Abstract
Heterobasidion irregulare and H. occidentale are two closely related conifer root rot pathogens in the H. annosum sensu lato (s.l.) species complex. The two species H. irregulare and H. occidentale have different host preference with pine and non-pine tree species favored, respectively. The comparison of transcriptomes of H. irregulare and H. occidentale growing in Norway spruce bark, a susceptible host non-native to North America, showed large differences in gene expression. Heterobasidion irregulare induced more genes involved in detoxification of host compounds and in production of secondary metabolites, while the transcriptome induced in H. occidentale was more oriented towards carbohydrate degradation. Along with their separated evolutionary history, the difference might be driven by their host preferences as indicated by the differentially expressed genes enriched in particular Gene Ontology terms.
Subject terms: Fungal genomics, Fungal pathogenesis
Introduction
Fungal pathogens use a range of strategies and mechanisms to infect and colonize plants1. The degree of specialization vary, from those that infect a wide range of host plants and/or many different tissues, to those that are restricted to a limited number of host species/cultivars or tissues1–4. Furthermore, closely related pathogens can utilize different strategies to infect and colonize plants and/or specialize on different hosts5. The genetic differences found between two closely related species will be a result of both selection and random genetic drift following reproductive isolation. Although not experimentally proven, differential selection is predicted to have a major impact during speciation in sympatry while both differential selection and random genetic drift is expected to contribute to genetic differences found between species evolving in allopatry. Differences expected to be found in sister taxa could be changes in gene sequences, changes in gene expression, genomic reorganization and gene copy variation2.
In plant pathogens, selection has been shown to contribute to the diversification of genes involved in infection, colonization and specialization on host plants, such as genes involved in biosynthesis of secondary metabolites or toxins and genes encoding cell wall degrading enzymes2,6,7. Certain effectors, small secreted proteins molecules that facilitating infection and/or triggering defense responses, that participate in determining the host specificity of the closely related oomycete pathogens Phytophthora infestans and P. mirabilis have been found to be under positive selection3. Adaption due to changes in gene expression has been studied to a much lesser degree. In a comparative transcriptome study of the Brassicaceae pathogen Colletotrichum higginsianum and the cereal pathogen C. graminicola, C. higginsianum strongly induced secondary metabolism genes while C. graminicola did not, suggesting a diversification driven by host interaction4.
The basidiomycete Heterobasidion annosum sensu lato (s.l.) is a species complex of devastating necrotrophic fungal pathogens that cause root and butt rot to conifers in the northern hemisphere8,9. Heterobasidion annosum s.l. consist of three European and two North American species with partly overlapping geographic distribution and host preferences10. In Europe, the species H. annosum s.s. is mostly found attacking Pine spp. but is able to infect other conifers and broad leaved trees as well. In contrast, H. parviporum lives almost exclusively on Picea abies, but attacks Abies sibirica in north-eastern Europe11,12, while H. abietinum is a pathogen or saprophyte on Abies species in southern and central Europe11,12. The North American species H. irregulare mostly infects Pinus and Juniperus species but also Abies balsamea while H. occidentale has a host range excluding Pinus spp. but including species in the genera Abies, Picea, Pseudotsuga, Tsuga, Sequoiadendron13. Although there is a patter in the host preferences among the H. annosum s.l. species the specialization has not been driven by co-speciation together with the host10. The speciation of H. annosum s.l. complex has been suggested to start with a split between an ancestor of the pine infecting species H. annosum s.s. and H. irregulare and the ancestor of the non-pine infecting species H. parviporum, H. abietinum and H. occidentale in Lauraisa10. The common ancestor of H. annosum s.s./H. irregulare migrated from west Europe to eastern North America. After the Atlantic land bridge disappeared the two species H. annosum s.s. and H. irregulare evolved in allopatry for several millions of years in Europe and North America, respectively. While the ancestor of H. parviporum, H. abietinum and H. occidentale may originate in east Asia or west North America10. In North America the H. irregulare continued to spread west over the continent while the spread of H. occidentale was restricted to the east by an arid region which probably was colonized by pines, which are a nonhost for H. occidentale10. After original speciation between pine and non-pine infecting species, H. irregulare and H. occidentale have had a long period to evolve in allopatry before today’s overlapping geographic distribution in the western North America. How the evolutionary history with speciation in sympatry and a long period of allopatric evolution have shaped their genomes and genetic differences found between H. irregulare and H. occidentale is not well understood.
Comprehensive studies of H. irregulare have revealed a trade off in gene expression between saprotrophic and parasitic lifestyle14. During the saprotrophic growth H. irregulare use various cellulose and pectin degrading enzymes and many cellulose oxidoreductase genes are up-regulated during wood degradation while genes related to secondary metabolism are more commonly activated during growth in bark14. Transcripts identified from H. annosum s.s. and Norway spruce interaction showed similar gene induction patterns as seen in H. irregulare15. Furthermore, a global gene expression study of H. annosum s.s. found that the glyoxylate cycle might be important for fungal adaptation to change of nutrients availability in the environment, melanization for adapting to salt stress, and the pathway related to DNA repair for oxidative stress16.
We hypothesize that H. irregulare and H. occidentale evolved distinct genetic machineries for infection and colonization of trees. To analyze the genetic bases for the difference in adaption we performed massive gene expression profiling using deep RNA sequencing of the two pathogens during infection of Norway spruce, a susceptible conifer host not native to North America.
Results
Virulence of H. irregulare and H. occidentale on Norway spruce
Both H. occidentale and H. irregulare were able to induce necrosis and colonize the sapwood of four-year-old branches of Norway spruce. The success rate of infections was slightly higher for H. occidentale than for H. irregulare inoculations, 87%, and 73%, respectively. There was no significant difference (One-way ANOVA) between H. occidentale and H. irregulare when comparing growth in sapwood and expansion of lesions in the inner bark (Table 1). However, for both species there was a significant difference in growth in sapwood and expansion of lesions in the inner bark from two weeks compared to 4 and 6 weeks (Tukey test, P < 0.05).
Table 1.
