Abstract
Purpose
To use conflict resolution analysis on the conflict between proponents and opponents of preimplantation genetic testing for aneuploidy (PGT-A), previously called preimplantation genetic screening (PGS).
Methods
Considered in conflict analysis a case study, we reviewed the English literature based on key-word searches at www.pubmed.com and www.google.com, and interviewed professional opinion leaders and other actor-representatives. This analysis was the product of a mandated externship by L.M. at the Foundation for Reproductive Medicine (FRM), as part of the Master of Science Program in Negotiations and Conflict Resolution at Columbia University, New York, NY.
Results
Initially a typical difference of opinion, conflict evolved after proponents rejected studies that failed to confirm expected benefits, and authors felt demeaned by their criticism. Becoming “destructive,” the conflict evolved according to Glasl’s escalation model stages. Proponents became continuous attractors. Unable to produce validations for PGT-A, proponents moved goal posts through 3 stages (PGS 1.0–PGS 3.0). Ultimately concurring that pregnancy and live birth rates are unaffected, they started claiming new benefits.
Conclusions
The FRM underwrote this study as a starting tool for a conflict resolution process. A consensus building conference of stakeholders appears as of this point to represent the most promising potential intervention. The goal of such a conference should be sustainable consensus about clinical utilization of PGS/PGT-A in IVF, based on transparent and validated criteria. A potential date for such a conference is set for 2020.
Keywords: Preimplantation genetic testing for aneuploidy (PGT-A), Preimplantation genetic screening (PGS), Conflict analysis, Conflict resolution
Introduction
Disagreements in medicine are so common that one must consider them part of the normal medical landscape. They, however, almost never advance to formal conflict status. A true conflict has now, however, been accelerating in intensity for over a decade between proponents and opponents of a diagnostic test, initially given the name preimplantation genetic screening (PGS), and since 2016 renamed preimplantation genetic testing for aneuploidy (PGT-A). As part of in vitro fertilization (IVF), the test involves the assessment of human embryos for chromosomal abnormalities before transfer into the uterus. It, therefore, determines which embryos are transferred and which are disposed of.
A conflict exists when at least two in some ways linked parties (individuals or groups), often also called “actors,” hold incompatible opinions concerning one or more issues of importance and, concomitantly, also experience strong emotions relating to the area(s) of disagreement. The field of conflict resolution believes that, per se, conflicts are not necessarily bad, as they can have positive as well as negative results. Positive results include reconciliations between parties, improved interactions between individuals and/or groups, sometimes internal changes within groups, or clarifications of what the real problems may be. Potential negative consequences are, of course, only too obvious: Minor differences of opinion can escalate to major conflicts, single-subject conflicts can expand into multiple subject disputes, the number of involved actors may increase, thereby complicating any attempts at resolution (https://www.maxwell.syr.edu/uploadedFiles/parcc/cmc/Conflict%20Resolution%20NK.pdf). In here discussed conflict, almost all of these negative developments were observed.
We here present a conflict analysis, which in the field of conflict resolution has been defined as an objective systemic study of profile, causes, actors, and dynamics of conflicts. Because of the often highly political nature of gathered information, this can be a very sensitive process (http://www.guillaumenicaise.com/wp-content/uploads/2014/09/resumé-du-cours_analyse-de-conflits.pdf). The conflict resolution literature describes three possible outcomes for conflicts: A conflict can be resolved by (i) dominance and/or imposition of will by one party on the other, often leading to resentment and, at times, destructive consequences; (ii) withdrawal of one side, also leading to resentment and lowered self-image for the other side; and (iii) some form of compromise, resulting in at least some positive outcomes for both sides. It is the last of these three possible resolutions that in this conflict (and likely in most others) appears desirable. Based on scientifically solid and validated data, we, therefore, will strive for a conflict resolution process based on compromise before the conflict develops more confusing features, adversely affecting patients as well as medical professionals. More continuous resentment of “losing” parties increases the risks of recidivism and, therefore, reignition of conflict.
Materials and methods
This manuscript is the final work product of a mandated field-externship at the not-for-profit Foundation for Reproductive Medicine (FRM) by one of the authors (L. M.) during her Master of Science program in Negotiation and Conflict Resolution at Columbia University’s School of Professional Studies, in New York City.
