Abstract
Purpose
To investigate the associations of previous pregnancy failures, including implantation failures (IFs), biochemical pregnancy losses (BPLs), and early (EMs) and late miscarriages (LMs), with blastocyst aneuploidy and pregnancy outcomes after PGT-A.
Methods
This study included 792 couples who underwent PGT-A after multiple pregnancy failures. Subgroup analyses were used to compare the blastocyst aneuploidy rate (BAR), implantation rate (IR), early miscarriage rate (EMR), and live birth rate (LBR). Multiple linear and logistic regression models were used to evaluate the associations. The control group comprised couples with ≤ 2 IFs, ≤ 1 BPL, ≤ 1 EM, and no LM.
Results
Notably, a history of ≥ 4 IFs was significantly associated with an increase in aneuploid blastocysts (42.86% vs. 33.05%, P = 0.044, B = 10.23 for 4 IFs; 48.80% vs. 33.05%, P = 0.002, B = 14.43 for ≥ 5 IFs). Women with ≥ 4 prior EMs also harbored more aneuploid blastocysts (41.00% vs. 33.05%, P = 0.048; B = 9.23). Compared with the control group, women with ≥ 4 prior EMs had a significantly higher EMR (6.58% vs. 31.11%, P < 0.001, OR = 6.49) and a lower LBR (53.49% vs. 34.18%, P = 0.007, OR = 0.56) after euploid transfer. Moreover, a history of LM(s) was associated with adverse pregnancy outcomes after PGT-A (OR for EM = 3.16; OR for live birth = 0.48). However, previous BPLs and 2 EMs were not associated significantly with blastocyst aneuploidy and pregnancy outcomes after PGT-A.
Conclusion
A history of high-order IFs or EMs and existence of LM(s) were significantly associated with blastocyst aneuploidy and adverse pregnancy outcomes after PGT-A, whereas no such associations were observed with BPLs or 2 EMs.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01722-9) contains supplementary material, which is available to authorized users.
Keywords: Implantation failure, Miscarriage, Embryonic aneuploidy, Live birth, Preimplantation genetic testing
Introduction
Miscarriage is a common adverse pregnancy outcome of both spontaneously conceived pregnancies and those resulting from assisted reproduction technology (ART), with a prevalence of 14.7–20% [1, 2]. With the rapid development of ART, implantation failure after in vitro fertilization (IVF) also gains great attention. Several observational studies have identified the number of previous miscarriages and unsuccessful IVF treatments as important prognostic factors for subsequent pregnancy outcomes [3–6]. Moreover, a history of late miscarriage (LM) appeared to be associated with a subsequent poor pregnancy prognosis [7–9]. However, some of the conclusions drawn from these studies were inconsistent and apparently contradictory. There are different types of pregnancy failures, and they might vary between women and within women with respect to kind, severity, and impact. Hence, it remains unclear whether and how previous pregnancy losses might affect future outcomes.
Although the definitions of recurrent pregnancy loss (RPL) and recurrent implantation failure (RIF) were firstly proposed more than 10 years ago [10, 11], no unified definition has been reached. The controversy of RPL definition lies in the minimum number of prior miscarriages (2 or 3) [12–14], while that of RIF definition mainly focuses on the minimum number of failed transfer cycles and the number of transferred good-quality embryos [11, 15]. Furthermore, it remains undetermined whether biochemical pregnancy loss (BPL) should be included in the definition for RPL or RIF [15–17]. It is therefore vital to elucidate the potential associations of the number of previous pregnancy failures with future pregnancy outcomes and embryonic viability.
Preimplantation genetic testing for aneuploidies (PGT-A) enables genetic selection of embryos and is thought to improve pregnancy outcomes, especially for women over 35 years old [18, 19]. It has been established that aneuploidy is the leading cause of pregnancy failures [1, 20], and RPL and RIF have been listed as common indications for PGT-A treatment worldwide. However, the application of PGT-A to patients younger than 38 years old and to those who do not meet the diagnostic standards of RPL or RIF still remains highly controversial [21–23].
In the present study, our main objective was to investigate the associations of the number of previous pregnancy failures (including implantation failures, biochemical pregnancy losses, and early and late miscarriages) with blastocyst aneuploidy and pregnancy outcomes after PGT-A.
Materials and methods
Study population
This retrospective analysis was conducted in 792 couples who underwent PGT-A from January 2013 to October 2018 at the Reproductive Medical Center of Shandong University. The analysis included women aged 20–38 years old who underwent PGT-A for RPL, RIF, or multiple pregnancy failures. Implantation failure (IF) was defined as the failure to achieve a biochemical pregnancy (positive serum hCG test) after the transfer of good-quality embryos in previous fresh or frozen cycles. This definition did not include biochemical pregnancy loss (BPL). The control group comprised patients who insisted on PGT-A after a single spontaneous abortion and whose villus tissues yielded abnormal genetic testing results, as well as women with ≤ 2 IFs and ≤ 1 BPL. Couples who fulfilled the following exclusion criteria were not included in the analysis: Couples underwent PGT for structural rearrangements (PGT-SR) or PGT for monogenic (PGT-M); Couples underwent PGT-A only for advanced age, with no history of previous pregnancy failures; Prior pregnancy failures occurred during the cycles of PGT-A; Couples were diagnosed with clear causes for implantation failures or miscarriages, such as submucous myoma, hydrosalpinx, or antiphospholipid syndrome.