Virulence of H. irregulare and H. occidentale measured as fungal growth in the spruce sapwood, and lesion expansion in the inner bark.
| Growth (mm) | Lesion (mm) | |||||
|---|---|---|---|---|---|---|
| 2 w | 4 w | 6 w | 2 w | 4 w | 6 w | |
| H. occidentale | 16.7 ± 12.6 | 42.0 ± 28.4 | 41.0 ± 36.0 | 7.7 ± 4.2 | 21.2 ± 16.6 | 28.2 ± 11.0 |
| H. irregulare | 23.8 ± 11.1 | 58.3 ± 7.6 | 62.5 ± 20.6 | 9.5 ± 7.6 | 11.0 ± 5.0 | 20.5 ± 7.2 |
Growth = Fungal growth in the sapwood, Lesion = lesion length in the inner bark, 2 w, 4 w and 6 w = 2, 4 and 6 weeks after inoculation (n = 3–5)a.
aNo significant difference between H. occidentale and H. irregulare (One-way ANOVA, P > 0.5) but a significant difference in growth in sapwood and expansion of lesions in the inner bark from two weeks compared to 4 and 6 weeks (Tukey test, P < 0.05).
Genome annotation and gene orthologue identification
The genome sequences of H. irregulare and H. occidentale were acquired from previous published data14,17. By re-annotating the genomes using MAKER, 9462 gene models were identified in H. irregulare and 10295 in H. occidentale. Although, we identified 2002 fewer gene models with the MAKER annotation than the published version of the H. irregulare genome the majority 8412 (89%) gene models were shared with the H. irregulare genome available at Joint Genome Institute (JGI) website (http://genome.jgi.doe.gov/Hetan2/Hetan2.home.html). The two species, H. irregulare and H. occidentale share 7545 one-to-one orthologous gene models. The gene models of the two species could be further grouped together into 8306 orthoMCL clusters.
Genes induced in H. irregulare and H. occidentale during infection
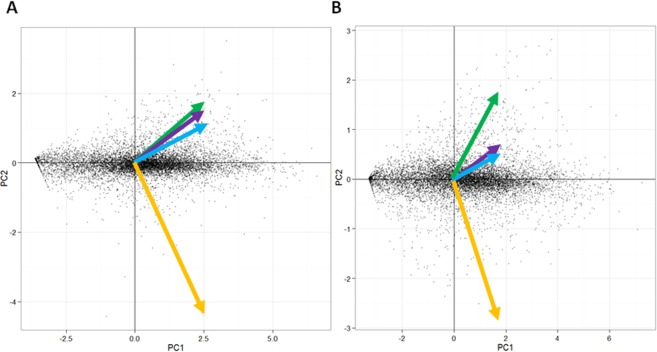
In total 179 GB RNA sequence data from 2, 4 and 6 weeks (2 w, 4 w and 6 w) of infected bark and liquid (L) culture control samples were generated by the Illumina Hiseq (European Nucleotide Archive with accession number: PRJEB33431). After length and quality filtering 30.5 to 154.8 million reads per bark sample and 10 to14 million reads per pure fungal sample were retained (Table 2). The sequence reads from the samples were mapped against their respective reference genome. From the pure fungal samples, 77 to 84% of the read pairs mapped, while from the bark samples 0.4 to 21.9% of the read pairs mapped (Table 2). The numbers of mapped pairs from the colonized bark samples were more than 500 000 except for one replicate of one sample which generated 229 570 mapped pairs (Table 2). The assembly of mapped reads identified 9491 gene models in H. irregulare which is 17 more than the gene models annotated in the genome by MAKER while in H. occidentale 10220 gene models were identified which are 75 less than the MAKER annotation of the genome. A PCA analysis of the expression fragments per kilobase of exon per million mapped reads (FPKM) of all genes in the four conditions for each species suggested that, the L samples are substantially different from the interaction samples taken from bark (Fig. 1).
Table 2.
Total number of sequence reads generated and aligned sequence pairs from H. irregulare and H. occidentale liquid cultures and 2, 4, and 6 weeks infected Norway spruce bark.
| Samples names* | Total reads | Aligned pairs | Mapped pairs (%) |
|---|---|---|---|
| TC32-1-L-rep1 | 11809378 | 9114879 | 77.2 |
| TC32-1-L-rep2 | 10555531 | 8764947 | 83.0 |
| TC32-1-L-rep3 | 13240830 | 11124807 | 84.0 |
| TC32-1-2w-rep1 | 39748040 | 1148233 | 2.9 |
| TC32-1-2w-rep2 | 43180655 | 1637664 | 3.8 |
| TC32-1-2w-rep3 | 40750502 | 1282462 | 3.1 |
| TC32-1-4w-rep1 | 121996688 | 583954 | 0.5 |
| TC32-1-4w-rep2 | 49434129 | 1241663 | 2.5 |
| TC32-1-4w-rep3 | 95247997 | 3219921 | 3.4 |
| TC32-1-6w-rep1 | 58357874 | 229570 | 0.4 |
| TC32-1-6w-rep2 | 53266103 | 681649 | 1.3 |
| TC32-1-6w-rep3 | 55675183 | 975852 | 1.8 |
| TC122-12-L-rep1 | 13661132 | 11135225 | 81.5 |
| TC122-12-L-rep2 | 12389419 | 10207752 | 82.4 |
| TC122-12-L-rep3 | 11098200 | 9092986 | 81.9 |
| TC122-12-2w-rep1 | 30863928 | 6764247 | 21.9 |
| TC122-12-2w-rep2 | 39222132 | 1928245 | 4.9 |
| TC122-12-2w-rep3 | 33431531 | 2346657 | 7.0 |
| TC122-12-4w-rep1 | 154786107 | 1519679 | 1.0 |
| TC122-12-4w-rep2 | 78321161 | 1714337 | 2.2 |
| TC122-12-4w-rep3 | 32287019 | 4234747 | 13.1 |
| TC122-12-6w-rep1 | 30472557 | 5817262 | 19.1 |
| TC122-12-6w-rep2 | 73805957 | 3078694 | 4.2 |
| TC122-12-6w-rep3 | 142671388 | 3277956 | 2.3 |
H. irregulare = TC32-1 and H. occidentale = TC122-12; L = liquid culture, 2 w, 4 w and 6 w = 2, 4, and 6 weeks after inoculating Norway spruce bark with the fungus; rep1, rep2 and rep3 = replicate 1, 2, and 3.
Figure 1.