It was based on review of the English medical literature on PGS/PGT-A based on keyword searches at PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Google Scholar (https://scholar.google.com/) and searches of lay literature at Google (https://www.google.com/), including phrases, like “chromosomal testing of embryos” and “genetic testing of embryos,” in addition to keywords like preimplantation genetic screening (PGS) and preimplantation genetic testing for aneuploidy (PGT-A). Reference lists of selected manuscripts were further reviewed for additional relevant articles. L.M., in addition, conducted a number of personal interviews with selected opinion leaders in PGS/PGT-A, administrators of IVF facilities, and some patients who either did or did not use PGS/PGT-A in a past IVF cycles in an attempt to understand motivations. Here presented analysis follows standard guidelines for conflict analysis and resolution [1].
Opinion leaders were identified based on published articles in the medical literature, whether advocating for or against PGS/PGT-A. An initial attempt was made to contact some by phone and/or e-mail within the USA as well as overseas. Since proponents of PGS/PGT-A were mostly unresponsive, personal interviews were deemphasized, recognizing potential biases that may arise from interviewing primarily opponents only. Instead, opinions expressed by these opinion leaders in their writings were analyzed and here described.
A number of patients opting for and against transfer of embryos, by PGS/PGT-A declared “abnormal” (mosaic or aneuploid), were interviewed at The Center for Human Reproduction (CHR), a fertility center in New York City, which has been offering transfers of “abnormal” embryos since 2014. Since CHR has never recommended PGS/PGT-A to its patients, all such transfers involved patients who had PGS/PGT-A performed at other IVF centers and had moved their allegedly chromosomal “abnormal” embryos to CHR. A more formal investigation by an independent ethicist from another institution, involving CHR’s PGS/PGT-A patients, is currently underway.
Ultimately, in excess of 120 medical articles were reviewed, with a large majority (over 80) authored by proponents of PGS/PGT-A. The final reference list of 42 citations, however, represents a more balanced selection: nine first authors, each, with a pro- and con-PGS/PGT-A view and 10 articles that can be considered to be authored by neutral writers.
The history of PGS/PGT-A
The 1st phase of PGS (PGS 1.0)
Based on the believe that chromosomal aneuploidy of embryos represented the most frequent cause of IVF failure, Yuri Verlinsky, Ph.D., a brilliant reproductive geneticist, proposed in the mid-1990s the testing of embryos for chromosomal abnormalities before replacing them into the uterus [2]. He hypothesized that, by discarding chromosomally abnormal (aneuploid) embryos and transferring only chromosomally normal (euploid) embryos, better pregnancy and live birth rates would be achieved and fewer miscarriages would occur.
Though unproven, the hypothesis quickly gained followers. The procedure of preimplantation genetic screening (PGS), as this test was later named, was ultimately, however, not based on testing of first and second polar body biopsies of zygotes, as originally suggested by Verlinsky [1], but on the technically much simpler blastomere biopsy of day-3 embryos. The first PGS procedure offered commercially to IVF centers and their patients (PGS 1.0) thus involved cleavage-stage embryo biopsy at 6–8-cell stage.
One of the most basic ethical rules of medical practice is to introduce treatments into routine clinical medicine only after their safety and efficacy have been established. PGS should, therefore, have been introduced into general clinical IVF practice only after appropriate validation studies. This is, however, not what happened: By the late 1990s, PGS 1.0 increasingly entered routine IVF practice as an add-on without prior clinical validations.
The first to seriously investigate the utility of PGS via prospectively randomized studies were Belgian investigators. As a total surprise to the whole IVF community, they failed to produce the widely expected confirmatory outcomes of improved pregnancy and lower miscarriage rates [3–5]. The surprise was so pronounced because, at that point, there was virtual unanimity in the field that the PGS hypothesis was correct. US investigators who reanalyzed some of the Belgian data, because the study’s authors had not looked at patient age as a potential confounder, were the first to voice concern that, besides not improving IVF outcomes, PGS 1.0 may in older women actually reduce IVF pregnancy chances. That possibility at that time period was considered so farfetched and outside of consensus, that their manuscript was initially rejected by all leading medical journals in the field. It was accepted only almost 2 years later [6] after Dutch investigators published in a leading general medical journal a prospectively randomized study that confirmed both of these suspicions [7].
