Abstract
Lignicolous freshwater fungi represent one of the largest groups of Ascomycota. This taxonomically highly diverse group plays an important role in nutrient and carbon cycling, biological diversity and ecosystem functioning. The diversity of lignicolous freshwater fungi along a north-south latitudinal gradient is currently being studied in Asia. In this paper, we introduce two novel freshwater taxa viz. Tingoldiago hydeisp. nov. and T. clavatasp. nov. which were collected from freshwater substrates in Eastern Thailand. Morphological comparison based on the size of ascomata, asci and ascospores, as well as multi-gene phylogenetic analyses based on LSU, SSU, ITS and TEF1-α DNA sequences, supports their placement in Tingoldiago (Lentitheciaceae). Descriptions and illustrations of these two new species are provided.
Keywords: 2 new species, Lentitheciaceae , Freshwater fungi, phylogeny, taxonomy
Introduction
Freshwater fungi are those which the whole or part of their life cycle is found in a freshwater habitat (Thomas 1996, Wong et al. 1998) and they are an evolutionary important group (Vijaykrishna et al. 2006). The members of freshwater fungi can be saprobes, parasites, endophytes and mutualistic taxa (Vijaykrishna et al. 2005, Zhang et al. 2008, Swe et al. 2009, Jones et al. 2014, Huang et al. 2018). There is a wide range of organisms that can be freshwater fungi hosts, such as wood, plants, alga, foams, fish etc. (Sparrow 1960, Ellis and Ellis 1985, Jones et al. 2014). However, a lot of studies on freshwater fungi have focused on lignicolous freshwater fungi (Tsui et al. 2000, Cai et al. 2002, Luo et al. 2004, 2018, Jones et al. 2014, Hyde et al. 2016, Yang et al. 2017), which were defined as those fungi that grow on submerged woody debris in freshwater streams, ponds, lakes and tree hollows (Hyde et al. 2016). They also grow on submerged wood in peat swamps and dams (Pinnoi et al. 2006, Pinruan et al. 2007, 2014, Hu et al. 2010). Lignicolous freshwater fungi are a diverse group comprising species from different phyla (Aphelidiomycota, Ascomycota, Basidiomycota, Blastocladiomycota, Chytridiomycota, Monoblepharomycota, Mortierellomycota and Rozellomycota) (Shearer et al. 2007, Kagami et al. 2012, Zhang et al. 2012, Jones et al. 2014, Wijayawardene et al. 2018). The dominant groups of lignicolous freshwater fungi are Dothideomycetes and Sordarialmycets (Jones et al. 2014, Hyde et al. 2016, Wijayawardene et al. 2017, 2018).
We are studying the diversity of lignicolous freshwater fungi in Thailand, in order to establish the phylogenetic relationships of lignicolous freshwater fungi, understanding the natural classification of this group and contributing to the biogeographical diversity of fungi (Hyde et al. 2016). The study on freshwater fungi in Thailand was first investigated by Tubaki et al. (1983) and they reported 40 freshwater fungal species from foam. Subsequently, mycologists started to study lignicolous freshwater fungi in Thailand and several taxa have been reported (Sivichai et al. 1998, 1999, 2000, 2002, 2010, Jones et al. 1999, Marvanová et al. 2000, Hu et al. 2010, Zhang et al. 2013, Luo et al. 2015, 2016, Bao et al. 2018).
Lentitheciaceae was introduced by Zhang et al. (2012) to accommodate Massarina-like species in the order Pleosporales. Presently, 13 genera are accepted in this family (Dayarathne et al. 2018, Hyde et al. 2018). Species in this family are widely distributed in the world (China, Egypt, Hungary, Italy, Japan, Russia, Saudi, Thailand, UK, Uzbekistan) and are commonly saprobic on stems and twigs of herbaceous and woody plants in terrestrial or aquatic habitats (Wanasinghe et al. 2014, 2018, Knapp et al. 2015, Wijayawardene et al. 2015, Luo et al. 2016, Tibpromma et al. 2017, Hyde et al. 2018). The genus Tingoldiago was established by Hirayama et al. (2010) with a single species Tingoldiago graminicola K. Hiray. & Kaz. Tanak, this species being originally treated as Massarina ingoldiana. Later, Hirayama et al. (2010) re-assessed the phylogeny of Massarina ingoldiana and introduced two new genera Tingoldiago and Lindgomyces to accommodate Massarina ingoldianasensu lato, based on phylogenetic analyses. Currently, only one species is accepted in this genus.
In this paper, we introduce two new freshwater species of Tingoldiago (Lentitheciaceae), based on morpho-molecular studies. Detailed descriptions and illustrations of these two new species are provided.
Materials and methods
Collection, Isolation and morphological studies
Submerged decaying wood samples were collected from That Phanom, Nakhon Phanom, Thailand and brought to the laboratory in plastic bags. The samples were incubated in plastic boxes lined with moistened tissue paper at room temperature for one week. Specimen observations and morphological studies were conducted, following the protocols provided by Luo et al. (2018).
Pure cultures were obtained by single spore isolation followed by Chomnunti et al. (2014). Germinating ascospores were transferred aseptically to potato dextrose agar (PDA) plates and grown at 16–25 °C in daylight. Colony colour and other characters were observed and measured after three weeks. The specimens were deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand. Living cultures are deposited in the Culture Collection of Mae Fah Luang University (MFLUCC). Facesoffungi numbers and Index Fungorum numbers were obtained, following Jayasiri et al. (2015) and Index Fungorum (2019). New species have been established as recommended by Jeewon and Hyde (2016).
DNA extraction, PCR amplification and sequencing
Fungal mycelium was scraped from the surface of colonies grown on a PDA plate or MEA plate at 25 °C for 4 weeks, transferred into a 1.5 ml centrifuge tube and ground using liquid nitrogen. The EZ geneTM fungal gDNA kit (GD2416) was used to extract DNA from the ground mycelium according to the manufacturer’s instructions. The gene regions of the large subunit of the nuclear ribosomal DNA (LSU), the internal transcribed spacers (ITS), the small subunit of the nuclear ribosomal DNA (SSU) and the translation elongation factor (TEF1-α) RNA were amplified using the primer pairs LR0R/LR7 (Vilgalys and Hester 1990), ITS5/ITS4, NS1/ NS4 (White et al. 1990) and 983F/2218R (Liu et al. 1999), respectively. The amplification reactions were performed in 25 μl of PCR mixtures containing 9.5 μl ddH2O, 12.5 μl 2× PCR MasterMix (Tsingke Co., China), 1 μl DNA sample and 1μl of each primer. The PCR thermal cycle programme for LSU, ITS, SSU and TEF1-α amplification were as follows: 94 °C for 3 minutes, followed by 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 56 °C for 50 seconds, elongation at 72 °C for 1 minute and a final extension at 72 °C for 10 minutes and finally kept at 4 °C. PCR amplification was confirmed on 1% agarose electrophoresis gels stained with ethidium bromide. PCR products were sequenced using the same set of primers used in PCR in Beijing Tsingke Biological Engineering Technology and Services Co. Ltd. (Beijing, P.R. China).
