Abstract
Bariatric surgery, an emerging treatment for severely obese youth with and without T2D, provides marked improvement in insulin resistance, beta-cell function, and central adiposity. Further, preliminary data suggest that bariatric surgery also results in significant improvement in markers of obesity-related nephropathy and DKD, beyond that which can be achieved with current medical interventions. Yet, the mechanisms whereby bariatric surgery attenuates kidney disease remain unclear. This review summarizes the data on the effects of bariatric surgery on obesity-related nephropathy and DKD in youth with and without T2D, in addition to potential mechanisms underlying the nephroprotective effects of weight loss surgery and how these may differ in Roux-en-Y gastric bypass vs. vertical sleeve gastrectomy. Finally, we discuss potential future non-surgical therapies to mitigate kidney disease.
Introduction:
Severe obesity now affects 4–6% of youth in the United States and predicts poor health outcomes, including increased risk of chronic kidney disease (CKD) and premature death (1). Obesity is one of the strongest risk factors for incident CKD in adults and obesity-related nephropathy is increasingly recognized in youth. The increasing prevalence of obesity also has implications for lifetime risk for type 2 diabetes (T2D), cardiovascular disease and premature death (1). In fact, the prevalence of youth-onset type 2 diabetes (Y-T2D) is increasing in parallel with the rise in obesity in the United States, and world-wide (2, 3). Y-T2D now represents a substantial percentage of new cases of diabetes in children and adolescents, ranging from 14% in non-Hispanic Whites to 86% in American Indians (2–4). Almost 40% of people with diabetes will develop diabetic kidney disease (DKD) (5), and 50% of Y-T2D will develop early DKD during young adulthood (6–9). Compared to adult-onset T2D, Y-T2D has a more aggressive phenotype with a greater degree of insulin resistance (IR), mitochondrial dysfunction, and higher prevalence of DKD, increasing the risk for early mortality and arguing for dedicated studies in youth (2, 10, 11). Despite the high prevalence and gravity of DKD and obesity-related nephropathy, Y-T2D is characterized by a suboptimal response to currently approved medical therapies, and major challenges in adherence and management. Although major therapeutic advances have been made in diabetes care for adults with T2D, the only FDA approved medications as of June 2019 for youth-onset T2D were metformin and insulin, with the recent addition of GLP-1 receptor agonist. Further, the recently completed Restoring Insulin Secretion (RISE) study, where youth and adult participants were randomly assigned to receive either 12 months of metformin or 3 months of insulin glargine followed by 9 months of metformin, early insulin glargine and metformin both failed to improve β-cell function in Y-T2D (12, 13). Moreover, RISE demonstrated that IR and insulin secretion were markedly higher in Y-T2D in comparison to adults, calling for novel approaches to Y-T2D (12, 13).
Metabolic bariatric surgery is now increasingly recognized as an accepted treatment option for severe obesity in youth that has beneficial effects on long-term obesity-related comorbidities, including kidney disease (14, 15). However, the mechanisms whereby bariatric surgery confers kidney protection remains incompletely understood. Although metabolic bariatric surgery is most commonly performed in later adulthood, data indicate that the longstanding effects of severe obesity from adolescence to adulthood increase the likelihood of complications including hypertension, nephropathy and cardiovascular disease (16).
Accordingly, this review will summarize existing data on the effects of metabolic bariatric surgery on obesity-related nephropathy and DKD in adolescents and adults with and without T2D, in addition to potential mechanisms underlying the nephroprotective effects of weight loss surgery and how these may differ in Roux-en-Y gastric bypass (RYGB) vs. vertical sleeve gastrectomy (VSG). Finally, we discuss promising future non-surgical strategies to mimic the nephroprotective effects mediated by metabolic bariatric surgery for people who are deemed unfit for surgery or lack insurance coverage.
Obesity-related nephropathy and DKD in children
The epidemic of obesity witnessed in recent decades has been paralleled by an increase in kidney disease, with the incidence of end-stage kidney disease (ESKD) nearly quadrupling from 1980–2000 (17). Recent estimates indicate that 24–33% of all kidney disease in the United States is related to obesity (18). Although quantifying the contribution of childhood obesity and obesity-related Y-T2D to the overall burden of ESKD in adulthood is challenging, recent evidence suggests these conditions in childhood carry significant risk of poor kidney outcomes. A recent analysis investigated early kidney disease in participants in the Teen-LABS cohort, a prospective observational study of 242 adolescents ages 13–19 who underwent bariatric surgery. Preoperatively, 17% had elevated albuminuria and 3% had impaired GFR (estimated glomerular filtration rate [eGFR] < 60 ml/min/1.73m2), indicating an alarmingly high prevalence of preexisting kidney damage in adolescents with severe obesity (19). Another study investigated biomarkers of tubular kidney injury in 22 severely obese adolescents without clinical evidence of kidney disease (i.e., normoalbuminuria and preserved eGFR). Urinary biomarkers, including NGAL, KIM-1, and IL-18, were increased compared with lean participants, indicating the presence of subclinical kidney injury in severe obesity (20). To investigate long-term outcomes of childhood obesity, Vivante et al. investigated the association of obesity at age 17 with ESKD in 1.2 million adolescents followed for an average of 25 years. Obesity was associated with a 6-fold increased risk of developing ESKD and a 19-fold risk of ESKD related to DKD (21). Therefore, severe obesity in childhood confers substantial risk for early kidney disease and progression to ESKD in adulthood.
