16 mg Corg cm−3 in seagrass meadows in the upper parts, declining to 1.06 mg Corg cm−3 in seagrass meadows at the estuary mouth, despite a general gradient of increasing seagrass cover and seagrass habitat extent in the opposite direction. The sedimentary Corg underneath seagrass meadows came principally from allochthonous (non-seagrass) sources (~70–90 %), while the contribution of seagrasses was low (~10–30 %) throughout the entire estuary. Our results showed that Corg stored in sediments of seagrass meadows can be highly variable within an estuary, attributed largely to accumulation of fine sediments and inputs of allochthonous sources. Local features and the existence of spatial gradients must be considered in Blue Carbon estimates in coastal ecosystems.
Subject terms: Marine biology, Carbon cycle, Climate-change mitigation, Ecosystem services
Introduction
Seagrass ecosystems are among the most significant natural carbon sinks worldwide, since they can sequester significant amounts of carbon, store it as organic carbon (Corg) in the sediments for long periods of time, and have a worldwide distribution1–3. It is estimated that seagrass ecosystems store globally up to 19.9 petagrams (Pg) of Corg in sediments2, or between 4.2 and 8.4 Pg of Corg from a more conservative approach2, where Corg could be stored in sediments for hundreds of years and even millennia4.
Due to the high mitigation potential for seagrass ecosystems to help offset carbon dioxide (CO2) emissions, there has been a major effort in recent years to improve the accuracy of estimates of carbon stored in seagrass sediments, and so, include seagrass ecosystems within greenhouse gases (GHG) abatement schemes5,6. Consequently, an increasing number of studies have attempted to quantify sedimentary Corg stocks associated with seagrass meadows from direct measurements7–9, demonstrating an enormous variability in the seagrass sedimentary Corg stocks reported worldwide.
Variability of sedimentary Corg stocks has been associated with multiple interrelated biological and environmental factors: type of seagrass species7, depth and light availability10,11, landscape configuration12, physical disturbances13, wave height and turbidity14, and even faunal presence such as bioturbators and top predators15,16 Most of these studies highlighted the type of carbon sources and the sediment grain size as the main factors influencing carbon storage in seagrass sediments, suggesting that the processes affecting these factors explain the high variability found in seagrass sedimentary Corg stocks17.
Variability in seagrass sedimentary Corg stocks has been described at a range of spatial scales: from cm and meters, at the seagrass patch and meadow scale18–20 to bioregions and latitudinal scales7,9. Studies at small scales (up to ~1′s km) have shown how sedimentary Corg can be spatially distributed inside seagrass meadows18,20,21. However, in studies at higher spatial scales (~100′s to ~1000′s km), comparisons and estimates are usually made based on a relatively low number of sediment cores from each site22–24, therefore, not accounting for the potential spatial variability in seagrass sedimentary Corg stocks among meadows in the same area. Just a few studies focus on the variability at intermediate spatial scales (~10′s km), comparing among seagrass meadows within a local system14,25,26, such as estuaries, where seagrasses can make up a large proportion of available habitat27.
Estuaries are dynamic transition zones acting as pathways for the transfer of sediments and organic materials from land to the sea. Estuaries are characterized by a high variability in geo-morphology, and the presence of spatial gradients, influenced by large tidal cycles, longer water residence times and poorer mixing compared to the open ocean. Carbon fluxes to estuarine sediments can be conceptualized in two main axes28. The horizontal axis is determined by a hydrodynamic control, exerted between the river and the ocean and/or following estuary geo-morphology, where terrestrial carbon is directly delivered and rapidly deposited in the sediments29, thus creating a strong spatial gradient of Corg in sediments from the upper parts of the estuary to the ocean. The vertical axis is determined by a biological control, where Corg in sediments is the result of direct deposition and burial of in situ photosynthetic production between the surface (i.e. phytoplankton) and the benthos (i.e. seagrasses and/or algae if present). The prevalence of one control or the other will largely determine the magnitude and origin of Corg stocks in the sediments within estuaries, and also its spatial distribution, thus, expecting a more homogeneous spatial distribution of Corg in sediments of seagrass meadows with a prevalence of biological control, and a more heterogeneous distribution where hydrodynamic control dominates.
Variation also exists among and within estuaries, in terms of turbidity and water flow, which may determine seagrass meadows’ habitat extent or landscape configuration30,31 and also species composition and structural traits, such as shoot density and cover32. Other coastal vegetated habitats, such as mangroves and saltmarshes can be present in estuaries also contributing to Corg stocks33,34 and to seagrass sedimentary Corg stocks35. Variation is also reflected in sedimentation rates, which can be influenced by seagrass canopies trapping particulate organic materials, thus decreasing turbidity and increasing sedimentary Corg stocks36. Some of these factors may determine spatial patterns of Corg storage in sediments of seagrass meadows. However, despite the large number of studies that have appeared recently providing empirical evidence of processes explaining Corg storage in seagrass sediments, there are a lack of studies providing the spatial component needed to improve accuracy of Blue Carbon assessments, particularly at the within estuary scale. The current work aimed to contribute to understanding the spatial variability of Corg storage in seagrass sediments by providing a comprehensive assessment of the spatial distribution of sedimentary Corg stocks and carbon sources in seagrass meadows within a coastal plain estuary and exploring the site-specific factors driving the patterns found.
We expected that sedimentary Corg storage in seagrass meadows within the estuary will present a high spatial heterogeneity due to the presence of spatial gradients of environmental and biological factors. Therefore, we investigated how sediment Corg content (%), Corg stocks (mg Corg cm−3) and carbon sources (δ13C) vary as a function of seagrass cover, meadow extent or landscape configuration (hereinafter “meadow type”), sediment particle size, water turbidity and the proximity to mangroves, as the main environmental and biological factors present within the estuary studied that have been already described to be affecting Corg storage in seagrass sediments12,14,26. Specifically, we hypothesized that (1) seagrass sedimentary Corg storage will increase with seagrass cover and will be higher in continuous meadows due to a greater accumulation of seagrass carbon sources and fine sediments; (2) proximity to mangroves and a higher water turbidity will increase sedimentary Corg storage due to higher contribution of allochthonous carbon sources.
Results
All sediment parameters and environmental and biological drivers are summarized in Table 1. The sediment Corg content in seagrass meadows within the estuary ranged from 0.08% to 6.01%, and Corg stocks ranged from 1.06 mg Corg cm−3 to 52.16 mg Corg cm−3, where in both cases the lowest values were found in seagrass meadows at the mouth of the estuary and the highest values in seagrass meadows at the upper estuary reaches. Values of δ13C were in general highly depleted along the estuary, from −18.32‰ to −26.20‰, with enriched (more positive) values in sediments of seagrass meadows at the mouth of the estuary and more depleted values in sediments in the upper estuary. Similar patterns were found at all depths analysed. All three sediment carbon variables assessed presented spatial autocorrelation on the original data (Moran I test p < 0.01; Supplementary Table S1). Sediment dry bulk density ranged from 0.38 g cm−3 to 3.08 g cm−3, with the lowest values in seagrass meadows at the upper parts of the estuary and the highest in seagrass meadows at the mouth. The proportion of fine sediments (<63 µm) ranged from 3.16% to 93.92%, with the lowest values in seagrass meadows at the mouth of the estuary and the highest in seagrass meadows at the upper estuary.
Table 1.
