Abstract
Purpose
To evaluate clinical outcomes after frozen-thawed blastocyst transfer (TBT) with blastocysts which were derived from different cell numbers on day 3.
Methods
The study included 1444 patients undergoing single autologous frozen-thawed blastocyst transfer cycles, which were allocated to five groups according to the cell numbers on day 3 of the transferred blastocysts: ≤ 6-cell (n = 109), 7-cell (n = 169), 8-cell (n = 811), 9-cell (n = 136), and ≥ 10-cell (n = 219).
Results
The LBR of the ≤ 6-cell group was found to be statistically lower than that of the 8-cell group in single TBT cycles which had been transferred with fair quality blastocysts (defined as 4BB according to Gardner’s grading scale) (41.28% vs 55.73%, P = 0.004), while the miscarriage rate was significantly higher for the ≤ 6-cell group compared with the 8-cell group (25.00% vs 13.74%, P = 0.02). No differences were found between the two groups in terms of cPR (P = 0.06). However, for blastocysts categorized as high quality according to Gardner’s classification (defined as 4AA/4AB/4BA), cPR, LBR, and early miscarriage rates did not differ between the two groups (P = 0.76, P = 0.44, P = 0.40, respectively).
Conclusions
When transferring blastocysts, an evaluation of the cleavage stage should be performed along with blastocyst morphology to shorten the time of conceiving.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01664-x) contains supplementary material, which is available to authorized users.
Keywords: Frozen blastocyst transfer, Live birth rate, Cell number, Clinical pregnancy rate, Neonatal outcomes
Introduction
The ultimate goal of in vitro fertilization (IVF) is to select the most competent embryo to lead to a healthy newborn. In 2009, Centers for Disease Control and Prevention reported that just 19% of transferred embryos resulted in successful delivery [1]. In order to improve the clinical outcomes of females with advanced age and repeated IVF failures, two embryos on day 3 are transferred in some assisted reproductive centers. However, multiple pregnancies may lead to increased maternal and neonatal complications such as hypertension, hemorrhage, chorioamnionitis, preterm delivery, and low birth weight [2, 3]. Therefore, it is crucial to decrease the number of embryos transferred by selecting embryos with the highest developmental competence.
It has been well known that single embryo transfer (SET) is the best practice for reducing multiple pregnancies in Assisted Reproductive Technology (ART) [4]. In addition, elective single blastocyst transfers have advantages over elective single cleavage-stage embryo transfers [5]. Extended in vitro culture and blastocyst transfer is considered a successful approach in IVF as they allow better embryo selection following activation of the embryonic genome, which results in higher implantation rates [6, 7]. Although preimplantation genetic testing for aneuploidies (PGT-A) and time-lapse systems are frequently used in many labs, morphological evaluation systems are still the most widely used method in embryo selection. Morphological criteria, such as grading of inner cell mass (ICM), trophectoderm cells (TE), and blastocele expansion have been utilized for blastocyst evaluation. It has been reported that blastocysts which have the better grading of ICM and TE are related to a higher euploidy rate as well as a higher implantation rate when compared with blastocysts which have lower morphology scores [7]. Even for euploid blastocysts, morphological scores have been used to select blastocysts of higher implantation potential [8]. However, in clinical practice, it is common that there are some blastocysts with the same morphological grading for one patient, leading to the question of which is the optimal blastocyst to be transferred first. It has been asked whether the cell number of the cleavage-stage embryos on day 3 has an effect on the developmental competence of the homologous blastocysts. A theoretical solution to this problem will be beneficial for optimizing the sequence of freezing blastocysts which, in turn, helps minimize waiting time for a live birth. It is still largely unknown whether day 3 cleavage-stage embryos with different cell numbers relates to the implantation potential of blastocysts derived from them. Hence, the aim of this study was to assess whether day 3 cell numbers are associated with the final clinical outcomes of the homologous blastocysts.