Y-T2D frequently complicates childhood obesity, and children with this condition represent a particularly vulnerable population for adverse outcomes. Compared to type 1 diabetes (T1D), children and young adults with Y-T2D have significantly higher long-term risk of developing CVD and ESKD (22, 23). Moreover, Y-T2D is associated with increased renal disease and mortality compared with adult-onset T2D (2). The prevalence of DKD in youth with T2D, defined as elevated albuminuria, has been reported to range from 28% to 40% within 0–5 years of diagnosis in single center studies (23–26). The prevalence and trajectory of DKD in Y-T2D has been investigated in two large multicenter studies in children: the SEARCH for Diabetes in Youth study (hereafter, the SEARCH study) and the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study. The SEARCH study enrolled 272 Y-T2D from 5 US sites and investigated renal outcomes over 7.9 years. DKD was present in 19.9% of those with T2D compared to only 5.8% in T1D (10). The TODAY study enrolled 669 adolescents at 15 centers across the US with T2D in a randomized trial to investigate the effect of medical management strategies on diabetes outcomes. In this study, microalbuminuria was present in 6.3% at baseline and increased to 16.6% at the end of the trial (average follow-up 3.9 years) (11). The participants of the TODAY clinical trial were enrolled in two longitudinal observational follow-up studies, TODAY 2 Phase 1 and TODAY 2 Phase 2, which sought to detail the rates of complications in Y-T2D as they transition to young adulthood. Data from Today 2 Phase 2 demonstrated that the 12-year cumulative incidence of elevated albuminuria was 40% in Y-T2D, and 48% for hyperfiltration (27). Finally, in an observational study of 242 youth with T2D in Manitoba, Canada, 6.7% developed ESKD at a mean follow-up of 9 years (28). Genetic factors of First Nations youth may, however, contribute to the higher risk than that which has been observed in more diverse cohorts. The rapid rise of kidney disease in this population is alarming, and these data indicate that a significant proportion of Y-T2D have existing DKD that will progress to ESKD in adulthood.
Bariatric surgery to treat obesity-related kidney disease and DKD in children
Considering the limited efficacy of lifestyle and pharmacologic interventions to treat severe obesity and obesity-related T2D, bariatric surgery has become an increasingly accepted treatment option for severely obese adolescents (29, 30). Bariatric surgical procedures most commonly performed include VSG, RYGB, and adjustable gastric banding. All three procedures have proven efficacy and are becoming increasingly used for adolescents with severe obesity and obesity-related comorbidities (1). Short-term outcomes have been excellent, including 25–35% weight loss following surgery and improvement in many obesity-related comorbidities, such as insulin resistance, dyslipidemia, and hypertension (31–37). Moreover, bariatric surgery in adolescents with obesity and T2D has resulted in improved glycemic control and sustained remission compared to medical management (38, 39). Accordingly, evidence-based best practice guidelines for pediatric/adolescent weight loss surgery recommend that adolescents with severe obesity and comorbid conditions should be strongly considered for weight loss surgery (40). Kidney disease, however, was not mentioned in these guidelines as a selection criterion for bariatric surgery, but mounting adolescent data would support inclusion of kidney disease as a comorbidity that should justify consideration of surgery, as we outline below.
In adult literature, consistent improvements in albuminuria and GFR following bariatric surgery has been reported (14, 41, 42). We recently performed a three-year longitudinal study of the Teen-LABS cohort to investigate kidney outcomes in youth following bariatric surgery (15). Similar to adult data, improvement in albuminuria and GFR was observed in those with pre-operative kidney impairment. In participants with a preoperative eGFR < 90 ml/min/1/73m2, eGFR improved from a mean of 76 ml/min/1.73m2 to 102 ml/min/1.73m2 at three years follow up. Similarly, in those with elevated albuminuria at baseline (albumin-to-creatinine ratio [ACR] ≥30mg/g), the median ACR decreased from 74 mg/g to 17 mg/g. GFR and albuminuria remained stable in those without any evidence of pre-operative kidney disease. To investigate the effect of bariatric surgery on DKD, we recently investigated and compared 5-year kidney outcomes in adolescents with severe obesity and Y-T2D in the Teen-LABS and TODAY studies. Elevated albuminuria was present in 21% of TODAY participants receiving medical management at baseline and increased to 43% at 5 years. Conversely, albuminuria decreased from 27% of Teen-LABS participants prior to surgery to 5% at 5 years follow-up. TODAY participants had a 27-fold higher odds of DKD at 5 years compared to Teen-LABS participants in adjusted analyses (43). Additionally, a recent combined analysis of Longitudinal Assessment of Bariatric Surgery (LABS) adults and Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) adolescents showed that adolescents experienced earlier remission of DKD than adults in response to bariatric surgery (44). These data indicate that bariatric surgery improves kidney outcomes in youth with severe obesity youth, including those with T2D. Bariatric surgery should therefore be strongly considered as a treatment option for adolescents with severe obesity and evidence of early kidney disease. However, it is important to underscore that Teen-LABS and LABS studies almost exclusively employed RYGB whereas VSG is now the procedure of choice in youth. Accordingly, there is an urgent need for studies defining the renal effects of VSG.
Potential mechanisms of nephroprotection with bariatric surgery
It is unclear how metabolic bariatric surgery effects the dramatic attenuation of kidney disease in those with obesity and T2D. Proposed mechanisms of action include improvements renal energetics, hemodynamic stress (hyperfiltration), and improvements in metabolic factors such as insulin sensitivity, reversal of inflammation, lipid metabolism, and other hormonal changes associated with weight loss (45–50). In the paragraphs below, the potential nephroprotective effects of these proposed mechanisms will be discussed.
A. Improvement in renal hemodynamic function
Elevated GFR, or hyperfiltration, is perhaps the earliest manifestation of kidney disease in obesity and Y-T2D. Hypefiltration is thought to represent a precursor to single-nephron increases in glomerular pressure, which eventually leads to podocyte injury, proteinuria, and glomerulosclerosis (51). Hyperfiltration at the histologic level is evident by increased glomerular size, podocyte hypertrophy, and in severe cases perihilar glomerular sclerosis (52, 53). The exact cause of hyperfiltration in obesity and Y-T2D is uncertain, but may relate to increased sodium retenion, sympathetic activation, or upregulation of the renin-angiotensin-aldosterone system (RAAS) (54). Insulin resistance also predicts hyperfiltration in Y-T2D (55). Several studies have demonsrated that hyperfiltration improves following bariatric sugery and weight loss, indicating that this phenotype of early kidney disease may be a reversible physiologic adapation (56–59). Therefore, bariatric surgery may reverse progressive increases in intraglomerlar pressure by improving the putative mechanisms of hyperfiltration. As hyperfiltration is associated with the development of proteinuria (51), reversal of maladaptive intraglomerular hemodynamics may partially explain the regression of albuminuria in adolescents who undergo bariatric surgery.