Principle component analysis (PCA) of significantly differentially expressed genes of H. irregulare (A) and H. occidentale (B). Arrows indicated the directions of components: orange arrow represented fungus grow in liquid culture, green, blue, purple arrows represented 2, 4, and 6 weeks after infection.
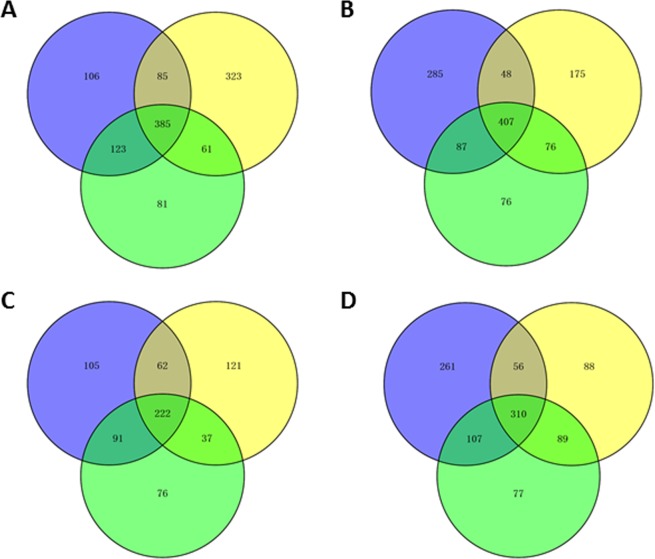
The total number of significant differentially expressed genes (DEGs) between any two of the treatments: pure fungal culture (L) and fungal colonization of Norway spruce bark (2 w, 4 w and 6 w) of H. irregulare and H. occidentale were 2081 and 2360, respectively. The number of DEGs in any of the three time points in bark compared to L were between 1076 and 1561 while the number of DEGs between the three time points in bark was much lower for both species (Table S1). In H. irregulare there are 385 genes consistently up-regulated and 222 consistently down-regulated genes in 2 w, 4 w and 6 w compared to L (Fig. 2A,C). Similarly, 407 and 310 genes were consistently up- and down-regulated, respectively, in H. occidentale when comparing infection with liquid culture control (Fig. 2B,D).
Figure 2.
Venn diagram comparing the number of significantly differentially up- or down-regulated genes during infection of Norway spruce in H. irregulare or H. occidentale. The purple, green and pink represent the number of significant up- or down-regulated genes from 2, 4 and 6 weeks after infection compare to liquid culture. The number of common and different up-regulated genes at 2, 4, and 6 weeks after inoculation compared with liquid culture of H. irregulare (A). The number of common and different up-regulated genes at 2, 4, and 6 weeks after inoculation compared with liquid culture of H. occidentale (B). The number of common and different down-regulated genes at 2, 4, and 6 weeks after inoculation compared with liquid culture of H. irregulare (C). The number of common and different down-regulated genes at 2, 4. and 6 weeks after inoculation compared with liquid culture of H. occidentale (D).
Annotation and enrichment test of DEGs from H. irregulare and H. occidentale
Gene ontology (GO) annotation were provided for 5685 out of 9474 gene models for H. irregulare and 5759 out of 10295 gene models for H. occidentale. There were enrichment of genes in 11, 12 and 15 categories for biological processes among the DEGs up-regulated for H. irregulare in 2 w, 4 w and 6 w and 13, 6 and 6 categories for H. occidentale (Fisher’s exact test with a FDR threshold of 5%) (Table 3, S2 and S3). Genes associated with oxidation-reduction process (GO:0055114), transmembrane transport (GO:0055085), amino acid transmembrane transport (GO:0003333) and small molecule catabolic process (GO:0044282) are consistently up-regulated and enriched in both species (Table S2). Among the genes found in categories uniquely enriched in H. irregulare, and that were consistently up-regulated during infection, several were related to detoxification, such as alpha-amino acid catabolic process (GO:1901606), drug transport (GO:0015893), benzoate metabolic process (GO:0018874) and xenobiotic catabolic process (GO:0042178). In contrast, genes associated with carbohydrate metabolism and transport were uniquely enriched in H. occidentale, especially genes in the categories carbohydrate transport (GO:0008643), polysaccharide metabolic process (GO:0005976), cellular carbohydrate metabolic process (GO:0044262) (Table S2).
Table 3.
The numbers of GO terms enriched in H. irregulare and H. occidentale.
| GO categories | H. irregulare | H. occidentale | Shared | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 w | 4 w | 6 w | Consistent | 2 w | 4 w | 6 w | Consistent | Consistent | |
| Cellular Localization | 2 | 3 | 1 | 1 | 2 | 0 | 2 | 0 | 0 |
| Molecular Function | 9 | 13 | 10 | 8 | 15 | 9 | 9 | 9 | 3 |
| Biological Process | 11 | 12 | 15 | 8 | 13 | 6 | 6 | 8 | 4 |
The down-regulated DEGs are associated with a smaller number of GO categories (Table S3). In H. irregulare, the down-regulated DEGs are related to fungal cell wall biogenesis and certain type of carbohydrate metabolism, such as fungal-type cell wall (GO:0009277) and serine-type carboxypeptidase activity (GO:0004185). In H. occidentale, the down-regulated DEGs are more diverse and includes both nutrient metabolism related to growth like amino sugar metabolic process (GO:0006040) and flavin adenine dinucleotide binding (GO:0050660). Although genes in the categories GO:0050660 and GO:0016614 are enriched in the up-regulated genes of H. irregulare and enriched among the down-regulated DEGs in H. occidentale the actual genes under these GO categories are not orthologous genes.
Genes commonly expressed for H. irregulare and H. occidentale during infection
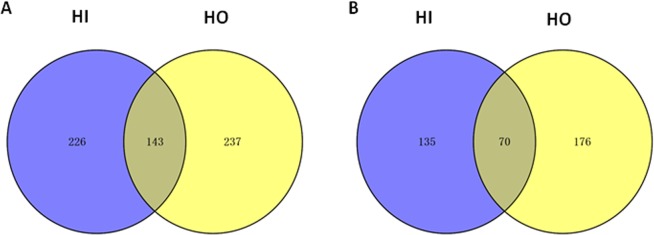
Among the consistently, over the three time point, up-regulated DEGs, 369 in H. irregulare and 380 in H. occidentale share an orthologue in the other genome. However, only 143 gene models with orthologues are consistently up-regulated in bark in the two species (Fig. 3, Table S4). Among the consistently down-regulated genes in bark there were 70 orthologous gene pairs (Fig. 3, Table S5). Additionally, nine H. irregulare genes correspond to 11 H. occidentale genes which do not have reciprocal orthologues but are in the same gene families, were also among the shared up-regulated genes. Among the 143 shared DEGs up-regulated in bark, a number of host material degradation enzymes, transmembrane transporters, and genes involved in metabolism are identified (Table S4).