Sequencing and sequence alignment
The sequence was assembled by using BioEdit and sequences with high similarity indices were determined from a BLAST search to find the closest matches with taxa in Lentitheciaceae and from recently published data (Dayarathne et al. 2018). All consensus sequences and the reference sequences were aligned using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html) (Katoh and Standley 2013), then checked visually and manually optimised using BioEdit v.7.0.9 (Hall 1999). Ambiguous regions were excluded from the analyses and gaps were treated as missing data. The phylogeny website tool “ALTER” (Glez-Peña et al. 2010) was used to convert the alignment fasta file to Phylip format for RAxML analysis and Clustalx BETA and PAUP 4.0 were used to convert the alignment fasta file to a Nexus file for Bayesian analysis. Phylogenetic analyses were obtained from Maximum Likelihood (ML), Maximum Parsimony (MP) and Bayesian analysis.
Phylogenetic analyses
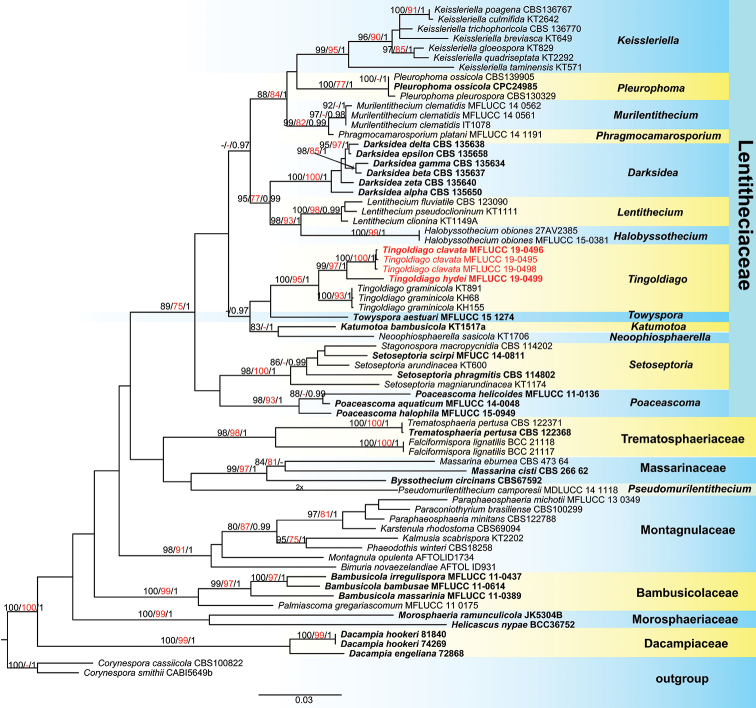
Maximum likelihood trees were generated using the RAxML-HPC2 on XSEDE (8.2.8) (Stamatakis 2006, Stamatakis et al. 2008) in the CIPRES Science Gateway platform (Miller et al. 2010) using GTR+ I + G model of evolution which was estimated by MrModeltest 2.2 (Nylander et al. 2008). Maximum likelihood bootstrap values (ML), equal to or greater than 75%, are given above each node (Figure 1).
Figure 1.
Phylogenetic tree based on RAxML analyses of combined LSU, SSU, ITS and TEF1-α sequence data. Bootstrap support values for maximum likelihood (ML, black) and maximum parsimony (MP, red) higher than 75% and Bayesian posterior probabilities (PP, black) greater than 0.95 are indicated above the nodes as MP / ML /PP. The ex-type strains are in bold and the newly obtained isolates are in red. The tree is rooted at Corynespora smithii (CABI5649b) and Corynespora cassiicola (CBS100822).
MP analyses were performed using the heuristic search option with 1000 random taxa addition and tree bisection and reconnection (TBR) as the branch-swapping algorithm. All characters were unordered and of equal weight and gaps were treated as missing data. Maxtrees were unlimited, branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. Clade stability was assessed using a bootstrap (BS) analysis with 1000 replicates, each with ten replicates of random stepwise addition of taxa (Hillis and Bull 1993).
The Bayesian analysis was performed with MrBayes v3.2 (Ronquist et al. 2012), with the best-fit model of sequence evolution estimated with MrModeltest 2.2 (Nylander et al. 2008) to evaluate posterior probabilities (PP) (Rannala and Yang 1996, Zhaxybayeva and Gogarten 2002) by Markov Chain Monte Carlo (MCMC) sampling. Six simultaneous Markov chains were run for 10,000,000 generations, trees were sampled every 1000th generation and 1,0000 trees were obtained. Based on the tracer analysis, the first 1,000 trees representing 10% were discarded as the burn-in phase in the analysis. The remaining trees were used to calculate posterior probabilities in the majority rule consensus tree (critical value for the topological convergence diagnostic set to 0.01).
The phylograms were visualised in FigTree 1.4.2 (Rambaut 2014) and made in Adobe Illustrator CS5 (Adobe Systems Inc., USA). All newly generated sequences of this study have been submitted in GenBank.
Table 1.
Taxa used in this study and their GenBank accession numbers, the newly generated sequences are indicated wirh * and the type strains are indicated in bold.
| Taxa | strain | GenBank accession number | |||
|---|---|---|---|---|---|
| LSU | SSU | ITS | TEF1 | ||
| Bambusicola bambusae | MFLUCC 11–0614 | JX442035 | JX442039 | NR121546 | KP761722 |
| B. irregulispora | MFLUCC 11–0437 | JX442036 | JX442040 | NR121547 | KP761723 |
| B. massarinia | MFLUCC 11–0389 | JX442037 | JX442041 | NR121548 | – |
| Bimuria novaezelandiae | AFTOL ID931 | – | – | – | DQ471087 |
| Byssothecium circinans | CBS67592 | GU205217 | GU205235 | – | GU349061 |
| Corynespora cassiicola | CBS100822 | GU301808 | GU296144 | – | GU349052 |
| C. smithii | CABI5649b | GU323201 | – | – | GU349018 |
| Dacampia engeliana | 72868 | KT383791 | – | – | – |
| D. hookeri | 74269 | KT383793 | – | – | – |
| D. hookeri | 81840 | KT383795 | – | – | – |
| Darksidea alpha | CBS 135650 | KP184019 | KP184049 | NR137619 | KP184166 |
| D. beta | CBS 135637 | KP184023 | KP184049 | NR137957 | KP184189 |
| D. delta | CBS 135638 | – | – | NR137075 | – |
| D. epsilon | CBS 135658 | KP184029 | KP184070 | NR137959 | KP184186 |
| D. gamma | CBS 135634 | KP184031 | KP184073 | NR137587 | KP184188 |
| D. zeta | CBS 135640 | KP184013 | KP184071 | NR137958 | KP184191 |
| Falciformispora lignatilis | BCC 21117 | GU371826 | GU371834 | KF432942 | GU371819 |
| F. lignatilis | BCC 21118 | GU371827 | GU371835 | KF432943 | GU371820 |
| Halobyssothecium obiones | 27AV2385 | – | – | KX263864 | – |
| H. obiones | MFLUCC 15–0381 | MH376744 | MH376745 | MH377060 | MH376746 |
| Helicascus nypae | BCC36752 | GU479789 | GU479755 | – | GU479855 |