From a conflict resolution viewpoint, the Dutch study became the event that converted a difference of opinion among medical professionals into a formal conflict, driven by personal antagonisms and emotional involvements of individual key actors. Continuing to insist that the promised outcome benefits would have been observed if the published research only been planned and executed better, proponents of PGS, at times quite viciously, attacked every published study that was unable to confirm expected outcomes of PGS 1.0., starting with above-noted Belgian studies. For all practical purposes, they, thus, transferred their own obligation of validating PGS to opponents of the procedure, who now were challenged to proof that PGS did not work. When similar attacks were also mounted on the Dutch study [7], PGS 1.0 for the first time faced some questioning from within the IVF community since this study was adequately powered, a rare prospectively randomized study in the field of reproductive medicine and was published by a leading general medical journal with very high impact factor.
Under such circumstances, attractors of conflict are omnipresent. Competently executed clinical studies in medicine usually, however, resolve such disputes before attractors succeed in converting scientific disagreements into formal conflicts. In many ways, well-executed studies are, indeed, perfect conflict resolution tools which resolve differences of opinion quickly since opposing actors usually accept results of appropriately designed studies as arbitrators of disagreements.
The medical dispute over PGS, however, for two principal reasons clearly differed from most others: Proponents of PGS refused to accept even well-executed studies, like the above-noted Dutch study [7], as arbitrators. Even more importantly, however, proponents of PGS continued to fail in offering validating evidence for the procedure, while opponents, increasingly successfully, produced evidence that PGS 1.0. really did not improve IVF outcomes.
Failure to acknowledge winners of a conflict, as the conflict resolution literature well documents, is almost universally an accelerant. The reason is that winners perceive such behavior as unfair denigration and withholding of respect and legitimacy. These dynamics were further aggravated by proponents of PGS often using challenging language in describing critical studies as biased or otherwise incompetent, while, themselves failing to generate believable evidence in favor of PGS. Emotionally increasingly committed to their respective positions, both sides started viewing their antagonists as deliberately attempting to conceal the truth. In the process, as we further demonstrate below, unable to validate PGS’ claims, proponents kept moving goal posts. Until today, they still have to reach steady state.
Resentment of opponents grew with every additional attack on yet another publication that refuted the PGS hypothesis. The conflict, therefore, accelerated, assuming many typical conflict characteristics. Even at that point, neither side of the divide would, however, have predicted how ugly the tone in this discourse would become in coming years.
Conflict escalation
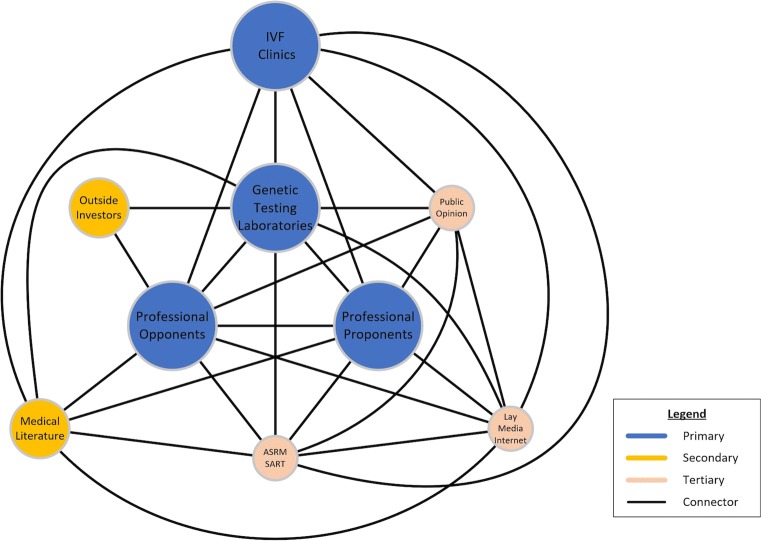
Added involvements of more primary, secondary, and tertiary actors (see conflict map in Fig. 1) further increased conflict intensity. Besides initial groups of clinical proponents and opponents of PGS within the IVF field, outside interests started joining the fray: A rapidly growing genetic laboratory industry quickly became an additional primary actor. Its growth speed was, indeed, remarkable, considering that PGS at that point was only an unvalidated hypothesis.