Materials and methods
Study design and participant cohort
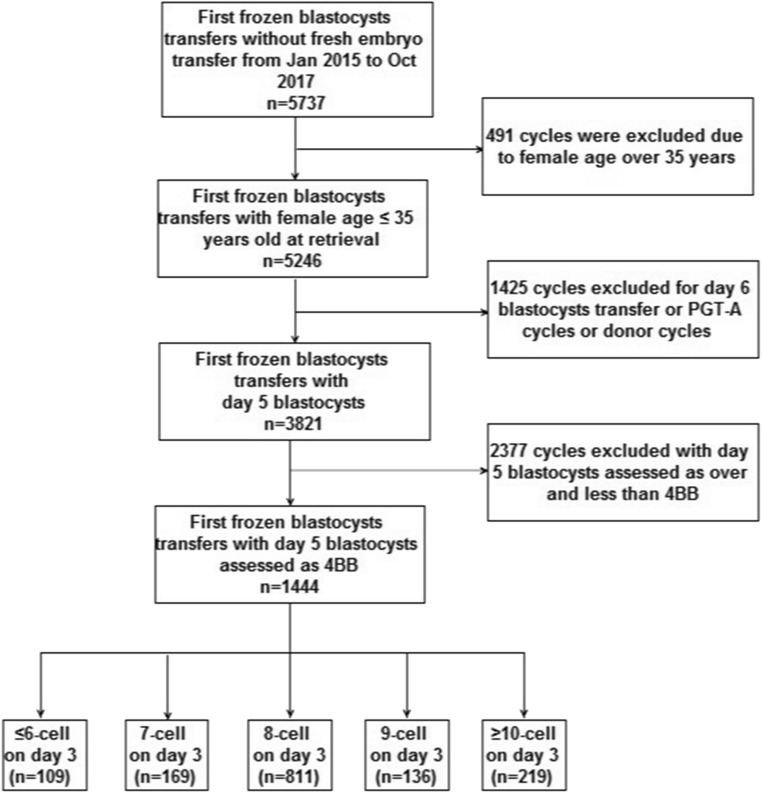
We performed a retrospective cohort study which included all first single autologous TBT cycles carried out between January 2015 and October 2017 at the Center for Reproductive Medicine in anonymity. The patients were divided into five groups according to the cell number on day 3 of the transferred blastocysts: (i) ≤ 6-cell; (ii) 7-cell; (iii) 8-cell; (iv) 9-cell; (v) ≥ 10-cell. During the study, 5737 first TBT embryo transfers without fresh cycles were performed. Blastocysts were drawn from “freeze all” strategy cycles for canceled embryo transfers in fresh cycles. Inclusion criteria were female age ≤ 35 years at retrieval, day 5 blastocysts assessed as 4BB according to criteria by Gardner and Lane [9], and participants undergoing a single autologous TBT. Exclusion criteria were donor cycles, preimplantation genetic testing for aneuploidies (PGT-A) cycles, day 6 blastocysts, day 5 blastocysts assessed as over or less than 4BB. The participant selection process is detailed in Fig. 1. The study’s primary outcome was LBR, while secondary outcomes included cPR, miscarriage rate, multiple pregnancy rate, and neonatal outcomes. This study was carried out in accordance with the guidelines of the International Conference on Harmonization and was approved by the Institutional Review Board of Reproductive Medicine at Shandong University (registration NO. 2013 IRB NO. 19).
Fig. 1.
Flow of patients through the trial
Ovarian stimulation, oocyte insemination, embryo culture, and scoring
There were no restrictions on ovarian stimulation protocols. Ovarian stimulation and oocyte collection were performed as has been previously described [10]. COCS were either inseminated in 4-well plates with approximately 100,000 motile spermatozoa for each oocyte (IVF), or they underwent introcytoplasmic sperm injection (ICSI). Sequential culture media from Vitrolife (G-IVF, G1, and G2; Scandinavian IVF Science, Sweden; 10,135, 10,127, and 10,131) was applied for all procedures. On day 3, two good quality embryos were routinely selected for transfer. Good quality embryos on day 3 (observation time: IVF, 68 hpi-68.5 hpi; ICSI, 67 hpi-67.5 hpi), were defined as ≥ 7 cells with a grade ≥ 3 according to criteria from Puissant [11]. All supernumerary embryos containing ≥ 4 cells with grade two and over were cultured until day 5 or six. On day 5/6 (observation time 119 hpi–120 hpi/143 hpi–144hpi), the quality of the blastocysts was evaluated using Gardner and Lane’s classification. Based on this classification, only high (4AA/4AB/4BA, 5AA/5AB/5BA), fair (4BB/5BB), and weak (4AC/4BC, 5AC/5BC) blastocysts were selected for cryopreservation (Supplementary Fig. 1). All other blastocysts were destroyed. Several equally skilled embryologists took turns at performing embryo grading and freezing to reduce the cases of discordance among them.
Blastocyst vitrification and thawing procedures
Vitrification was carried out using the Mukaida protocol with cryoloop [12]. The base medium contained 5 mg/ml HSA in HEPES-buffered–modified hTF medium. Blastocysts were firstly placed into the base medium before being transferred into the base medium containing 7.5% (v/v) DMSO and 7.5% (v/v) EG (vitrification solution I) for 2 min. Finally, blastocysts were suspended in the base medium containing 15% (v/v) DMSO and 15% (v/v) EG as well as 10 mg/ml Ficoll 70 (Pharmacia Biotech, Sweden, CB9248463) and 0.65 mol/l sucrose (vitrification solution II) for 30 s before being quickly plunged into liquid nitrogen. Warming was performed in a four-well multi-dish following the Mukaida protocol. Blastocysts were incubated in a base medium containing 0.33 mol/l sucrose (thawing solution I), a base medium containing 0.2 mol/l sucrose (thawing solution II), and a base medium for 2, 3, and 5 min at 37 °C, respectively.