Ethical approval for the use and analysis of information and data from patients who underwent PGT was obtained from the Ethics Committee of Reproductive Medicine Center of Shandong University. Informed consent was obtained from all of the patients included in the study.
Study procedures
Appropriate ovarian stimulation protocols were given according to each patient’s ovarian reserve function and prior response to gonadotropin. These protocols included long and short GnRH agonist, antagonist, and mild stimulation protocols. An hCG (Pregnyl, Livzon, Guangdong, China) trigger for final oocyte maturation was administrated when at least 2 follicles with diameters ≥ 18 mm were detected, and transvaginal ultrasound-guided oocyte retrieval was performed 34–36 h later. For all patients, fertilization was achieved by intracytoplasmic sperm injection (ICSI). All of the embryos were cultured in the sequential media to the blastocyst stage.
Good-quality blastocysts were selected according to Gardner criteria [24] on Day 5 or 6 of embryo culture for trophectoderm biopsy, which was completed by an experienced embryologist with laser method. A total of 2975 blastocysts were genetically screened, of which there were 1598 blastocysts tested using array comparative genomic hybridization (array-CGH), and 1377 blastocysts tested using next-generation sequencing (NGS).
All of the embryos were subsequently frozen, and it was suggested that only a single euploid blastocyst can be transferred after the second menses following oocyte retrieval. However, 13 couples insisted on the transfer of 2 embryos in a single transfer cycle. No mosaic embryos were transferred in this study. The endometrium was prepared via either a natural ovulation cycle or an artificial regimen, depending on the individual patient’s condition. Luteal-phase support with twice-daily oral dydrogesterone (20 mg; Duphaston, Abbott, USA) and either daily vaginal progesterone capsules (200 mg; Utrogestan, Besins Manufacturing, Belgium) or vaginal progesterone gel (90 mg; Crinone, Merck Serono, Switzerland) was initiated when the endometrial thickness reached at least 7 mm and continued until 12 weeks of gestation.
The serum hCG concentration was measured 2 weeks after embryo transfer, at which time conception was diagnosed if the hCG level was ≥ 25 mIU/mL. A transvaginal ultrasound scan was performed 5 weeks after embryo transfer, and clinical pregnancy was diagnosed if an intrauterine gestational sac was observed. A repeat ultrasound scan was performed 10 weeks after embryo transfer, and an ongoing pregnancy was diagnosed following the ultrasonographic detection of a fetal heartbeat. Follow-up evaluations were implemented at fixed time intervals until the termination of the cycle following a single oocyte retrieval (live birth, no available embryos, or abandonment). Live birth was defined as the delivery of a viable infant at ≥ 28 weeks of gestation after embryo transfer.
Statistical analysis
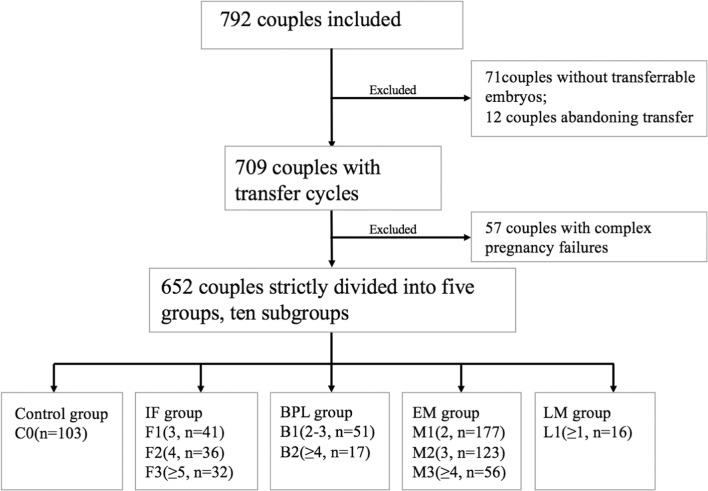
After excluding the couples who had suffered complex pregnancy failures, 652 out of 709 couples with transfer cycles were initially categorized into 5 groups, as well as 10 subgroups, according to the number of prior pregnancy failures and the definitions of RIF and RPL, as depicted in Fig. 1. The following groups were designated: the control group C0 (n = 103), defined as couples with ≤ 2 IFs, ≤ 1 BPL, ≤ 1 EM, and no LM; the IF group, including the F1 (n = 41), F2 (n = 36), and F3 (n = 32) subgroups, defined as couples with 3, 4, or ≥ 5 IFs, respectively; the BPL group, including the B1 (n = 51) and B2 (n = 17) subgroups, defined as couples with 2–3 or ≥ 4 BPLs, respectively; the early miscarriage (EM) group, including M1 (n = 177), M2 (n = 123), and M3 (n = 56) subgroups, defined as couples with 2, 3, or ≥ 4 EMs, respectively; and the LM group, including the L1 (n = 16) subgroup of couples with ≥ 1 LM. The populations with 2 and 3 BPLs had very similar blastocyst aneuploidy rates (BARs) and pregnancy outcomes and were combined into the B1 group. The BAR, implantation rate (IR), early miscarriage rate (EMR), live birth rate (LBR) per transfer cycle, and LBR per retrieval were calculated for each subgroup, and these values were compared separately with those of group C0. The LBR per retrieval referred to the cumulative LBR after a complete IVF cycle with PGT-A. A complete cycle was defined as all transfer cycles in an IVF cycle initiated by a single oocyte retrieval and ended with a live birth, no available embryos, or abandonment. Differences were examined using a non-parametric test or Pearson chi-square test.