B. Improvement in metabolic and lipid factors
Obesity is characterized by aberrant accumulation of fatty acids and their metabolites in non-adipose tissues such as the liver, heart, skeletal muscle, and kidneys. This results in various lipotoxic effects that are believed to contribute to inflammation and insulin resistance, (60) both of which are causally related to kidney disease (61). Data also suggest that lipotoxic effects directly mediate chronic kidney injury in obesity and metabolic syndrome. Intracellular accumulation of saturated fatty acids, most specifically palmitic acid, causes injury to both glomerular and tubular epithelial cells (62, 63). This may occur via production of reactive oxygen species and subsequent mitochondrial damage, which in turn leads to impaired fatty oxidation and further increase in fatty acid accumulation (64, 65). Obesity is also associated with increased production and accumulation sphingolipids, including ceramides and sphingomyelin species, which are composed of a sphingosine and fatty acid (66). Sphingolipids have recently been shown to mediate glomerular changes observed in diabetes, including mesangial hypertrophy and glomerular fibrosis (67, 68).
Improvement in lipid metabolism may be a mechanism of improved kidney outcomes following bariatric surgery. Improvement in clinical lipid parameters, including decreased total cholesterol and increased HDL, have consistently been observed following bariatric surgery (69). Recent studies have also demonstrated decreased levels of the nephrotoxic lipid species mentioned above. Fatty acid profiles are improved 1 year following bariatric surgery, with decreased saturated fatty acids and increased levels of anti-atherogenic polyunsaturated fatty acids (70, 71). Interestingly, in the immediate months following bariatric surgery, a transient increase in serum fatty acids has been observed, which may represent augmented lipolysis and mobilization of fatty acids from adipose tissue and other organs (72–74). Improvement in sphingolipid profiles, specifically in people undergoing RYGB and biliopancreatic diversion procedures (BPD), has also been observed 3–6 months post operatively (75–77). In a recent analysis of urinary sphingolipids in adolescents undergoing bariatric surgery, we reported that almost all species investigated were significantly elevated preoperatively and improved 1 year postoperatively (78). Collectively, these studies suggest that improvement in lipotoxic fatty acids and other lipid metabolites may at least partially mediate the nephroprotective effects of bariatric surgery,
In addition to reduced levels of potentially nephrotoxic lipid species following bariatric surgery, improved adipocyte function per se may also exert nephroprotective effects. Obesity is a state of adipokine dysregulation, or adiposopathy, which is defined as pathologic adipose tissue that promotes functional disturbances in susceptible individuals. Recently, adiposopathy has been directly implicated as a cause of kidney injury in obesity (79, 80). Altered levels of fat-derived adipokines, including leptin and adiponectin, has been associated with initiation and progression of CKD (79, 80). In murine models of obesity, decreased adiponectin levels cause increased proteinuria, podocyte dysfunction, and interstitial fibrosis (81, 82). High leptin levels that are present in obesity may directly affect kidney function by stimulating glomerular cell proliferation (83) and mesangial cell hypertrophy (84). Several studies have shown that this adipocyte dysregulation improves following bariatric surgery, with resultant increases in adiponectin and decreases in leptin levels (85–87). These metabolic effects may have a directly beneficial effect in reversing kidney injury that occurs in obesity and Y-T2D.
C. Improvement in renal energetics
Albeit speculative, the net effect of the renal hemodynamic and metabolic changes would likely be improved substrate utilization and adenosine triphosphate generation, which when coupled with reduced renal energy expenditure from attenuated hyperfiltration, glucosuria and sodium reabsorption could collectively mitigate renal hypoxia risk.
The initial metabolic and molecular derangements underlying the development of early DKD are poorly understood. Renal hypoxia, potentially stemming from a mismatch between increased renal energy demand and impaired substrate utilization, is emerging as a unifying early pathway in the development of DKD and a potential therapeutic target (88–91) (Figure 1). T2D is characterized by mechanisms that 1) increase renal ATP consumption, including elevated glomerular filtration rate (GFR, i.e. hyperfiltration), effective renal plasma flow (ERPF) and filtered Na+ load, and 2) decrease ATP generation, including insulin resistance and mitochondrial dysfunction (Fig. 1). Insulin resistance shifts renal fuel utilization towards free fatty acid oxidation, which has a low ATP yield per O2 consumed (92, 93). Further, insulin resistance limits ATP synthesis by inhibiting AMP-kinase (AMPK) (94, 95), and is associated with mTOR complex 1 (mTORC1) hyperactivation which results in mitochondrial dysfunction (96, 97) and reduced electron transport chain efficiency (98). It has been hypothesized that the imbalance between renal hypermetabolism and impaired substrate utilization leads to increased renal O2 consumption, since the majority of ATP produced in the kidneys is through aerobic metabolism (99). The high O2 demand ultimately leads to renal hypoxia, to which a diabetic kidney may be unable to adapt due to an impaired hypoxia-inducible factor (HIF) system (88–90). Bariatric surgery may correct the metabolic mismatch (Figure 2). Bariatric surgery improves insulin resistance (45) and provides remission of T2D in 86% of youth (100). Further, bariatric surgery is associated with greater attenuation of hyperfiltration and albuminuria than standard medical therapy (43). Accordingly, bariatric surgery, while not the ideal treatment for all Y-T2D, may be the ideal model to enhance our understanding of the potential metabolic imbalance between renal energy expenditure and substrate utilization underlying early DKD.
Figure 1.

Renal energetics dysfunction underlying DKD
Figure 2.