Fig. 1.
The conflict map of here discussed conflict
Unsurprisingly, the rapid growth of the worldwide PGS industry, therefore, raised ethical and regulatory questions about how states and the federal government in the USA controlled utilization of important diagnostic tests. That PGS within such a context had to be considered an “important” test stems from the fact that its outcome determined whether an embryo was transferrable or to be disposed of. How all over the world regulatory bodies allowed commercial interests to bring to market an unvalidated test like PGS, therefore, became an ethical as well as regulatory issue of considerable importance.
In the USA, the answer was simple: Laboratories exploited a loophole in US federal law, permitting so-called in-house-developed tests to come to market without government approvals [8]. As long as Food and Drug Administration (FDA) and/or other federal or state regulators do not see legal reasons to intervene, indeed, almost any clinical test can be offered commercially without FDA approval if designated as “in-house-developed.” During the research phase of this project, we also learned that IVF centers generally are unaware of the fact that PGS is not an FDA-approved diagnostic test.
Previously noted shared economic interests between IVF centers and the genetic testing community created a highly successful coalition network of proponents of PGS that also included outside investors. This network was formed by exploring yet another loophole in the law, as in medicine fee-splitting is generally considered unethical and, often, illegal (https://www.ama-assn.org/delivering-care/ethics/fee-splitting). Under federal law, physicians are even prohibited from referring patients to ancillary service providers in which they hold ownership (http://starklaw.org/).
Despite such prohibitions, PGS/PGT-A fees (in the USA between ~ $4000–5000 per cycle) are, nevertheless, almost routinely split between IVF centers, where patients are encouraged (in some centers even mandated) to use PGS/PGT-A, and PGS/PGT-A laboratories. Legal jeopardy is, however, circumvented by dividing procedure costs between IVF clinic, where the embryo biopsy is performed, and laboratory, where the genetic analysis takes place. In this way of creating an “equal partnership,” strong emotional as well as economic connections are created between IVF centers and PGS/PGT-A laboratories. In addition, such partnerships, of course, create mutually beneficial economic incentives for IVF centers to order these tests.
After 2010, with the switch from PGS 1.0 to PGS 2.0, the decision to utilize this test in association with IVF cycles also led to additional IVF cycle outcome consequences: PGS/PGT-A automatically mandated extended embryo culture to blastocyst stage and so-called all-freeze cycles. The latter is required since genetic testing results cannot quickly enough be obtained to transfer embryos in the same cycle. Though still controversial subjects, both of these practice changes have, independently, in general patient populations been associated with lowered IVF pregnancy and live birth rates. Yet, IVF clinics gain additional revenue from embryo freezing/thawing and the need for an additional thaw-cycle, after test results have been returned that demonstrate at least one potentially transferrable embryo.
The combination of potentially diminished cycle outcome success and additional frozen-thawed cycle costs, for patients, therefore, do not appear cost-effective and clearly exceed cost of an, otherwise necessary, fresh embryo transfer. Financial considerations for IVF centers, in contrast, are, however, very favorable and significantly increase overall IVF cycle revenue. Since PGS/PGT-A, as an unvalidated procedure, is not covered by third-party insurers, costs, even in patients with full IVF coverage, will come out of pocket and will be undiscounted. Especially where insurance coverage for IVF (often under state-law mandates) is widely available (and cycle reimbursement rates, therefore, are relatively low), this additional income stream increases overall cycle fees to significant degrees. For many IVF centers, PGS/PGT-A, therefore, has become an essential income source.
Some US IVF centers are now practically mandating PGS/PGT-A for all IVF cycles, even including donor egg cycles. Because young egg donor cycles even without PGS/PGT-A universally offer excellent pregnancy chances and very low miscarriage rates, ethics of such utilization have been for even outspoken proponents of the procedure somewhat uncomfortable. The possibility of financial conflicts of interest, therefore, added to the complexity of the conflict, with opponents accusing proponents of inappropriate financial motivations, while proponent felt indignant about such accusations. From a single-issue dispute, whether PGS/PGT-A could or could not influence IVF outcomes, a much more complex and multifactorial conflict arose that sprouted into a multitude of hubs and spokes with diverging agendas and interests. Added actors of significance now also included professional organizations, like the American Society for Reproductive Medicine (ASRM) and its sister society, the Society for Assisted Reproductive Technologies (SART), and professional medical journals, including their editors and editorial boards. Because the PGS industry also started pursuing direct-to-consumer marketing, general public and media also became actors in the conflict (see conflict map in Fig. 1).