Environmental and biological drivers, and sediment parameters.
| Site | Environmental and Biological drivers | Sediment parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seagrass species and number of cores sampled in brackets | Seagrass cover (%) | Meadow type | Turbidity level | Distance to mangroves (m) | <63 µm (%) | DBD (g cm−3) | Corg (%) | δ13C (‰) | Corg stocks (mg Corg cm−3) | ||||||
| Redcliffe | Z. muelleri (1); H. ovalis (1); H. decipiens (1) | 0–1 | patchy | high | 140–140 | 80.17 | 85.19 | 0.38 | 0.87 | 2.81 6.01 | −26.00 | −24.46 | 12.91 | 52.16 |  |
| Black Swan | Z. muelleri (3) | 0–10 | patchy | high | 200–300 | 27.82 | 75.92 | 0.86 | 1.48 | 0.87 2.92 | −25.67 | −24.72 | 11.26 | 35.58 | |
| Fishermans Landing | Z. muelleri (2); H. ovalis (1) | 0–1 | patchy | medium | 515–565 | 47.16 | 85.47 | 0.82 | 1.47 | 0.44 2.51 | −25.37 | −23.32 | 4.60 | 25.11 | |
| Channel Islands | Z. muelleri (3) | 5–20 | patchy | medium | 100–200 | 8.29 | 35.60 | 1.25 | 2.97 | 0.28 2.03 | −25.09 | −22.51 | 4.55 | 31.81 | |
| Wiggins Island | Z. muelleri (3) | 0–10 | patchy | high | 610–680 | 50.62 | 73.73 | 0.90 | 1.45 | 0.19 0.99 | −24.10 | −22.99 | 2.08 | 10.79 | |
| Grahams Creek | H. decipiens (3) | 0–5 | patchy | high | 35–35 | 56.37 | 93.92 | 0.71 | 1.04 | 1.50 4.55 | −26.20 | −25.29 | 11.61 | 33.84 | |
| Pelican Banks South 1 | Z. muelleri (3) | 0–15 | continuous | medium | 3500–3500 | 9.49 | 12.47 | 1.38 | 2.12 | 0.39 1.45 | −22.07 | −20.90 | 7.07 | 21.62 | |
| Pelican Banks South 2 | Z. muelleri (2); H. ovalis (1) | 10–20 | continuous | medium | 3400–3400 | 7.73 | 11.56 | 1.37 | 1.93 | 0.37 2.08 | −21.83 | −19.11 | 5.21 | 34.57 | |
| Pelican Banks North 1 | Z. muelleri (3) | 30–65 | continuous | low | 1230–1230 | 13.69 | 16.55 | 1.13 | 1.83 | 0.44 1.03 | −19.99 | −18.51 | 5.96 | 17.15 | |
| Pelican Banks North 2 | Z. muelleri (3) | 45–70 | continuous | low | 1120–1120 | 15.02 | 18.43 | 1.06 | 1.63 | 0.39 1.17 | −19.44 | −18.61 | 5.65 | 12.41 | |
| Facing Island | Z. muelleri (2); H. ovalis (1) | 0–5 | patchy | medium | 3255–3280 | 7.45 | 28.87 | 1.25 | 1.91 | 0.24 1.42 | −23.35 | −19.87 | 4.47 | 19.67 | |
| Pelican Banks North 3 | Z. muelleri (3) | 57–60 | continuous | low | 1950–1950 | 9.91 | 16.11 | 1.33 | 1.75 | 0.3 1.72 | −19.17 | −18.32 | 4.70 | 24.86 | |
| South Trees | Z. muelleri (3) | 0–1 | variable | medium | 150–250 | 5.96 | 17.71 | 1.12 | 1.95 | 0.18 1.57 | −24.11 | −20.44 | 3.49 | 17.52 | |
| Boyne Island | H. uninervis (3) | 2–10 | variable | medium | 1000–1025 | 3.16 | 3.45 | 1.07 | 3.08 | 0.08 0.25 | −22.89 | −20.43 | 1.06 | 3.68 | |
| South Facing Island | Z. muelleri (3) | 0–10 | variable | medium | 250–250 | 12.50 | 17.35 | 1.45 | 2.54 | 0.09 1.41 | −21.99 | −20.40 | 1.30 | 22.18 | |
A total of 45 cores were collected in 15 sites along the estuary. For summary purposes data is shown per site but cores are treated independently on the spatial GLS models applied. Data show ranges of minimum and maximum values or category of each variable.
The non-spatial GLS models (except those with meadow type as explanatory variable and those assessing Corg (%) with proportion of fine sediments) presented spatial autocorrelation on the residuals (Moran I p < 0.01, Supplementary Table S2), showing that the explanatory variables were not removing the effect of spatial dependence among samples, and that error correlation structures were then necessary. All the spatial GLS models, except some cases assessing δ13C in core section 1–3 cm, did not present spatial autocorrelation in the residuals (Moran I p > 0.01, Supplementary Table S2). We will therefore describe just the results from spatial GLS models when spatial autocorrelation was removed by the correlation structure (Table 2).
Table 2.
Coefficient estimates for carbon sediment variables in the GLS spatial models.
| Depth | Dependent variable | Corg (%) | C stocks (mg Corg cm−3) | δ13C (‰) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Driver | ∆AIC | Estimate | SE | ∆AIC | Estimate | SE | ∆AIC | Estimate | SE | |
| 0–1 cm | Seagrass cover* | 38.75 | 0.00 | 0.00 | 30.61 | 0.00 | 0.00 | 63.93 | 0.03 | 0.01 |
| <63 µm* | 12.28 | 0.01 | 0.00 | 18.44 | 0.00 | 0.00 | 47.21 | −0.01 | 0.01 | |
| Distance from mangroves* | 36.74 | 0.00 | 0.00 | 33.64 | 0.00 | 0.00 | 71.45 | 0.00 | 0.00 | |
| Turbidity (low) | 22.78 | 0.55 | 0.34 | 19.26 | 2.23 | 0.64 | 36.68 | −20.14 | 0.83 | |
| Turbidity (medium) | 22.78 | −0.20 | 0.27 | 19.26 | −0.12 | 0.29 | 36.68 | −2.09 | 0.42 | |
| Turbidity (high) | 22.78 | 0.42 | 0.39 | 19.26 | 0.16 | 0.40 | 36.68 | −4.05 | 0.57 | |
| Meadow type (continuous) | 24.38 | 0.51 | 0.47 | 5.34 | 2.52 | 0.17 | 24.64 | −19.94 | 0.96 | |
| Meadow type (patchy) | 24.38 | 0.42 | 0.55 | 5.34 | 0.20 | 0.24 | 24.64 | −4.55 | 1.11 | |
| Meadow type (variable) | 24.38 | −0.19 | 0.56 | 5.34 | −0.90 | 0.27 | 24.64 | −1.12 | 1.24 | |
| 1–3 cm | Seagrass cover* | 28.51 | 0.00 | 0.00 | 7.64 | 0.00 | 0.01 | 61.09 | 0.04 | 0.01 |
| <63 µm* | 8.06 | 0.01 | 0.00 | 3.21 | 0.01 | 0.00 | 48.06 | 0.00 | 0.01 | |
| Distance from mangroves* | 30.54 | 0.00 | 0.00 | 8.57 | 0.00 | 0.00 | 60.62 | 0.00 | 0.00 | |
| Turbidity (low) | 17.52 | 0.34 | 0.32 | 9.27 | 1.61 | 1.29 | 29.26 | −19.11 | 0.76 | |
| Turbidity (medium) | 17.52 | 0.12 | 0.27 | 9.27 | 0.44 | 0.40 | 29.26 | −2.95 | 0.87 | |
| Turbidity (high) | 17.52 | 0.67 | 0.38 | 9.27 | 0.50 | 0.51 | 29.26 | −5.93 | 0.95 | |
| Meadow type (continuous) | 19.21 | 0.46 | 0.43 | 2.87 | 2.47 | 0.20 | 21.41 | −19.95 | 0.46 | |
| Meadow type (patchy) | 19.21 | 0.50 | 0.50 | 2.87 | 0.21 | 0.27 | 21.41 | −4.71 | 0.62 | |
| Meadow type (variable) | 19.21 | −0.19 | 0.52 | 2.87 | −0.65 | 0.31 | 21.41 | −1.67 | 0.74 | |
| 3–10 cm | Seagrass cover* | 26.84 | −0.01 | 0.00 | 13.19 | −0.01 | 0.01 | 48.87 | 0.04 | 0.01 |
| <63 µm* | 8.94 | 0.01 | 0.00 | 9.92 | 0.00 | 0.00 | 55.64 | 0.00 | 0.01 | |
| Distance from mangroves* | 27.19 | 0.00 | 0.00 | 11.85 | 0.00 | 0.00 | 69.80 | 0.00 | 0.00 | |
| Turbidity (low) | 16.28 | 0.38 | 0.31 | 13.04 | 1.62 | 1.53 | 31.19 | −18.97 | 1.10 | |
| Turbidity (medium) | 16.28 | 0.19 | 0.27 | 13.04 | 0.63 | 0.48 | 31.19 | −3.74 | 1.15 | |
| Turbidity (high) | 16.28 | 0.56 | 0.37 | 13.04 | 0.38 | 0.61 | 31.19 | −5.95 | 1.29 | |
| Meadow type (continuous) | 16.56 | 0.45 | 0.42 | 6.94 | 2.60 | 0.24 | 36.75 | −20.34 | 0.80 | |
| Meadow type (patchy) | 16.56 | 0.49 | 0.49 | 6.94 | 0.12 | 0.33 | 36.75 | −4.47 | 0.95 | |
| Meadow type (variable) | 16.56 | −0.19 | 0.47 | 6.94 | −0.89 | 0.37 | 36.75 | −1.69 | 1.08 | |
Significant terms (p-value < 0.05) are shown in bold. ∆AIC represents the AIC difference between the non-spatial GLS and the spatial GLS accounting for spatial autocorrelation on the response variable; positive value means lower AIC on the spatial GLS model. SE, standard error. (*) shows continuous explanatory variables.
