Introduction
Natural fecundity of women decreases gradually and more rapidly after age 37 years. This decrease is accompanied by rising aneuploidy rates of pregnancies and can also be observed in products of conception of spontaneous abortions [1]. These observations lead to the hypothesis that transferring only euploid embryos in association with in vitro fertilization (IVF) might decrease miscarriages and increase live birth rates (LBRs), attesting-procedure now called preimplantation genetic testing (of embryos) for aneuploidy (PGT-A), until recently generally referred to as preimplantation genetic screening (PGS).
Verlinsky and Kuliev further proposed that the removal of all aneuploid embryos prior to transfer would improve implantation rates and live birth rates and suggested that the diagnosis be made via biopsy of both polar bodies [2]. Polar body biopsy, however, proved technically too difficult for general IVF practice and would have revealed only meiotic aneuploidies. The procedure was, therefore, initially performed biopsying 1–2 blastomeres of day-3 cleavage-stage embryos, often given the acronym PGS 1.0.
This form of embryo testing has, since, been replaced by PGS 2.0, with the embryo biopsy being moved from day-3 cleavage stage to trophectoderm biopsy of blastocyst-stage embryos on days 5–6 after fertilization. In July 2016, another major change in PGT-A was announced, for the first time introducing the concept pf “mosaic” embryos (also called PGS 3.) (Preimplantation Genetic Diagnosis Society (PGDIS) position statement on chromosome mosaicism and preimplantation aneuploidy testing at the blastocyst stage, Chicago, IL; July 19, 2016 http://pgdis.org/docs/newsletter_071816.html).
After almost two decades of PGS 1.0 through PGS 3.0, the procedure has, however, still been unable to demonstrate the promised improvements in live births and anticipated declines in miscarriage rates [3–5]. Several studies, even summarized in a meta-analysis [6], have claimed improved clinical IVF outcomes following PGT-A. They, however, reported IVF outcomes with reference point embryo transfer rather than cycle start (intent-to-treat) and, therefore, by excluding poorer prognosis patients, were severely biased [7].
The STAR study
This is why the recently published STAR study [8] attracted special attention: It avoided at least some patient selection biases of earlier fresh-cycle studies by being prospectively randomized and reporting on IVF outcomes from transfers of only single frozen-thawed embryos at blastocyst stage. That qualifying patients required having at least two frozen embryos from a prior fresh cycle, however, still demonstrates a favorable patient selection bias. Importantly, however, the study at least analyzed outcomes for study and control groups with reference point initial first cycle start [7].
In doing so, the study convincingly revealed no improvements in live birth rates and no reduction in miscarriage rates when cycle outcomes were compared in singe-embryo transfers at blastocyst stage between women, randomized to either PGT-A or only morphological assessments of a single embryo prior to transfers [8].
For no declared reason, the authors then, however, performed a post hoc sub-group analysis based on age and reported, between ages 35 and 40 years, that PGT-A, still, offered significant increases in ongoing pregnancy rate (OPR). In the discussion of their manuscript, they emphasized this finding as “continuous evidence” for the clinical utility of PGT-A in at least that age group. Again, in contrast to the overall study that had been performed with reference point cycle start (intent-to-treat), their post hoc analysis was performed with reference embryo transfer and, therefore, statistically suspect.
Because results of the STAR study are already impacting IVF practice worldwide, we here offer a statistically corrected analysis of the STAR study, reaffirming the study’s overall findings by refuting the results of the post hoc analysis and its interpretation by the authors, claimed benefits for PGT-A utilization for all age groups.
Re-analysis of the STAR study
Further pointing at favorable patient selection, women were considered eligible for the STAR trial only between ages 25 and 40 years and if they demonstrated normal ovarian reserve, defined as follicle-stimulating hormone (FSH) < 10 IU/L on days 2–4 of their menstrual cycle and/or anti-Müllerian hormone (AMH) < 1.0–2.0 ng/mL. They, therefore, also demonstrated excellent oocyte and blastocyst yields (18.6/18.9 and 7.4/7.4, respectively, in study and control groups), and excellent OPR per embryo transfer (50% vs. 45.7%), as well as very low miscarriage rates (9.9% and 9.6%, respectively).
Furthermore, while enrollment was completed before oocyte retrieval, eligibility for randomization occurred 110–150 h after fertilization, when the IVF laboratory confirmed development of at least two blastocysts of sufficient quality for biopsy and vitrification. As already noted before, this further biased patient selection, as patients who produce two or more blastocysts will have better IVF outcomes than women with no or only 1 blastocyst-stage embryo.