Frozen-thawed blastocyst transfer
The protocols of endometrial preparation for frozen-thawed blastocyst transfer were carried out as previously described [10]. Vitrified blastocysts were thawed on the morning of transfer day. Assisted hatching was then applied to all thawed embryos half an hour after thawing. The blastocysts were incubated for 4 to 5 h before transfer, and only expanded blastocysts were transferred. In other cases, the transfer was canceled. Twelve days of luteal support was routinely provided after transfer, irrespective of pregnancy status. For patients with an ongoing pregnancy, progesterone was continued for 10 weeks of gestation, with the dosage gradually reduced after confirming the occurrence of the fetal heart.
Diagnosis of pregnancy and neonatal outcomes
An observation of fetal cardiac activity 7 weeks after transfer was defined as a clinical pregnancy. A live birth was defined as a delivery of a viable infant at ≥ 28 weeks of gestation. Early miscarriage was defined as complete pregnancy loss at < 20–28 weeks of gestation. Neonatal outcomes included gestational age at delivery (days), pre-term birth (< 37 weeks), and post-term birth (≥ 41 weeks), low birth weight (< 2500 g), and high birth weight (≥ 4000 g) [13].
Statistical analysis
The software package SPSS version 16.0 was used for statistical analysis. Student’s t distribution and chi-square (χ2) or Fisher’s exact test were used to analyze means and proportional values respectively. A P value of < 0.05 was considered statistically significant. In order to locate confounders that may be independently related to live birth, we performed a logistic regression analysis. Patient age (> 35 years old), endometrial thickness, day 3 cell number (≤ 6-cell versus 8-cell), blastocyst quality, and the day of blastocyst expansion (day 5 vs day 6) were entered into the multiple logistic regression model.
Results
Study population
The process of patients’ inclusion is detailed in Fig. 1. From the initial cohort of 5737 first TBT cycles without fresh embryo transfers which were conducted in our reproductive center during the study period, 4293 TBT cycles were excluded for the following reasons: female age over 35 years (n = 491), TBT cycles performed on day 6, PGT-A cycles and donor cycles (n = 1425), and TBT cycles with day 5 blastocysts assessed as over or less than 4BB according to Gardner and Lane’s criteria (n = 2377). In total, 1444 TBT cycles with single blastocyst transfers were included. Patients were divided into five groups based on the cell numbers on day 3 of the transferred blastocysts: ≤ 6-cell (n = 109), 7-cell group (n = 169), 8-cell group (n = 811), 9-cell group (n = 136), and ≥ 10-cell group (n = 219).
Baseline characteristics
Baseline characteristics were compared between the 8-cell (ref.) and ≤ 6-cell, 7-cell, 9-cell, and ≥ 10-cell groups, respectively. As shown in Table 1, no differences were found in any of the examined baseline characteristics between 8-cell groups and other groups. In addition, survival and expanding rates after vitrification-warming were 100% for all studied groups.
Table 1.
The demographics of TBT cycles with fair quality blastocysts derived from cleavage stage embryos with cell numbers of ≤ 6, 7, 8, 9, and ≥ 10 on day 3
| ≤6-cell derived | P | 7-cell derived | P | 8-cell derived (Ref.) | 9-cell derived | P | ≥ 10-cell derived | P | |
|---|---|---|---|---|---|---|---|---|---|
| NO. of cycles | 109 | – | 169 | – | 811 | 136 | – | 219 | – |
| Female age (years) (± SD) | 29.55 ± 3.46 | NS | 29.24 ± 3.40 | NS | 29.24 ± 3.34 | 28.88 ± 3.29 | NS | 29.47 ± 3.81 | NS |
| BMI (kg/m2) | 23.47 ± 4.17 | NS | 23.32 ± 3.71 | NS | 23.08 ± 3.47 | 23.11 ± 3.85 | NS | 23.61 ± 3.65 | NS |
| Type of infertility | |||||||||
| Primary | 52.29%(57/109) | NS | 56.21%(95/169) | NS | 59.31%(481/811) | 65.44%(89/136) | NS | 59.36% (130/219) | NS |