Fig. 1.
Inclusion and classification of couples in this study. IF implantation failure, BPL biochemical pregnancy loss, EM early miscarriage, LM late miscarriage
Subsequently, multiple linear regression and binary logistic regression models were constructed separately and used to evaluate the associations of previous pregnancy failures with BAR or pregnancy outcomes. The models were also used to adjust potential confounders, including maternal age, BMI, AMH, ovarian stimulation protocols, prior live birth, number of prior intrauterine operations, number of oocytes retrieved, number of D5 good-quality embryos, and semen quality on the day of oocyte retrieval. Different types of previous pregnancy failures were also considered as important confounding factors for each other. The ovarian stimulation protocols, number of prior pregnancy failures, and number of prior intrauterine operations were considered as multicategorical variables and transformed into dummy variables.
A P value < 0.05 was considered statistically significant. All of the statistical analyses were performed using SPSS version 20.0 (Chicago, IL, USA) and Graphpad Prism 6 software (Graphpad Software, San Diego, CA, USA).
Results
The study included 792 couples undergoing PGT-A. The mean age of the women in these couples was 32.36 ± 3.55 years. Overall, 2975 blastocysts were genetically tested using array-CGH or NGS, and 1216 blastocysts were confirmed to be aneuploid (40.87%). Seventy-one couples had no transferrable embryos (8.96%) because all of the embryos were aneuploid or did not survive the freezing and thawing process. Twelve couples abandoned the transfer despite the availability of genetically transferrable embryos. Finally, 932 blastocysts were transferred to 709 couples during 919 transfer cycles (out of 709 complete cycles). Overall, the IR was 58.91% (549/932), the EMR among clinical pregnancies was 13.28% (72/542), and the LBRs per transfer and per retrieval were 48.42% (445/919) and 62.76% (445/709), respectively.
The baseline characteristics, including age, BMI, AMH, basal FSH, number of prior intrauterine operations, thickness of the endometrium on the trigger day, sperm quality on the day of oocyte retrieval, number of oocytes retrieved, and number of good-quality blastocysts on Day 5 of embryo culture, were compared between the 10 individual subgroups and the C0 group. Most of the baseline data in the subgroups were comparable with those of C0 (Supplement Table 1). Notably, we observed a gradual increase in the number of prior intrauterine operations and a gradual decrease in the thickness of the endometrium on the trigger day as the number of prior EMs increased (P < 0.05).
Association of prior IFs with blastocyst aneuploidy and pregnancy outcomes after PGT-A
As illustrated in Table 1 and Supplement Fig. 1, the BAR increased gradually as the number of prior IFs increased. Specifically, groups F2 and F3 had significantly higher BARs than group C0 (42.86% and 48.80% vs. 33.05%, P = 0.044 and P = 0.002; respectively). As shown in Table 2, the multiple linear regression analysis revealed that both 4 and ≥ 5 prior IFs were associated with an increased BAR (B = 10.23, 95% confidence interval [CI] 1.48–18.98, P = 0.022 and B = 14.43, 95% CI 4.91–23.96, P = 0.003, respectively).
Table 1.