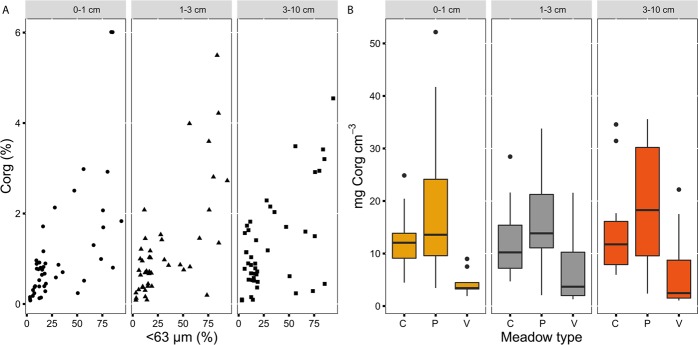
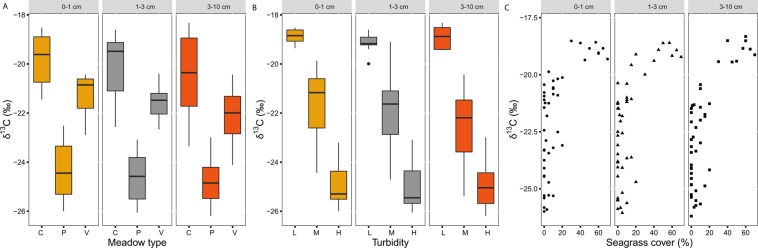
The Corg (%) content in the sediment for all core sections was positively related with the proportion of fine fraction of sediments (Fig. 1a, p ≤ 0.02, Table 2). The Corg stocks (mg Corg cm−3) in the sediment for all core sections were significantly related to meadow type (p ≤ 0.02, Table 2). The highest Corg stocks were associated with continuous meadow types and patchy meadows, although there was a high variability present in patchy meadows as shown by the model estimates’ standard errors and confidence intervals (Supplementary Table S3, Fig. 1b). The δ13C values in the sediments for all core sections were significantly related to meadow type, turbidity and seagrass cover (p ≤ 0.01, Table 2). The most depleted values were associated with patchy meadows (Fig. 2a) and to high water turbidity (Fig. 2b); and these were positively related with seagrass cover (Fig. 2c).
Figure 1.
Relationships among sediment carbon variables and drivers per each depth core section. (a) Biplot of Corg content (%) and sediment grain size <63 µm (%); (b) Boxplot of Corg stocks (mg Corg cm−3) and meadow type. Symbols for grain size: circle, depth section 0–1 cm; triangle, depth section 1–3 cm; quadrat, depth section 3–10 cm. Legend for meadow type: C, permanent continuous meadows; P, permanent patchy meadows; V, variable meadows.
Figure 2.
Relationships among sediment carbon sources and drivers per each depth core section. (a) Boxplot of δ13C (‰) and meadow type; (b) Boxplot of δ13C (‰) and turbidity; (c) δ13C (‰) and seagrass cover. Legend for meadow type: C, permanent continuous meadows; P, permanent patchy meadows; V, variable meadows. Legend for water turbidity: H, high; M, medium; L, low. Symbols for seagrass cover: circle, depth section 0–1 cm; triangle, depth section 1–3 cm; quadrat, depth section 3–10 cm.
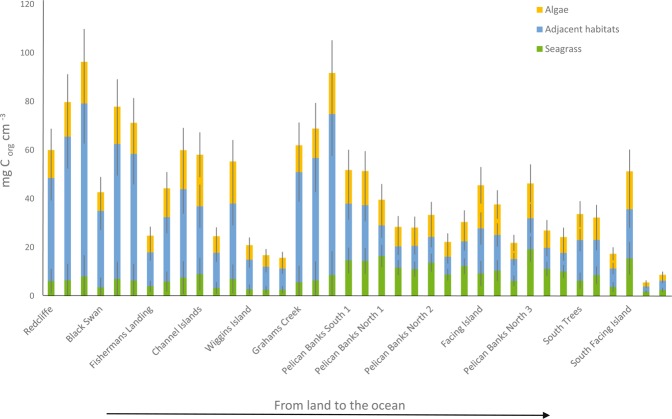
The mixing models applied indicated that allochthonous, non-seagrass, sources were the most important source of sediment Corg (contribution range from 58% to 92%; Table 3), followed by seagrass (contribution range from 8% to 42%; Table 3). In general, seagrass presented a minor contribution to sedimentary Corg stocks along the estuary (~8 mg Corg cm−3) (Fig. 3), although this contribution increased in the lower regions of the estuary (up to ~20 mg Corg cm−3), coincident with the presence of the largest seagrass meadows in the estuary (Table 1). The contribution from adjacent habitat sources and marine algae was higher (~35 mg Corg cm−3 on average) and also decreasing along a gradient from the upper (with up to ~92 mg Corg cm−3) to the lower parts of the estuary (Fig. 3).
Table 3.
Results from the mixing models.
| Adjacent habitats (mangroves & saltmarshes) | Algae (benthic algae & seston) | Seagrass | Allochthonous sources | Autochthonous sources | |
|---|---|---|---|---|---|
| Mean | 50.57 | 26.65 | 22.79 | 77.21 | 22.79 |
| SD | 15.50 | 5.50 | 12.37 | 12.37 | 12.37 |
| Min | 27.45 | 17.59 | 8.12 | 58.39 | 8.12 |
| Max | 74.07 | 39.04 | 41.61 | 91.88 | 41.61 |
Values represent summary statistics of the proportion (%) of each source (mean, standard deviation and range by minimum and maximum values). Autochthonous sources represent the seagrass contribution, while allochthonous sources represent the sum of non-seagrass sources.
Figure 3.
Contribution of the different carbon sources to total sediment Corg stocks (mg Corg cm−3) of seagrass meadows on each core within the estuary. Sources: adjacent habitats sources (including mangroves and saltmarshes); marine algae (including benthic algae and seston); seagrass sources. This figure is based on the mean results and standard deviation of each source fraction from mixing models (see Table 3). Total sediment Corg stocks (mg Corg cm−3) represent the cumulative amount of Corg on each 10 cm depth core.