| Secondary | 47.71%(52/109) | NS | 43.79%(74/169) | NS | 40.69%(330/811) | 34.56%(47/136) | NS | 40.64% (89/219) | NS |
| Patient’s ovarian reserve | |||||||||
| Basal serum FSH (IU/l) | 5.96 ± 1.19 | NS | 5.87 ± 1.17 | NS | 6.14 ± 1.61 | 5.99 ± 1.33 | NS | 6.01 ± 1.50 | NS |
| Basal serum LH (IU/l) | 6.51 ± 4.05 | NS | 6.00 ± 3.91 | NS | 6.55 ± 4.26 | 6.48 ± 4.19 | NS | 7.07 ± 4.40 | NS |
| AMH (ng/ml) | 7.48 ± 4.30 | NS | 7.19 ± 4.49 | NS | 7.51 ± 4.68 | 6.89 ± 4.19 | NS | 7.94 ± 5.23 | NS |
| E2 on hCG trigger day (pg/ml) | 4976.04 ± 2507.70 | NS | 4968.50 ± 2191.64 | NS | 4870.56 ± 2279.81 | 4748.06 ± 2273.87 | NS | 4734.18 ± 2352.92 | NS |
| Duration of stimulation (days) | 10.12 ± 1.76 | NS | 10.08 ± 1.57 | NS | 10.12 ± 1.67 | 10.14 ± 1.47 | NS | 10.04 ± 1.59 | NS |
| Total dose of Gn administrated (IU) | 1819.23 ± 608.19 | NS | 1845.18 ± 501.24 | NS | 1874.15 ± 753.26 | 1833.21 ± 625.61 | NS | 1805.17 ± 527.65 | NS |
| Endometrial thickness (cm) | 0.95 ± 0.15 | NS | 0.97 ± 0.17 | NS | 0.97 ± 0.16 | 0.96 ± 0.17 | NS | 0.96 ± 0.15 | NS |
Ref. reference; BMI body mass index; FSH follicle-stimulating hormone; LH luteinizing hormone; AMH anti mullerian hormone; NS not significant
Values are presented as number (%) or mean ± SD
P value was compared with 8-cell derived blastocysts group by the chi-square (χ2) test or t test
Clinical and neonatal outcomes
As can be seen in Table 2, there were no significant differences in cPR between the ≤ 6-cell and 8-cell groups (55.05% vs 64.61%, P = 0.06). However, LBR for the ≤ 6-cell group was significantly lower than that of the 8-cell group (41.28% vs 55.73%, P = 0.004), while the miscarriage rate was statistically higher for the ≤ 6-cell group than the 8-cell group (25.00% vs 13.74%, P = 0.02). In addition, cPR, LBR, and miscarriage rates did not differ between the 8-cell group and the other groups (that is, the 7-cell, 9-cell, and ≥ 10-cell groups) (see Table 2). Regarding neonatal outcomes, infants in the 8-cell group had a significantly lower mean birth weight when compared to those from the ≥ 10-cell group (3.40 kg ± 0.54 vs 3.52 kg ± 0.49, P = 0.03). Additionally, the percentage of infants in the 8-cell group with a birth weight of ≤ 2500 g was significantly higher than that in the ≥ 10-cell group (5.29% vs 0.86%, P = 0.04), and the same trend was found for the rate of infants with premature deliveries (< 37 weeks GA) (8.05% vs 2.59%, P = 0.04) (Table 2).
Table 2.
Clinical outcomes of TBT cycles with fair quality blastocysts derived from cleavage stage embryos with cell numbers of ≤ 6, 7, 8, 9, and ≥ 10 on day 3
| ≤ 6-cell derived | P | 7-cell derived | P | 8-cell derived (Ref.) | 9-cell derived | P | ≥10-cell derived | P | |
|---|---|---|---|---|---|---|---|---|---|
| No. of cycles | 109 | – | 169 | – | 811 | 136 | – | 219 | – |
| Clinical pregnancy rate | 55.05% (60/109) | NS | 66.27% (112/169) | NS | 64.61% (524/811) | 58.82% (80/136) | NS | 65.30% (143/219) | NS |
| Miscarriage rate | 25.00% (15/60) | 0.02 | 16.96% (19/112) | NS | 13.74% (72/524) | 21.25% (17/80) | NS | 16.78% (24/143) | NS |
| Multiple pregnancy rate | 1.67% (1/60) | NS | 1.79% (2/112) | NS | 3.24% (17/524) | 5.00% (4/80) | NS | 2.10% (3/143) | NS |
| Total Live birth rate | 41.28% (45/109) | 0.004 | 55.03% (93/169) | NS | 55.73% (452/811) | 46.32% (63/136) | NS | 54.34% (119/219) | NS |
| Singleton live birth rate | 40.37% (44/109) | 0.009 | 53.85% (91/169) | NS | 53.64% (435/811) | 43.38% (59/136) | 0.03 | 52.97% (116/219) | NS |
| Mean birth weight, kg | 3.39 ± 0.53 | NS | 3.47 ± 0.71 | NS | 3.40 ± 0.54 | 3.54 ± 0.56 | NS | 3.52 ± 0.49 | 0.03 |
| Birth weight ≥ 4000 g | 15.91% (7/44) | NS | 15.38% (14/91) | NS | 14.25% (62/435) | 25.42% (15/59) | 0.03 | 20.69% (24/116) | NS |
| Birth weight ≤ 2500 g | 2.27% (1/44) | NS | 7.69% (7/91) | NS | 5.29% (23/435) | 5.08% (3/59) | NS | 0.86% (1/116) | 0.04 |