Subgroup analysis of blastocyst anueploidy rate and pregnancy outcomes in 652 couples with prior pregnancy failures
| Groups | Subgroups (no. of prior pregnancy failures) | Blastocyst aneuploidy rate (%) | Pe | Implantation rate (%)a | Pe | Early miscarriage rate (%)b | Pe | Live birth rate per transfer cycle (%)c | Pe | Live birth rate per retrieval (%)d | Pe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | C0 | 33.05 (117/354) | 58.91 (76/129) | 6.58 (5/76) | 53.49 (69/129) | 66.99 (69/103) | |||||
| IF | F1(3) | 39.49 (62/157) | 0.16 | 50.91 (28/55) | 0.32 | 3.57 (1/28) | 1.00 | 49.09 (27/55) | 0.59 | 65.85 (27/41) | 0.90 |
| F2(4) | 42.86 (57/133) | 0.044* | 63.64 (28/44) | 0.58 | 10.71 (3/28) | 0.77 | 52.27 (23/44) | 0.89 | 63.89 (23/36) | 0.74 | |
| F3(≥ 5) | 48.80 (61/125) | 0.002* | 55.00 (22/40) | 0.66 | 13.64 (3/22) | 0.53 | 47.5 (19/40) | 0.51 | 59.38 (19/32) | 0.43 | |
| BPL | B1(2–3) | 28.64 (59/206) | 0.28 | 53.62 (37/69) | 0.47 | 10.81 (4/37) | 0.68 | 46.38 (32/69) | 0.34 | 62.75 (32/51) | 0.60 |
| B2(≥ 4) | 43.84 (32/73) | 0.078 | 61.90 (13/21) | 0.80 | 7.69 (1/13) | 1.00 | 57.14 (12/21) | 0.76 | 70.59 (12/17) | 0.77 | |
| EM | M1(= 2) | 37.57 (266/708) | 0.15 | 62.08 (149/240) | 0.55 | 10.34 (15/145) | 0.35 | 53.02 (123/232) | 0.93 | 69.49 (123/177) | 0.66 |
| M2(= 3) | 38.61 (195/505) | 0.10 | 58.75 (94/160) | 0.98 | 14.13 (13/92) | 0.115 | 47.77 (75/157) | 0.34 | 60.98 (75/123) | 0.35 | |
| M3(≥ 4) | 41.00 (98/239) | 0.048* | 56.25 (45/80) | 0.70 | 31.11 (14/45) | <0.001* | 34.18 (27/79) | 0.007* | 48.21 (27/56) | 0.021* | |
| LM | L1(≥ 1) | 32.20 (19/59) | 0.90 | 83.33 (15/18) | 0.05 | 13.33 (2/15) | 0.71 | 61.11 (11/18) | 0.54 | 68.75 (11/16) | 0.89 |
IF implantation failure, BPL biochemical pregnancy loss, EM early miscarriage, LM late miscarriage
*Significant difference
aIR in subgroups was calculated as number of embryos implanted divided by number of total embryos transferred
bEMR in subgroups was calculated as number of early miscarriages divided by number of clinical pregnancies
cLBR per transfer cycle in subgroups was calculated as number of live births by number of total transfer cycles
dLBR per retrieval in subgroups was calculated as number of live births by number of total cycles after an oocyte retrieval
eP value was yield from comparison between individual subgroups and C0
Table 2.
Multiple linear regression analysis for associations of demographic and clinical variables with blastocyst aneuploidy in 709 couples with prior pregnancy failures
| Variable | B | Standard error | 95%CI | P |
|---|---|---|---|---|
| Age | 0.65 | 0.29 | 0.08–1.23 | 0.026* |
| BMI | − 0.20 | 0.28 | − 0.75–0.34 | 0.46 |
| AMH | 0.31 | 0.27 | − 0.21–0.84 | 0.25 |
| Ovarian stimulation protocols | ||||
| Long | ||||
| Antagonist | − 0.99 | 3.00 | − 6.89–4.90 | 0.74 |
| Short | − 3.71 | 2.39 | − 8.41–0.99 | 0.12 |
| Ultra-long | 3.43 | 5.42 | − 7.22–14.07 | 0.53 |
| Others | − 3.60 | 7.22 | − 17.77–10.58 | 0.62 |
| Prior live birth | − 1.41 | 2.71 | − 6.74–3.92 | 0.61 |
| IF | ||||
| ≤ 2 | ||||
| 3 | 4.53 | 3.99 | − 3.32–12.37 | 0.26 |
| 4 | 10.23 | 4.46 | 1.48–18.98 | 0.022* |
| ≥ 5 | 14.43 | 4.85 | 4.91–23.96 | 0.003* |
| BPL | ||||
| ≤ 1 | ||||
| 2–3 | − 1.10 | 3.43 | − 7.83–5.64 | 0.75 |
| ≥ 4 | 11.75 | 6.13 | − 0.27–23.78 | 0.06 |
| EM | ||||
| ≤ 1 | ||||
| 2 | 0.96 | 2.94 | − 4.82–6.74 | 0.75 |
| 3 | 5.40 | 3.53 | − 1.54–12.34 | 0.13 |
| ≥ 4 | 9.23 | 4.60 | 0.20–18.27 | 0.045* |
| Prior LM | 7.71 | 3.99 | − 0.13–15.54 | 0.05 |
IF implantation failure, BPL biochemical pregnancy loss, EM early miscarriage, LM late miscarriage, B observed coefficient, CI confidence interval
*Significant difference
After the transfer of optimal blastocysts, the IF and C0 group had comparable IR, EMR, and LBR (Table 1 and Supplement Figs. 2–5), consistent with the results of the logistic regression model (shown in Table 3).
Table 3.
