Abstract
Youth-onset type 2 diabetes (T2D) is a formidable threat to the health of obese adolescents because of its potential for early-onset and aggressive co-morbidities and complications. The physiology of youth-onset T2D differs from T2D in adults and is associated with a greater degree of insulin resistance, a more rapid decline in pancreatic β-cell function, and a poorer response to medications. Medical management in youth is focused on combining lifestyle intervention and pharmacological treatment, but these therapies have yet to demonstrate improvements in disease progression. Metabolic bariatric surgery (MBS) is now recommended for the treatment of T2D in adults largely because of the beneficial effects on weight, ability to improve glycemic control, and, in a large proportion of people, induce diabetes remission. MBS is now being performed in adolescents with severe obesity and T2D, with initial results also showing high rates of T2D remission. Here, we review the state of medical management of youth-onset T2D and the outcomes of MBS studies in youth with T2D published to date.
Keywords: Type 2 diabetes, Severe Obesity, Bariatric surgery, Insulin resistance
Introduction
Youth-onset type 2 diabetes (T2D) incidence is on the rise and associated with early morbidity and mortality. This review will outline the pathophysiology and natural progression of youth-onset T2D, briefly review the evidence base for treatment of adult-onset T2D with metabolic bariatric surgery (MBS), and discuss what is known about the use of MBS for management of youth-onset T2D.
Pathophysiology and Epidemiology of Youth-onset T2D
Epidemiology of Youth-onset T2D
Youth-onset T2D has been steadily rising since the 1980s [1, 2] such that, in some ethnic and racial populations, it represents 50% or more of the incident cases of diabetes in adolescents[3]. In the U.S., youth-onset T2D is most common in Native Americans, followed by Non-Hispanic Black, Hispanic, and Asian Pacific Islander adolescents. It is also more common in girls than in boys[3, 4], a sex difference not seen in adult-onset T2D. T2D is rarely, if ever, seen prior to puberty and is confined to pubertal youth with overweight or obesity, with very rare exceptions[4, 5]. Other commonly seen features in youth with T2D are a strong family history of T2D, exposure to gestational diabetes in utero, physical and metabolic characteristics of insulin resistance, and significant psychosocial stressors, including low socioeconomic status. Data from the SEARCH for Diabetes in Youth Study estimates that the U.S. prevalence of youth-onset T2D was 0.46/1,000 in 2009, equating to approximately 20–22 thousand youth[3, 6]. Thus, while youth-onset T2D is on the rise, it is still a relatively rare disease. By comparison, approximately 4-fold more (1.93/1,000) youth have T1D. This is important to note in the context of MBS, as diagnostic confusion can occur in youth with clinical features of T2D (such as obesity and metabolic syndrome characteristics), resulting in missed cases of T1D. Because all patients with T1D require treatment with insulin, it is critical to test for diabetes auto-antibodies associated with T1D in all youth with diabetes[7].
Pathophysiology and Clinical Presentation
Normal glucose homeostasis requires insulin secretion by pancreatic β-cells to match changes in insulin sensitivity; if insulin sensitivity declines, there should be an increase in insulin secretion[8]. Prediabetes and T2D result from an inability to maintain β-cell insulin secretion (i.e. relative insulin deficiency) in the face of insulin resistance. While most youth with obesity have some degree of insulin resistance, only a subset will develop T2D. In fact, most of the genetic risk markers that have been identified are variants related to β-cell function [9, 10]. Other hormone abnormalities are also seen in the progression to β-cell decline, including a decrease in incretins (e.g. glucagon-like peptide-1[GLP-1])[11, 12] and an increase in glucagon secretion[11].
Youth-onset T2D is rare prior to puberty. Data from both the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) and SEARCH studies show a mean age of onset of 14 years, typically occurring during mid- to late-puberty[4, 5]. This is likely in part related to the transient insulin resistance seen in all youth during puberty[13–15], which is exaggerated in youth with obesity[16, 17]. This results in a critical window of increased risk for those with a predisposition to T2D, such that they are unable to maintain β-cell function and develop diabetes. This risk is highest in youth with predisposing factors described above; in addition, youth with a history of atypical antipsychotic treatment are at greater risk for developing T2D[18].