Figure 3.
The number of common and different consistently up-regulated (A) and down-regulated (A) genes of H. irregulare and H. occidentale (HI = H. irregulare, HO = H. occidentale).
Differently expressed genes in H. irregulare and H. occidentale in bark
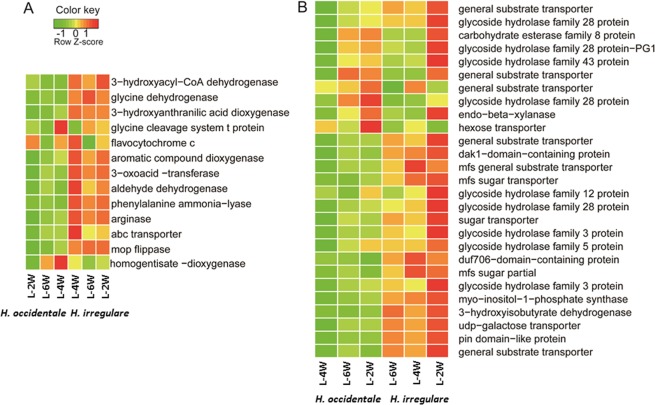
Out of the genes specifically differentially up-regulated in H. irregulare and H. occidentale 226 and 237 have orthologues in the other species were the gene was not up-regulated. In addition, there were 15 H. irregulare and 22 H. occidentale genes specifically induced for which we could not identify an orthologue in the other species. Consequently, a set of 241 H. irregulare gene and 259 H. occidentale genes corresponding to two third of the consistently up-regulated DEGs were differentially regulated between the two species, and were used for further analysis. In total 91 of 241 H. irregulare genes were assigned Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology terms and were mapped to 102 KEGG pathways, as well as 87 of 259 H. occidentale genes that were mapped to 85 KEGG pathways. Approximately half of the KEGG pathways identified that contains species specifically differentially regulated genes in H. irregulare and H. occidentale were unique to either species (Table S8). The genes assigned to the enriched GO terms in H. irregulare (alpha-amino acid process, GO:1901606; drug transport, GO:0015893 and benzoate metabolic process, GO:0018874) and H. occidentale (carbohydrate transport, GO:0008643; polysaccharide metabolic process, GO:0005976; and cellular carbohydrate metabolic process, GO:0044262) show different expression patterns compared to orthologues in the other species(Fig. 4A,B).
Figure 4.
Differentially regulated genes within the enriched GO catalogue of H. irregulare (alpha-amino acid process, GO:1901606; drug transport, GO:0015893 and benzoate metabolic process, GO:0018874) and H. occidentale (carbohydrate transport, GO:0008643; polysaccharide metabolic process, GO:0005976; and cellular carbohydrate metabolic process, GO:0044262) as well as their corresponding genes from another species. The heat map of genes expression of log2 fold change from H. irregulare of enriched GO terms comparing with their orthologous in H. occidentale (A) and H. occidentale enriched GO terms comparing with their orthologous in H. irregulare (B).
Discussion
The closely related species H. irregulare and H. occidentale, in the species complex H. annosum s.l., showed surprisingly small difference in gene content, a majority of genes could be found as one-to-one orthologous or in groups of gene families. However, the species show large differences in gene expression patterns in planta when inoculated on the same host species (which is not the natural host for either species). Approximately 2/3 of the genes consistently differentially expressed during growth in bark in either species are specifically induced in that species. The difference in enriched GO terms of gene expression in spruce bark between H. irregulare and H. occidentale indicate that the differences found are likely to be a result of adaption to their particular infection strategy. If the changes in gene expression between two species are a result of a random process or a result of stabilizing selection, the expression pattern would show random variation or little variation rather than variation of genes enriched in certain orthology groups. A previous study of the evolutionary history of H. annosum s.l. shows that separation of the H. irregulare and H. occidentale common ancestor to modern species is associated with the host preference of pine infection and non-pine infection10. Thus the difference in gene expression patterns between pine infecting H. irregulare and non-pine infecting H. occidentale most likely is associated with this hallmark trait in the evolutionary history of these taxa.
The comparisons of the transcriptomes of H. irregulare and H. occidentale growing in Norway spruce bark give insights into the different strategies the two species have for infecting their respective main host tree species. The transcriptional patterns shown by H. irregulare accentuate tolerance to the harsh environment created by host defense and production of secondary metabolites for attacking its host (Fig. 4A). When fungal pathogen invasion is detected by plants, the plant mounts a defense that among other mechanisms, involve production of specialized toxic metabolites. Those chemicals are often terpenes, phenolics and nitrogen containing compounds18. To successfully colonize their host, pathogens have to overcome the antimicrobial effects of such chemicals by employing their xenobiotic metabolizing enzymes such as cytochrome P450s or glucosyltransferases19,20. We show that both H. irregulare and H. occidentale induce expression of the nitrogen compound metabolism, benzoate metabolic and xenobiotic catabolic enzymes as well as the multidrug transporters to detoxify host defense chemicals to grow in spruce bark. However, the transcriptome of H. irregulare showed induction of more genes involved in detoxification than did H. occidentale, GO terms related to detoxification were only found to be enriched in H. irregulare’s interaction with the Norway spruce. An aromatic compound dioxygenase is particularly up-regulated in H. irregulare. Aromatic compound dioxygenase catalyzes the oxidative ring cleavage of catechol and might be involved in detoxification of phenolic compounds produced by the host. In H. annosum s.s. infected tissues, stilbenes converted to ring-opened, de-glycosylated, and dimeric products are found21. Furthermore, analyses of Endoconidiophora polonica protein and metabolite extracts have shown that these stilbene metabolites arise from fungal enzyme activities22 and that E. polonica most likely use these small metabolites as carbon sources for growth23. Possibly, H. irregulare uses the aromatic compound dioxygenase to generate linearized stilbene metabolites for its nutrition. To avoid the effects of antimicrobial compounds, effluxing them out of the fungal cells is another important mechanism for handling them.