| Kalmusia scabrispora | KT2202 | AB524594 | AB524453 | – | AB539107 |
| Karstenula rhodostoma | CBS69094 | GU301821 | GU296154 | – | GU349067 |
| Katumotoa bambusicola | KT1517a | AB524595 | AB524454 | LC014560 | AB539108 |
| Keissleriella breviasca | KT649 | AB807588 | AB797298 | – | AB808567 |
| K. culmifida | KT2642 | AB807592 | AB797302 | LC014562 | – |
| K. gloeospora | KT829 | AB807589 | AB797299 | LC014563 | – |
| K. poagena | CBS136767 | KJ869170 | – | KJ869112 | – |
| K. quadriseptata | KT2292 | AB807593 | AB797303 | AB811456 | AB808572 |
| K. taminensis | KT571 | AB807595 | AB797305 | LC014564 | AB808574 |
| K. trichophoricola | CBS 136770 | KJ869171 | – | KJ869113 | – |
| Lentithecium clionina | KT1149A | AB807540 | AB797250 | LC014566 | AB808515 |
| L. fluviatile | CBS 123090 | FJ795450 | FJ795492 | – | – |
| L. pseudoclioninum | KT1111 | AB807544 | AB797254 | AB809632 | AB808520 |
| Massarina cisti | CBS 266 62 | FJ795447 | FJ795490 | LC014568 | AB808514 |
| M. eburnea | CBS 473 64 | GU301840 | GU296170 | – | GU349040 |
| Montagnula opulenta | AFTOLID1734 | DQ678086 | AF164370 | – | – |
| Morosphaeria ramunculicola | JK5304B | GU479794 | GU479760 | – | – |
| Murilentithecium clematidis | IT1078 | KM408758 | KM408760 | KM408756 | – |
| M. clematidis | MFLUCC 14–0562 | KM408759 | KM408761 | KM408757 | KM454445 |
| Neoophiosphaerella sasicola | KT1706 | AB524599 | AB524458 | LC014577 | AB539111 |
| Palmiascoma gregariascomum | MFLUCC 11–0175 | KP744495 | KP753958 | KP744452 | – |
| Paraconiothyrium brasiliense | CBS100299 | JX496124 | AY642523 | JX496011 | – |
| Paraphaeosphaeria michotii | MFLUCC 13–0349 | KJ939282 | KJ939285 | KJ939279 | – |
| P. minitans | CBS122788 | EU754173 | EU754074 | – | GU349083 |
| Phaeodothis winteri | CBS18258 | – | GU296183 | – | – |
| Phragmocamarosporium platani | MFLUCC 14–1191 | KP842915 | KP842918 | – | – |
| Pleurophoma ossicola | CBS139905 | KR476769 | – | KR476736 | – |
| P. ossicola | CPC24985 | KR476770 | – | NR137992 | – |
| Pleurophoma pleurospora | CBS130329 | JF740327 | – | – | – |
| Poaceascoma aquaticum | MFLUCC 14–0048 | KT324690 | KT324691 | – | – |
| P. halophila | MFLUCC 15–0949 | MF615399 | MF615400 | – | – |
| P. helicoides | MFLUCC 11–0136 | KP998462 | KP998463 | KP998459 | KP998461 |
| Pseudomurilentithecium camporesii | MDLUCC 14-1118 | MN638846 | MN638850 | MN638861 | – |
| Setoseptoria arundinacea | KT600 | AB807575 | AB797285 | LC014595 | AB808551 |
| S. magniarundinacea | KT1174 | AB807576 | AB797286 | LC014596 | AB808552 |
| S. phragmitis | CBS 114802 | KF251752 | – | KF251249 | – |
| S. scirpi | MFUCC 14–0811 | KY770982 | KY770980 | MF939637 | KY770981 |
| Stagonospora macropycnidia | CBS 114202 | GU301873 | GU296198 | – | GU349026 |
| Tingoldiago graminicola | KH155 | AB521745 | AB521728 | LC014599 | AB808562 |
| T. graminicola | KH68 | AB521743 | AB521726 | LC014598 | AB808561 |
| T. graminicola | KT891 | AB521744 | AB521727 | – | AB808563 |
| *T. hydei | MFLUCC 19-0499 | MN857177 | – | MN857181 | – |
| *T. clavata | MFLUCC 19-0496 | MN857178 | MN857186 | MN857182 | – |
| *T. clavata | MFLUCC 19-0498 | MN857179 | MN857187 | MN857183 | – |
| *T. clavata | MFLUCC 19-0495 | MN857180 | MN857188 | MN857184 | – |
| Towyspora aestuari | MFLUCC 15–1274 | KU248852 | KU248853 | NR148095 | – |
| Trematosphaeria pertusa | CBS 122368 | FJ201990 | FJ201991 | NR132040 | KF015701 |
| Trematosphaeria pertusa | CBS 122371 | GU301876 | GU348999 | KF015669 | KF015702 |
Results
Phylogenetic analyses
The aligned sequence matrix comprises LSU, SSU, ITS and TEF1-α sequence data for 69 taxa, with Corynespora smithii and Corynespora cassiicola as out-group taxa. The dataset comprises 3334 characters after alignment including gaps (LSU: 1–897; SSU: 898–1920; ITS: 1921–2522; TEF1-α: 2523–3479). The topologies of RAxML, MP and Bayesian are similar and the bootstrap support values for Maximum Likelihood (ML), Maximum Parsimony (MP) higher than 75% and Bayesian posterior probabilities (PP) greater than 0.95 are given above the nodes. Maximum parsimony analyses indicated that 2,442 characters were constant, 232 variable characters parsimony uninformative and 805 characters are parsimony-informative. The RAxML analysis of the combined dataset yielded the best scoring tree (Figure 1) with a final ML optimisation likelihood value of -21568.713178. The matrix had 1322 distinct alignment patterns, with 30.89% undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.238228, C = 0.248262, G = 0.272670, T = 0.240839; substitution rates AC = 1.161111, AG = 2.490274, AT = 1.596115, CG = 1.194931, CT = 7.261814, GT = 1.000000; gamma distribution shape parameter α = 0.183824.
The novel species Tingoldiago hydei and T. clavata, introduced in this paper, are supported by multi-phylogenetic analyses. Four newly generated strains clustered together within Tingoldiago with strong statistical support (100 ML/95 MP/1.00 PP, Figure. 1). Three strains of T. clavata clustered together and sister to T. hydei with strong bootstrap support (99 ML/97 MP/1 PP, Figure 1).
Taxonomy
Tingoldiago hydei
D.F. Bao, Z.L. Luo & H.Y. Su sp. nov.