It is important to note that, while pediatric patients with T2D do progress through a phase of prediabetes in the progression to T2D, youth with prediabetes do not progress to T2D as consistently as adults do. Studies suggest that a significant proportion of youth with a diagnosis of prediabetes, based on the adult American Diabetes Association (ADA) standards, will revert to normoglycemia in a second test[19–21]. Retrospective clinical data suggest that risk progression is highest with an HbA1c of ≥ 6%, particularly if there is ongoing weight gain [22]. For this reason, and because little is known about the impact of pubertal insulin resistance on glycemia in normal weight youth, prediabetes is challenging to define in youth; however, current ADA recommendations are to use the same laboratory criteria as for adults [23].
The presentation of youth-onset T2D ranges from asymptomatic detection during routine screening for obesity-associated comorbidities, to mild symptomatic presentation (polyuria, polydipsia, unexplained weight loss), to diabetic ketoacidosis (5–11%) or hyperosmolar state [24, 25]. The ADA criteria for diagnosing T2D require repeat confirmatory testing for asymptomatic patients, with laboratory criteria defined as: a fasting glucose ≥ 126 mg/dL, 2-hour glucose ≥ 140 mg/dL during oral glucose tolerance testing, or an HbA1c of ≥6.5% [23].
Treatment of Prediabetes and Diabetes in Youth
The majority of what is known about long-term medical management of youth-onset T2D comes from the TODAY study, a randomized control trial of ethnically diverse youth (n=699) who were aged 10–17 years and had a BMI ≥ 85th percentile at enrollment [26]. These youth were randomized to receive metformin monotherapy, metformin + intensive lifestyle intervention, or metformin + rosiglitazone, with a primary outcome of loss of glycemic control (HbA1c ≥ 8% for at least 6 months or failure to wean from temporary insulin started after metabolic decompensation) [27]. Loss of glycemic control was seen in almost half of participants (45.6%), after a median treatment duration of 11.5 months [26]. Failure rates were significantly lower in the metformin + rosiglitazone group (38.6%) than in the metformin monotherapy group (51.7%, p=0.006). There were some significant sex and racial/ethnic differences in response to treatment: rosiglitazone was associated with significantly less glycemic failure in girls than boys and metformin was particularly ineffective in non-Hispanic Blacks. Loss of glycemic control was most strongly associated with ongoing β-cell failure, as opposed to decline in insulin sensitivity [28]. Notably, at the time of randomization, the median duration of T2D was 7.8% months and average HbA1c was 5.9% on treatment with metformin monotherapy [5]. Thus, almost half of youth with T2D had rapid progression of their disease, despite initial good control on metformin alone [29]. This is significantly different from the natural history of T2D in adults. By comparison, in the A Diabetes Outcome and Progression Trial (ADOPT), the glycemic failure rate of metformin monotherapy was only 20% in adults at the end of 5 years of follow up [30]. Similarly, the failure rate of metformin and rosiglitazone from the U.S. Department of Defense Database was <20% [31]. Unfortunately, evidence-based treatment to prevent this rapid progression is limited. Until the recent approval of liraglutide for use in pediatric T2D [32], the only two FDA-approved medications in youth were insulin and metformin. Treatment with insulin has been associated with weight gain, which could contribute to worsening of insulin resistance and comorbid conditions such as hypertension and musculoskeletal problems. Furthermore, in TODAY, the addition of insulin after glycemic failure was not associated with any further improvement in glycemic control [33]. Clinical trials are currently underway to study other agents in youth-onset diabetes, including other GLP-1 agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, and sodium glucose transporter 2 (SGLT2) inhibitors.
Youth-onset T2D is also associated with early complications. In TODAY, cardiovascular risk worsened as the study progressed, with increasing incident hypertension, dyslipidemia, and inflammation [34, 35]. TODAY also found evidence of endothelial dysfunction [36], vascular stiffness[37] and cardiac structure and functional defects [38]. Several TODAY participants have also had cardiovascular events before the age of 30 in ongoing follow-up (presented at ADA Annual Meeting, 2019). Both TODAY and SEARCH found a relatively high prevalence (10–20%) of retinopathy [39, 40], nephropathy [34], and neuropathy [41]. Youth-onset T2D was also associated with high rates of obstructive sleep apnea and non-alcoholic fatty liver disease [42]. In TODAY, those girls who became pregnant experienced significantly higher rates of stillbirth, spontaneous abortions, prematurity, and congenital anomalies as compared with the general adolescent population [43]. Finally, both TODAY [44] and SEARCH [45] showed significant mental health comorbidity associated with a diagnosis of T2D, including depression and disordered eating.