Production of low-molecular weight toxins is proposed to be an important virulence factor of many necrotrophic plant pathogens and H. annosum s.l. is known to produce toxins such as, oosponol, fomannosin, fomannoxin, and the fomjorins24. However, secondary metabolite profiling has showed that there is a lager difference in compound composition between the pine infecting species and the non-pine infecting species than with in each of the groups25. Here we show that differential up-regulated enzymatic genes from the two species to a large extent mapped to different KEGG pathways related to secondary metabolism. This result suggests that compounds produced during infection were different in H. irregulare and H. occidentale.
Interestingly, we also found a differential shift of primary metabolism in H. irregulare during infection. The glyoxylate cycle is only induced in H. irregulare, which is similar to what has been reported for Fusarium graminearum growing in wheat26. Two key enzymes, an isocitrate lyase and a malate synthase, in the glyoxylate cycle were highly induced and with high expression level. The glyoxylate cycle requires a mitochondrial inner membrane carriers to transport isocitrate to the cytosol26. Mitochondrial carrier genes have also been shown to be important for virulence of Candida albicans27. The glyoxylate cycle could be important for H. irregulare growing in host and it could also be related to previous observation of mitochondrial involvement in H. annosum s.l. virulence28. In addition, genes of the glyoxylate cycle have been found up-regulated in H. annosum s.s. when facing nutrient starvation and they might be important for survival of the fungus during stress conditions26,27,29. There are three mitochondrial carriers and one mitochondrial inner membrane carrier up-regulated in H. irregulare during infection. Knock out one of mitochondrial carrier gene (CIC1) or (FOW1) in F. graminearum or F. oxysporum to disrupt the glyoxylase cycle, resulted in mutants that performed well in vitro but had a 2/3 reduced lesion size in infected coleoptiles26,29. This suggest that as in many plant pathogens mitochondrial function is important for successful colonization of hosts.
Several quantitative trait loci (QTL) for virulence of H. irregulare have been identified30. By re-mapping the virulence data from Lind et al.30, 619 gene models have been annotated in the QTL regions14. With our updated annotation, there are 389 gene models still found in the QTL regions. Totally, 13 genes in the QTL regions have been shown to be consistently significantly up-regulated and 10 down-regulated. The up-regulated genes included three transporters and two transferases which further supported our previous conclusions that detoxification of anti-fungal substrates from host resistance responses, is important for the virulence of H. irregulare. A gene mentioned by Olson et al.14, the putative Flavin containing Baeyer-Villiger monooxygenase (JGI proteinID 58532) was found up-regulated around four times at two weeks after inoculation. The product of this gene is potentially involved in the biosynthesis of the toxin fomannosin, which is an important virulence factor in H. annosum s.l.31.
Compared to H. irregulare, H. occidentale appears to use more cell wall degrading enzymes (CWDEs) during infection, possibly to utilize the local nutrient source more efficiently and exploit the substrate more intensively than H. irregulare (Fig. 4B). The emphasis on cell wall degradation in the H. occidentale transcriptome could be interpreted as H. occidentale invests more in biomass production than H. irregulare, a difference in colonization strategy that might partly explain why we found higher proportion of fungal RNA sequence reads in H. occidentale infected bark samples than in H. irregulare infected bark samples. Although there were genes of glycoside hydrolyze (GH) families significantly up-regulated in both in H. irregulare and H. occidentale, many more of them were highly expressed in H. occidentale than in H. irregulare, especially at two weeks after the infection. This indicates that H. occidentale employ those enzymes to degrade the host cell structure at earlier infection stage for use as nutrient and carbon source. This type of virulence behavior has been found in other necrotrophic pathogens2. There is some evidence that the GH28 family of CWDEs act as virulence factors in B. cinerea32, Alternaria citri33 and Aspergillus flavus34 where they are involved in cell wall decomposition and tissue maceration. Importantly, a Claviceps purpurea strain carrying a deletion of two GH28 genes renders the fungus nearly non-pathogenic on rye without affecting its vegetative growth properties35. Here, one GH28 was found up-regulated in both H. irregulare and H. occidentale, and three additional GH28s and a number of other GHs for degrading cellulose and hemi-cellulose showed higher induction levels in H. occidentale than in H. irregulare, which indicate that GH28 might play an important role for H. occidentale to survive and spread throughout woody tissues during infection of Norway spruce. Hu et al.36 characterized one GH28 gene encoding endo-rhamnogalacturonase (HIRHG) located in the virulence QTLs of H. irregulare14,30. They found that HIRHG was up-regulated during infection and maybe important for the pathogen to grow in a pectin containing environment. Liu et al.37 compared the transcriptomes of two different H. occidentale isolates grown on apple fruits. Their results suggest that the capacity of H. occidentale to colonize apple fruit correlated with the expression of potential carbohydrate active enzyme genes37.
Even though there are marked differences in consistently up-regulated genes between H. irregulare and H. occidentale during infection, there are still common features for the pathogenicity. There are large numbers of transporters up-regulated in both species. Transporters are important for pathogens to acquire nutrients from host tissue and efflux of toxic compounds. One ABC transporter belonging to subgroup G (ABC-G) was highly induced in both H. irregulare and H. occidentale, but with higher fold change in H. irregulare. ABC transporters have been showed important for tolerance to phytoalexins and to achieve full virulence in many fungal plant pathogens, such as, Fusarium sambucinum, Mycosphaerella graminicola and B. cinerea38–40. The ABC-Gs involved in efflux of toxic compounds are referred to as multidrug resistance-associated proteins. Especially in the fungal-fungal interaction of the mycoparasite Clonostachys rosea, the ABC-Gs are highly induced by particular mycotoxin and ABC-G5 have been confirmed important for xenobiotic tolerance by gene disruption and complementation41,42. The activity of aromatic compound biosynthetic processes is possibly related to production of toxins. Similarly, a clavaminate synthase-like protein belonging to the alpha-ketoglutarae-dependent oxygenases was highly expressed. This enzyme has been observed in interaction between H. annosum s.s and Norway spruce, and was predicted be involved in secondary metabolism for fungal toxin productions15.