71CF27F5-B5F2-5627-8728-FAA5BBB81D1B
Index Fungorum No: IF557047
Facesoffungi No: FoF07082
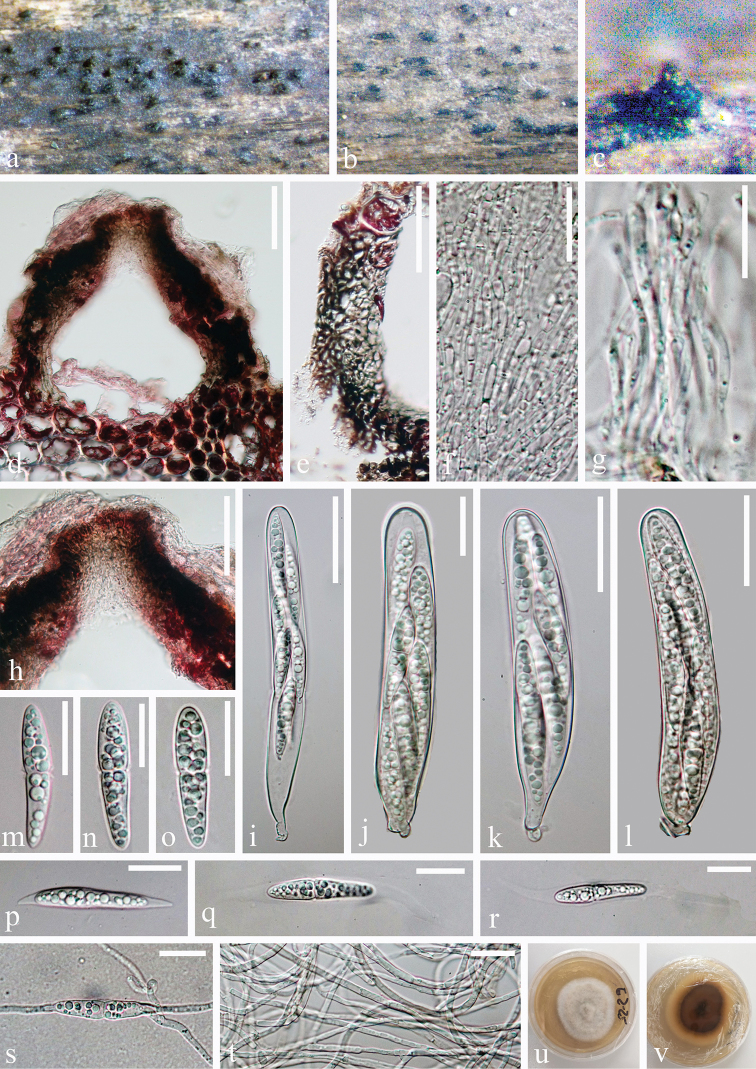
Figure 2.
Tingoldiago hydei (MFLU 19–2842, holotype). a–c Ascomata on wood d section of ascoma e peridium f, g pseudoparaphyses h ostiole i–l asci m–r ascospores s germinating ascospore t vegetative hyphae in culture u, v culture on PDA from surface and reverse. Scale bars: 50 μm (d, e, h), 20 μm (f–g, m–t), 30 μm (i–l).
Etymology.
Referring to Kevin D. Hyde for his contributions in fungal taxonomy.
Holotype.
Thailand, That Phanom, Nakhon Phanom, on submerged decaying wood, 13 November 2018, D.F. Bao, B-126 (MFLU 19–2842, holotype), ex-type living culture, MFLUCC 19–0499.
Description.
Saprobic on submerged decaying wood. Sexual morph: Ascomata 180–280 × 330–470 μm (x̄ = 400 × 420 μm, n = 10), immersed to semi-immersed, erumpentia, gregarious, scattered, depressed globose to conical with a flattened base, dark brown to black, as dark spots on host surface. Ostioles central, papillate, short, crest-like, dark brown. Peridium 33.5–50 μm wide, comprising 4–6 layers, brown to dark brown cells of textura anngularis. Hamathecium comprising 2–2.5 μm (n = 30) wide, numerous, branched, septate, hyaline, cellular pseudoparaphyses. Asci 95–164 × 18–22 μm (x̄ = 129 × 20 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical-clavate, rounded at apex, with a short pedicellate. Ascospores 37.5–42 × 7.5–9 μm (x̄ = 40 × 8 μm, n = 30), overlapping, 2–3-seriate, clavate with round ends, straight, uniseptate, deeply constricted at septum, with broad and short upper cells 17.5–20 × 7–8.7 μm (x̄ = 18.7 × 7.9 μm, n = 30), narrow and long lower cells 20.6–23.3 × 5.9–7.4 μm (x̄ = 21.9 × 6.7 μm, n = 30), tapering towards the end, with short appendages at the septum, hyaline, guttulate, smooth, surrounded by a fusiform gelatinous sheath. Asexual morph: Undetermined.
Culture characteristics.
Ascospores germinating on PDA within 24 hours. Colonies on MEA effuse, greyish-white to dark brown from above and below, reaching 3–4 cm diameter within 30 days at room temperature under natural light, composed of subhyaline to pale brown, septate, smooth hyphae.
Notes.
Phylogenetic analysis showed that Tingoldiago hydei is related to T. clavata; however, they are in different lineages with significant support (99 ML/97 MP/1.00 PP, Figure 1). Tingoldiago hydei resembles T. clavata in having bitunicate, cylindrical-clavate asci and clavate, hyaline, uniseptate, ascospores with broad and short upper cells, narrow and long lower cells, tapering towards the end, surrounded by a gelatinous sheath. However, Tingoldiago hydei can be distinguished from T. clavata in having longer and narrower asci (95–164 × 18–22 vs. 110–148 × 20–27 μm) and smaller ascospores (37.5–42 × 7.5–9 vs. 48–51 × 7.5–8.5 μm). Moreover, ascospores of T. clavata have longer appendages at the septum, while the appendages of T. hydei are much shorter than T. hydei.
Tingoldiago clavata is similar to the type species, T. raminicola in having immersed to semi-immersed, depressed globose to conical ascomata with flattened base, bitunicate, fissitunicate, cylindrical-clavate asci and clavate, straight, uniseptate ascospores. However, T. clavata differs from T. raminicola in having longer asci (95–164 × 18–22 vs. 87.5–122 × 18.25–25 μm) and smaller ascospores (37.5–42 × 7.5–9 vs. 43.5–53 × 7.5–11 μm). Moreover, ascopores of T. clavata have short appendages at the septum while ascospores of T. raminicola lack appendages. In addition, we compared the base pairs of ITS regions between these two species and there were 25 base pairs without gaps (5.1%) differences. Therefore, we introduce our isolate as a new species based on both phylogeny and morphological characters.
Tingoldiago clavata
D.F. Bao, L. Xu & H.Y. Su sp. nov.