| Gestational age at birth, days | 275.73 ± 9.68 | NS | 274.48 ± 12.00 | NS | 274.00 ± 13.44 | 274.25 ± 11.83 | NS | 275.94 ± 8.24 | NS |
| Number of premature deliveries (< 37 weeks GA) | 6.82% (3/44) | NS | 7.69% (7/91) | NS | 8.05% (35/435) | 6.78% (4/59) | NS | 2.59% (3/116) | 0.04 |
| Number of deliveries (> 41 weeks GA) | 11.36% (5/44) | NS | 6.59% (6/91) | NS | 9.66% (42/435) | 8.47% (5/59) | NS | 10.34% (12/116) | NS |
| Sex ratio (male/female) | 1.44 (26/18) | NS | 1.02(46/45) | NS | 1.14 (232/203) | 1.46 (35/24) | NS | 1.52 (70/46) | NS |
Ref. reference; NS not significant; GA gestational age
Values are presented as number (%) or mean ± SD
P value was compared with 8-cell derived blastocysts group by the chi-square (χ2) test or t test
In order to investigate clinical outcomes for high-quality blastocysts derived from the ≤ 6-cell group on day 3, we compared these blastocysts with high, fair, and weak quality blastocysts derived from 8-cell on day 3, respectively. Results showed that the LBR of high blastocysts derived from the ≤ 6-cell on day 3 was markedly higher than that of weak blastocysts derived from the 8-cell on day 3 (61.76% vs 44.44%, P = 0.04), while cPR and miscarriage rates were similar for the two groups (70.59% vs 55.56%, P = 0.06; 12.50% vs 20.00%, P = 0.33, respectively). In addition, cPR, LBR, and miscarriage rates did not differ between high blastocysts derived from the ≤ 6-cell and 8-cell on day 3 (Table 3).
Table 3.
Clinical outcomes of TBT cycles with high quality blastocysts derived from ≤ 6-cell versus high/fair/weak quality blastocysts derived from 8-celll on day 3
| ≤ 6-cell derived high quality blastocysts | 8-cell derived high quality blastocysts | P | 8-cell derived fair quality blastocysts | P | 8-cell derived weak quality blastocysts | P | |
|---|---|---|---|---|---|---|---|
| NO. of cycles | 102 | 1258 | – | 811 | – | 54 | – |
| Clinical pregnancy rate | 70.59% (72/102) | 69.16% (870/1258) | NS | 64.61% (524/811) | NS | 55.56% (30/54) | NS |
| Miscarriage rate | 12.50% (9/72) | 16.32% (142/870) | NS | 13.74% (72/524) | NS | 20.00% (6/30) | NS |
| Multiple pregnancy rate | 1.39% (1/72) | 2.18% (19/870) | NS | 3.24% (17/524) | NS | 6.67% (2/30) | NS |
| Total live birth rate | 61.76% (63/102) | 57.87% (728/1258) | NS | 55.73% (452/811) | NS | 44.44% (24/54) | 0.04 |
| Singleton live birth rate | 60.78% (62/102) | 56.36% (709/1258) | NS | 53.64% (435/811) | NS | 40.74% (22/54) | 0.02 |
| Mean birth weight, kg | 3.40 ± 0.49 | 3.47 ± 0.51 | NS | 3.40 ± 0.54 | NS | 3.46 ± 0.35 | NS |
| Birth weight ≥ 4000 g | 11.29% (7/62) | 15.23% (108/709) | NS | 14.25% (62/435) | NS | 9.09% (2/22) | NS |
| Birth weight ≤ 2500 g | 1.61% (1/62) | 3.39% (24/709) | NS | 5.29% (23/435) | NS | 0.00% (0/22) | NS |
| Gestational age at birth, days | 275.85 ± 9.80 | 275.10 ± 11.73 | NS | 274.00 ± 13.44 | NS | 277.77 ± 5.72 | NS |
| Number of premature deliveries (<37 weeks GA) | 9.68% (6/62) | 6.35% (45/709) | NS | 8.05% (35/435) | NS | 0.00% (0/22) | NS |
| Number of deliveries (>41 weeks GA) | 9.68% (6/62) | 9.17% (65/709) | NS | 9.66% (42/435) | NS | 4.55% (1/22) | NS |
| Sex ratio (male/female) | 1.38 (36/26) | 1.17 (382/327) | NS | 1.14 (232/203) | NS | 1.20 (12/10) | NS |
Note: NS = not significant; GA = gestational age
Values are presented as number (%)
P value was compared with ≤ 6-cell derived blastocysts group by the Chi-square (χ2) test
In order to determine the clinical outcomes of single blastocysts transfers derived from different degrees of fragmentation on day 3, we compared the clinical outcomes of TBT cycles with blastocysts derived from 7 to 10 cells with fragmentation of < 10% and 30–50% on day 3. As can be seen in Table 4, cPR, LBR, and miscarriage rates were similar for the two groups (64.76% vs 64.31%, P = 0.93; 55.24% vs 54.47%, P = 0.88; 14.71% vs 15.30%, P = 0.90 respectively).