Proponents and opponents increasingly behaved like typical actors in a complex conflict. As Deutsch, a leading conflict resolution theoretician, first demonstrated, early stages of conflicts are characterized by a destructive phase where opposing parties, uncompromisingly, view their conflict as a win-lose proposition [9]. This is, indeed, exactly how proponents and opponents of PGS initially behaved. Considering that research is always an inherently competitive endeavor between scientists, laboratories, universities, and even countries, such a desire “to win” should not surprise. If, especially when it comes to economic motivations, one further considers the distinctly different needs of both sides of the conflict, Deutsch’s concept of motivation is similarly applicable.
The conflict in addition perfectly followed Glasl’s escalation model stages [10]: positions hardened, polarization of thinking and feelings advanced, and “black and white thinking” took over. Parties reached the conclusion that speaking no longer helped and actions were mandated instead. Both sides formed their own coalitions, trying to outmaneuver opponents while seeking support from uninvolved parties. This was followed by more direct and public attacks, even in medical journals, questioning the opponents’ integrity, causing further escalation. Threats and counter threats represent the current phase of the conflict. An essential objective of this analysis is to help avoid the last stage of Glasl’s model, the stages of dehumanization and fragmentation of opponents.
Both sides initiated additional recruitments of secondary and tertiary actors in support of their positions. Proponents of PGS, at that point in a large majority within the IVF community, literally took control of a large part of the peer review process on PGS/PGT-A, thereby determining which scientific papers were or were not published on the subject in medical journals. Considered to be the best qualified “experts” on the subject, editors preferentially chose them as reviewers. Unsurprisingly, they then favored publications of proponents, while routinely rejecting publications from opponents of PGS/PGT-A. The medical literature surrounding PGS/PGT-A, therefore, for a good number of years was clearly biased. Previously addressed important Dutch study on PGS 1.0 was, tellingly, not published in a journal in the field but by a prominent general medical journal [7].
The 2nd generation of PGS (PGS 2.0)
When only a few months following publication of this study, the ASRM published a first formal opinion on what then was still called PGS, concluding that the procedure so-far had not fulfilled any of the promises of the PGS hypothesis [11], for the first time clinical utilization of the procedure appeared threatened. At that point, the genetic testing industry, as somewhat of a surprise, suddenly acknowledged that, as performed, PGS 1.0 had, indeed, been ineffective. The industry, however, also claimed that the past no longer mattered since a greatly improved version of PGS (going forward, here described as PGS 2.0) was now available for immediate clinical launch and would, finally, fulfill the expectations of the PGS hypothesis. This would be accomplished with improved diagnostic techniques and technologies, thus attributing the failure of PGS 1.0 exclusively to inadequate techniques and technologies. Changes brought to the procedure, therefore, continued to overlook relevant aspects of basic biology of human preimplantation-stage embryos.
Changes brought on by PGS 2.0 were, indeed, substantial, moving the embryo biopsy from cleavage-stage on day 3 after fertilization (6–8 cell embryos) to days-5/6 embryos (blastocyst stage). New diagnostic laboratory platforms not only were much more accurate in determining aneuploidies but also, for the first time, allowed the testing of all 46 chromosomes (PGS 1.0 allowed only testing of up to ca. 8 most frequently abnormal chromosomes). Technically, PGS 2.0 was, thus, indeed, clearly improved.
Geneticists who introduced PGS 2.0, however, failed to realize that he principal reasons why PGS 1.0 had failed were not technical inadequacies but a basic misunderstanding of biological realities affecting preimplantation-stage embryos. The IVF field, in addition, failed to understand the impact PGS 2.0 would have on general IVF practice by encouraging extended embryo culture to all IVF cycles, requiring all-freeze cycles with embryo-banking because PGS 2.0 results were not available in time for fresh embryo transfers, and by fostering eSET, all IVF practice changes associated with reduced pregnancy and live birth rates. Since, like PGS 1.0 before, PGS 2.0 was brought to market without prior validation studies, these oversights cannot surprise.