2905DCA7-92E5-5C62-A22E-AE1058358E0E
Index Fungorum No: IF557048
Facesoffungi No: FoF07083
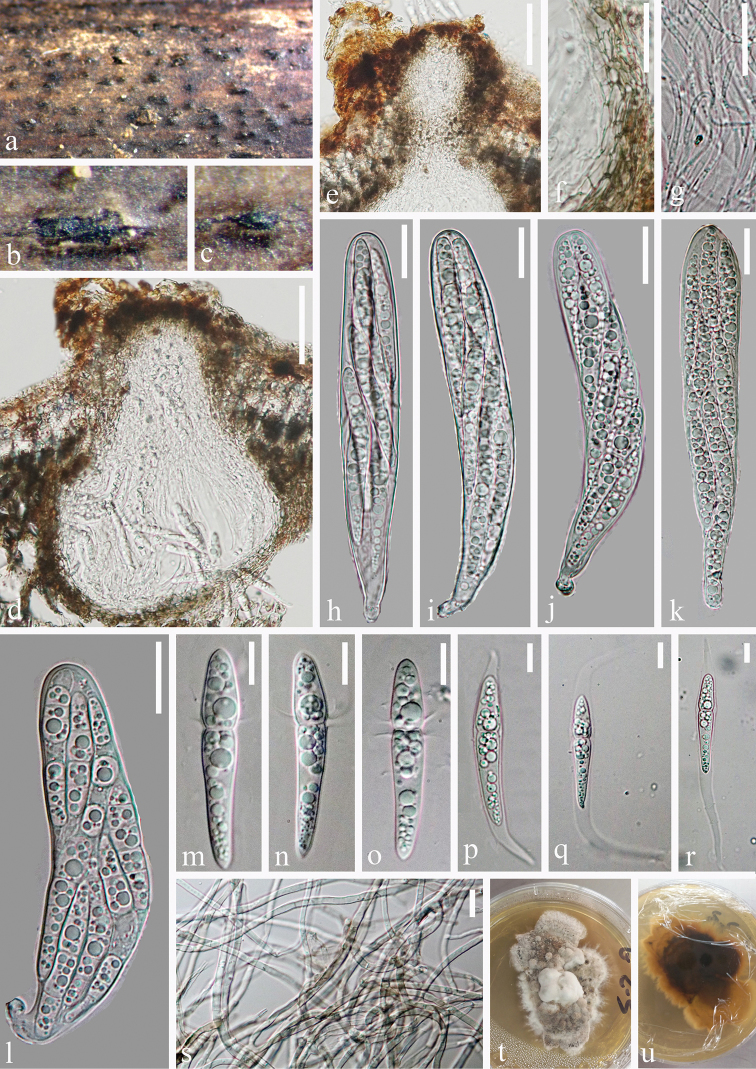
Figure 3.
Tingoldiago clavata (MFLU 19–2843, holotype). a–c ascomata on wood d section of ascoma e ostiole f peridium g pseudoparaphyses h–l asci m–r ascospores s vegetative hyphae in culture t, u culture on PDA from surface and reverse. Scale bars: 50 μm (d, e), 20 μm (f–l), 10 μm (m–s).
Etymology.
Referring to the clavate ascospores of this fungus.
Holotype.
Thailand, That Phanom, Nakhon Phanom, on submerged decaying wood, 13 November 2018, D.F. Bao, B-161 (MFLU 19–2843, holotype), ex-type culture, MFLUCC 19–0496.
Description.
Saprobic on submerged decaying wood. Sexual morph: Ascomata 145–210 × 145–195 μm (x̄ = 175 × 169 μm, n = 10), immersed to semi-immersed, gregarious, scattered, erumpentia, depressed globose to conical with a flattened base, dark brown to black, as dark spots on host surface. Ostiole central, round to papillate, short, crest-like, dark brown. Peridium 28–47 μm wide, comprising several layers, pale brown to brown cells of textura anngularis. Hamathecium comprising 1.5–2.0 μm (n = 30) wide, numerous, branched, septate, hyaline, cellular pseudoparaphyses. Asci 110–148 × 20–27 μm (x̄ = 129 × 23 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical-clavate, rounded at apex, with a short pedicellate. Ascospores 48–51 × 7.5–9 μm (x̄ = 50.5 × 8.5 μm, n = 30), overlapping, 2–3-seriate, clavate, with round ends, straight, uniseptate, deeply constricted at septum, hyaline, with broad and short upper cells 16.6–18.9 × 7.8–9.0 μm (x̄ = 17.7 × 8.4 μm, n = 30), narrow and long lower cells 30–32.9 × 6.5–8.0 μm (x̄ = 31.5 × 7.3 μm, n = 30), tapering towards the end, guttulate, smooth, 2–4 equatorial appendages at the septum and surrounded by a fusiform gelatinous, sheath. Asexual morph:Undetermined.
Culture characteristics.
Ascospores germinating on PDA within 24 hours. Colonies on MEA effuse, velvety, greyish-white to dark brown from above and below, reaching 2.5–3 cm diameter within 30 days at room temperature under natural light, composed of subhyaline to brown, septate, smooth hyphae.
Additional specimens examined.
Thailand, That Phanom, Nakhon Phanom, on submerged decaying wood, 13 November 2018, D.F. Bao, B160 (paratype: MFLU 19–2844; living culture, MFLUCC 19–0498); Thailand, That Phanom, Nakhon Phanom, on submerged decaying wood, 13 November 2018, D.F. Bao, B136 (paratype: MFLU 19–2845; living culture, MFLUCC 19–0495)
Notes.
Tingoldiago clavata resembles the type species, T. graminicola in having bitunicate, cylindrical-clavate asci with a short pedicellate and clavate, hyaline, 1-septate, ascospores with broad upper cells, narrow lower cells. However, we can distinguish them by the size of ascomata and asci and the colour, septate and appendages of ascospores. Tingoldiago clavata has smaller ascomata (110–148 ×145–195 vs. 150–250 × 250–450 μm) and larger asci (110–148 × 20–27 vs. 87.5–122 × 18.25–25 μm). Moreover, ascopsores of T. clavata are hyaline, uniseptate, with 2–4 equatorial appendages at the septum, while ascopspores of T. graminicola are brown and 3-septate at maturity and lacking appendages at the septum. In addition, a comparison of the 491 nucleotides across the ITS gene region of T. clavata and T. graminicola reveals 25 base-pair differences and therefore provides further evidence to introduce T. clavata as a new species as recommended by Jeewon and Hyde (2016).
Discussion
During the last decade, freshwater fungi in Thailand have been mainly reported from north, south and northeast of Thailand (Jones et al. 1999, Marvanová and Hywel-Jones 2000, Sivichai and Boonyuen 2010, Sivichai and Hywel-Jones 1999, Sivichai et al. 1998, 2000, Sri-indrasutdhi et al. 2010). No freshwater fungi from Eastern Thailand have been reported so far. In this study, two new freshwater species, viz. Tingoldiago hydei and T. clavata from Eastern Thailand, are introduced, based on morphology and phylogeny. Tingoldiago hydei and T. clavata satisfied the generic concept of the genus Tingoldiago (Hirayama et al. 2010). They comprise globose to conical, immersed to erumpent ascomata, cellular pseudoparaphyses, bitunicate, fissitunicate asci and clavate ascospores with a median primary septum and a large fusiform gelatinous sheath around the ascospore (Hirayama et al. 2010). Morphologically, T. hydei and T. clavata are quite similar as they have similar shape of asci and ascospores; however, we can distinguish them by the size of ascomata, asci and ascospores (Table 2). In addition, we also compared the morphological differences of these two species with the type species, T. graminicola. Ascopores of T. hydei and T. clavata are hyaline, uniseptate, with appendages at the septum and the upper cells are broader and shorter than the lower cells, while the ascopsores of T. graminicola are hyaline, uniseptate, but becoming brown and 3-septate with age, lacking appendages at the septum, upper cells and lower cells are similar lengths. Phylogenetic analyses showed that our two new isolates clustered together and are sister to the type species, Tingoldiago graminicola with strong bootstrap support (100 ML/92 MP/1.00 PP). This evidence strongly supports our two isolates to be the new species.