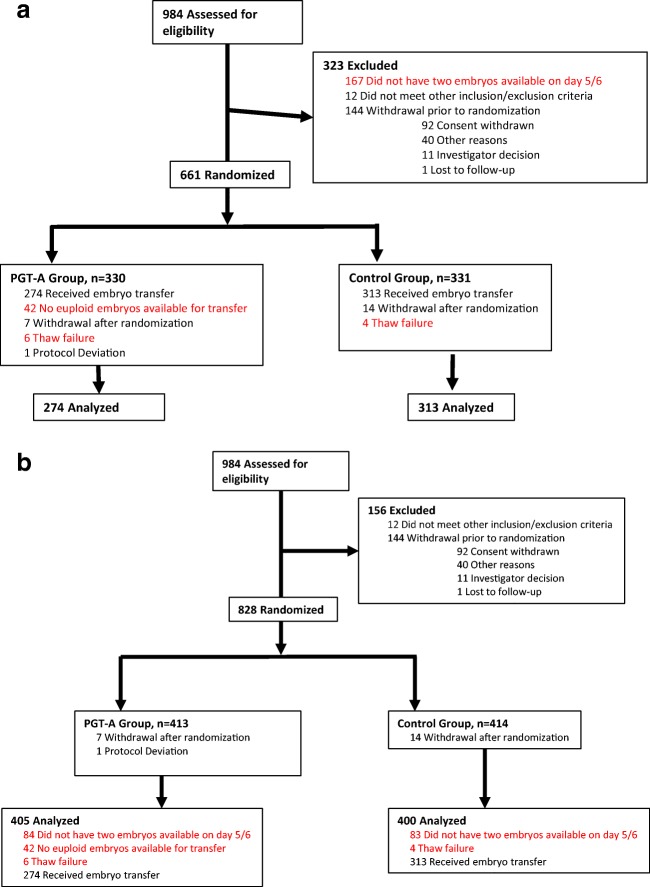
Details of the STAR study design are important, to understand outcomes and reach appropriate conclusions (Fig. 1a): The initially eligible patient population included 984 women; but only 661 were randomized to study (n = 330) and control groups (n = 331). Among the 323 excluded patients, 167 failed in obtaining at least 2 blastocyst-stage embryos on post-fertilization days 5 and 6. In an intent-to-treat analysis, they should not have been excluded from study and analysis. Half (n = 83), therefore, must be added to the denominator in the study and control groups. In addition, the study excluded 42 women who did have blastocyst-stage embryos; but since not even one was euploid, they, too, were incorrectly excluded, as were 10 women (6 study and 4 control patients, respectively), who’s embryos did not survive thawing. All of these unjustified excluded patients must be added to the denominators of the study and control groups. The revised denominator is, therefore, n = 400 in the control study group (83 + 4 + 313) and n = 405 (83 + 42 + 6 + 274) in the PGT-A group (Fig. 1b). Considering these numbers, recalculated OPRs for the whole study population were 137/405 (33.8%) and 143/400 (35.8%) (P = 0.56), in the PGT-A and control groups, respectively, therefore demonstrating no outcome difference between the study and control groups, as also concluded in the STAR study [8] (Table 1).
Fig. 1.
Enrollment, assignment, treatment, and analysis process of study and control patients. a Original START study. b Corrected analysis in this submission. PGT-A: preimplantation genetic testing for aneuploidy
Table 1.
Original STAR study result and the corrected analysis according to this submission
| All patients | 35–40 years | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | STAR | Corrected analysis | STAR | Corrected analysis | ||||
| PGT-A (n = 274) | Control (n = 313) | PGT-A (n = 405.5) | Control (n = 400.5) | PGT-A (n = 122) | Control (n = 145) | PGT-A (n = 196.5) | Control (n = 193) | |
| Miscarriage | 27 (9.9) | 30 (9.6) | 27 (6.7) | 30 (7.5) | 10 (8.2) | 16 (11.0) | 10 (5.1) | 16 (8.3) |
| p value | 0.9 + 1 | 0.64 | 0.43 | 0.2 | ||||
| OPR | 137 (50.0) | 143 (45.7) | 137 (33.8) | 143 (35.8) | 62 (50.8) | 54 (37.2) | 62 (31.6) | 54 (28.0) |
| p value | 0.29 | 0.47 | 0.025 | 0.44 | ||||
Reanalysis of STAR study for women at age 35–40 years
Performing the same reanalysis only for women at age 35–40 years established a denominator of n = 196 in the PGT-A group (48 + 26 + 122) and of n = 193 (48 + 145) in the control group. In contrast to the claim made in the manuscript [8], recalculating OPRs, however, demonstrates none of the claimed outcome difference at 62/196 (31.6%) and 54/193 (28.0%) (P = 0.43), between the PGT-A and control groups, respectively (Table 1).