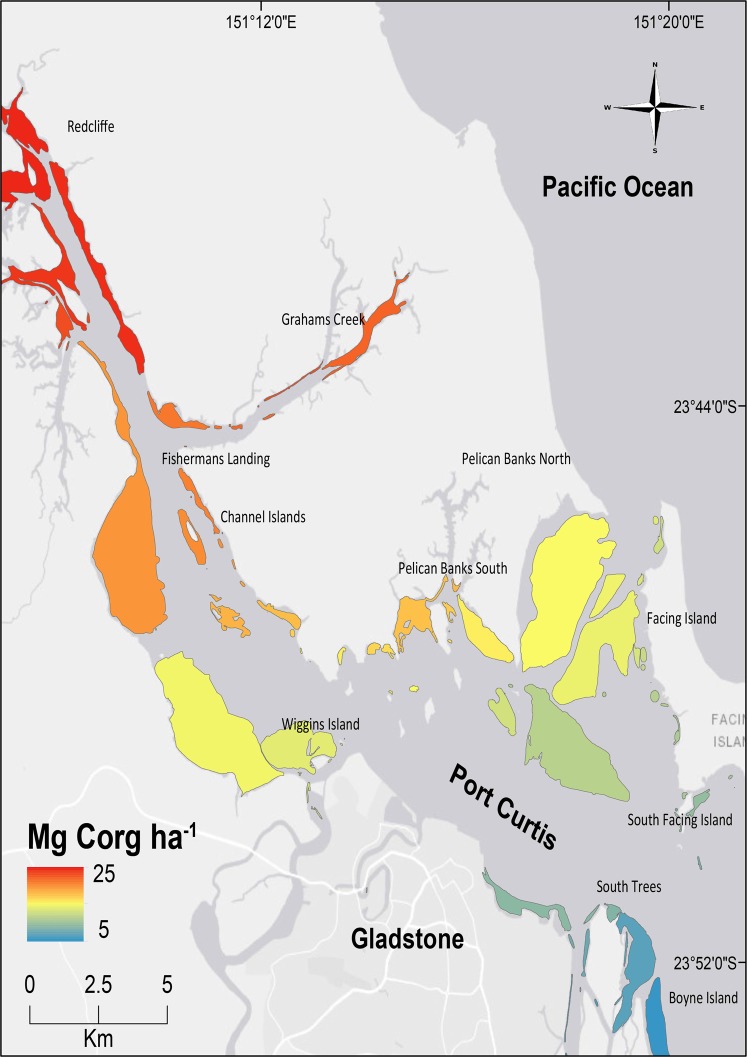
Predicted seagrass Corg stocks within the estuary were five times higher in the upper regions than in the lower regions, with Corg stocks ranging from 25 Mg Corg ha−1 to 5 Mg Corg ha−1 in the first 10 cm of sediment (Fig. 4).
Figure 4.
Predictive map of Corg stocks (Mg Corg ha−1) in the first 10 cm of sediment of seagrass meadows within the Port Curtis estuary, Queensland, Australia, based on data from this study. The map was built using Esri ArcGIS 10.4 (www.esri.com/software/arcgis).
Discussion
The distribution of sedimentary Corg storage in seagrass meadows within the Port Curtis estuary, Queensland, Australia, was spatially heterogeneous, following a general pattern of higher Corg accumulation in sediments of seagrass meadows in the upper reaches of the estuary than in seagrass meadows at the estuary mouth. Contrary to our expectations, seagrass sedimentary Corg content (%) or Corg stocks (mg Corg cm−3) did not increase with seagrass cover. Although, Corg content (%) was significantly related with a higher proportion of fine sediments and sedimentary Corg stocks (mg Corg cm−3) were related with the meadow type. Water turbidity was related with carbon sources, as well as seagrass cover and meadow type. Thus, a higher seagrass contribution was found in sedimentary Corg in continuous meadows, which also presented higher seagrass cover and low water turbidity. Despite this, the contribution from seagrasses (as a source) to Corg stored in sediments was still very low through all seagrass meadows in the estuary. Overall, sedimentary Corg storage in seagrass meadows within the estuary studied seems to be governed by the accumulation of fine sediments and allochthonous carbon sources along a gradient from land to the ocean, suggesting that these patterns should be considered when quantifying Corg stocks at similar scales.
Sedimentary Corg beneath seagrass meadows in the upper parts of the estuary, were more than ten times higher than in the lower estuary. The highest values in the upper estuary are comparable to those from long-lived seagrass species of the Posidonia genus, while values for the same seagrass species (Zostera muelleri) in the lower estuary are comparable to other fast-growing seagrass species7 including those from estuaries within the same region14,37. The strong spatial gradient on sedimentary Corg stocks in seagrass meadows exerted from the upper to the lower estuary suggests that the estuary studied is governed by a strong hydrodynamic control of carbon deposition processes28. Therefore, it is likely that a higher deposition of fine sediments, carrying particulate organic materials through freshwater inputs (e.g. land runoff), is occurring in the upper parts of the estuary. This explains the higher carbon levels in these seagrass meadows and is corroborated also by the more depleted values of δ13C in these areas38. These results highlight the necessity to account for spatial gradients when assessing Corg stocks within estuaries to adequately account for the variability present. Our results also support that spatial autocorrelation is an important consideration when assessing drivers of Corg stocks where strong spatial gradients are present20.
Among the main environmental and biological factors explaining the variability found within the estuary, the proportion of fine sediments appeared as the only significant explanatory variable for Corg content (%) in sediments. Seagrass sediment carbon storage has been widely related with a high proportion of fine sediments12,39 as fine sediments will slow down decomposition processes by avoiding oxygenation40. In agreement with our results, recent studies suggest that in fast growing seagrass species, such as the ones in this study, this relationship is even stronger when the contribution of seagrass-derived carbon to the sedimentary Corg pool is relatively low41.
Seagrass meadow type (i.e. landscape configuration) was among the main factors associated with sedimentary Corg stocks (mg Corg cm−3) and carbon sources within the estuary. As shown by the spatial models applied Corg stocks were positively related with continuous seagrass meadows and negatively related with variable non-permanent meadows, while results on patchy meadows were uncertain due to the high variability present. In fact, in the different patchy meadows sampled in this study, sedimentary Corg storage varied by one order of magnitude depending on the location within the estuary. In addition, the cores with the highest records of Corg stocks on this study were from patchy meadows in the upper extreme of the estuary, demonstrating that the spatial location of seagrass meadows within the estuary matters in Blue Carbon assessments. On the other hand, continuous meadows showed a low variability in sedimentary Corg stocks, and a higher contribution from seagrass sources. This could be related to higher, and probably more constant, burial of seagrass-derived carbon in continuous meadows, compared to patchy and non-permanent variable meadows, promoting more accumulation and burial of seagrass-derived material18, that are generally less labile and will decompose at lower rates promoting long lasting Corg stocks42. This relationship could also be related with the capacity of seagrass meadows to avoid resuspension and mixing of sediments36,43–45, which is presumably higher in continuous meadows18. This fact is also corroborated by the positive relationship found here among seagrass cover and carbon isotopic signature, and the low isotopic signature of Corg in patchy and non-permanent variable seagrass meadows suggesting less seagrass contribution in these meadows.