In general, the pine infecting species, H. annosum s.s. and H. irregulare are more effective than the other species within the species complex in colonizing the cambial layer and sapwood of their hosts, both in the root system and at or just above the root collar43. In comparison, infection by non-pine infecting species, such as H. parviporum, is normally confined to the heart wood in the vast majority of root rot infected Norway spruce in Europe12. Since a trade-off between saprotrophic decomposition and necrotrophic parasitism in H. irregulare have been suggested14 it is tempting to suggest that the transcriptional differences found between H. irregulare and H. occidentale could indicate that H. irregulare might be more prone to use necrotrophic parasitism in interactions with a host compared to H. occidentale that mainly grow in the heart wood of trees and rely on its saprobic capacity to invade its host.
Material and Methods
Fungal isolates, plants and inoculation method
The H. irregulare strain TC32-1 and H. occidentale strain TC122-12 were used in this study and maintained on Hagem agar (HA) medium9 at 4 °C for storage, and 20 °C for growth. For inoculation of Norway spruce, pieces of spruce wood (∅5 × 5 mm) were placed on one-week-old mycelia grown on HA medium. The plates were placed at 20 °C in darkness, and incubated for four weeks to allow the fungi to colonize the wood pieces.
Six trees were used for inoculations, three ramets from two progeny lines each, of the age of eight years, form the Norway spruce family S21H982000544. Prior to inoculation, the trees were grown for one month in a greenhouse at 20–22 °C temperature at day time, 16–20 °C at night time with 16 hours’ light. Nine branches of the age of four years were selected from each tree for inoculation. Each branch was inoculated at three points with H. irregulare strain TC32–1 and H. occidentale strain TC122-12 and one isolate not included in this study. At each inoculation site, a round piece of ∅5 mm tree bark tissue was removed and colonized wood dowels inserted into the wound and attached by wrapping tightly with Parafilm® according to previous established method45. The distances between inoculation sites within the same branch were kept to a minimum of 15 cm to avoid cross contamination. Three branches per tree was harvested at each time point and the bark samples from two sites closest to the main stems were taken for RNA extraction and the remaining tissue was used for virulence test after two, four and six weeks. The virulence tests of H. irregulare and H. occidentale were scored according established methods45.
RNA extraction and sequencing
Strains of H. irregulare and H. occidentale grown in liquid HA for two weeks and spruce bark infected by H. annosum s.l. for 2, 4 and 6 weeks as mentioned previously were harvested for RNA isolation. The total RNA was isolated as described by Chang et al.46 and stored at −80 °C until used. The RNA 6000 Nano Kit (Agilent Technologies) was used to evaluate quantity and integrity of total RNA with Bio-analyzer 2001. Total RNA was treated with DNase I (Sigma-Aldrich) to eliminate contamination of genomic DNA. Library construction and cDNA synthesis was performed at the SNP&SEQ Technology Platform of Uppsala University Hospital. High-throughput sequencing was also performed at the same place using the Illumina Hiseq (Illumina, San Diego, CA, USA) according to standard protocols. The samples were sequenced for ‘paired-end’ reads across one Illumina lane.
RNAseq data analysis
Nesoni (https://github.com/Victorian-Bioinformatics-Consortium/nesoni) was used to filter the Illumina reads based on a phred-scale quality score cut off of 20 and reads length cut off 50 basepairs. All the filtered reads from the H. irregulare and H. occidentale samples were mapped to their own genomes by Tophat247. The reads were mapped to their respective genome without any reads mismatches, and adjusted the mate-inner- distance to 20, min-intron-length, min-coverage-intron and min-segment-intron to 5, max-intron-length, max-coverage-intron and max-segment-intron to 5000, and read-realign-edit-dist to 0, and set library type to fr-firststrand. Differential expression analysis was performed with the software package Cufflinks (2.1.1) to quantify transcript abundance in terms of fragments per kilobase of exon per million mapped reads (FPKM). The transcriptomes were assembled in each mapped alignment strictly according the reference genome annotation, using activation of the multi-read-correct and setting the overlap-radius to 1, intron and library parameters as well as in Tophat2. Further, the individual assemblies were merged to a final annotation for each species by cuffmerge and quantification of the gene and transcript expression were done using cuffquant. The differentially expressed genes (DEGs) were finally identified by cuffdiff based on q-value (false discovery rate (FDR)) < 0.05 and visualized using CummeRbund.
Annotation, Gene Ontology (GO) enrichment test and identification of gene orthologous between H. irregulare and H. occidentale
In order to obtain comparable gene sets from the two species, the genome sequences of H. irregulare and H. occidentale acquired from previous published data14,17 were both annotated using the MAKER2 genome annotation pipeline (version 2.31.8)48. Maker was configured to use the Augustus, SNAP and GeneMarkES ab initio gene predictors. To support the annotations, RNASeq data from the liquid cultures was assembled using Trinity49 and provided to MAKER. Orthology of gene models between the two studies species was established by determining the best reciprocal blast matches between the two sets of predicted transcripts.
BLAST2GO v3.1050 was used to improve the annotation of H. irregulare and H. occidentale transcripts as well as to assess GO term enrichment. Functional categories were assigned to the differentially expressed genes according to the GO system using Blast2GO, enabling the integrated Interproscan and ANNEX functions for further improvement of annotations. Enrichment of GO terms in biological process, molecular function, and cellular component categories of genes significantly up- and down-regulated in 2w, 4w and 6w, respectively, compared to liquid culture of each species were evaluated by comparing to the gene models of each genome using Fisher’s exact test with a FDR threshold of 5%. In addition, protein sequence were submitted in KAAS (KEGG automatic annotation server) and KEGG orthology (KO) assignments51 were obtained based on bi-directional best-hit of BLAST searching to a threshold of 60 with the GENES data set of KAAS defaulted Eukaryotes plus Neurospora crassa, Magnaporthe oryzae, Fusarium graminearum, Sclerotinia sclerotiorum, Botrytis cinerea, Aspergillus nidulans, Aspergillus fumigatus, Aspergillus oryzae, Aspergillus niger, Phaeosphaeria nodorum, Cryptococcus neoformans JEC21, Postia placenta and Ustilago maydis52.