Logistic regression analysis of the associations of demographic and clinical variables with pregnancy outcomes after PGT-A in 709 couples with prior pregnancy failures
| Variables | Implantation | Early miscarriage | Live birth | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | aOR | 95%CI | P | aOR | 95%CI | P | aOR | 95%CI | |
| Age | 0.09 | 0.95 | 0.90–1.01 | 0.17 | 0.95 | 0.88–1.02 | 0.34 | 0.98 | 0.93–1.03 |
| BMI | 0.38 | 1.02 | 0.97–1.08 | 0.07 | 1.07 | 0.99–1.14 | 0.90 | 1.00 | 0.96–1.05 |
| AMH | 0.03* | 1.06 | 1.01–1.13 | 0.81 | 0.99 | 0.92–1.06 | 0.07 | 1.05 | 0.99–1.10 |
| Ovarian stimulation protocols | |||||||||
| Long | – | ||||||||
| Antagonist | 0.82 | 0.94 | 0.54–1.63 | 0.54 | 1.26 | 0.59–2.70 | 0.50 | 0.84 | 0.51–1.38 |
| Short | 0.96 | 0.99 | 0.65–1.52 | 0.93 | 0.97 | 0.51–1.85 | 0.67 | 0.92 | 0.62–1.36 |
| Ultra-long | 0.68 | 0.82 | 0.32–2.11 | 0.67 | 0.71 | 0.15–3.46 | 0.65 | 0.82 | 0.34–1.96 |
| Others | 0.26 | 0.51 | 0.16–1.64 | 0.91 | 0.88 | 0.10–7.55 | 0.29 | 0.54 | 0.17–1.7 |
| Prior live birth | 0.71 | 1.10 | 0.68–1.79 | 0.42 | 1.33 | 0.67–2.68 | 0.62 | 1.12 | 0.72–1.76 |
| IF | |||||||||
| ≤ 2 | – | ||||||||
| 3 | 0.23 | 0.66 | 0.34–1.30 | 0.70 | 0.74 | 0.16–3.47 | 0.62 | 0.85 | 0.44–1.64 |
| 4 | 0.54 | 1.30 | 0.56–3.01 | 0.19 | 2.27 | 0.66–7.76 | 0.62 | 0.83 | 0.40–1.73 |
| ≥ 5 | 0.65 | 0.82 | 0.36–1.90 | 0.09 | 2.99 | 0.84–10.73 | 0.28 | 0.65 | 0.30–1.42 |
| BPL | |||||||||
| ≤ 1 | – | ||||||||
| 2–3 | 0.55 | 0.83 | 0.45–1.53 | 0.37 | 1.58 | 0.59–4.25 | 0.32 | 0.75 | 0.43–1.32 |
| ≥ 4 | 0.83 | 1.14 | 0.35–3.67 | 0.35 | 2.17 | 0.43–10.92 | 0.53 | 0.72 | 0.27–1.97 |
| EM | |||||||||
| ≤ 1 | – | ||||||||
| 2 | 0.91 | 1.03 | 0.60–1.77 | 0.22 | 1.72 | 0.725–4.1 | 0.65 | 0.89 | 0.55–1.46 |
| 3 | 0.91 | 1.04 | 0.54–2.00 | 0.07 | 2.51 | 0.95–6.64 | 0.29 | 0.73 | 0.41–1.31 |
| ≥ 4 | 0.69 | 1.18 | 0.51–2.74 | 0.001* | 6.49 | 2.17–19.46 | 0.120 | 0.56 | 0.26–1.17 |
| Prior LM | 0.90 | 1.05 | 0.51–2.18 | 0.007* | 3.16 | 1.36–7.35 | 0.024* | 0.48 | 0.25–0.91 |
| No. of prior intrauterine operations | |||||||||
| ≤ 1 | – | ||||||||
| 2 | 0.49 | 1.19 | 0.73–1.95 | 0.55 | 1.24 | 0.62–2.50 | 0.58 | 1.13 | 0.73–1.76 |
| 3 | 0.90 | 1.04 | 0.54–2.02 | 0.68 | 0.82 | 0.32–2.13 | 0.79 | 1.08 | 0.60–1.95 |
| ≥ 4 | 0.06 | 0.50 | 0.25–1.01 | 0.73 | 0.85 | 0.33–2.18 | 0.038* | 0.51 | 0.27–0.96 |
IF implantation failure, BPL biochemical pregnancy loss, EM early miscarriage, LM late miscarriage, aOR adjusted odds ratio
*Significant difference
Association of prior BPLs with blastocyst aneuploidy and pregnancy outcomes after PGT-A
In a subgroup analysis, the BPL and C0 groups did not differ significantly with respect to the BAR and pregnancy outcomes (shown in Table 1 and Supplement Figs. 1–5). The BAR was higher in group B2 than in group C0, although this difference was not significant (43.84% vs. 33.05%, P = 0.078). The multiple linear regression and logistic regression analyses yielded no significant correlations between the number of prior BPLs and blastocyst aneuploidy or pregnancy outcomes after PGT-A (shown in Tables 2 and 3).
Association of prior EMs with blastocyst aneuploidy and pregnancy outcomes after PGT-A
The BAR varied little as the number of prior EMs increased (shown in Table 1 and Supplement Fig. 1), although group M3 had a significantly higher BAR than group C0 (41.00% vs. 33.05%, P = 0.048). Furthermore, the coefficient estimated from the multiple linear regression analysis indicated that couples with ≥4 EMs exhibited a significant tendency towards aneuploid blastocysts (B = 9.23, 95% CI 0.20–18.27, P = 0.045), whereas no significant correlation was observed between 2 EMs and blastocyst aneuploidy (Table 2).