Although we found a significant relationship between carbon sources and water turbidity, but not water turbidity and Corg stocks (mg Corg cm−3), our results support the hypothesis14 that high sediment carbon storage occurs in areas of high turbidity due to high levels of allochthonous carbon. High water turbidity was related with depleted values of δ13C, suggesting a high contribution of allochthonous sources, while low water turbidity was related with δ13C values close to seagrass sources. The latter suggests two potential mechanisms: (1) in low turbidity conditions, higher levels of light promote seagrass growth, and so, more seagrass contribution to sediment Corg; and (2) that the seagrass canopies filtering capacity decreases water turbidity, thus relating seagrass contribution and low turbidity (see also results for seagrass cover and meadow type). In fact, low water turbidity occurred only in areas with presence of continuous seagrass meadows. However, in this study we did not assess the relative importance of each driver compared to the others, making it difficult to make conclusions about the particular processes involved, which should be further studied. In addition, if measured during the rainy season, water turbidity could have led to different results in the surface sediments, by affecting seagrass physiology (e.g. photosynthetic processes), sedimentation and eventually Corg storage. Finally, despite the presence of mangroves being highlighted as an important factor promoting seagrass carbon storage in other studies35, our results showed no relationship between proximity to mangroves and seagrass sedimentary Corg for any variable assessed, probably due to the low carbon exchange between those habitats within the estuary.
These results suggest that, within this estuary, the seagrass plant itself is not governing the amount of Corg stored in the sediment, and that multiple other interacting factors are involved (e.g. sediment deposition). Light availability is known to be a major control of seagrass growth46 and seems to be dominating seagrass meadows distribution within the estuary. The upper parts of the estuary presented higher water turbidity, a low seagrass cover, and a higher presence of patchy meadows when compared with the lower parts of the estuary, usually with less turbid water, more seagrass cover and where continuous meadows are found. Although seagrass cover is usually associated with higher Corg storage12, in this estuary, seagrass cover values found were small, and seagrass sedimentary Corg seems overwhelmingly driven by the proximity to the upper estuary. This leads to an inverse correlation between Corg and seagrass cover that we suggest is coincidental and driven by the light environment rather than a causal link between seagrass cover and low Corg storage.
In this study plant traits other than seagrass cover were not measured. However, annual seagrass monitoring in Port Curtis estuary since 2009 show that aboveground biomass follows the same spatial pattern as seagrass cover in the same study sites used here, ranging from 0.88 g DW m−2 in the upper estuary to 18.02 g DW m−2 in continuous meadows at the estuary mouth47–49. All seagrass species found in the estuary are considered fast growing, characterized by a small size and a small above- and below-ground fraction, with roots and rhizomes that do not penetrate deeper than the first few centimetres. This could be related with the small seagrass contribution to carbon storage found in this estuary. Small intra-specific or inter-specific variation in plant traits could potentially influence spatial patterns of carbon storage14,20 and should be included in further studies.
Although seagrass cover was a determinant of the type of carbon sources entering into the sediments, the contribution of seagrasses as carbon sources along the estuary was low. The increase of seagrass contribution to sedimentary Corg in the lower parts of the estuary could be related to a lower deposition of allochthonous carbon (compared to the upper parts), and also a major presence of continuous meadows that could facilitate the trapping and burial of seagrass materials into the sediments and a higher local production contributing autochthonous carbon50. Despite this, allochthonous sources were the most important contributors, and followed the same spatial pattern as Corg stocks within the estuary.
Overall, fine sediments and allochthonous carbon sources accumulating more in the upper parts of the estuary seem to be driving spatial heterogeneity found in sedimentary Corg storage in seagrass meadows within the estuary studied. This highlights the necessity to account for the river-ocean continuum gradients on Blue Carbon assessments within-estuaries. We therefore recommend, when assessing carbon stocks in seagrass meadows within estuaries, to identify the main sources of variation, and, in order to adequately capture the highest variability, sample sediments in different seagrass meadows following the main spatial gradients. Comprehensive and accurate assessments of sediment Corg stocks are required to understand the role of estuaries as carbon sinks, and ultimately disentangle their total carbon budget and climate change mitigation potential.
Materials and methods
Study site and sampling design
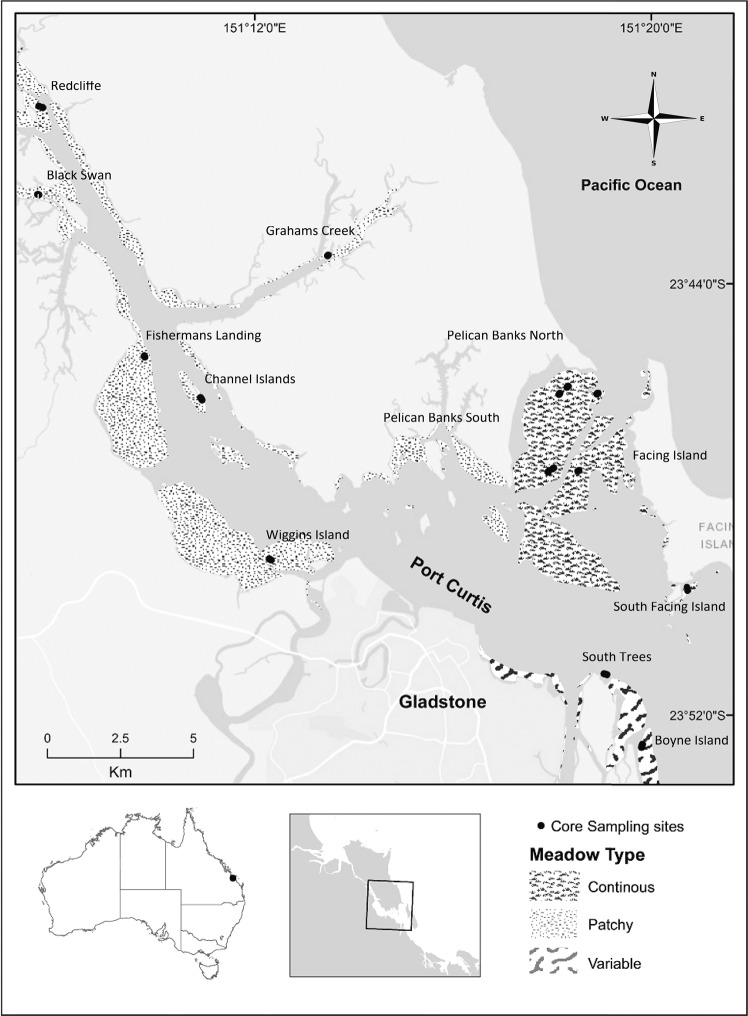
The study was conducted in Port Curtis (23°46′57″S; 151°18′0″E), a macro-tidal estuary on the central Queensland coast of north-eastern Australia. Port Curtis is a large natural harbour that has been industrialized and urbanized over the last half century. Despite this, much of it remains in a relatively natural state with a forested catchment dominating, and mangrove vegetation followed by saltmarshes landward of the mangroves in the upper estuary. The estuary contains large intertidal and subtidal sand and mud flats, which support seagrass meadows dominated mainly by the species Zostera muelleri, with Halodule uninervis, Halophila decipiens, Halophila ovalis and Halophila spinulosa also present. Seagrass appears sparsely distributed in the upper reaches of the estuary while meadows become larger (and more continuous) on the lower parts47–49. These relatively low cover seagrass meadows are typical of much of the tropical and sub-tropical Queensland coast where they play key roles in supporting megagrazers, such as dugong and green turtles, and as a fish habitat for juvenile commercial and recreationally important species51.
To study the Corg storage variability of seagrass sediments within the estuary, and to identify the main factors promoting it, we sampled across all areas of seagrass distribution within the estuary (Fig. 5)47,48. We sampled a total of 45 sediment cores in order to cover the main environmental and biological gradients within the estuary. Thus, sediment cores were taken in 15 sites along the estuary in groups of three. In each site, cores were sampled with a minimum distance of 50 m among them and sampling all the seagrass species present inside each site in order to embrace all potential variability present (Table 1). Cores of sediments were sampled at low tide by manually inserting open-barrel PVC pipes (20 cm length, 5 cm internal diameter) into soils to a depth of 10 cm and using a piston to provide suction as cores were withdrawn. Compaction during coring was low (<10%). Once extracted, cores were capped at both ends and transported to the laboratory. Cores were kept upright during transport to prevent mixing of sediment layers within the core. GPS coordinates and a 50 × 50 cm photo quadrat were taken at each core location. Seagrass cover (%) and species composition in each quadrat were estimated visually following Seagrass-Watch percent cover standards52.