Conclusions
Presented reanalysis here of the recently published STAR study [8], which already has been affecting IVF practice worldwide, reveals significant shortcomings in the study’s statistical analyses. Those, however, do not change the principal conclusion of the STAR study that PGT-A does not favorably affect IVF outcomes by increasing pregnancy chances or reducing miscarriage risks.
Presented revised analyses here, however, do conclusively demonstrate that the study’s post hoc analysis, claiming outcome benefits from PGT-A in beneficially affecting OPRs in women between age 35 and 40 years, was mistaken as a consequence of the above noted errors in statistical methods utilized.
The STAR study thus reveals that PGT-A does not beneficially affect IVF outcomes in confirmation of another relatively recent study in women 37 years and older by Kang et al. [9]. Like the STAR study, Kang et al. reported seemingly improved live birth rates following PGT-A but this outcome advantage, actually, reversed itself after correct intent-to-treat analysis of outcomes with reference cycle start: Pregnancy as well as live birth rates, indeed, ended up to be significantly higher in control non-PGT-A patients (49.5 vs 21.5% and 39.8 vs 19.9%).
Since the Kang study did not favorably select out participating patients to the degree the STAR study did, both of these studies further suggest that in unselected IVF patient PGT-A, actually, likely exerts adverse effects on IVF outcomes.
Within this context, Rubio et al. also randomized good-prognosis patients of mildly advanced maternal age (38–41 years) to either PGT-A or blastocyst transfer without PGT-A [10]. To be included in the study, women had to produce five or more metaphase II oocytes in one or two cycles. The authors here again reported higher delivery rates for the first embryo transfer in the PGT-A group, when outcomes were analyzed with reference embryo transfer. Yet, there was absolutely no difference in cumulative pregnancy rates between PGT-A and control patients, when the data were analyzed per patient.
This additional example further highlights how, historically, inaccurate statistical assumptions, and false conclusions based on these assumptions, have for decades marred PGT-A studies and misled physicians and the public. Moreover, by demonstrating no difference in outcome in relative good-prognosis patients, it is again reasonable to assume that in truly poor prognosis women who produce only small egg and embryo numbers, PGT-A will actually reduce pregnancy chances.
Such a conclusion was also supported by statistical modeling [11]: Relying on evidence-based data in the literature on blastulation and aneuploidy rates, the prevalence of mosaicism, technical errors, and implantation/live birth rates of PGT-A and non-PGT-A cycles at cleavage and blastocyst stages, a model concluded that highest live birth rates could be expected in patients having day-3 embryo transfers without prior PGT-A (21.4–50.0%), followed by day-5 blastocyst-stage cycles without PGT-A (18.2–22.2%), and that women undergoing PGT-A and blastocyst-stage transfers after all-freeze cycles achieved the lowest live birth rates (7.6–12.6%).
Considering all presented evidence here, it is difficult to understand what further argument can be made for the continuous routine clinical utilization of PGT-A to improve IVF outcomes.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101:633–634. doi: 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Verlinsky Y, Cieslak J, Ivakhnenko V, Evsikov S, Wof G, White M, et al. Preimplantation diagnosis of common aneuploidies by the first- and second-polar body FISG analysis. J Assist Reprod Genet. 1998;15:285–289. doi: 10.1023/A:1022592427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM, Buys CH, Heineman MJ, Repping S, van der Veen F. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 4.Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review. Hum Reprod Update. 2011;17:454–466. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 5.Orvieto R, Gleicher N. Should preimplantation genetic screening (PGS) be implemented to routine IVF practice? J Assist Reprod Genet. 2016;33(14):1445–1448. doi: 10.1007/s10815-016-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104:1503–1512. doi: 10.1016/j.fertnstert.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Gleicher N, Kushnir V, Barad DH. The impact of patient preselection on reported IVF outcomes. J Assist Reprod Genet. 2016;33:455–459. doi: 10.1007/s10815-016-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M, Wells D, Gordon T, Stock-Myer S, Willman S, STAR Study Group Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;S0015–0282(19):31979. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 9.Kang HJ, Melnick AP, Stewart JD, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106:597–602. doi: 10.1016/j.fertnstert.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J, Pellicer A, Simón C. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107(5):1122–1129. doi: 10.1016/j.fertnstert.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Orvieto R. Preimplantation genetic screening- the required RCT that has not yet been carried out. Reprod Biol Endocrinol. 2016;14:35. doi: 10.1186/s12958-016-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]