Next, a subgroup analysis of pregnancy outcomes after PGT-A was conducted (shown in Table 1 and Supplement Figs. 2–5). Within the EM group, the EMR increased gradually and the LBR decreased gradually as the number of prior EMs increased, whereas the IR remained fairly steady. Group M3 had a significantly higher EMR than group C0 (31.11% vs. 6.58%, P < 0.001) but a significantly lower LBR per transfer cycle (34.18% vs. 53.49%, P = 0.007) and LBR per retrieval (48.21% vs. 66.99%, P = 0.021). After adjusting potential confounders, particularly the number of prior intrauterine operations, we confirmed that ≥ 4 prior EMs led to a high risk of subsequent EM (OR = 6.49, 95% CI 2.17–19.46, P = 0.001) but were not associated directly with the likelihood of live birth (Table 3). Notably, no distinct relationship was observed between a history of 2 prior EMs and pregnancy outcomes after the transfer of euploid embryos (Tables 1 and 3 and Supplement Figs. 2–5).
The logistic regression analysis revealed that a history of ≥ 4 prior intrauterine operations correlated negatively with the outcome of live birth (OR = 0.51, 95% CI 0.27–0.96, P = 0.038; Table 3). The results of a subgroup analysis of pregnancy outcomes within the EM group after stratification by the number of prior intrauterine operations are shown in Supplement Table 2. In group M3, couples with ≥ 4 prior intrauterine operations had a significantly lower LBR than those in group C0 (LBR per transfer, 24.44% vs. 53.49%, P = 0.001; LBR per retrieval, 34.38% vs. 66.99%, P = 0.001).
Association of prior LMs with blastocyst aneuploidy and pregnancy outcomes after PGT-A
The results of the logistic regression analysis revealed that prior LMs could potentially increase the risk of EM and adversely affect the outcome of live birth (OR for EM = 3.16, 95% CI 1.36–7.35, P = 0.007; OR for live birth = 0.48, 95% CI 0.25–0.91, P = 0.024; Table 3).
Discussion
To the best of our knowledge, this was the first comprehensive study to determine the associations of various prior pregnancy failures with blastocyst aneuploidy and pregnancy outcomes after PGT-A via NGS or array-CGH. Notably, we observed that a history of BPLs and two EMs had no significant association with blastocyst aneuploidy or pregnancy outcomes after PGT-A, a history of high-order IFs (n ≥ 4) or EMs (n ≥ 4) was significantly associated with an elevated risk of blastocyst aneuploidy and a history of ≥ 4 EMs or LM(s) was shown to correlate strongly with an increase in EMR and a decrease in LBR after a PGT-A cycle.
Initially, we confirmed that blastocyst aneuploidy increased gradually as the number of prior IFs increased and that a history of ≥ 4 IFs was significantly associated with a greater number of aneuploid blastocysts in PGT-A cycles. After euploid embryo transfer, the IF and control groups achieved similar pregnancy outcomes, consistent with the conclusion from a 2014 pilot RCT study that determined similar clinical pregnancy and implantation rates in the RIF PGS and NO RIF PGS groups (68.3% and 70.5%, respectively) [25]. This provides evidence for the establishment of a more accurate definition of RIF and the application of PGT-A for these patients.
One novel finding was the relative lack of significant relationships between the number of prior BPLs and blastocyst aneuploidy and pregnancy outcomes after PGT-A. Although a relatively higher BAR was observed among women with ≥ 4 prior BPLs, this difference was non-significant. A 2014 retrospective study of 587 women with ≥ 3 consecutive clinical miscarriages or non-visualized pregnancy losses showed that each additional non-visualized pregnancy loss decreased the RR of live birth by 10%, equivalent to the risk conferred by a clinical miscarriage [16]. The reasons for this inconsistency could be the limited sample size in the group B1 and B2 (n = 51 and 17, respectively), which compromised the reliability of this outcome, especially for the population with ≥ 4 prior BPL. Thus, the correlation of prior BPL with blastocyst aneuploidy and pregnancy outcomes will require further investigation in a large sample. We note further that both the 2014 study [16] and the 2017 Guideline published by the European Society of Human Reproduction and Embryology (ESHRE) [14] explicitly included BPL into the definition of RPL, whereas a 2014 review [15] classified BPL as an implantation failure. We observed an obvious distinction between the BPL and IF or EM groups in terms of both blastocyst aneuploidy and PGT-A pregnancy outcomes, of which the latter reflected other etiologies except embryonic factor. We believe that the impact and classification of BPL warrant further clarification and discussion.
After subdividing the EM group according to the number of prior EMs, we observed that patients with a history of 2 spontaneous EMs had a similar BAR to that of the control group, as well as a good prognosis after PGT-A. A recent prospective register-based study of a large sample in Norway evaluated the association between recurrence risk of miscarriage and pregnancy history and showing that there was an OR of 2.21 (2.03 to 2.41) for subsequent miscarriage after 2 miscarriages [5]. However, another prospective cohort study of 5465 subjects has reported that depression was common (OR = 1.65, 1.01–2.70) among women with 2 prior miscarriages, and they had higher scores for limiting/resting behavior in pregnancy [26]. With psychological supportive care and selective medical management, women with a history of 2 consecutive EMs achieved a good prognosis with LBR of 72.7% [27]. Currently, the definitions of RPL still varies from 2 miscarriages according to the American Society for Reproductive Medicine (ASRM) [13] and ESHRE [14] to 3 consecutive pregnancy losses according to the Royal College of Obstetricians and Gynecologists (RCOG) [12]. As the diagnosis of RPL tends to result in excessive investigation and management, with associated financial burden on the patients, the inclusion of 2 prior miscarriages in the definition of RPL and the application of PGT-A to these patients require serious consideration.