Statistical analysis
Phenotypic data were analyzed by analysis of variance (ANOVA) using a general linear model implemented in using Minitab Statistical software 16 (Minitab Inc., State Collage, PA). Pairwise comparisons were made using Tukey´s test at the 95% significant level.
Supplementary information
Acknowledgements
We thank the Swedish Foundation for Strategic Research (grant number R8b08-0011) and Zhenjiang National Natural Science Foundation of China (No. LQ20C160003) for financial supporting of this study. The experiments reported here also feature in the doctoral thesis of Y.H (https://pub.epsilon.slu.se/12861/1/hu_y_151126.pdf). Open access funding provided by Swedish University of Agricultural Sciences.
Author contributions
Y.H., A.O., M.E. and J.S. conceived and designed the experiments. Y.H. performed the experiments. Y.H. and M.B.D. analyzed the data. Y.H. and Å.O. wrote the paper. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62521-x.
References
- 1.Knogge W. Fungal infection of plants. Plant Cell. 1996;8:1711–1722. doi: 10.2307/3870224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner PC, Torriani SFF, Croll D, Stukenbrock EH, McDonald BA. Coevolution and life cycle specialization of plant cell wall degrading enzymes in a hemibiotrophic pathogen. Mol. Biol. Evol. 2013;30:1337–1347. doi: 10.1093/molbev/mst041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong S, et al. Effector specialization in a lineage of the Irish potato famine pathogen. Sci. 2014;343:552–555. doi: 10.1126/science.1246300. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell RJ, et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genet. 2012;44:1060–1065. doi: 10.1038/ng.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhillon B, et al. Horizontal gene transfer and gene dosage drives adaptation to wood colonization in a tree pathogen. Proc. Natl. Acad. Sci. USA. 2015;112:3451–3456. doi: 10.1073/pnas.1424293112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stukenbrock EH, McDonald BA. Geographical variation and positive diversifying selection in the host-specific toxin SnToxA. Mol. Plant Pathol. 2007;8:321–332. doi: 10.1111/j.1364-3703.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 7.Schardl CL, et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. Plos Genet. 2013;9:e1003323. doi: 10.1371/journal.pgen.1003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huettermann, A. & Woodward, S. In Heterobasidion annosum, Biology, Ecology, Impact and Control. (eds. Woodward, S. Stenlid, J. Karjalainen & R. Huetterman, A.) 1–25, CAB International, Wallingford, UK (1998).
- 9.Stenlid J. Population structure of Heterobasidion annosum as determined by somatic incompatibility, sexual incompatibility, and isozyme patterns. Can. J. Bot. 1985;63:2268–2273. doi: 10.1139/b85-322. [DOI] [Google Scholar]
- 10.Dalman K, Olson A, Stenlid J. Evolutionary history of the conifer root rot fungus Heterobasidion annosumsensu lato. Mol. Ecol. 2010;19:4979–4993. doi: 10.1111/j.1365-294X.2010.04873.x. [DOI] [PubMed] [Google Scholar]
- 11.Korhonen, K. Capretti, P. Karjalainen, R. & Stenlid, J. In Heterobasidion annosum, Biology, Ecology, Impact and Control. (eds. Woodward, S. Stenlid, J. Karjalainen, R. Huetterman, A.) 93–104. CAB International, Wallingford, UK (1998).
- 12.Korhonen, K. & Stenlid, J. In Heterobasidion annosum, Biology, Ecology, Impact and Control. (eds. Woodward, S. Stenlid, J. Karjalainen, R. Huetterman, A.) 43–70. CAB International, Wallingford, UK (1998).
- 13.Otrosina WJ, Garbelotto M. Heterobasidion occidentale sp nov and Heterobasidion irregulare nom. nov.: A disposition of North American Heterobasidion biological species. Fungal Biol. 2010;114:16–25. doi: 10.1016/j.mycres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Olson A, et al. Insight into trade-off between wood decay and parasitism from the genome of a fungal forest pathogen. New Phytologist. 2012;194:1001–1013. doi: 10.1111/j.1469-8137.2012.04128.x. [DOI] [PubMed] [Google Scholar]
- 15.Lunden K, et al. Transcriptional responses associated with virulence and defence in the interaction between Heterobasidion annosum s.s. and Norway Spruce. Plos One. 2015;10:e0131182. doi: 10.1371/journal.pone.0131182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raffaello T, Chen H, Kohler A, Asiegbu FO. Transcriptomic profiles of Heterobasidion annosum under abiotic stresses and during saprotrophic growth in bark, sapwood and heartwood. Environ. Microbiol. 2014;16:1654–1667. doi: 10.1111/1462-2920.12321. [DOI] [PubMed] [Google Scholar]
- 17.Lind, M. van der Nest, M. Olson, A. Brandstrom-Durling, M. & Stenlid, J. A II generation linkage map of Heterobasidion annosum s.l. based on in silico anchoring of AFLP markers. Plos One7 (2012). [DOI] [PMC free article] [PubMed]
- 18.Neilson EH, Goodger JQD, Woodrow IE, Moller BL. Plant chemical defense: at what cost? Trends in. Plant Sci. 2013;18:250–258. doi: 10.1016/j.tplants.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Sexton AC, Minic Z, Cozijnsen AJ, Pedras MSC, Howlett BJ. Cloning, purification and characterisation of brassinin glucosyltransferase, a phytoalexin-detoxifying enzyme from the plant pathogen Sclerotinia sclerotiorum. Fungal Genet. Biol. 2009;46:201–209. doi: 10.1016/j.fgb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Lah L, et al. The versatility of the fungal cytochrome P450 monooxygenase system is instrumental in xenobiotic detoxification. Mol. Microbiol. 2011;81:1374–1389. doi: 10.1111/j.1365-2958.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- 21.Danielsson M, et al. Chemical and transcriptional responses of Norway spruce genotypes with different susceptibility to Heterobasidion spp. infection. BMC Plant Biol. 2011;11:154. doi: 10.1186/1471-2229-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammerbacher A, et al. A Common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 2013;162:1324–1336. doi: 10.1104/pp.113.218610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadke N, et al. The bark-beetle-associated fungus, Endoconidiophora polonica, utilizes the phenolic defense compounds of its host as a carbon source. Plant Physiol. 