Given the rapid progression of β-cell failure and early-onset comorbidities in TODAY, the pediatric arm of the Restoring Insulin Secretion (RISE) study was designed to determine whether early treatment with metformin alone (for 12 months) or insulin (for 3 months) followed by metformin (for 9 months) in youth with impaired glucose toelrance or recently-diagnosed T2D (n=91) would help preserve β-cell function. In this study, pediatric participants had ongoing β-cell failure during the treatment phase, regardless of treatment arm, which worsened after treatment withdrawal [46]. These results were different from the parallel adult RISE study, in which adult participants demonstrated preservation of β-cell function while on treatment [47]. Due to the similarity in enrollment criteria for youth and adults in the RISE study, a direct head-to-head comparison could be made in terms of insulin sensitivity, which was more than 2 times lower, insulin secretion, which was more than twice as high, and insulin clearance which was approximately 50% lower in youth than in adults at baseline [48].
Metabolic Bariatric Surgery for Treatment of T2D: Adult Evidence
The poor responses to medical treatment observed in youth-onset T2D demands alternative approaches to improve glycemic control, slow the progression of pancreatic β-cell decline, and reduce long term co-morbidities. MBS is now recommended for the treatment of T2D in adults largely based on data showing the ability to induce substantial weight loss, lead to remission of T2D, and reduce co-morbidities [49, 50]. A consensus statement endorsed by 48 medical and surgical organizations now recommends MBS in adults with T2D who have class III obesity (BMI≥40 kg/m2) or class II obesity (BMI 35–39.9 kg/m2) and inadequate glycemic control [51]. Clinical practice guidelines specify that MBS should also considered for adults with T2D and BMI 30–34.9 kg/m2 if hyperglycemia is inadequately controlled despite optimal treatment with either oral or injectable medications [51].
Some of the most robust data examining T2D outcomes after MBS have come from the multi-center adult Swedish Obese Subjects (SOS) study, a prospective, controlled observational study comparing MBS to conventional medical treatment. At baseline, 343 of 2,010 surgical patients and 260 of 2,037 control patients had T2D. At two years, T2D remission rates were 72.3% (95% CI, 66.9%−77.2%) in the MBS group vs. 16.4% (95% CI, 11.7%−22.2%) in the controls. At 15 years, T2D remission rates remained higher in the MBS group than controls (30.4% vs. 6.5% with an odds ratio of 6.3 [95% CI, 2.1–18.9], p< 0.001) [52]. The SOS study also demonstrated reduction in the incidence of T2D in those without T2D at baseline (28.4 per 1,000 in the MBS group compared to 6.8 cases per 1,000 patients in the control arm over 15 years) [53]. Importantly, the risk of microvascular complications from T2D in the MBS group was one-half of that observed in the control group [54]. The anti-diabetic effect of MBS appears long lasting and has been documented with 16 years of follow-up [55], although the incidence of reoperation for conversion from less effective operations to more modern and more effective procedures is not well described. While some MBS patients experienced a slow and steady recurrence of T2D, the rates of T2D were still less than in non-operated controls [56].
T2D remission rates were also examined in the STAMPEDE randomized clinical trial, wherein adults with uncontrolled T2D were randomized to intensive medical therapy (IMT) alone, IMT plus Roux-en-Y gastric bypass (RYGB) or IMT plus vertical sleeve gastrectomy (VSG) [49, 50]. Remission of T2D at years one and three in those undergoing MBS were ~ 40% (42% RYGB; 37% VSG) and 31% (38% RYGB; 24% VSG), respectively; compared to 12% and 5% for the medical group [49, 57]. At 5-years of follow-up, remission rates for RYGB and VSG were 29% and 23%, respectively and 5% for patients treated with IMT alone[49]. T2D remission after MBS is likely the result of improved insulin sensitivity, an augmented incretin response, and overall improvements in pancreatic β- cell responsiveness to glucose [55, 58, 59].