We further confirmed an association of ≥ 4 prior EMs with an increase in embryo aneuploidy and a significantly higher risk of subsequent miscarriage and a low LBR, even after the transfer of optimal embryos. Possibly, repetitive curettages due to recurrent miscarriages could seriously impair the endometrial receptivity and cause a thin endometrium. Moreover, high-order miscarriages may reflect a highly incompetent uterine environment and disrupted endometrium-embryo crosstalk per se. To investigate the specific reasons underlying these adverse post-PGT pregnancy outcomes, we performed a further subgroup analysis after stratifying women according to the number of prior intrauterine operations and performed a logistic regression analysis. We determined that a history of ≥ 4 prior EMs was an independent prognostic factor for subsequent miscarriage in our sample, while a history of excessive (≥ 4) intrauterine operations contributed to the low LBR after PGT-A. Two previous cohort studies emphasized the importance of the number of previous miscarriages in patients with RPL, which was a prognostic factor for subsequent pregnancy outcomes and significantly associated with time to live birth [4, 6]. As the interaction between recurrent miscarriages and endometrial receptivity generates a vicious cycle, PGT-A can be used as a shortcut to achieve a live birth and reduce further impairments as much as possible [28]. Meanwhile, more emphasis should be placed on developing methods to improve endometrial receptivity, which is another key to success of ART [29].
Despite the lack of an association between the number of LMs and blastocyst aneuploidy, we observed that LM significantly increased the risk of EM and consequently reduced the LBR of the subsequent pregnancies. This result was consistent with the conclusion reported by Egerup et al. [7], in which the number of prior LM was confirmed as an adverse prognostic factor through both univariate analyses with chi-square test and multivariate analyses with Poisson regression. The authors of that study ascribed the impact of LMs to the associated inflammatory and/or thrombotic factor.
One strength of the present study was the inclusion of patients who underwent PGT cycles as research subjects. This enabled us to investigate the prognostic impacts of different types of pregnancy failures from both the embryonic and uterine perspectives. Another strength was the complete and strict classification of various pregnancy failures, which minimized mutual interference and enabled studies of the individual impacts. Furthermore, the LBR per retrieval was calculated as the primary endpoint because this is the most relevant outcome and the ultimate goal of infertility treatment. However, our study also had some limitations. First, the control group comprised couples with ≤ 2 IFs, ≤ 1 BPL or EM, and no LM, rather than those with no history of pregnancy failure. The latter, except patients with advanced age, is not the common indication of PGT-A in our country. Second, the sample sizes were relatively limited in some subgroups, particularly F2, B2, and L1. Finally, the study was subject to the inherent limitations of the retrospective design.
In conclusion, the associations of different types of previous pregnancy failures with embryonic viability and pregnancy outcomes are extremely complicated. We determined that a history of high-order IFs or EMs and the existence of LM(s) were significantly associated with blastocyst aneuploidy and adverse pregnancy outcomes after PGT-A, whereas a history of BPLs or 2 EMs was not a significant factor. Nowadays, PGT has become an important tool for selecting embryos, but whether previous BPLs and low-order pregnancy failures should be listed as the indications for PGT-A needs serious consideration. More importantly, endometrial factors should also be considered as a history of high-order pregnancy failures, especially ≥ 4 EMs, was associated with a poor pregnancy prognosis even after the transfer of selected euploid embryos. However, the improvement of endometrial receptivity remains an intractable clinical problem. Finally, our findings provide evidence to support more unified definitions of RIF and RPL.
Electronic supplementary material
(DOCX 611 kb)
(DOCX 28 kb)
(DOCX 17 kb)
Acknowledgments
The authors would like to thank Jingfu Yang for his invaluable help during data collection. The authors are indebted to every member from the IVF and PGT laboratory for their tremendous contributions to laboratory procedures.
Author contribution
All authors contributed to the study conception and design. Junhao Yan and Zi-Jiang Chen conceived and designed the study. Tianxiang Ni analyzed the data and drafted the manuscript. Qianqian Wu, Yueting Zhu and Wenjie Jiang collected and verified the data; Qian Zhang and Yan Li revised the manuscript. All authors were involved in interpreting the data and approved the final manuscript.
Funding information
The study was funded by the National Key Research and Development Program of China (2016YFC1000202, 2018YFC1002804) and the National Natural Science Foundation of China (81671522).