2016;171:914–931. doi: 10.1104/pp.15.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asiegbu FO, Adomas A, Stenlid J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant Pathol. 2005;6:395–409. doi: 10.1111/j.1364-3703.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 25.Hansson D, et al. Secondary metabolite comparison of the species within the Heterobasidion annosum s.l. complex. Phytochem. 2014;108:243–251. doi: 10.1016/j.phytochem.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X-W, et al. In planta stage-specific fungal gene profiling elucidates the molecular strategies of Fusarium graminearum growing inside wheat coleoptiles. Plant Cell. 2012;24:5159–5176. doi: 10.1105/tpc.112.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature. 2001;412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 28.Olson A, Stenlid J. Plant pathogens - Mitochondrial control of fungal hybrid virulence. Nature. 2001;411:438–438. doi: 10.1038/35078147. [DOI] [PubMed] [Google Scholar]
- 29.Inoue I, Namiki F, Tsuge T. Plant colonization by the vascular wilt fungus Fusarium oxysporum requires FOW1, a gene encoding a mitochondrial protein. Plant Cell. 2002;14:1869–1883. doi: 10.1105/tpc.002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind M, Dalman K, Stenlid J, Karlsson B, Olson A. Identification of quantitative trait loci affecting virulence in basidiomycete Heterobasidion annosum s.l. Curr. Genet. 2007;52:35–44. doi: 10.1007/s00294-007-0137-y. [DOI] [PubMed] [Google Scholar]
- 31.Sonnenbicher J, Bliestle IM, Peipp H, Holdenrieder O. Secondary fungal metabolites and their biological activities, I. Isolation of antibiotic compounds from cultures of Heterobasidion annosum synthesized in the presence of antagonistic fungi or host plant cells. Biol Chem Hoppe Seyler. 1989;370:1295–1303. doi: 10.1515/bchm3.1989.370.2.1295. [DOI] [PubMed] [Google Scholar]
- 32.ten Have A, Mulder W, Visser J, van Kan JAL. The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant Microbe Int. 1998;11:1009–1016. doi: 10.1094/MPMI.1998.11.10.1009. [DOI] [PubMed] [Google Scholar]
- 33.Isshiki A, Akimitsu K, Yamamoto M, Yamamoto H. Endopolygalacturonase is essential for citrus black rot caused by Alternaria citri but not brown spot caused by Alternaria alternata. Mol. Plant Microbe Int. 2001;14:749–757. doi: 10.1094/MPMI.2001.14.6.749. [DOI] [PubMed] [Google Scholar]
- 34.Shieh MT, et al. Molecular genetic evidence for the involvement of a specific polygalacturonase, P2c, in the invasion and spread of Aspergillus flavus in cotton bolls. App. Environ. Microbiol. 1997;63:3548–3552. doi: 10.1128/AEM.63.9.3548-3552.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oeser B, Heidrich PM, Muller U, Tudzynski P, Tenberge KB. Polygalacturonase is a pathogenicity factor in the Claviceps purpureal rye interaction. Fungal Genet. Biol. 2002;36:176–186. doi: 10.1016/S1087-1845(02)00020-8. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, et al. Characterization of a Heterobasidion irregulare endo-rhamnogalacturonase that mediate growth on pectin. J. Phytopathol. 2018;166:34–43. doi: 10.1111/jph.12657. [DOI] [Google Scholar]
- 37.Liu J, et al. Characterization of Heterobasidion occidentale transcriptomes reveals candidate genes and DNA polymorphisms for virulence variations. Microbial. Biotechnol. 2018;11:537–550. doi: 10.1111/1751-7915.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander NJ, McCormick SP, Hohn TM. TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: gene isolation and expression in yeast. Mol. General Genet. 1999;261:977–984. doi: 10.1007/s004380051046. [DOI] [PubMed] [Google Scholar]
- 39.Schoonbeek HJ, van Nistelrooy JGM, de Waard MA. Functional analysis of ABC transporter genes from Botrytis cinerea identifies BcatrB as a transporter of eugenol. Euro. J. Plant Pathol. 2003;109:1003–1011. doi: 10.1023/B:EJPP.0000003936.61182.14. [DOI] [Google Scholar]
- 40.Stergiopoulos I, Zwiers LH, de Waard MA. The ABC transporter MgAtr4 is a virulence factor of Mycosphaerella graminicola that affects colonization of substomatal cavities in wheat leaves. Mol. Plant Microbe Int. 2003;16:689–698. doi: 10.1094/MPMI.2003.16.8.689. [DOI] [PubMed] [Google Scholar]
- 41.Dubey MK, Jensen DF, Karlsson M. An ATP-binding cassette pleiotropic drug transporter protein is required for xenobiotic tolerance and antagonism in the fungal biocontrol agent Clonostachys rosea. Mol. Plant Microbe Int. 2014;27:725–732. doi: 10.1094/MPMI-12-13-0365-R. [DOI] [PubMed] [Google Scholar]
- 42.Karlsson M, et al. Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. Genome Biol. Evol. 2015;7:465–480. doi: 10.1093/gbe/evu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garbelotto, M. & Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. (eds. VanAlfen, N. K.) Ann Rev. Phytopathol. 51, 39-59 (2013). [DOI] [PubMed]
- 44.Arnerup J, Swedjemark G, Elfstrand M, Karlsson B, Stenlid J. Variation in growth of Heterobasidion parviporum in a full-sib family of Picea abies. Scand. J. Forest Res. 2010;25:106–110. doi: 10.1080/02827581003730799. [DOI] [Google Scholar]
- 45.Dalman K, et al. A genome-wide association study identifies genomic regions for virulence in the non-model organism Heterobasidion annosum s.s. Plos One. 2013;8:e53525. doi: 10.1371/journal.pone.0053525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993;11:113–116. doi: 10.1007/BF02670468. [DOI] [Google Scholar]
- 47.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics. 2011;12:491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conesa A, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 51.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Research. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raffaello T, Asiegbu FO. Small secreted proteins from the necrotrophic conifer pathogen Heterobasidion annosum s.l. (HaSSPs) induce cell death in Nicotiana benthamiana. Scientific Reports. 2017;7:8000. doi: 10.1038/s41598-017-08010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.