The effects of MBS on T2D remission in adolescents has been less studied but initial results mirror findings in adults where both weight loss and remission of diabetes have been observed [60–64]. In adolescents, MBS is considered for adolescents who meet clinical criteria for T2D and severe obesity (defined as an absolute BMI ≥35 kg/m2 or a BMI ≥120% of the 95th percentile for age and sex) [65].
Teen-LABS is one of the largest and longest observational studies of pediatric patients undergoing MBS with 3 and 5 years of follow-up data published. Participants in the Teen-LABS study were enrolled at a mean age of 17 years [66]. At one year, this cohort experienced a 31% reduction in weight (similar for both RYGB and VSG) with minimal weight regain by years 2 and 3. Of the 242 obese adolescents who underwent MBS, 29 had T2D[66]. A 95% (95% CI 85–100%) T2D remission rate occurred at 3 years with no incident T2D cases in the youth without T2D at baseline [60]. Similarly, 19 participants had prediabetes at baseline with a 76% (95% CI 56–97) remission rate observed at 3 years [60]. Recently, these T2D remission rates in Teen-LABS were compared to LABS, a related but independent study of adults who underwent MBS. Among those who underwent RYGB, adolescents were more likely than adults to have remission of T2D (86 vs. 53%, risk ratio 1.27, 95%CI 1.21–1.88) at 5 years [67]. Other pediatric studies have reported improved glycemic control and T2D remission after MBS but each included few participants with T2D, and the case definitions used for T2D at baseline and the methodology used to assess response to surgery were not standardized, making inferences difficult [63, 66, 68–70].
Adult studies estimate remission rates of T2D to be approximately 40–70%. Thus, the T2D remission rates of 88–95% in adolescents with T2D undergoing MBS appear to be similar if not greater than adults [60, 63, 64, 67, 71]. The SOS study showed that shorter T2D duration at baseline was associated with higher T2D remission rates in MBS patients after 2, 10, and 15 years of follow-up [54]. In contrast, older age, higher baseline HbA1c, and treatment with insulin or other diabetes medications at baseline were associated with decreased likelihood of T2D remission over a 5-year period in another cohort [72]. Thus, MBS at a younger age, earlier in the disease, or before insulin dependence may improve T2D remission rates and explain the better treatment responses in adolescents vs. adults.
While direct comparisons of MBS to medical therapy in youth has yet to occur, a retrospective comparison of outcomes from youth studied in the TODAY clinical trial were compared to youth from Teen-LABS [71]. Despite a higher starting BMI, over 2 years HbA1c fell from 6.8% on medication to 5.5% off medication in Teen-LABS, but increased from 6.2% to 7.8% on medication in TODAY. T2D-related comorbidities including hypertension decreased from 45% at baseline to 20% at 2 years in the MBS group vs. a near doubling in TODAY from 22 to 41%[71]. Similarly, dyslipidemia decreased from 72% to 24% in Teen-LABS with no appreciable change in TODAY [71] and the proportion of youth in Teen-LABS with elevated urinary albumin excretion decreased from 26% to 5% over 2 years with no change in TODAY [73].
While these initial results suggests a beneficial effect of MBS on glycemia and T2D related co-morbidities in youth, it is important to note that most results in youth to date have evaluated RYGB, which is no longer the preferred MBS in adolescents sue to higher rates of complications with RYGB [74]. VSG now accounts for more than 80% of MBS procedures in the US [74], but only a limited number of youth with T2D to date have been prospectively evaluated after VSG [60] and no studies have prospectively compared VSG to medical management. Thus, the superiority of VSG compared to medical therapy has not been established nor have the mechanisms underlying changes in glycemic control in response to VSG in youth with T2D been explored.