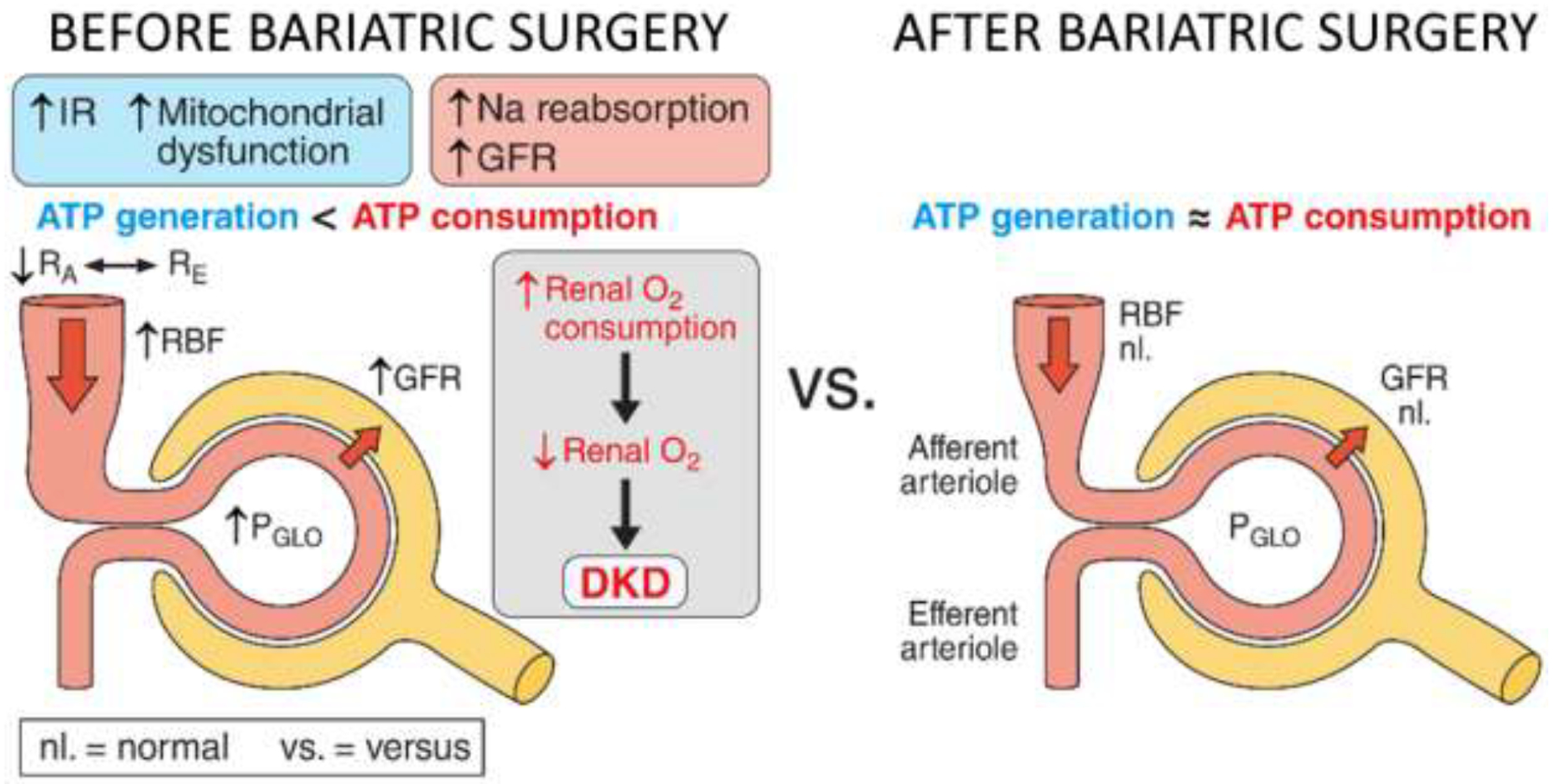
Potential Mechanisms for Improvement in Kidney Disease after Bariatric Surgery
Roux-en-Y gastric bypass vs. vertical sleeve gastrectomy
VSG and RYGB represent the most common bariatric procedures today, and likely have distinct metabolic effects. RYGB appears to exert a more pronounced effect on gastrointestinal hormonal secretions (101). Recent data indicate that kidney outcomes may also be differentially affected depending on the type of bariatric surgery. Using propensity score matching, Imam et al. compared kidney outcomes between patients receiving RYGB and VSG. Over a 3-year follow-up period, patients who underwent RYGB experienced a 6.6 ml/min/1.73m2 improvement in eGFR compared to those who underwent VSG (42). Similarly, in the Teen-LABS cohort, we demonstrated that RYGB, compared to VSG, was associated with 7.0 ml/min/1.73m2 improvement in eGFR following surgery (15). Both analyses were adjusted for clinically important covariates, including BMI and baseline eGFR, indicating an independent beneficial effect in those undergoing RYGB. While the cause of comparatively improved kidney function following RYGB is not known with certainly, differential changes in lipid profiles may offer a plausible explanation. Specifically, RYGB has been shown to have a much more pronounced effect in reducing levels of potentially nephrotoxic lipid species compared to VSG. In two recent studies investigating the effect bariatric surgery on lipidomic profiles, patients receiving RYGB experienced an overall marked reduction in serum sphingolipids, including many ceramides and sphingomyelin species, compared to those undergoing VSG (75, 76). Moreover, decreases in these lipids following RYGB were associated with decreases in body weight and increased insulin sensitivity (76). Improved levels of nephrotoxic sphingolipids may similarly contribute to the renoprotective effects of RYGB, though future studies are needed to investigate this.
Potential non-surgical interventions to mimic effects of bariatric surgery
Although data demonstrate a marked attenuation of obesity-related nephropathy and DKD in response to bariatric surgery, the potential risks associated with metabolic bariatric surgery needs to be emphasized. Complications of metabolic bariatric surgery are described in detail elsewhere (38), but include the risk of repeat surgery, dietary deficiencies, potential negative effects on bone health, offspring, as well as mental health. Clinical adverse events previously reported in participants in Teen-LABS show that 23% experienced complications that required subsequent operation and/or readmission that were related or possibly related (e.g., cholecystectomy for gallstones) to their prior bariatric surgery (38). However, RYGB, the surgery done predominantly in Teen-LABS, appears to have more complications than the currently preferred VSG procedure. We do not yet know the extent of complications observed with VSG in severely obese adolescents with T2D. Additionally, bariatric surgery incurs a substantial initial cost and may not be available to all due to inequitable insurance coverage. Accordingly, there is a critical need to better understand the molecular and metabolic mechanisms of nephroprotection underlying bariatric surgery to uncover novel targetable pathways for the development of novel non-surgical therapeutic targets.