Table 2.
The morphological comparisons of Tingoldiago species discussed in this study.
| Taxa | Distribution | Ascomata (μm) | Pseudoparaphyses (μm) | Asci (μm) | Ascospores (μm) | References |
|---|---|---|---|---|---|---|
| Tingoldiago graminicola | Japan, UK | 150–250 × 250–450 | 1.5–4 | 87.5–122 × 18.25–25 | 43.5–53 × 7.5–11 | Hirayama et al. 2010 |
| T. hydei | Thailand | 180–280 × 330–470 | 1.8–2.5 | 95–164 × 18–22 | 37.5–42 × 7.5–9 | This study |
| T. clavata | Thailand | 145–210 × 145–195 | 1.4–2.0 | 110–148 × 20–27 | 48–51 × 7.5–8.5 | This study |
Hyde et al. (2020) introduced a new genus, Pseudomurilentithecium in Lentitheciaceae. In their phylogenetic analysis, Pseudomurilentithecium clustered with Poaceascoma and was basal to Lentitheciaceae. However, in our phylogenetic analysis, Pseudomurilentithecium grouped with the members of Massarinaceae, rather than Lentitheciaceae. Therefore, further investigation is required to confirm the placement of the genus.
Tingoldiago is a well-resolved genus in this family with a stable clade within Lentitheciaceae. The genus can be distinguished from other genera in this family by having hyaline, uniseptate, upper cells are broad and basal cells are narrow ascospores with a large fusiform gelatinous sheath. The sheath is considered to be an adaptation by the genus that enables ascospores to attach to the substrates in moving water (Shearer 1993, Hyde and Goh 2003, Jones 2006, Devadatha et al. 2019). It is reported that the genus Tingoldiago is exclusively found in freshwater habitats (Hirayama et al. 2010) and our two new species were collected from lotic habitats of Mekong River.
Supplementary Material
Acknowledgements
We would like to thank the National Natural Science Foundation of China (NSFC 31860006, 31660008) and the Fungal Diversity Conservation and Utilization Innovation team of Dali University (ZKLX2019213) for financial and laboratory support. Dan-Feng Bao thanks Shaun Pennycook from Landcare Research, Auckland, New Zealand, for advising on the taxon names. Wen-Li Li and Yan-Mei Zhang are acknowledged for their help on DNA extraction and PCR amplification.
Citation
Xu L, Bao D-F, Luo Z-L, Su X-J, Shen H-W, Su H-Y (2020) Lignicolous freshwater ascomycota from Thailand: Phylogenetic and morphological characterisation of two new freshwater fungi: Tingoldiago hydei sp. nov. and T. clavata sp. nov. from Eastern Thailand. MycoKeys 65: 119–138. https://doi.org/10.3897/mycokeys.65.49769
References
- Bao DF, Luo ZL, Jeewon R, Nalumpang S, Su HY, Hyde KD. (2018) Neoastrosphaeriella aquatica sp. nov. (Aigialaceae), a new species from freshwater habitat in southern Thailand. Phytotaxa 391: 197–206. 10.11646/phytotaxa.391.3.3 [DOI] [Google Scholar]
- Cai L, Tsui CKM, Zhang KQ, Hyde KD. (2002) Aquatic fungi from Lake Fuxian, Yunnan, China. Fungal Diversity 9: 57–70. [Google Scholar]
- Dayarathne MC, Hyde KD, Wanasinghe DN, Jones EBG, Chomnunti P. (2018) A novel marine genus, Halobyssothecium (Lentitheciaceae) and epitypification of Halobyssothecium obiones comb. nov. Mycological Progress 17: 1161–1171. 10.1007/s11557-018-1432-3 [DOI] [Google Scholar]
- Devadatha B, Sarma VV, Jeewon R, Hyde KD, Jones EBG. (2019) Morosphaeria muthupetensis sp.nov. (Morosphaeriaceae) from India: Morphological characterisation and multigene phylogenetic inference. Botanica Marina 61: 395–405. 10.1515/bot-2017-0124 [DOI] [Google Scholar]
- Ellis MB, Ellis JP. (1985) Microfungi on Land Plants: An Identification Handbook (1st ed.). Macmillan Pub Co.
- Glez-Peña D, Gómez-Blanco D, Reboiro-Jato M, Fdez-Riverola F, Posada D. (2010) ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Research 38: 14–18. 10.1093/nar/gkq321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. 10.1093/nar/gkq321 [DOI] [Google Scholar]
- Hillis DM, Bull JJ. (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192. 10.1093/sysbio/42.2.182 [DOI] [Google Scholar]
- Hirayama K, Tanaka K, Raja HA, Miller AN, Shearer CA. (2010) A molecular phylogenetic assessment of Massarina ingoldianasensu lato. Mycologia 102: 729–746. 10.3852/09-230 [DOI] [PubMed] [Google Scholar]
- Hu DM, Cai L, Chen H, Bahkali AH, Hyde KD. (2010) Fungal diversity on submerged wood in a tropical stream and an artificial lake. Biodiversity and Conservation 19: 3799–3808. 10.1007/s10531-010-9927-5 [DOI] [Google Scholar]
- Hyde KD. (1995) Tropical Australia freshwater fungi VII. New genera and species of ascomycetes. Nova Hedwigia 61: 119–140. [Google Scholar]
- Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchikumbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang QJ, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat DJ, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, Mckenzie EHC, Wen TC, Yan JY, Zhao Q. (2018) Mycosphere notes 169–224. Mycosphere 9(2): 271–430. 10.5943/mycosphere/9/2/8 [DOI] [Google Scholar]
- Hyde KD, Fryar S, Tian Q, Bahkali AH, Xu JC. (2016) Lignicolous freshwater fungi along a north-south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecology 19: 190–200. 10.1016/j.funeco.2015.07.002 [DOI]
- Hyde KD, Goh TK. (2003) Adaptations for dispersal in filamentous freshwater fungi. Fungal Diversity 10: 231–258. [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M. (2013) Families of Dothideomycetes. Fungal Diversity 63: 1–313. 10.1007/s13225-013-0263-4 [DOI] [Google Scholar]
- Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ, Jones EBG, Liu NG, Abeywickrama PD, Mapook A, Wei DP, Perera RH, Manawasinghe IS, Pem D, Bundhun D, Karunarathna A, Ekanayaka AH, Bao DF, Li JF, Samarakoon MC, Chaiwan N, Lin CG, Phutthacharoen K, Zhang SN, Senanayake IC, Goonasekara ID, Thambugala KM, Phukhamsakda C, Tennakoon DS, Jiang HB, Yang J, Zeng M, Huanraluek N, Liu JK, Wijesinghe SN, Tian Q, Tibpromma S, Brahmanage RS, Boonmee S, Huang SK, Thiyagaraja V, Lu YZ, Jayawardena LS, Dong W, Yang EF, Singh SK, Singh SM, Rana S, Lad SS, Anand G, Devadatha B, Niranjan M, Sarma VV, Liimatainen K, Aguirre-Hudson B, Niskanen T, Overall A, Alvarenga RLM, Gibertoni TB, Pliegler WP, Horváth E, Imre A, Alves AL, Santos ACDS, Tiago RV, Bulgakov TS, Wanasinghe DN, Bahkali AH, Doilom M, Elgorban AM, Maharachchikumbura SSN, Rajeshkumar KC, Haelewaters D, Mortimer PE, Zhao Q, Lumyong S, Xu JC, Sheng J. (2020) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity In press.