Table 4.
Clinical outcomes of TBT cycles with fair quality blastocysts derived from 7- ≥ 10 cell cleavage stage embryos with fragmentation of <10% and 30%–50% on day 3
| Fair quality blastocysts derived from 7 to ≥ 10 cells with fragmentation of < 10% | fair quality blastocysts derived from 7 to ≥ 10-cell with fragmentation of 30–50% | P | |
|---|---|---|---|
| NO. of cycles | 1230 | 105 | – |
| Clinical pregnancy rate | 64.31% (791/1230) | 64.76% (68/105) | NS |
| Miscarriage rate | 15.30% (121/791) | 14.71% (10/68) | NS |
| Multiple pregnancy rate | 2.91% (23/791) | 4.41% (3/68) | NS |
| Total live birth rate | 54.47% (670/1230) | 55.24% (58/105) | NS |
| Singleton live birth rate | 52.60% (647/1230) | 52.38% (55/105) | NS |
| Mean birth weight (kg) | 3.44 ± 0.55 | 3.47 ± 0.80 | NS |
| Birth weight ≥ 4000 g | 16.54% (107/647) | 14.55% (8/55) | NS |
| Birth weight ≤ 2500 g | 4.95% (32/647) | 5.45% (3/55) | NS |
| Gestational age at birth, days | 274.24 ± 12.96 | 274.61 ± 11.14 | NS |
| Number of premature deliveries (< 37 weeks GA) | 6.80% (44/647) | 10.91% (6/55) | NS |
| Number of deliveries (> 41 weeks GA) | 9.43% (61/647) | 7.27% (4/55) | NS |
| Sex ratio (male/female) | 1.22 (355/292) | 1.16 (29/26) | NS |
NS not significant; GA gestational age
Values are presented as number (%)
P value was calculated by the Chi-square (χ2) test
Multivariate analysis
We performed a multivariate analysis to adjust for the following potential confounding factors: female age (> 35 years old), endometrial thickness, day 3 cell number (≤ 6-cell or 8-cell), day of blastocyst expansion (D5 or D6) and embryo quality (Table 5). The following variables were found to be independently associated with LBR: female age at the time of oocyte collection was associated with a significant decrease in the LBR compared to female age ≤ 35 years (OR = 0.40; 95% CI [0.32–0.51]; P < 0.001), as was ≤ 6-cell on day 3 (OR = 0.69; 95% CI [0.51–0.94]; P < 0.02). In contrast, endometrial thickness, a good quality embryo transfer (> 4BB), and blastocyst expansion on D5 all had a significant positive impact on LBR (OR = 2.08; 95% CI [1.34–3.22]; P = 0.001, OR = 1.77; 95% CI [1.29–2.42]; P < 0.001 and OR = 1.54; 95% CI [1.14–2.08]; P = 0.005 respectively).
Table 5.
Logistic regression analysis of the risk factors affecting the live birth rate after frozen-thawed blastocyst transfer
| Parameters | OR | 95% CI | P |
|---|---|---|---|
| Age > 35 y/o at retrieval | 0.40 | 0.32–0.51 | < 0.001 |
| Endometrial thickness | 2.08 | 1.34–3.22 | 0.001 |
| Day 3 cell number (≤ 6 cells versus 8 cells) | 0.69 | 0.51–0.94 | 0.02 |
| Good quality embryo transfer | 1.24 | 1.07–1.45 | 0.005 |
| Day 5 versus day 6 blastocyst | 1.54 | 1.14–2.08 | 0.005 |
y/o years old, OR odds ratio, CI confidence interval
A good-quality embryo was defined as a stage 4 or stage 5 blastocysts ≥ BB based on Gardner and Lane’s criteria
Discussion
Our findings suggest that LBR following TBT with fair quality blastocysts derived from ≤ 6 cell embryos on day 3 was significantly lower than from 8-cell on day 3. These results are in line with our previous studies, which indicated that 6-cell embryos had a markedly lower LBR rate than that of 8-cell embryos in day 3 single embryo transfer cycles [14] as well as literature confirming the disadvantages of slower embryos over typically progressing embryos [15]. There are several possible causes of embryos with low cell numbers: (1) prolonged cell cycles; (2) fragmentation resulting in fewer surviving blastomeres; (3) developmental arrest or unexplained developmental delays [16]. Interestingly, we found LBR did not differ between high quality blastocysts derived from ≤ 6-cell and 8-cell embryos on day 3 in TBT cycles. We hypothesize that embryos with low cell numbers on day 3 could be accelerated and repaired during subsequent development. If partially repaired, slow embryos will progress to become fair quality blastocysts. High quality blastocysts could then be obtained after complete repair. To the best of our knowledge, this is the first paper to specifically compare LBR after TBT with blastocysts derived from different cell numbers on day 3. Our results could be beneficial for optimizing the sequence of freezing blastocysts on day 5 and so decreasing the time of conceiving for patients undergoing IVF/ICSI treatment.