First direct evidence against the PGS hypothesis
As proponents of PGS/PGT-A continued to fail in developing supportive validation studies for PGS 2.0, evidence grew that the PGS hypothesis for biological reasons, simply, could not work: (i) Mathematical modeling demonstrated that a single embryo biopsy averaging 5–7 trophectoderm (TE) cells could not with adequate statistical probability determine whether an embryo would end up chromosomally normal or not. Assuming the highly unlikely scenario of even distribution of aneuploidy within an embryo, in a statistical best-case scenario, such a biopsy would require at least 28 cells [12]. With clonal distribution of aneuploidy, as usual the case in mitotic aneuploidies, the required cell number would be even bigger. (ii) Embryos with mitotic clusters of aneuploid cells, downstream from blastocyst stage, often self-correct after implantation. This ability is stronger in the embryonic cell lineage (inner cell mass) than the extra-embryonic cell lineage (TE) [13, 14]. The remaining rational for testing embryos upstream at blastocyst stage is, therefore, unclear.
Underlying disputes to the conflict started to shift: Opponents of PGS now for the first time not only pointed out that the predictions of the PGS hypothesis had not been met, but demonstrated convincingly that, for apparent biological reasons, those goals were, simply, unachievable. They, thus, for the first time actually fulfilled the rather unreasonable previously noted demand of proponents of PGS/PGT-A to demonstrate proof that the PGS hypothesis could not work.
Proof of significant false-positive results from PGS
By 2014, clinical investigators in New York City and Rome, Italy, independently, initiated, at the time seemingly somewhat daring, an experiment in women who only produced chromosomal “abnormal” embryos in IVF cycles. Since “abnormal” embryos at that point in time were still automatically disposed of, such patients would not reach embryo transfer. Both groups of investigators, under experimental protocols and with detailed informed consents, now, however, initiated transfers of selected chromosomal “abnormal” embryos.
The New York group in October of 2015 reported 5 healthy chromosomally normal pregnancies following 12 transfers of seemingly “abnormal” embryos at the Annual Conference of the ASRM in Baltimore, MD [15]. Only weeks later, the Italian group published a research letter, reporting 6 healthy deliveries in 18 attempts [16]. Considering the unexpectedly high pregnancy and live birth rates in both of these reports, a highly significant false-positive rate for the PGS procedure was as of that point no longer deniable. With 5/12 (41.7%) and 6/18 (33.3%) of such transfers producing normal pregnancies, no other explanation was possible. Paulson, thereafter, published a mathematical modeling study, suggesting a false-positive rate as high as 40% [17].
These findings had clinical as well as ethical relevance: Clinically, they meant that patients were routinely discarding significant pregnancy chances by disposing of large numbers of apparently normal embryos, capable of producing normal pregnancies and live births. Ethically, it meant that the field was discarding perfectly normal human embryos in apparently large quantities and, therefore, depriving their” parents” of significant pregnancy chances. Both concerns attracted increasing attention in the clinical IVF community.
The 3rd generation of PGS (PGS 3.0, also renamed PGT-A)
Following these two groundbreaking reports by US and Italian investigators, the trajectory of PGS again quickly changed, as PGS no longer could deny a significant false-positive rate in embryo diagnosis. Within a short time period, the Preimplantation Genetic Diagnosis International Society (PGDIS), a professional organization representing primarily the genetic testing industry, published again radically new guidelines for all aspects of the procedure (concomitantly also renaming PGS to PGT-A), going forward here referred to as PGS 3.0. These new guidelines, which systematically restructured how PGS has since been practiced worldwide, were only published anonymously on the PGDIS website [18] and, indeed, never peer reviewed.
PGT-A (PGS 3.0), thus, for a second time, acknowledged its own inadequacy as practiced up to that point in July of 2016; yet, it continued to maintain the same claims of clinical efficacy it espoused during PGS 1.0 and PGS 2.0. Moreover, the switch from PGS 2.0 to PGS 3.0 was accompanied by almost identical arguments to those used during the earlier replacement of PGS 1.0 by PGS 2.0 and, for the third time, a new form of PGS was brought to market without any prior validation studies.