Figure 5.
Map of the Port Curtis estuary, Queensland, Australia, and areas of seagrass distribution. Sediment core locations are indicated with black dots. The map was built using Esri ArcGIS 10.4 (www.esri.com/software/arcgis).
Laboratory procedures
In the laboratory, the sediments were sliced into three sections at 0–1, 1–3, 3–10 cm intervals. Coarse inorganic particles (i.e. large carbonate materials) and living plant material were manually removed7,9. Depth sediment sections were dried at 60°C and weighed in order to calculate bulk density. Each depth section was then homogenized by mixing the sediments with a clean stainless-steel spoon thoroughly or until visually homogeneous, and split into two sub-samples, with grain size particle distribution analysed from the first subsample using a Malvern Mastersizer 2000 laser microgranulometer. Prior to grain size analysis, organic matter in this sub-sample was removed by addition of hydrogen peroxide 10%. Particle size distribution was expressed as percentage (%) of volume for particle diameters from 0 to 2000 µm. The second sub-sample was ground to a fine powder with a laboratory ball grinding mill and split again into two sub-samples for Corg and N elemental and isotopic analysis. Half were washed with acid for Corg analysis, and the other half remained untreated, as this chemical procedure has been reported to alter δ15N values53. Sub-samples were acidified drop by drop with HCl 1M, until there was no visual evidence of effervescence to remove any carbonates and re-dried without rinsing54,55. After drying, samples were re-ground, placed in tin capsules and analysed for Corg and N elemental and isotopic composition. Measurements of Corg and N elemental composition and stable isotope ratios were performed using a continuous-flow isotope-ratio mass spectrometer MAT253 (Thermo Finnigan) coupled to an elemental analyser EA1108 (Carlo Erba Instruments) through a Conflo III interface (ThermoFinnigan). The C and N isotope ratios are expressed as δ values in parts per thousand (‰) relative to Vienna Pee Dee Belemnite and the atmospheric air standard, respectively, according to standard notation (δX = [(Rsample/Rstandard) − 1] × 1000, where R is the ratio 13C/12C or 15N/14N). International Atomic Energy Agency standards were inserted every 12 samples for calibration. Replicate assays of standards indicated measurement errors of ± 0.1 and ± 0.2 ‰ for C and N, respectively. Standing Corg stocks per volume unit were calculated using dry bulk density data and Corg content and expressed as mg Corg cm−3.
Environmental and biological drivers of seagrass carbon storage
Environmental and biological drivers of seagrass carbon storage were characterized using categorical and numerical variables as a function of the seagrass cover, meadow type, sediment particle size, water turbidity and their proximity to other vegetated habitats such as mangroves (Table 1). We did not compare among the different seagrass species due to the low number of cores for some of them (Table 1) and cores from different species were integrated in data analysis (see below). Meadow type was classified based on definitions by47,49 using three categories, permanent continuous meadows, permanent patchy meadows and variable meadows, the last defining non-permanent meadows that have been reported to appear intermittently as continuous or as isolated or aggregated patches. The proportion (%) of the smallest sediment fraction (silt and clay < 63 µm) was used for further statistical analysis. Water turbidity was classified using three categories, based on monthly averaged data from Gladstone Ports Corporation56 in Nephelometric Turbidity Units (NTU) measured in the bottom of the water column during the dry season (May to October) where: low was <6 NTU, medium <7–15>, high 15 NTU>. Finally, we calculated the shortest distance from each core to mangroves using aerial photographs (Google Earth, 2013) and Euclidean distance measures representing shortest travel paths (ArcGIS 10.4; ESRI Software Inc).
Data analysis
Due to the potential presence of spatial gradients within the estuary and the grouped sampling of the sediment cores, we initially estimated the spatial autocorrelation in carbon sediment variables Corg (%), δ13C (‰) and Corg stocks (mg Corg cm−3) for each core depth section by calculating Moran I statistics. We then used generalized least squares (GLS) to model carbon sediment variables for each core depth in relation to the environmental and biological explanatory variables (i.e. seagrass cover (%), meadow type, <63 µm (%), distance to mangroves (m) and turbidity). Each explanatory variable was assessed individually in separate models given the high correlation among them. All GLS models were developed with and without spatial correlation structure in the error term of the regression model (GLS spatial models and GLS non-spatial models, respectively) to model spatially autocorrelated residuals when present57. Exponential, rational quadratic, gaussian, linear and spherical correlation structures were assessed for each spatial GLS model. The best fitting model and correlation structure were defined by the minimum Akaike’s Information Criterion (AIC)58–61. The non-spatial GLS model is equivalent to Ordinary Least Squares (OLS), and the spatial GLS extends OLS by providing for possibly unequal residual variances and covariance of residuals between locations62. Finally, we tested for spatially autocorrelated residuals visualizing semivariograms of normalized residuals and calculating Moran’s I statistic for the GLS non-spatial and GLS spatial models. Normality and homoscedasticity were explored in final models via visual estimation of trends of model residuals (errors associated with homogeneity of variance, independence, and normality). We also checked if the 95% confidence intervals for parameter estimates of numerical explanatory variables were reasonable or included zero, which indicates fitting problems63. We fit all the models using restricted maximum likelihood with the nlme package64, and Moran tests and semivariograms were done with package ape65 and spdep66 in the R Statistical Computing Environment. Non-transformed values (means ± SE) are shown in the figures and tables. For all analyses, core section values of Corg (%) and Corg stocks (mg Corg cm−3) were log transformed.
The Bayesian mixing model SIAR 4.267 in the R Statistical Computing Environment was used to estimate the contribution of potential sources to the sedimentary Corg pool. The model was run with two isotopes (δ13C and δ15N) and three sources (see Supplementary Fig. S1): seagrasses (δ13C −12.94 ‰ ± 3.08 SD; δ15N 4.06 ‰ ± 1.31 SD), adjacent habitat sources (including mangroves and saltmarshes) (δ13C −26.23 ‰ ± 1.03 SD; δ15N 3.4 ‰ ± 1.1 SD), and marine algae (including benthic algae and seston) (δ13C −21.34 ‰ ± 3.29 SD; δ15N 4.69 ‰ ± 1.47 SD). Isotopic signatures of sources were collected and averaged from previous studies in the same area (see Supplementary Table S4). Separate mixing models were computed for each core. The isotopic values for all sources were assumed to be constant for each model. We did not consider any fractionation with aging (0 ‰) in the model because previous studies suggest small diagenetic shifts for δ13C and δ15N during decomposition68,69. The proportion of each source on the mixing model outputs was used to calculate the total contribution of each source to the Corg stocks accumulated per core (the sum of mg Corg cm−3 in 10 cm of core depth).
Finally, to elaborate a predictive map of Corg stocks, and their variability, at the estuary scale the total Corg stock in the first 10 cm soil depth in tonnes per hectare (Mg Corgha−1) was interpolated across known areas of seagrass distribution47–49 in the estuary extent using ArcGIS and the Empirical Bayesian kriging tool Geostatistical Analyst extension (ArcGIS 10.4; ESRI Software Inc). Areas of seagrass distribution integrated information of all seagrass species within the estuary because of the non-monospecific nature of most seagrass meadows. Empirical Bayesian kriging is an interpolation method that accounts for the error in estimating the underlying semivariogram through repeated simulations70. The standard deviation from each site (see Table 1) was interpolated to provide a measure of variability in the values and used as error terms. For each seagrass defined area or seagrass patch we calculated the mean and error term per hectare.