In this study, we found that high-quality blastocysts derived from ≤ 6 cell embryos on day 3 had an advantage over weak blastocysts derived from 8-cell embryos on day 3. Both of these were day 5 blastocysts which were first transferred in TBT cycles therefore eliminating the day of blastocyst expansion bias. We suggest that blastocysts with a better grading of inner cell mass (ICM) and trophoderm cells (TE) are associated with higher euploidy rates and thus a higher LBR compared with blastocysts with lower morphology scores, regardless of cell numbers on day 3 of the blastocysts [7].
We found no significant difference in the clinical outcomes of blastocysts derived from cleaved embryos with fragmentation of < 10% vs 30–50%. This result is in agreement with that reported by Mizobe et al. (2016). Those authors reported that the blastocyst formation rate did not differ between normal cleaved embryos with a fragmentation of < 10% and normal cleaved embryos with fragmentation of 10–50% [17]. It seemed that embryos could self-repair and develop into blastocysts during the process of development. It has also been reported that recent improvement of culture media and environment mean that typically cleaved embryos are able to develop into blastocysts despite the presence of fragmentation [18–20]. Therefore, the presence or absence of fragmentation on day 3 does not appear to affect the blastocyst pregnancy rate.
A degree of controversy remains in literature discussing the developmental potential of fast cleaving embryos, defined as with 10 or more blastomeres on day 3. Alikani et al. suggested that fast cleaving embryos on day 3 should not be selected for transfer due to the possibility of chromosomal or epigenetical abnormity [21]. In contrast, Luna et al. (2008) reported that these embryos had been associated with a high rate of progression to blastocyst stage as well as high LBR. In this study, we found that the clinical outcomes of blastocysts derived from ≥ 10-cell embryos on day 3 did not differ from those of blastocysts derived from typically progressing embryos on day 3 in TBT cycles. These findings are consistent with those which suggest that increased day 3 cell number is related to improved blastocysts formation rate, LBR, and embryo morphology [22–25]. In addition, blastocysts derived from fast cleaving embryos on day 3 had improved neonatal outcomes including high mean birth weight, low rate of infants with low-birth weight (≤2500 g), and low rate of premature deliveries. Our explanation for the phenomenon is that the fast cleaving embryos on day 3 may have maintained the rapid development speed until delivery compared with normal cleaving embryos on day 3. But, this is just our speculation, further studies would be required to fully confirm these findings.
In keeping with much existing research, the results of our study suggest that embryo quality has a significant positive effect on LBR [26–28]. In addition, after multivariate logistic regression analysis, blastocyst expansion on day 5 is independently associated with an increase in LBR compared with day 6 blastocyst expansion. This supports a study by Ferreux et al. which found that LBR following TBT is higher with blastocysts expanded on day 5 than day 6 [29].
This study has several limitations. Firstly, the morphological method is a poor predictive value in embryo selection, although blastocyst quality was uniform among the groups. Secondly, the study is limited by the subjective nature of morphological assessment, even though inter-embryologist variability is minimal in our practice. Thirdly, the nature of retrospective studies is a limitation. Further prospective studies are needed to confirm our findings.
In conclusion, this study provides strong evidences that fair blastocysts which have been derived from ≤ 6 cell embryos on day 3 exhibit disadvantages over those derived from 8-cell embryos on day 3. However, these disadvantages disappear in high quality blastocysts. Therefore, when transferring blastocysts, an evaluation of the cleavage stage should be performed along with blastocyst morphology to shorten the time of conceiving.
Electronic supplementary material
(JPG 370 kb)
(DOC 21 kb)
(DOC 43 kb)
Acknowledgments
The authors thank all of the members of laboratory at the Center for Reproductive Medicine, Shandong University for providing assistance. This study is funded by The National Key Research and Development Program of China (2017YFC1001600), The Fundamental Research Funds of Shandong University, and National Natural Science Foundation of China (81871168).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention (2009) National summary report. Assisted reproductive technology (ART) report. Atlanta: CDC.
- 2.Lemos EV, Zhang D, van Voorhis BJ, Hu XH. Healthcare expenses associated with multiple vs. singleton pregnancies in the United States. Am J Obstet Gynecol. 2013;209:586.e1–586.11. doi: 10.1016/j.ajog.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni AD, Jamieson DJ, Jones HW, Kissin DM, Gallo MF, Macaluso M. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–2225. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of Society for Assisted Reproductive Technology, ASRM, Medicine. PCoASfR. Elective single-embryo transfer. Fertil Steril 2012;97.