Non-surgical strategies might be most effective if they simultaneously address both sides of the mismatch equation, i.e. ATP consumption and ATP generation (Figure 3). A promising example includes combining agents that may lower renal ATP consumption [e.g. SGLT2 inhibitors (88) or vasopressin receptor blockers (102)] with interventions to improve ATP generation [e.g. mitochondrial peptides and bioavailable-small molecule activators of AMPK and mTORC1 inhibitors (95, 97, 103, 104)]. Even small enhancements in fuel utilization minute to minute may translate into large improvements in kidney function and ultimately clinical outcomes. Hypoxia-adaptability is also a promising therapeutic target. The HIF system induces cell-type specific gene expression changes to promote cell survival in response to hypoxia, including increased erythropoietin (EPO) production in the kidney. Data also suggest that EPO has direct renoprotective effects beyond improving hematocrit and oxygen carrying capacity. For example, EPO has been shown to prevent podocyte injury (105, 106), improve endothelial function and attenuate albuminuria (107). Further, it is hypothesized that activation of HIF system and increased EPO may explain some of the cardio-renal benefits of SGLT2 inhibitors. Indeed, a mediation analysis of the EMPA-REG OUTCOME trial found that change in hematocrit explained 51.8% of the effect of empagliflozin versus placebo on the risk of cardiovascular death (108). HIFs can also be directly increased by Prolyl Hydroxylase (PH) inhibitors which prevent degradation of these proteins. PH inhibitors are novel drug agents, which are designed to increase erythropoietin production and phase 3 trials are underway to evaluate their role in treatment of anemia of CKD (# NCT02876835 (109–111)). If changes in HIF1α expression in response to bariatric surgery relate to nephroprotection, PH inhibitors may be promising agent to repurpose to impede DKD in Y-T2D.
Figure 3.
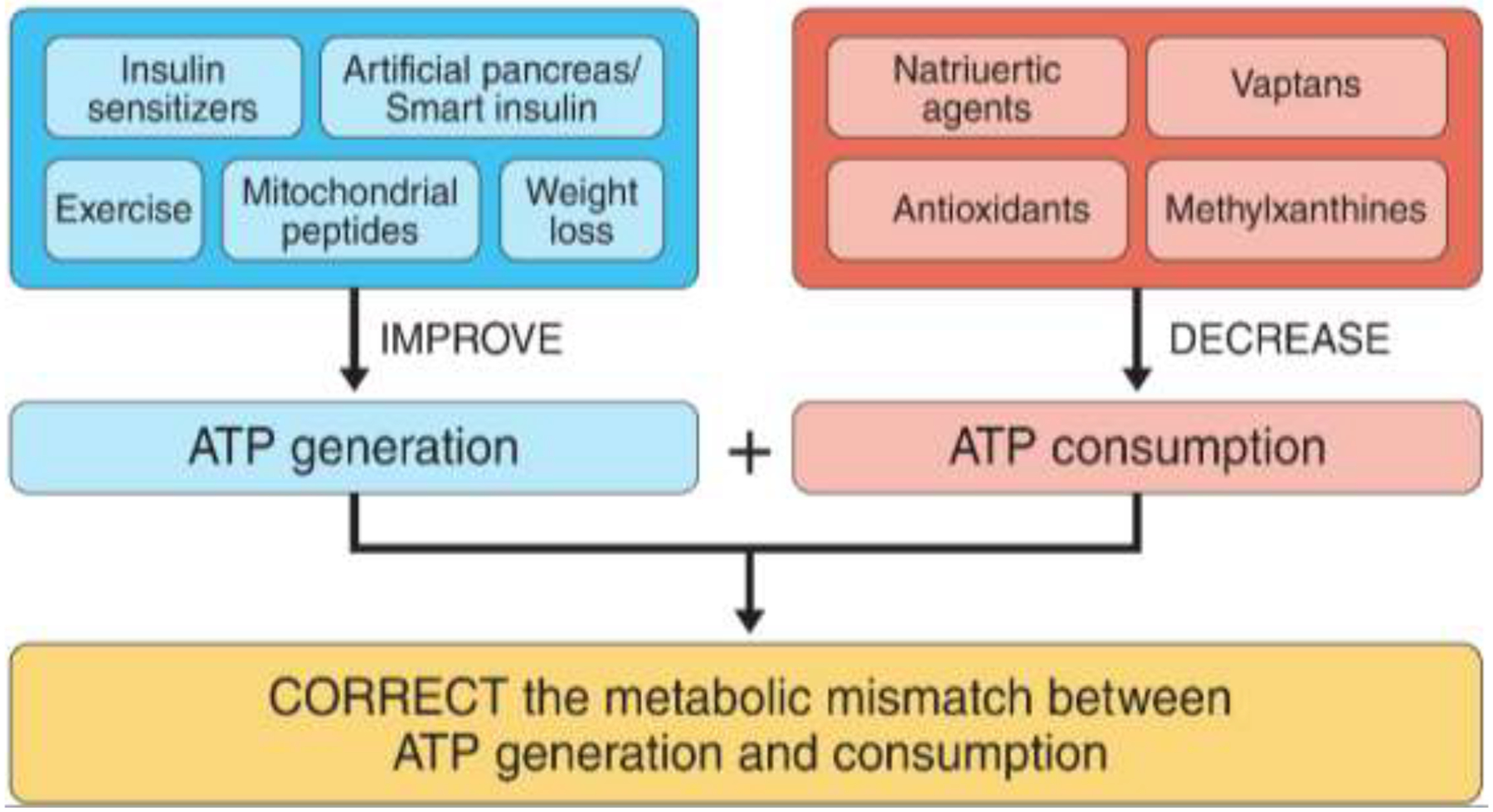
Potential non-surgical strategies
Conclusions and Future Directions:
Obesity-related nephropathy is increasingly recognized as one of the leading causes of CKD, and over 40% of people with diabetes will develop DKD during their lifetime which continues to be the leading cause of renal failure (5). Bariatric surgery is now increasingly recognized as a treatment option for severely obese youth with and without T2D, and data suggest that bariatric surgery mitigates DKD risk to a greater extent than what is observed with standard medical therapy (14, 15). Additionally, less invasive surgical procedures are now available including VSG which may have a superior safety profile to RYGB, but it remains unclear whether it exerts similar nephroprotective effects. Accordingly, studies comprehensively profiling the renal effects of VSG is needed for severely obese youth with and without T2D. Furthermore, studies with bariatric surgery may provide the ideal model to better understand the metabolic and molecular pathophysiology underlying early DKD which is needed to combat this accelerating public health problem. Precision medicine and an integrated biological approach to obesity-related nephropathy and DKD integrating rigorous physiology procedures, biomarker discovery and tissue-based morphometrics, metabolomics and transcriptomics are likely needed to achieve this objective. Such technologies offer a platform to deeply investigate potential molecular mechanisms activated by bariatric surgery and define innovative non-surgical interventional targets to impede early DKD and obesity-related nephropathy.