Supplementary information
Acknowledgements
AMR was supported financially by the Spanish government (project CTM2010-22273-C02-01 and scholarship BES-2011-046849). MR, PY & PM were supported through an Australian Research Council grant (LP160100492).
Author contributions
A.M.R., P.H.Y., C.V.B., M.A.R. and P.I.M. conceived and designed the study. A.M.R., P.H.Y., M.A.R., P.I.M. and C.V.B. performed field research and laboratory analyses. A.M.R. and D.I. analysed the data. A.M.R. led the paper with substantial input from all the authors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62639-y.
References
- 1.Nellemann, C. et al. Blue carbon. A rapid response assessment. United Nations Environ. Program. GRID-Arendal, www.grida.no (2009).
- 2.Fourqurean J, et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012;5:1–7. doi: 10.1038/ngeo1477. [DOI] [Google Scholar]
- 3.Duarte CM, Kennedy H, Marbà N, Hendriks I. Assessing the capacity of seagrass meadows for carbon burial: Current limitations and future strategies. Ocean Coast. Manag. 2013;83:32–38. doi: 10.1016/j.ocecoaman.2011.09.001. [DOI] [Google Scholar]
- 4.Mateo MA, Romero J, Pérez M, Littler MM, Littler DS. Dynamics of millenary organic deposits resulting from the growth of the mediterranean seagrass Posidonia oceanica. Estuar. Coast. Shelf Sci. 1997;44:103–110. doi: 10.1006/ecss.1996.0116. [DOI] [Google Scholar]
- 5.Macreadie PI, Baird ME, Trevathan-Tackett SM, Larkum AWD, Ralph PJ. Quantifying and modelling the carbon sequestration capacity of seagrass meadows - A critical assessment. Mar. Pollut. Bull. 2014;83:430–439. doi: 10.1016/j.marpolbul.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Duarte CM. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences. 2017;14:301–310. doi: 10.5194/bg-14-301-2017. [DOI] [Google Scholar]
- 7.Lavery PS, Mateo MA, Serrano O, Rozaimi M. Variability in the carbon storage of seagrass habitats and its implications for global estimates of blue carbon ecosystem service. PLoS One. 2013;8:e73748. doi: 10.1371/journal.pone.0073748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Röhr ME, Boström C, Canal-Vergés P, Holmer M. Blue Carbon stocks in Baltic Sea eelgrass (Zostera marina) meadows. Biogeosciences. 2016;13:6139–6153. doi: 10.5194/bg-13-6139-2016. [DOI] [Google Scholar]
- 9.Miyajima T, et al. Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Global Biogeochem. Cycles. 2015;29:397–415. doi: 10.1002/2014GB004979. [DOI] [Google Scholar]
- 10.Serrano O, Lavery P, Rozaimi M, Mateo M. Influence of water depth on the carbon sequestration capacity of seagrasses. Global Biogeochem. Cycles. 2014;28:1–12. doi: 10.1002/2014GB004872. [DOI] [Google Scholar]
- 11.Serra no O, et al. Key biogeochemical factors affecting soil carbon storage in Posidonia meadows. Biogeosciences. 2016;13:4581–4594. doi: 10.5194/bg-13-4581-2016. [DOI] [Google Scholar]
- 12.Ricart AM, Pérez M, Romero J. Landscape configuration modulates carbon storage in seagrass sediments. Estuar. Coast. Shelf Sci. 2017;185:69–76. doi: 10.1016/j.ecss.2016.12.011. [DOI] [Google Scholar]
- 13.Macreadie PI, et al. Losses and recovery of organic carbon from a seagrass ecosystem following disturbance. Procedings R. Soc. Biol. Sci. 2015;282:20151537. doi: 10.1098/rspb.2015.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samper-Villarreal J, Lovelock CE, Saunders MI, Roelfsema C, Mumby PJ. Organic carbon in seagrass sediments is influenced by seagrass canopy complexity, turbidity, wave height, and water depth. Limnol. Oceanogr. 2016;61:938–952. doi: 10.1002/lno.10262. [DOI] [Google Scholar]
- 15.Atwood, T. B. et al. Predators help protect carbon stocks in Blue Carbon ecosystems. Nat. Clim. Chang. 1–8 (2015).
- 16.Thomson, A. C. G., Trevathan-Tackett, S. M., Maher, D. T., Ralph, P. J. & Macreadie, P. I. Bioturbator-stimulated loss of seagrass sediment carbon stocks. Limnol. Oceanogr. 1–15 (2018).
- 17.Mazarrasa, I. et al. Habitat characteristics provide insights of carbon storage in seagrass meadows. Mar. Pollut. Bull. 0–1 (2018). [DOI] [PubMed]
- 18.Ricart AM, et al. Variability of sedimentary organic carbon in patchy seagrass landscapes. Mar. Pollut. Bull. 2015;100:476–482. doi: 10.1016/j.marpolbul.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, et al. Effects of nutrient load on microbial activities within a seagrass-dominated ecosystem: Implications of changes in seagrass blue carbon. Mar. Pollut. Bull. 2017;117:214–221. doi: 10.1016/j.marpolbul.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Oreska MPJ, McGlathery KJ, Porter JH, Bost M, McKee BA. Seagrass blue carbon accumulation at the meadow-scale. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0176630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos R, et al. Superficial sedimentary stocks and sources of carbon and nitrogen in coastal vegetated assemblages along a flow gradient. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard, J., Hoyt, S., Isensee, K., Pidgeon, E. & Telszewski, M. Coastal blue carbon methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrass meadows. (Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature, 2015).
- 23.Kindeberg T, Ørberg SB, Röhr ME, Holmer M, Krause-Jensen D. Sediment stocks of carbon. nitrogen, and phosphorus in Danish eelgrass meadows. Front. Mar. Sci. 2018;5:1–14. [Google Scholar]
- 24.Young MA, et al. Optimal soil carbon sampling designs to achieve cost-effectiveness: a case study in blue carbon ecosystems. Biol. Lett. 2018;14:20180416. doi: 10.1098/rsbl.2018.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Githaiga MN, Kairo JG, Gilpin L, Huxham M. Carbon storage in the seagrass meadows of. PLoS One. 2017;12:1–13. doi: 10.1371/journal.pone.0177001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gullström, M. et al. Blue Carbon storage in tropical seagrass meadows relates to carbonate stock dynamics, plant–sediment processes, and landscape context: Insights from the Western Indian Ocean. Ecosystems 1–16 (2017).
- 27.Ierodiaconou DA, Laurenson LJB. Estimates of Heterozostera tasmanica, Zostera muelleri and Ruppia megacarpa distribution and biomass in the Hopkins Estuary, western Victoria, by GIS. Aust. J. Bot. 2002;50:215–228. doi: 10.1071/BT00093. [DOI] [Google Scholar]
- 28.Matson EA, Brinson MM. Stable carbon isotopes and the C-N Ratio in the estuaries of the Pamlico and Neuse Rivers, North-Carolina. Limnol. Oceanogr. 1990;35:1290–1300. doi: 10.4319/lo.1990.35.6.1290. [DOI] [Google Scholar]
- 29.Cai WJ. Estuarine and coastal ocean carbon paradox: CO 2 sinks or sites of terrestrial carbon incineration? Ann. Rev. Mar. Sci. 2011;3:123–145. doi: 10.1146/annurev-marine-120709-142723. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca M, Bell S. Influence of physical setting on seagrass landscapes near Beaufort, North Carolina, USA. Mar. Ecol. Prog. Ser. 1998;171:109–121. doi: 10.3354/meps171109. [DOI] [Google Scholar]
- 31.Bell, S., Fonseca, S. & Stafford, N. B. Seagrass ecology: new contributions from a landscape perspective. Seagrasses Biol. Ecol. Conserv. 625–645 (2006).