NIH funded new trial in 2019: Surgical or Medical Treatment for Pediatric T2D (ST2OMP)
These gaps in the literature led to the authors of this review to propose of a prospective, open labelled clinical trial, Surgical or Medical Treatment for Pediatric T2D, ST2OMP (Figure). ST2OMP will compare VSG to advanced medical therapy (AMT) for the treatment of adolescent-onset T2D. Funded by the National Institutes of Health, ST2OMP investigators will recruit 90 post-pubertal adolescents with a of BMI ≥ 35 kg/m2 at two large pediatric centers in the US, Cincinnati Children’s Hospital Medical Center and Children’s Hospital Colorado. A BMI of ≥35 kg/m2 will be required for inclusion because it follows the current best-practice clinical guidelines to consider MBS in adolescents [65], is low enough to allow for BMI-comparable surgical and medical study groups at enrollment, and it ensures all participants will have the potential option of surgery, as insurance companies will not presently cover MBS at a BMI <35 kg/m2.
Figure: Design of Surgical or Medical Treatment for Pediatric T2D (ST2OMP) Trial.
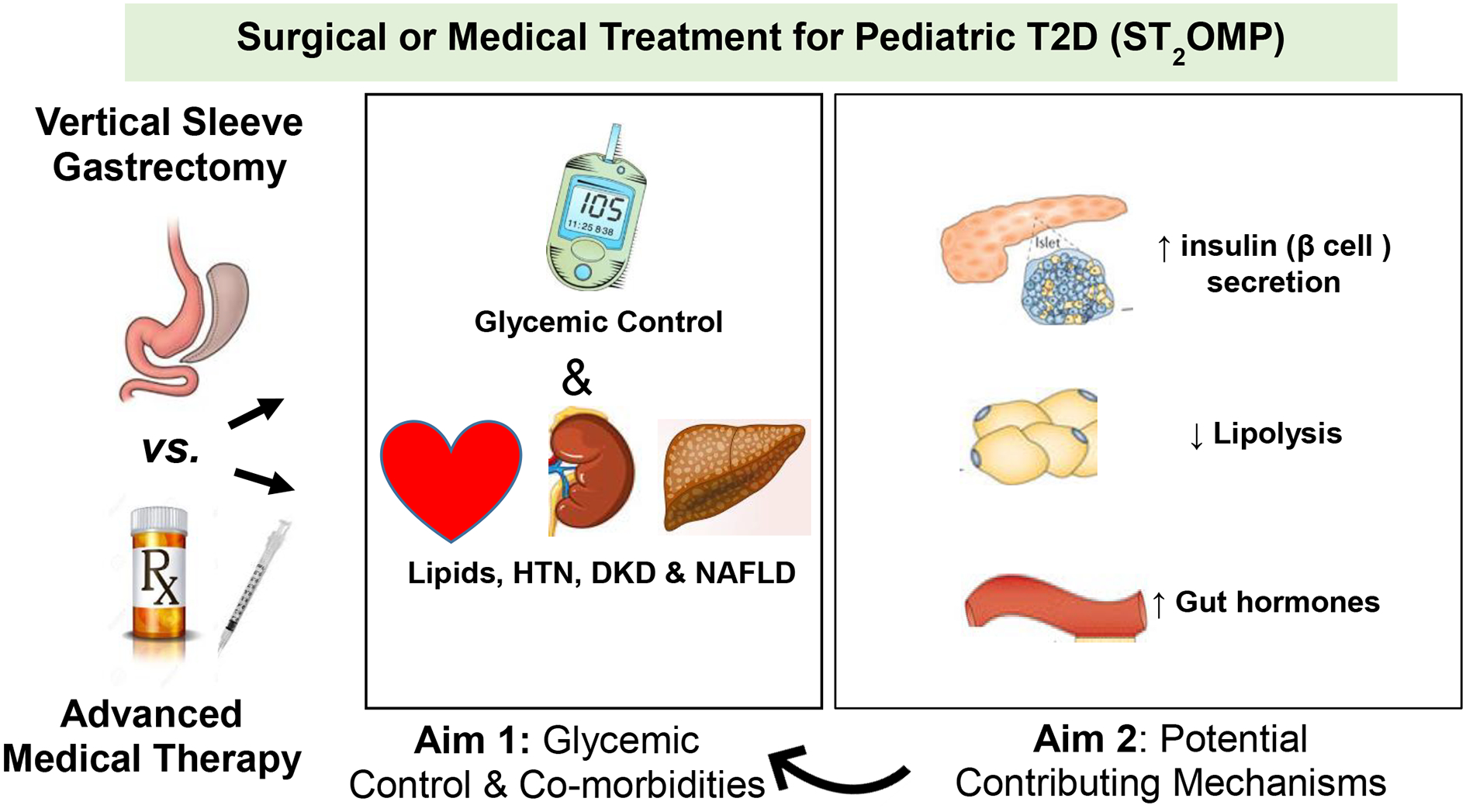
The ST2OMP trial, led by the authors of this review article, aims to directly compare vertical sleeve gastrectomy and advanced medical therapy for control of T2D in adolescents. The primary endpoint will be the proportion of participants in each group with hemoglobin A1c values less than 6.5% at 1 year. Secondary endpoints will be mechanistic in nature, including differences in β cell function, insulin sensitivity, and other metabolic and cardiovascular abnormalities at baseline, one, and two years after enrollment.