- 5.Grady R, Alavi N, Vale R, Khandwala M, McDonald SD. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril. 2012;97:324e31. doi: 10.1016/j.fertnstert.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro BS, Richter KS, Harris DC, Daneshmand ST. Implantation and pregnancy rates are higher for oocyte donor cycles after blastocyst-stage embryo transfer. Fertil Steril. 2002;77:1296–1297. doi: 10.1016/S0015-0282(02)03086-8. [DOI] [PubMed] [Google Scholar]
- 7.Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31:2245–2254. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 8.Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107:664–670. doi: 10.1016/j.fertnstert.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 10.Wu KL, Zhao HB, Liu H, Li M, Ma S, Li C, Liu C, Chen ZJ. Day 3 ET, single blastocyst transfer (SBT) or frozen-thawed embryo transfer (FET): which is preferable for high responder patients in IVF/ICSI cycles? J Assist Reprod Genet. 2014;31:275–278. doi: 10.1007/s10815-013-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puissant F, Van RM, Barlow P. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–708. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 12.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, Hoang L, Kotelchuck M, Stern JE, Hornstein MD. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts outcomes study of assisted reproductive technologies (MOSART) Fertil Steril. 2015;103:888–895. doi: 10.1016/j.fertnstert.2014.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao HB, Liu H, Li M, Ma SY, Li C, Wu KL. Over ten-cell good embryo transfers on day three have equivalent clinical outcomes with those of eight-cell embryos in female patients aged ≤35 years: a retrospective cohort study. Gynecol Obstet Investig. 2019;2:1–7. doi: 10.1159/000495407. [DOI] [PubMed] [Google Scholar]
- 15.Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15:2190–2196. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 16.Kong X, Yang S, Gong F, Lu C, Zhang S, Lu G, Lin G. The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: a time-lapse study. PLoS One. 2016;11(4):e0153697. doi: 10.1371/journal.pone.0153697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizobe Y, Oya N, Iwakiri R, Yoshida N, Sato Y, Miyoshi K, Tokunaga M, Ezono Y. Effects of early cleavage patterns of human embryos on subsequent in vitro development and implantation. Fertil Steril. 2016;106(2):348–353. doi: 10.1016/j.fertnstert.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28:569–73. [DOI] [PMC free article] [PubMed]
- 19.Mizobe Y, Akiyoshi T, Minami S, Matsuo K, Fukushima R, Yamaguchi A. Effect of a time-lapse incubator (EmbryoScope®) on in vitro culture of human embryos. J Mamm Ova Res. 2014;31:40–44. doi: 10.1274/jmor.31.40. [DOI] [Google Scholar]
- 20.Waldenstrom U, Engstrom AB, Hellberg D, Nilsson S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil Steril. 2009;91:2461–2465. doi: 10.1016/j.fertnstert.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15:2634–2643. doi: 10.1093/humrep/15.12.2634. [DOI] [PubMed] [Google Scholar]
- 22.Luna M, Copperman AB, Duke M, Ezcurra D, Sandler B, Barritt J. Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (≥10 cells), bringing into question current embryological dogma. Fertil Steril. 2008;89:358–363. doi: 10.1016/j.fertnstert.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro BS, Harris DC, Richter KS. Predictive value of 72-hour blastomere cell number on blastocyst development and success of subsequent transfer based on the degree of blastocyst development. Fertil Steril. 2000;73:582–586. doi: 10.1016/S0015-0282(99)00586-5. [DOI] [PubMed] [Google Scholar]
- 24.Check JH, Summers-Chase D, Yuan W, Horwath D, Wilson C. Effect of embryo quality on pregnancy outcome following single embryo transfer in women with a diminished egg reserve. Fertil Steril. 2007;87:749–756. doi: 10.1016/j.fertnstert.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95:1985–1989. doi: 10.1016/j.fertnstert.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–3296. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 27.Gardner DK. Blastocyst culture: toward single embryo transfers. Hum Fertil (Camb) 2000;3:229–237. doi: 10.1080/1464727002000199051. [DOI] [PubMed] [Google Scholar]
- 28.Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJG, Klein BM, Helmgaard L, Arce JC. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod BioMed Online. 2013;27:353–361. doi: 10.1016/j.rbmo.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ferreux L, Bourdon M, Sallem A, Santulli P, Barraud-Lange V, Le Foll N, Maignien C, Chapron C, de Ziegler D, Wolf JP, Pocate-Cheriet K. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expandedon day 5 than on day 6. Hum Reprod. 2018;33(3):390–398. doi: 10.1093/humrep/dey004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPG 370 kb)
(DOC 21 kb)
(DOC 43 kb)