- Huang SK, Jeewon R, Hyde KD, Bhat JD, Wen TC. (2018) Novel taxa within Nectriaceae: Cosmosporella gen. nov. and Aquanectria sp. nov. from freshwater habitats in China. Crytogamie Mycologie 39: 169–192. 10.7872/crym/v39.iss2.2018.169 [DOI] [Google Scholar]
- Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat DJ, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, Ghobad-Nejhad M, Nilsson H, Pang KA, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao RL, Zhao Q., Kang JC, Promputtha I. (2015) The faces of fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Diversity 74: 3–18. 10.1007/s13225-015-0351-8 [DOI] [Google Scholar]
- Jeewon R, Hyde KD. (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7: 1669–1677. 10.5943/mycosphere/7/11/4 [DOI] [Google Scholar]
- Jones EBG. (2006) Form and function of fungal spore appendages. Mycoscience 47: 167–183. 10.1007/S10267-006-0295-7 [DOI] [Google Scholar]
- Jones EBG, Hyde KD, Pang KL. (2014) Freshwater Fungi and Fungal-like Organisms. De Gruyter, Germany. 10.1515/9783110333480 [DOI]
- Jones EBG, Wong SW, Sivichai S, Au DWT, Hywel-Jones NL. (1999) Lignicolous freshwater ascomycota from Thailand: Micropeltopsis quinquecladiopsis sp. nov. Mycological Research 103: 729–735. 10.1017/S0953756298007618 [DOI] [Google Scholar]
- Kagami M, Amano Y, Ishii N. (2012) Community structure of planktonic fungi and the impact of parasitic chytrids on phytoplankton in Lake Inba, Japan. Microbial Ecology 63: 358–368. 10.1007/s00248-011-9913-9 [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DG, Kovács GM, Zajta E, Groenewald JZ, Crous PW. (2015) Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 35: 87–100. 10.3767/003158515X687669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Luo J, Yin JF, Cai L, Zhang KQ, Hyde K D. (2004) Freshwater fungi in Lake Dianchi, a heavily polluted lake in Yunnan, China. Fungal Diversity 16: 93–112. [Google Scholar]
- Luo ZL, Maharachchikumnura SSN, Liu XY, Li SH, Chen LJ, Su HY, Zhou DQ, Hyde KD. (2015) Annulatascus saprophyticus sp. nov. and Pseudoannulatascus gen. nov. to accommodate Annulatascus biatriisporus (AnnulatascalesSordariomycetes) from Thailand. Phytotaxa 239(2): 174–182. 10.11646/phytotaxa.239.2.6 [DOI] [Google Scholar]
- Luo ZL, Bahkali AH, Liu XY, Phookamsak R, Zhao YC, Zhou DQ, Su HY, Hyde KD. (2016) Poaceascoma aquaticum sp. nov. (Lentitheciaceae), a new species from submerged bamboo in freshwater. Phytotaxa 253(1): 71–80. 10.11646/phytotaxa.253.1.5 [DOI] [Google Scholar]
- Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY. (2018) Lignicolous freshwater fungi from China II: Novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 9: 444–461. 10.5943/mycosphere/9/3/2 [DOI] [Google Scholar]
- Marvanová L, Hywel-Jones NL. (2000) Sigmoidea conforta sp. nov. and two rare hyphomycete species from streams in Thailand. Cryptogamie Mycologie 21: 13–26. 10.1016/S0181-1584(00)00101-9 [DOI] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the 2010 Gateway Computing Environments Workshop (GCE): 1–8. 10.1109/GCE.2010.5676129 [DOI]
- Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583. 10.1093/bioinformatics/btm388 [DOI] [PubMed] [Google Scholar]
- Pinnoi A, Lumyong S, Hyde KD, Jones EBG. (2006) Biodiversity of fungi on the palm Eleiodoxa conferta in Sirindhorn peat swamp forest, Narathiwat, Thailand. Fungal Diversity 22: 205–218. [Google Scholar]
- Pinruan U, Lumyong S, Hyde KD, Jones EBG. (2007) Occurrence of fungi on tissues of the peat swamp palm Licuala longecalycata. Fungal Diversity 25: 157–173. [Google Scholar]
- Pinruan U, Lumyong S, Hyde KD, Jones EBG. (2014) Tropical peat swamp fungi with special reference to palms. In: Jones EBG, Hyde KD, Pang KL. (Eds) Freshwater Fungi.De Gruyter, Germany, 371–388. 10.1515/9783110333480.371 [DOI]
- Rambaut A. (2014) FigTree v1.4: tree figure drawing tool. http://tree.bio.ed.ac.uk/software/figtree/
- Rannala B, Yang Z. (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43(3): 304–311. 10.1007/BF02338839 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference andmodel choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaer CA. (1993) The freshwater ascomycetes. Nova Hedwigia 56: 1–33. [Google Scholar]
- Shearer CA, Descals E, Kohlmeyer B, Kohlmeyer J, Marvanová L, Padgett D, Porter D, Raja HA, Schmit JP, Thorton HA, Voglmayr H. (2007) Fungi biodiversity in aquatic habitats. Biodiversity and Conservation 16: 49–67. 10.1007/s10531-006-9120-z [DOI] [Google Scholar]
- Sivichai S, Boonyuen N. (2010) Jahnula morakotii sp. nov. and J. appendiculata from a peat swamp in Thailand. Mycotaxon 112: 475–481. 10.5248/112.475 [DOI] [Google Scholar]
- Sivichai S, Hywel-Jones N. (1999) Biflagellospora (aero-aquatic hyphomycetes) from submerged wood in Thailand. Mycological Research 103: 908–914. 10.1017/S0953756298007928 [DOI] [Google Scholar]
- Sivichai S, Hywel-jones NL, Jones EBG. (1998) Lignicolous freshwater Ascomycota from Thailand: 1. Ascotaiwania sawada and its anamorph state Monotosporella. Mycoscience 39: 307–311. 10.1007/BF02464013 [DOI] [Google Scholar]
- Sivichai S, Hywel-jones NL, Somrithipol S. (2000) Lignicolous freshwater Ascomycota from Thailand: Melanochaeta and Sporoschisma anamorphs. Mycological Research 104: 478–485. 10.1017/S0953756299001604 [DOI] [Google Scholar]
- Sivichai S, Jones EBG, Hywel-Jones NL. (2000) Fungal colonisation of wood in a freshwater stream at Khao Yai National Park, Thailand. Fungal Diversity 5: 71–88. [Google Scholar]
- Sivichai S, Jones EBG, Hywel-Jones NL. (2002) Fungal colonisation of wood in a freshwater stream at Tad Ta Phu, Khao Yai National Park, Thailand. Fungal Diversity 10: 113–129. [Google Scholar]
- Sparrow FK. (1960) Aquatic Phycomycetes (Second Rev). The University of Michigan Press, Ann Arbor. 10.5962/bhl.title.5685 [DOI]
- Sri-indrasutdhi V, Boonyuen N, Suetrong S, Chuaseeharonnachai C, Sivichai S, Jones EBG. (2010) Wood-inhabiting freshwater fungi from Thailand: Ascothailandia grenadoidia gen. et sp. nov., Canalisporium grenadoidia sp. nov. with a key to Canalisporium species (Sordariomycetes, Ascomycota). Mycoscience 51: 411–420. 10.1007/S10267-010-0055-6 [DOI] [Google Scholar]
- Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22(21): 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Swe A, Jeewon R, Pointing SB, Hyde KD. (2009) Diversity and abundance of nematode-trapping fungi from decaying litter in terrestrial, freshwater and mangrove habitats. Biodiversity and Conservation 18: 1695–1714. 10.1007/s10531-008-9553-7 [DOI] [Google Scholar]
- Thomas K. (1996) Australian freshwater fungi. In: Grgurinovic CA (Ed.) Introductory Volume to the Fungi (Part2). Fungi of Australian. Canberra, Australia: Australian Biological Resources Study 1–37.
- Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo Santiago ALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li JF, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Meiras-Ottoni A, Mešić A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, Plautz HL, Ariyawansa HA, Lee H, Kušan I, Song J, Sun JZ, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšík M, Samarakoon MC, Wijayawardene NN, Kim NK, Matočec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Marathe SD, Khan S, Hongsanan S, Adhikari S, Mehmood T, Bandyopadhyay TK, Svetasheva TY, Nguyen TTT, Antonín V, Li WJ, Wang Y, Indoliya Y, Tkalčec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, Luangsaard J, Xu JC, Yan JY, Kang JC, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen T-C, Karunarathna SC. (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83(1): 1–261. 10.1007/s13225-017-0378-0 [DOI] [Google Scholar]
- Tsui CKM, Hyde KD, Hodgkiss IJ. (2000) Biodiversity of fungi on submerged wood in Hong Kong streams. Aquatic Microbial Ecology 21: 289–298. 10.3354/ame021289 [DOI] [Google Scholar]
- Tubaki K, Watanabe K, Manoch L. (1983) Aquatic hyphomycetes from Thailand. Transactions of the Mycological Society of Japan 24: 451–457. 10.3354/ame021289 [DOI] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/JB.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishna D, Jeewon R, Hyde KD. (2005) Fusoidispora aquatica: New freshwater ascomycetes from Hong Kong based on morphology and molecules. Sydowia 57: 267–280.
- Vijaykrishna D, Jeewon R, Hyde KD. (2006) Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Diversity 23: 367–406. [Google Scholar]
- Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Hyde KD. (2014) An Exciting Novel Member of Lentitheciaceae in Italy from Clematis Vitalba. Cryptogamie Mycologie 35(4): 323–337. 10.7872/crym.v35.iss4.2014.323 [DOI] [Google Scholar]
- Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Jones EG, Tibpromma S, Tennakoon DS, Dissanayake AJ, Jayasiri SC, GafforovErio Y, Camporesi E, Bulgakov TS, Ekanayake AH, Perera RH, Samarakoon SC, Goonasekara ID, Mapook A, Li WJ, Senanayake IC, Li JF, Norphanphoun C, Doilom M, Bahkali AH, Xu JC, Mortimer PE, Tibell L, Tibell S, Karunarathna SC. (2018) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Diversity 89(1): 1–236. 10.1007/s13225-018-0395-7 [DOI] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis GM, Shinsky D, White T. (Eds) PCR protocols: a guide to methods and applications.Academic, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wijayawardene NN, Hyde KD, Bhat DJ, Goonasekara ID, Nadeeshan D, Camporesi E, Schumacher RK, Yong W. (2015) Additions to Brown Spored Coelomycetous Taxa in Massarinae, Pleosporales: Introducing Phragmocamarosporium gen. nov. and Suttonomyces gen. nov. Cryptogamie Mycologie 36: 213–224. 10.7872/crym/v36.iss2.2015.213 [DOI] [Google Scholar]
- Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lűcking R, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch HT, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castanéda-Ruiz RF, Bahkali AH, Pang KL, Tanaka K, Dai DQ, Sakayaroj J, Hujslová M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Cáceres MES, Pérez-Ortega S, Fiuza PO, Monteiro JS, Vasilyeva LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel MA, Takamatsu S, Bensch K, Silva NI, De Kesel A, Karunarathna A, Boonmee S, Pfister DH, Lu YZ, Luo ZL, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng XY, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera PH, Tang LZ, Phukhamsakda C, Hernández-Restrepo M, Ma XY, Tibpromma S, Gusmao LFP, Weerahewa D, Karunarathna SC. (2017) Notes for genera: Ascomycota. Fungal Diversity 86: 1–594. 10.1007/s13225-017-0386-0 [DOI] [Google Scholar]
- Wijayawardene NN, Pawłowska J, Letcher PM, Kirk PM, Humber RA, Schüßler A, Wrzosek M, Muszewska A, Okrasińska A, Istel Ł, Gęsiorska A, Mungai P, Lateef AA, Rajeshkumar KC, Singh RV, Radek R, Walther G, Wagner L, Walker C, Wijesundara DSA, Papizadeh M, Dolatabadi S, Shenoy BD, Tokarev YS, Lumyong S, Hyde KD. (2018) Notes for genera: basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Diversity 92: 43–129. 10.1007/s13225-018-0409-5 [DOI] [Google Scholar]
- Wong MKM, Goh TK, Hodgkiss IJ, Hyde KD, Ranghoo VM, Tsui CKM, Ho WH, Wong WSW, Yuen TK. (1998) Role of fungi in freshwater ecosystems. Biodiversity and Conservation 7: 1187–1206. 10.1023/A:1008883716975 [DOI] [Google Scholar]
- Yang J, Liu JK, Hyde KD, Jones EBG, Liu Z. (2017) Two new species in Fuscosporellaceae from freshwater habitat in Thailand. Mycosphere 8: 1893–1903. 10.5943/mycosphere/8/10/12 [DOI] [Google Scholar]
- Zhang H, Jones EBG, Zhou DQ, Bahkali AH, Hyde KD. (2011) Checklist of freshwater fungi in Thailand. Cryptogamie, Mycologie 32: 199–217. 10.7872/crym.v32.iss2.2011.199 [DOI] [Google Scholar]
- Zhang H, Hyde KD, Abdel-Wahab MA, Abdel-Aziz F A, Ariyawansa HA, KoKo TW, Zhao RL, Alias SA, Bahkali AH, Zhou DQ. (2013) A modern concept for Helicascus with a Pleurophomopsis-like asexual state. Sydowia 65: 147–166. [Google Scholar]
- Zhang Y, Jeewon R, Fournier J, Hyde KD. (2008) Multi-gene phylogeny and morphotaxonomy of Amniculicola lignicola: novel freshwater fungus from France and its relationships to the Pleosporales. Fungal Biology 112: 1186–1194. 10.1016/j.mycres.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012) Pleosporales. Fungal Diversity 53: 1–221. 10.1007/s13225-011-0117-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaxybayeva O, Gogarten JP. (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics 3: 4. 10.1186/1471-2164-3-4 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.