HTN, hypertension; DKD, diabetic kidney disease; NAFLD, non-alcoholic fatty liver disease
AMT in ST2OMP is defined as use of lifestyle intervention and multi-drug therapy for T2D and its related co-morbidities aimed at aggressive lowering of HbA1c to <6.5%. Given the previous results of the TODAY clinical trial and the RISE study demonstrating metformin alone or basal insulin followed by metformin are insufficient to achieve or maintain glycemic control and prevent β cell function, ST2OMP was designed to use combination therapy including newer medications such as glucagon-like peptide (GLP-1) agonists and sodium-glucose transport-2 (SGLT-2) inhibitors with the goal to attempt to achieve HbA1c levels similar to those achieved after MBS.
The primary outcome of ST2OMP is glycemic control at one year. Secondary endpoints include the effects of VSG vs. AMT on T2D-related co-morbidities including kidney function, fatty liver disease, dyslipidemia and hypertension as well as pancreatic β-cell function at one and two years. Mechanisms underlying the impact of MBS will also be explored. ST2OMP will begin enrollment in October of 2019.
In conclusion, treatment with metformin and basal insulin do not result in achievement of glycemic control, reduction in youth-onset T2D related co-morbidities, or slow the progression of pancreatic β-cell decline. GLP-1 agonists have been shown to lower glycemic control in youth, but the ability to induce substantial weight loss or slow pancreatic β-cell decline have not yet been demonstrated [32]. Newer pharmacological therapy such as DPP-4 inhibitors and SGLT-2 inhibitors are currently being studied. Currently, MBS is the only treatment that results in significant weight loss and remission of T2D in adults, with ongoing data collection on VSG in youth-onset T2D. Direct comparisons to aggressive medical therapy are needed and are also currently underway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fazeli Farsani S, et al. , Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia, 2013. 56(7): p. 1471–88. [DOI] [PubMed] [Google Scholar]
- 2.Pinhas-Hamiel O and Zeitler P, The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr, 2005. 146(5): p. 693–700. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, et al. , Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Jama, 2014. 311(17): p. 1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabelea D, et al. , Incidence of diabetes in youth in the United States. JAMA: The Journal of the American Medical Association, 2007. 297(24): p. 2716–2724. [DOI] [PubMed] [Google Scholar]
- 5.Copeland KC, et al. , Characteristics of Adolescents and Youth with Recent-Onset Type 2 Diabetes: The TODAY Cohort at Baseline. Journal of Clinical Endocrinology & Metabolism, 2011. 96(1): p. 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettitt DJ, et al. , Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care, 2014. 37(2): p. 402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arslanian S, et al. , Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care, 2018. 41(12): p. 2648–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn SE, et al. , Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes, 1993. 42(11): p. 1663–72. [DOI] [PubMed] [Google Scholar]
- 9.Kwak SH and Park KS, Genetics of type 2 diabetes and potential clinical implications. Arch Pharm Res, 2013. 36(2): p. 167–77. [DOI] [PubMed] [Google Scholar]
- 10.Sartorius T, et al. , Association of common genetic variants in the MAP4K4 locus with prediabetic traits in humans. PLoS One, 2012. 7(10): p. e47647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaliszyn SF, et al. , β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes, 2014. 63(11): p. 3846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahrani AA, et al. , Management of type 2 diabetes: new and future developments in treatment. Lancet, 2011. 378(9786): p. 182–97. [DOI] [PubMed] [Google Scholar]
- 13.Amiel SA, et al. , Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. New England Journal of Medicine.315(4):215–9, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Goran MI and Gower BA, Longitudinal study on pubertal insulin resistance. Diabetes, 2001. 50(11): p. 2444–50. [DOI] [PubMed] [Google Scholar]
- 15.Hannon TS, Janosky J, and Arslanian SA, Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res, 2006. 60(6): p. 759–63. [DOI] [PubMed] [Google Scholar]
- 16.Kelly LA, et al. , Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr, 2011. 158(3): p. 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran A, et al. , Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes, 1999. 48(10): p. 2039–44. [DOI] [PubMed] [Google Scholar]