- 32.Alcoverro T, Cebrian E, Ballesteros E. The photosynthetic capacity of the seagrass Posidonia oceanica: influence of nitrogen and light. J. Exp. Mar. Bio. Ecol. 2001;261:107–120. doi: 10.1016/S0022-0981(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 33.Kelleway JJ, et al. Seventy years of continuous encroachment substantially increases ‘Blue Carbon’ capacity as mangroves replace intertidal salt marshes. Glob. Chang. Biol. 2016;22:1097–1109. doi: 10.1111/gcb.13158. [DOI] [PubMed] [Google Scholar]
- 34.Sousa AI, et al. ‘Blue Carbon’ and nutrient stocks of salt marshes at a temperate coastal lagoon (Ria de Aveiro, Portugal) Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, et al. Mangroves as a major source of soil carbon storage in adjacent seagrass meadows. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gacia E, Duarte CM. Sediment retention by a mediterranean Posidonia oceanica meadow: The balance between deposition and resuspension. Estuar. Coast. Shelf Sci. 2001;52:505–514. doi: 10.1006/ecss.2000.0753. [DOI] [Google Scholar]
- 37.Samper-Villarreal J, Mumby PJ, Saunders MI, Roelfsema C, Lovelock CE. Seagrass organic carbon stocks show minimal variation over short time scales in a heterogeneous subtropical seascape. Estuaries and Coasts. 2018;41:1732–1743. doi: 10.1007/s12237-018-0381-z. [DOI] [Google Scholar]
- 38.Kennedy H, et al. Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochem. Cycles. 2010;24:GB4026–GB4026. doi: 10.1029/2010GB003848. [DOI] [Google Scholar]
- 39.Dahl, M. et al. Sediment properties as important predictors of carbon storage in Zostera marina meadows: A comparison of four european areas. PLoS One (2016). [DOI] [PMC free article] [PubMed]
- 40.Burdige DJ. Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem. Rev. 2007;107:467–485. doi: 10.1021/cr050347q. [DOI] [PubMed] [Google Scholar]
- 41.Serrano O, et al. Can mud (silt and clay) concentration be used to predict soil organic carbon content within seagrass ecosystems? Biogeosciences. 2016;13:4915–4926. doi: 10.5194/bg-13-4915-2016. [DOI] [Google Scholar]
- 42.Trevathan-Tackett, S. M. et al. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 150511125256001 (2015). [DOI] [PubMed]
- 43.Ward LG, Michael Kemp W, Boynton WR. The influence of waves and seagrass communities on suspended particulates in an estuarine embayment. Mar. Geol. 1984;59:85–103. doi: 10.1016/0025-3227(84)90089-6. [DOI] [Google Scholar]
- 44.Gacia E, et al. Sediment deposition and production in SE-Asia seagrass meadows. Estuar. Coast. Shelf Sci. 2003;56:909–919. doi: 10.1016/S0272-7714(02)00286-X. [DOI] [Google Scholar]
- 45.Dahl, M. et al. Increased current flow enhances the risk of organic carbon loss from Zostera marina sediments: Insights from a flume experiment. Limnol. Oceanogr. 1–13 (2018).
- 46.Green, E P., Short, F. T. World atlas of seagrasses. (University of California Press, 2003).
- 47.Rasheed, M. A., Thomas, R., Roelofs, A. J., Neil, K. M. & Kerville, S. P. Port Curtis and Rodds Bay seagrass and benthic macro-invertebrate community baseline survey November / December 2002, (2002).
- 48.Bryant, C., Davies, J., Sankey, T., Jarvis, J. & Rasheed, M. Long term seagrass monitoring in Port Curtis: Quarterly seagrass assessments & permanent transect monitoring progress report 2009 to 2013, 10.13140/RG.2.1.3689.5204 (2014).
- 49.Carter, A. et al. Seagrasses in Port Curtis & Rodds Bay Seagrasses in Port Curtis & Rodds Bay Annual long‐term monitoring, biannual Western Basin, and updated baseline survey, (2015).
- 50.Ricart AM, Dalmau A, Pérez M, Romero J. Effects of landscape configuration on the exchange of materials in seagrass ecosystems. Mar. Ecol. Prog. Ser. 2015;532:89–100. doi: 10.3354/meps11384. [DOI] [Google Scholar]
- 51.Coles RG, et al. The Great Barrier Reef World Heritage Area seagrasses: Managing this iconic Australian ecosystem resource for the future. Estuarine, Coast. Shelf Sci. 2015;153:A1–A12. doi: 10.1016/j.ecss.2014.07.020. [DOI] [Google Scholar]
- 52.Mckenzie, L. J., Campbell, S. J. & Roder, C. A. Seagrass-Watch: Manual for Mapping & Monitoring Seagrass Resources by Community (citizen) Volunteers. INFORMATION SERIES QI01094.
- 53.Bunn SE, Loneragan NR, Kempster MA. Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: Implications for food-web studies using multiple stable isotopes. Limnol. Oceanogr. 1995;40:622–625. doi: 10.4319/lo.1995.40.3.0622. [DOI] [Google Scholar]
- 54.Jacob U, Mintenbeck K, Brey T, Knust R, Beyer K. Stable isotope food web studies: a case for standardized sample treatment. Mar. Ecol. Prog. Ser. 2005;287:251–253. doi: 10.3354/meps287251. [DOI] [Google Scholar]
- 55.Carabel S, Godínez-Domínguez E, Verísimo P, Fernández L, Freire J. An assessment of sample processing methods for stable isotope analyses of marine food webs. J. Exp. Mar. Bio. Ecol. 2006;336:254–261. doi: 10.1016/j.jembe.2006.06.001. [DOI] [Google Scholar]
- 56.Aurecon Australia Pty, L. Western basin dredging and disposal (onshore and offshore) project water quality management plan Gladstone Ports Corporation, (2011).
- 57.Zuur, A., Ieno, E., Walker, N., Saveliev, A. & Smith, G. Mixed effects models and extensions in ecology with R. (Springer, 2009).
- 58.Littel, R., MIlliken, G., Stroup, W. & Wolfinger, R. SAS Systems for Mixed Models. (SAS Institute Inc, 1996).
- 59.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. (Springer, 2002).
- 60.Crawley, M. J. The R Book. (John Wiley & Sons, 2007).
- 61.Selmi S, Boulinier T. Ecological biogeography of Southern Ocean islands: The importance of considering spatial issues. Am. Nat. 2001;158:426–437. doi: 10.1086/321992. [DOI] [PubMed] [Google Scholar]
- 62.Fox, J. & Weisberg, S. Time-series regression and generalized least squares: an appendix to an R companion to applied regression. An R Companion to Applied Regression (SAGE, 2018).
- 63.Bolker BM, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team R. nlme: Linear and nonlinear mixed effects models. R package version. 2019;3:1–140. [Google Scholar]
- 65.Paradis E, Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2018;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 66.Bivand, R. S. & Wong, D. W. S. Comparing implementations of global and local indicators of spatial association. TEST27, 716–748.
- 67.Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: coping with too much variation. PLoS One. 2010;5:e9672. doi: 10.1371/journal.pone.0009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zieman J, Macko S, Mills A. Role of seagrasses and mangroves in estuarine food webs: temporal and spatial changes in stable isotope composition and amino acid content during decomposition. Bull. Mar. Sci. 1984;35:380–392. [Google Scholar]
- 69.Mateo MA, Renom P, Michener RH. Long-term stability in the production of a NW Mediterranean Posidonia oceanica (L.) Delile meadow. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010;291:286–296. doi: 10.1016/j.palaeo.2010.03.001. [DOI] [Google Scholar]
- 70.Gribov, A. & Krivoruchko, K. New flexible non-parametric data transformation for trans-gaussian kriging in Geostatistics Oslo, vol 17 (eds. Abrahamsen P., Hauge R., Kolbjørnsen (Springer, 2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.