Acknowledgement
PB receives salary and research support by NIH/NIDDK, Juvenile Diabetes Research Foundation, Thrasher Research Fund, International Society of Pediatric and Adolescent Diabetes (ISPAD), Colorado Clinical & Translational Sciences Institute (CCTSI) and Center for Women’s Health Research at University of Colorado. D.Hv.R. received research support from the Dutch Diabetes Foundation and EU Marie Curie program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
PB has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk and Horizon Pharma. PB serves on a clinical development board for Boehringer Ingelheim and the advisory board of XORTX. DHvR has acted as a consultant and received honoraria from Boehringer Ingelheim and Eli Lilly, Merck, Novo Nordisk, and Sanofi and has received research operating funds from AstraZeneca, Boehringer Ingelheim-Eli Lilly Diabetes Alliance, MSD, and Novo Nordisk.
References:
- 1.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–712. [DOI] [PubMed] [Google Scholar]
- 2.Al-Saeed AH, Constantino MI, Molyneaux L, D’Souza M, Limacher-Gisler F, Luo C, et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes care. 2016;39(5):823–9. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Group for the SfDiYSG, Dabelea D, Bell RA, D’Agostino RB Jr., Imperatore G, Johansen JM, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–24. [DOI] [PubMed] [Google Scholar]
- 5.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One. 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornstad P, Hughan K, Laffel L, Nadeau K, Rayas MS, Tollefsen S, et al. Diabetic Kidney Disease in Adolescents with Type 2 Diabetes: Results from Today 2 Phase 2. American Diabetes Association Scientific Sessions 2019. 2019. [Google Scholar]
- 7.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, et al. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes care. 2014;37(11):3033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group TS. TODAY 2 Phase 2: Diabetic Kidney Disease Data. In Preparation. 2018. [Google Scholar]
- 9.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R Jr., Dolan L, Imperatore G, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317(8):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes care. 2013;36(6):1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Consortium R. Impact of Insulin and Metformin Versus Metformin Alone on beta-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care. 2018;41(8):1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium R. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care. 2018;41(8):1696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90(1):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehus EJ, Khoury JC, Inge TH, Xiao N, Jenkins TM, Moxey-Mims MM, et al. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. 2017;91(2):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inge TH, King WC, Jenkins TM, Courcoulas AP, Mitsnefes M, Flum DR, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132(6):1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63(1 Suppl):A7. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73(1):19–33. [DOI] [PubMed] [Google Scholar]
- 19.Xiao N, Jenkins TM, Nehus E, Inge TH, Michalsky MP, Harmon CM, et al. Kidney function in severely obese adolescents undergoing bariatric surgery. Obesity (Silver Spring). 2014;22(11):2319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao N, Devarajan P, Inge TH, Jenkins TM, Bennett M, Mitsnefes MM. Subclinical kidney injury before and 1 year after bariatric surgery among adolescents with severe obesity. Obesity (Silver Spring). 2015;23(6):1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172(21):1644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk AO, Lau ES, So WY, Ma RC, Kong AP, Ozaki R, et al. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes care. 2014;37(1):149–57. [DOI] [PubMed] [Google Scholar]
- 23.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes care. 2014;37(2):436–43. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58(1):302–11. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger LM, Freeman K, DiMartino-Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr. 2005;147(1):67–73. [DOI] [PubMed] [Google Scholar]
- 26.Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes care. 2006;29(6):1300–6. [DOI] [PubMed] [Google Scholar]
- 27.Bjornstad P, Group TS. Today 2 Phase 2 Symposium at ADA Scientific Sessions 2019 ADA Scientific Sessions 2019. 2019;San Francisco, California. [Google Scholar]
- 28.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savoye M, Nowicka P, Shaw M, Yu S, Dziura J, Chavent G, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127(3):402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2012;256(2):266–73. [DOI] [PubMed] [Google Scholar]
- 32.Boza C, Viscido G, Salinas J, Crovari F, Funke R, Perez G. Laparoscopic sleeve gastrectomy in obese adolescents: results in 51 patients. Surg Obes Relat Dis. 2012;8(2):133–7; discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 33.de la Cruz-Munoz N, Messiah SE, Cabrera JC, Torres C, Cuesta M, Lopez-Mitnik G, et al. Four-year weight outcomes of laparoscopic gastric bypass surgery and adjustable gastric banding among multiethnic adolescents. Surg Obes Relat Dis. 2010;6(5):542–7. [DOI] [PubMed] [Google Scholar]
- 34.Inge TH, Jenkins TM, Zeller M, Dolan L, Daniels SR, Garcia VF, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156(1):103–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadler EP, Reddy S, Isenalumhe A, Youn HA, Peck V, Ren CJ, et al. Laparoscopic adjustable gastric banding for morbidly obese adolescents affects android fat loss, resolution of comorbidities, and improved metabolic status. Journal of the American College of Surgeons. 2009;209(5):638–44. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien PE, Sawyer SM, Laurie C, Brown WA, Skinner S, Veit F, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA. 2010;303(6):519–26. [DOI] [PubMed] [Google Scholar]
- 37.Olbers T, Gronowitz E, Werling M, Marlid S, Flodmark CE, Peltonen M, et al. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS). International journal of obesity. 2012;36(11):1388–95. [DOI] [PubMed] [Google Scholar]