- 18.Galling B, et al. , Type 2 Diabetes Mellitus in Youth Exposed to Antipsychotics: A Systematic Review and Meta-analysis. JAMA Psychiatry, 2016. 73(3): p. 247–59. [DOI] [PubMed] [Google Scholar]
- 19.Dolan LM, et al. , Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr, 2005. 146(6): p. 751–8. [DOI] [PubMed] [Google Scholar]
- 20.Giannini C, et al. , Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes, 2012. 61(3): p. 606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleber M, et al. , One-year follow-up of untreated obese white children and adolescents with impaired glucose tolerance: high conversion rate to normal glucose tolerance. Diabet Med, 2010. 27(5): p. 516–21. [DOI] [PubMed] [Google Scholar]
- 22.Love-Osborne KA, et al. , Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr Diabetes, 2018. 19(2): p. 199–204. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A, 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care, 2019. 42(Suppl 1): p. S13–S28. [DOI] [PubMed] [Google Scholar]
- 24.Klingensmith GJ, et al. , Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes, 2016. 17(4): p. 266–73. [DOI] [PubMed] [Google Scholar]
- 25.Pinhas-Hamiel O, et al. , Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr, 1996. 128(5 Pt 1): p. 608–15. [DOI] [PubMed] [Google Scholar]
- 26.Zeitler P, et al. , A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med, 2012. 366(24): p. 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeitler P, et al. , Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes, 2007. 8(2): p. 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacha F, et al. , Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes, 2012. 13(5): p. 376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelsey MM, et al. , Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn SE, et al. , Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med, 2006. 355(23): p. 2427–43. [DOI] [PubMed] [Google Scholar]
- 31.Rascati K, et al. , Progression to insulin for patients with diabetes mellitus on dual oral antidiabetic therapy using the US Department of Defense Database. Diabetes Obes Metab, 2013. 15(10): p. 901–5. [DOI] [PubMed] [Google Scholar]
- 32.Tamborlane WV, et al. , Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med, 2019. 381(7): p. 637–646. [DOI] [PubMed] [Google Scholar]
- 33.Levitt Katz LE, et al. , Lipid Profiles, Inflammatory Markers, and Insulin Therapy in Youth with Type 2 Diabetes. J Pediatr, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Group TS, Rapid Rise in Hypertension and Nephropathy in Youth With Type 2 Diabetes: The TODAY clinical trial. Diabetes Care, 2013. 36(6): p. 1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Group TS, Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care, 2013. 36(6): p. 1758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjornstad P, et al. , Cardiopulmonary Dysfunction and Adiponectin in Adolescents With Type 2 Diabetes. J Am Heart Assoc, 2016. 5(3): p. e002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah AS, et al. , Prevalence of arterial stiffness in adolescents with type 2 diabetes in the TODAY cohort: Relationships to glycemic control and other risk factors. J Diabetes Complications, 2018. 32(8): p. 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitt Katz L, et al. , Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes, 2015. 16(1): p. 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Group TS, Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care, 2013. 36(6): p. 1772–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer-Davis EJ, et al. , Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med, 2012. 29(9): p. 1148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal M, et al. , Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care, 2013. 36(12): p. 3903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newton KP, et al. , Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr, 2016. 170(10): p. e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klingensmith GJ, et al. , Pregnancy Outcomes in Youth With Type 2 Diabetes: The TODAY Study Experience. Diabetes Care, 2016. 39(1): p. 122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson BJ, et al. , Depressive symptoms and quality of life in adolescents with type 2 diabetes: baseline data from the TODAY study. Diabetes Care, 2011. 34(10): p. 2205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence JM, et al. , Prevalence and Correlates of Depressed Mood Among Youth With Diabetes: The SEARCH for Diabetes in Youth Study. Pediatrics, 2006. 117(4): p. 1348–1358. [DOI] [PubMed] [Google Scholar]