Compliance with ethical standards
Ethical approval for the use and analysis of information and data from patients who underwent PGT was obtained from the Ethics Committee of Reproductive Medicine Center of Shandong University. Informed consent was obtained from all of the patients included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schieve LA, Tatham L, Peterson HB, Toner J, Jeng G. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstet Gynecol. 2003;101(5 Pt 1):959–967. doi: 10.1016/s0029-7844(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 2.Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65(3):503–509. doi: 10.1016/S0015-0282(16)58144-8. [DOI] [PubMed] [Google Scholar]
- 3.Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8(1):e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaandorp SP, van Mens TE, Middeldorp S, Hutten BA, Hof MH, van der Post JA, et al. Time to conception and time to live birth in women with unexplained recurrent miscarriage. Hum Reprod. 2014;29(6):1146–1152. doi: 10.1093/humrep/deu052. [DOI] [PubMed] [Google Scholar]
- 5.Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ (Clinical research ed) 2019;364:l869. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund M, Kamper-Jorgensen M, Nielsen HS, Lidegaard O, Andersen AM, Christiansen OB. Prognosis for live birth in women with recurrent miscarriage: what is the best measure of success? Obstet Gynecol. 2012;119(1):37–43. doi: 10.1097/AOG.0b013e31823c0413. [DOI] [PubMed] [Google Scholar]
- 7.Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non-consecutive losses? Hum Reprod. 2016;31(11):2428–2434. doi: 10.1093/humrep/dew169. [DOI] [PubMed] [Google Scholar]
- 8.Kling C, Hedderich J, Kabelitz D. Fertility after recurrent miscarriages: results of an observational cohort study. Arch Gynecol Obstet. 2018;297(1):205–219. doi: 10.1007/s00404-017-4532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kling C, Magez J, Hedderich J, von Otte S, Kabelitz D. Two-year outcome after recurrent first trimester miscarriages: prognostic value of the past obstetric history. Arch Gynecol Obstet. 2016;293(5):1113–1123. doi: 10.1007/s00404-015-4001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauniaux E, Farquharson RG, Christiansen OB, Exalto N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21(9):2216–2222. doi: 10.1093/humrep/del150. [DOI] [PubMed] [Google Scholar]
- 11.Thornhill AR, deDei-Smulders CE, Geraedts JP, Harper JC, Harton GL, Lavery SA, et al. ESHRE PGD consortium 'Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS)'. Hum Reprod. 2005;20(1):35–48. doi: 10.1093/humrep/deh579. [DOI] [PubMed] [Google Scholar]
- 12.Regan LRR, Backos M. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. RCOG Green Top Guideline. 2011;17:1–17. [Google Scholar]
- 13.ASRM Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Atik RB, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Human Reproduction Open. 2018. 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed]
- 15.Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod BioMed Online. 2014;28(1):14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Kolte AM, van Oppenraaij RH, Quenby S, Farquharson RG, Stephenson M, Goddijn M, et al. Non-visualized pregnancy losses are prognostically important for unexplained recurrent miscarriage. Hum Reprod. 2014;29(5):931–937. doi: 10.1093/humrep/deu042. [DOI] [PubMed] [Google Scholar]
- 17.ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):hoy004. 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed]
- 18.Munne S. Status of preimplantation genetic testing and embryo selection. Reprod BioMed Online. 2018;37(4):393–396. doi: 10.1016/j.rbmo.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Rubio C, Rodrigo L, Garcia-Pascual C, Peinado V, Campos-Galindo I, Garcia-Herrero S, et al. Clinical application of embryo aneuploidy testing by NGS. Biol Reprod. 2019. 10.1093/biolre/ioz019. [DOI] [PubMed]
- 20.Sato T, Sugiura-Ogasawara M, Ozawa F, Yamamoto T, Kato T, Kurahashi H, et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. 2019;34(12):2340–8. 10.1093/humrep/dez22920. [DOI] [PubMed]
- 21.Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104(6):1503–1512. doi: 10.1016/j.fertnstert.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 22.Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36(4):629–636. doi: 10.1007/s10815-018-01399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munne S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019. 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed]
- 24.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Mortimer JR, editor. Toward reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. pp. 378–388. [Google Scholar]
- 25.Greco E, Bono S, Ruberti A, Lobascio AM, Greco P, Biricik A, Spizzichino L, Greco A, Tesarik J, Minasi MG, Fiorentino F. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. Biomed Res Int. 2014;2014:457913. doi: 10.1155/2014/457913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy FP, Moss-Morris R, Khashan AS, North RA, Baker PN, Dekker G, et al. Previous pregnancy loss has an adverse impact on distress and behaviour in subsequent pregnancy. BJOG. 2015;122(13):1757–1764. doi: 10.1111/1471-0528.13233. [DOI] [PubMed] [Google Scholar]
- 27.Green DM, O'Donoghue K. A review of reproductive outcomes of women with two consecutive miscarriages and no living child. Journal of Obstetrics and Gynaecology. 2019:1–6. 10.1080/01443615.2019.1576600. [DOI] [PubMed]
- 28.Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, et al. Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 2018;110(5):896–904. doi: 10.1016/j.fertnstert.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611–617. doi: 10.1016/j.fertnstert.2019.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 611 kb)
(DOCX 28 kb)
(DOCX 17 kb)