- 38.Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML, et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr. 2018;172(5):452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123(1):214–22. [DOI] [PubMed] [Google Scholar]
- 40.Pratt JS, Lenders CM, Dionne EA, Hoppin AG, Hsu GL, Inge TH, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring). 2009;17(5):901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28 Suppl 4:iv82–98. [DOI] [PubMed] [Google Scholar]
- 42.Imam TH, Fischer H, Jing B, Burchette R, Henry S, DeRose SF, et al. Estimated GFR Before and After Bariatric Surgery in CKD. Am J Kidney Dis. 2017;69(3):380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjornstad P, Hughan K, Kelsey MM, Shah A, Lynch J, Nehus E, et al. Effect of Surgical versus Medical Therapy on Diabetic Kidney Disease over 5 Years in Severely Obese Adolescents with Type 2 Diabetes. Diabetes Care. 2019;(under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA, et al. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N Engl J Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inge TH, Prigeon RL, Elder DA, Jenkins TM, Cohen RM, Xanthakos SA, et al. Insulin Sensitivity and beta-Cell Function Improve after Gastric Bypass in Severely Obese Adolescents. J Pediatr. 2015;167(5):1042–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chondronikola M, Harris LL, Klein S. Bariatric surgery and type 2 diabetes: are there weight loss-independent therapeutic effects of upper gastrointestinal bypass? Journal of internal medicine. 2016;280(5):476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fjeldborg K, Pedersen SB, Moller HJ, Richelsen B. Reduction in serum fibroblast growth factor-21 after gastric bypass is related to changes in hepatic fat content. Surg Obes Relat Dis. 2017;13(9):1515–23. [DOI] [PubMed] [Google Scholar]
- 49.Gastaldelli A, Iaconelli A, Gaggini M, Magnone MC, Veneziani A, Rubino F, et al. Short-term Effects of Laparoscopic Adjustable Gastric Banding Versus Roux-en-Y Gastric Bypass. Diabetes Care. 2016;39(11):1925–31. [DOI] [PubMed] [Google Scholar]
- 50.Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes (Lond). 2016;40(2):275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012;27(5):1708–14. [DOI] [PubMed] [Google Scholar]
- 52.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73(8):947–55. [DOI] [PubMed] [Google Scholar]
- 53.Choung HG, Bomback AS, Stokes MB, Santoriello D, Campenot ES, Batal I, et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int. 2019;95(3):647–54. [DOI] [PubMed] [Google Scholar]
- 54.Nehus E, Mitsnefes M. Childhood Obesity and the Metabolic Syndrome. Pediatr Clin North Am. 2019;66(1):31–43. [DOI] [PubMed] [Google Scholar]
- 55.Bjornstad P, Nehus E, El Ghormli L, Bacha F, Libman IM, McKay S, et al. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis. 2018;71(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serra A, Granada ML, Romero R, Bayes B, Canton A, Bonet J, et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin Nutr. 2006;25(3):400–8. [DOI] [PubMed] [Google Scholar]
- 57.Saliba J, Kasim NR, Tamboli RA, Isbell JM, Marks P, Feurer ID, et al. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147(2):282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro-Diaz M, Serra A, Romero R, Bonet J, Bayes B, Homs M, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17(12 Suppl 3):S213–7. [DOI] [PubMed] [Google Scholar]
- 59.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–6. [DOI] [PubMed] [Google Scholar]
- 60.Ertunc ME, Hotamisligil GS. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;57(12):2099–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–71. [DOI] [PubMed] [Google Scholar]
- 62.Katsoulieris E, Mabley JG, Samai M, Sharpe MA, Green IC, Chatterjee PK. Lipotoxicity in renal proximal tubular cells: relationship between endoplasmic reticulum stress and oxidative stress pathways. Free Radic Biol Med. 2010;48(12):1654–62. [DOI] [PubMed] [Google Scholar]
- 63.Kampe K, Sieber J, Orellana JM, Mundel P, Jehle AW. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am J Physiol Renal Physiol. 2014;306(4):F401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016;90(5):997–1011. [DOI] [PubMed] [Google Scholar]
- 65.Tang C, Cai J, Dong Z. Mitochondrial dysfunction in obesity-related kidney disease: a novel therapeutic target. Kidney Int. 2016;90(5):930–3. [DOI] [PubMed] [Google Scholar]
- 66.Russo SB, Ross JS, Cowart LA. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol. 2013(216):373–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subathra M, Korrapati M, Howell LA, Arthur JM, Shayman JA, Schnellmann RG, et al. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol. 2015;309(3):F204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison-Bernard LM. Sphingolipids, new kids on the block, promoting glomerular fibrosis in the diabetic kidney. Am J Physiol Renal Physiol. 2015;309(8):F685–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bays HE, Jones PH, Jacobson TA, Cohen DE, Orringer CE, Kothari S, et al. Lipids and bariatric procedures part 1 of 2: Scientific statement from the National Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: FULL REPORT. J Clin Lipidol. 2016;10(1):33–57. [DOI] [PubMed] [Google Scholar]
- 70.Hovland A, Nestvold T, Bohov P, Troseid M, Aukrust P, Berge RK, et al. Bariatric surgery reduces fasting total fatty acids and increases n-3 polyunsaturated fatty acids in morbidly obese individuals. Scandinavian journal of clinical and laboratory investigation. 2017;77(8):628–33. [DOI] [PubMed] [Google Scholar]
- 71.Walle P, Takkunen M, Mannisto V, Vaittinen M, Kakela P, Agren J, et al. Alterations in fatty acid metabolism in response to obesity surgery combined with dietary counseling. Nutr Diabetes. 2017;7(9):e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kullberg J, Sundbom M, Haenni A, Freden S, Johansson L, Bornert P, et al. Gastric bypass promotes more lipid mobilization than a similar weight loss induced by low-calorie diet. J Obes. 2011;2011:959601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson L, Roos M, Kullberg J, Weis J, Ahlstrom H, Sundbom M, et al. Lipid mobilization following Roux-en-Y gastric bypass examined by magnetic resonance imaging and spectroscopy. Obes Surg. 2008;18(10):1297–304. [DOI] [PubMed] [Google Scholar]