- 46.Impact of Insulin and Metformin Vs. Metformin Alone on β-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care, 2018. 41(8): p. 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Consortium R and Investigators RC, Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on β-Cell Function: Comparison of Responses In Youth And Adults. Diabetes, 2019. 68(8): p. 1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Consortium R, Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care, 2018. 41(8): p. 1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schauer PR, et al. , Bariatric Surgery vs. Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med, 2017. 376(7): p. 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schauer PR, et al. , Bariatric surgery vs. intensive medical therapy for diabetes--3-year outcomes. N Engl J Med, 2014. 370(21): p. 2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubino F, et al. , Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Surg Obes Relat Dis, 2016. 12(6): p. 1144–62. [DOI] [PubMed] [Google Scholar]
- 52.Sjostrom L, Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med, 2013. 273(3): p. 219–34. [DOI] [PubMed] [Google Scholar]
- 53.Carlsson LM, et al. , Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med, 2012. 367(8): p. 695–704. [DOI] [PubMed] [Google Scholar]
- 54.Sjostrom L, et al. , Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. Jama, 2014. 311(22): p. 2297–304. [DOI] [PubMed] [Google Scholar]
- 55.Schauer PR, et al. , Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg, 2003. 238(4): p. 467–84; discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arterburn DE, et al. , A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg, 2013. 23(1): p. 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schauer PR, et al. , Bariatric Surgery vs. Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med, 2017. 376(7): p. 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kashyap SR, et al. , Acute effects of gastric bypass vs. gastric restrictive surgery on β-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond), 2010. 34(3): p. 462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khanna V, et al. , Adults with long-duration type 2 diabetes have blunted glycemic and β-cell function improvements after bariatric surgery. Obesity (Silver Spring), 2015. 23(3): p. 523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inge TH, et al. , Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med, 2016. 374(2): p. 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vilallonga R, Himpens J, and van de Vrande S, Long-Term (7 Years) Follow-Up of Roux-en-Y Gastric Bypass on Obese Adolescent Patients (<18 Years). Obes Facts, 2016. 9(2): p. 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Sabah SK, et al. , The efficacy of laparoscopic sleeve gastrectomy in treating adolescent obesity. Obes Surg, 2015. 25(1): p. 50–4. [DOI] [PubMed] [Google Scholar]
- 63.Inge TH, et al. , Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics, 2009. 123(1): p. 214–22. [DOI] [PubMed] [Google Scholar]
- 64.Inge TH, et al. , Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol, 2017. 5(3): p. 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pratt JS, et al. , Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring), 2009. 17(5): p. 901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inge TH, et al. , Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inge TH, et al. , Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N Engl J Med, 2019. 380(22): p. 2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alqahtani AR, Elahmedi MO, and Al Qahtani A, Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis, 2014. 10(5): p. 842–50. [DOI] [PubMed] [Google Scholar]
- 69.Paulus GF, et al. , Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes Surg, 2015. 25(5): p. 860–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Messiah SE, et al. , Changes in weight and co-morbidities among adolescents undergoing bariatric surgery: 1-year results from the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis, 2013. 9(4): p. 503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inge TH, et al. , Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr, 2018. 172(5): p. 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dicker D, et al. , Long-Term Outcomes of Three Types of Bariatric Surgery on Obesity and Type 2 Diabetes Control and Remission. Obes Surg, 2016. 26(8): p. 1814–20. [DOI] [PubMed] [Google Scholar]
- 73.Inge TH, et al. , Surgical Therapy is More Effective than Medical Therapy for Type 2 diabetes in Severely Obese Adolescents. Diabetes Care (under review), 2017. [Google Scholar]
- 74.Inge TH, et al. , Comparative effectiveness of bariatric procedures among adolescents: the PCORnet bariatric study. Surg Obes Relat Dis, 2018. 14(9): p. 1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]