- 74.Campos GM, Rabl C, Havel PJ, Rao M, Schwarz JM, Schambelan M, et al. Changes in post-prandial glucose and pancreatic hormones, and steady-state insulin and free fatty acids after gastric bypass surgery. Surg Obes Relat Dis. 2014;10(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramos-Molina B, Castellano-Castillo D, Alcaide-Torres J, Pastor O, de Luna Diaz R, Salas-Salvado J, et al. Differential effects of restrictive and malabsorptive bariatric surgery procedures on the serum lipidome in obese subjects. J Clin Lipidol. 2018;12(6):1502–12. [DOI] [PubMed] [Google Scholar]
- 76.Kayser BD, Lhomme M, Dao MC, Ichou F, Bouillot JL, Prifti E, et al. Serum lipidomics reveals early differential effects of gastric bypass compared with banding on phospholipids and sphingolipids independent of differences in weight loss. International journal of obesity. 2017;41(6):917–25. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Kasumov T, Gatmaitan P, Heneghan HM, Kashyap SR, Schauer PR, et al. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity (Silver Spring). 2011;19(11):2235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis S, Nehus E, Inge T, Zhang W, Setchell K, Mitsnefes M. Effect of bariatric surgery on urinary sphingolipids in adolescents with severe obesity. Surg Obes Relat Dis. 2018;14(4):446–51. [DOI] [PubMed] [Google Scholar]
- 79.Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305(12):F1629–36. [DOI] [PubMed] [Google Scholar]
- 80.Ruster C, Wolf G. Adipokines promote chronic kidney disease. Nephrol Dial Transplant. 2013;28 Suppl 4:iv8–14. [DOI] [PubMed] [Google Scholar]
- 81.Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, Fujita K, et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(9):1910–7. [DOI] [PubMed] [Google Scholar]
- 82.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118(5):1645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, et al. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int. 1999;56(3):860–72. [DOI] [PubMed] [Google Scholar]
- 84.Lee MP, Orlov D, Sweeney G. Leptin induces rat glomerular mesangial cell hypertrophy, but does not regulate hyperplasia or apoptosis. International journal of obesity. 2005;29(12):1395–401. [DOI] [PubMed] [Google Scholar]
- 85.Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbely Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7(5):561–8. [DOI] [PubMed] [Google Scholar]
- 86.Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragon-Alonso A, Pascual-Diaz M, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22(6):950–5. [DOI] [PubMed] [Google Scholar]
- 87.Appachi S, Kashyap SR. ‘Adiposopathy’ and cardiovascular disease: the benefits of bariatric surgery. Curr Opin Cardiol. 2013;28(5):540–6. [DOI] [PubMed] [Google Scholar]
- 88.Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40(2):123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22(8):1429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia. 2004;47(7):1223–31. [DOI] [PubMed] [Google Scholar]
- 91.Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1(15):e86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2005;1706(1–2):1–11. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, Fry BC, Layton AT. Modeling glucose metabolism and lactate production in the kidney. Math Biosci. 2017;289:116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyamoto S, Hsu CC, Hamm G, Darshi M, Diamond-Stanic M, Decleves AE, et al. Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator. EBioMedicine. 2016;7:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cree-Green M, Gupta A, Coe GV, Baumgartner AD, Pyle L, Reusch JE, et al. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. Journal of diabetes and its complications. 2017;31(1):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol Cell. 2017;67(6):922–35 e5. [DOI] [PubMed] [Google Scholar]
- 98.Friederich M, Fasching A, Hansell P, Nordquist L, Palm F. Diabetes-induced up-regulation of uncoupling protein-2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim Biophys Acta. 2008;1777(7–8):935–40. [DOI] [PubMed] [Google Scholar]
- 99.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol. 1986;48:9–31. [DOI] [PubMed] [Google Scholar]
- 100.Inge TH, Courcoulas A, Jenkins T, Michalsky M, Brandt ML, Xanthakos A, et al. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N Engl J Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3–36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24(2):241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand. 2009;53(8):1052–9. [DOI] [PubMed] [Google Scholar]
- 103.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171(8):2017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22(6):1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aizawa K, Takeda S, Tashiro Y, Yorozu K, Hirata M, Kanada H, et al. Renoprotection by continuous erythropoietin receptor activator in puromycin aminonucleoside-induced nephrotic syndrome. Am J Nephrol. 2012;36(5):419–26. [DOI] [PubMed] [Google Scholar]
- 106.Eto N, Wada T, Inagi R, Takano H, Shimizu A, Kato H, et al. Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int. 2007;72(4):455–63. [DOI] [PubMed] [Google Scholar]
- 107.Serizawa K, Yogo K, Tashiro Y, Aizawa K, Kawasaki R, Hirata M, et al. Epoetin beta pegol prevents endothelial dysfunction as evaluated by flow-mediated dilation in chronic kidney disease rats. Eur J Pharmacol. 2015;767:10–6. [DOI] [PubMed] [Google Scholar]
- 108.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes care. 2018;41(2):356–63. [DOI] [PubMed] [Google Scholar]
- 109.Brigandi RA, Johnson B, Oei C, Westerman M, Olbina G, de Zoysa J, et al. A Novel Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitor (GSK1278863) for Anemia in CKD: A 28-Day, Phase 2A Randomized Trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67(6):861–71. [DOI] [PubMed] [Google Scholar]
- 110.Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of Molidustat in the Treatment of Anemia in CKD. Clinical journal of the American Society of Nephrology : CJASN. 2019;14(1):28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, et al. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(6):982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