The new PGDIS guidelines addressed every aspect of PGS/PGT-A, from how laboratory testing was to be performed, how results were to be interpreted and reported to IVF centers, and how IVF providers should manage patients based on reported results. The guidelines, however, remained silent on who created them, and what data and analysis formats they were based on; they, indeed, did not contain even one single reference [18].
Publication of medical guidelines must follow rules. They must be based on a fully transparent and evidence-based process, involving conflict-free experts and often also community representation. Proceedings must be formally adopted and published, describing in detail in methods of data acquisition, results, and evidence levels of reached conclusions/recommendations [19]. The new PGDIS guidelines did not fulfill any of these requirements. Yet, they, still, became the new foundation for how PGS/PGT-A has been practiced worldwide since July 2016 and up to this day.
The most important change was a switch from binary (normal/abnormal) to trinary reporting (normal-euploid, mosaic, and abnormal-aneuploid). The differentiation between allegedly euploid, mosaic, and aneuploid embryos was still based on an on average 5-cell TEB but now included the new possibility of a “mosaic” diagnosis if genetic analyses demonstrated more than one cell lineage within that biopsy.
Since only next-generation sequencing (NGS) was able to detect more than a single cell-line in a biopsy specimen, NGS under the new guidelines became the only recommended diagnostic platform for PGS/PGT-A. Though this new guideline automatically disqualified results obtained with other platforms and most in the literature reported results had been obtained with different platforms from NGS, so, obtained data are still widely quoted in the medical literature. Indeed, not a single study using “inadequate” platforms has been withdrawn.
The newly designated embryo diagnosis of “mosaicism” was carved out of what up to that point were considered “aneuploid-abnormal” embryos, likely almost automatically disposed. Under the new guidelines, “mosaic” embryos were now, however, considered potentially transferrable, thus to a degree reducing the number disposed of embryos due to false-positive diagnoses. To receive such a designation, a single trophectoderm biopsy had to contain at least two distinct cell-lines and between 20 and 80% aneuploid DNA. Below 20% aneuploid DNA, embryos were considered “normal-euploid” and available for unrestricted transfer. Only over 80% aneuploid DNA were embryos now designated as “aneuploid-abnormal” and, therefore, still subject to routine disposal, Aneuploid DNA below 40% was among “mosaic” embryos suggested to offer better pregnancy chances than higher abnormal DNA loads [20].
First proposed in a 2016 shortly ahead of publication of the new PGDIS guidelines [21], what data this “threshold concept,” however, was based on, remained mostly unexplained. Original proponents of the threshold concept, indeed, suggested that laboratories establish their own individual cut-offs [21].
This, of course, does not represent an acceptable solution for an important screening test and was not even considered feasible by the PGDIS. Limited information available on how these thresholds were established, however, is anything but reassuring, as cut-offs for aneuploid DNA percentages proposed by the PGDIS lack, any and all, scientific and/or biological underpinnings. They, therefore, cannot be expected to offer clinically valid differentiations between IVF outcomes.
Twenty percent DNA load, differentiating “normal” from “mosaic,” was chosen because it reflects the sensitivity limit of current NGS equipment. “Normal” is, thus, distinguished from “mosaic” not because of clinical or biological evidence but because of technical limitation of currently available diagnostic equipment. To consider an embryo with 19% aneuploid DNA load “normal” allows unrestricted embryo transfer; but this cut-off designates an embryo with 21% aneuploid DNA as “mosaic” and, therefore, as restricted in its transfer. That a difference of 2% in aneuploid DNA would disqualify an embryo from automatic transfer, however, appears to make little biological and/or statistical sense. In addition, because of the confusion surrounding “mosaic” embryos, many IVF centers, still, automatically dispose embryos with any degree of aneuploid DNA. Some US IVF centers, indeed, specifically request PGS laboratories not to distinguish between mosaic and aneuploid embryos in their reports.
The 80% aneuploid DNA threshold, separating “mosaic” from “aneuploid-abnormal,” is even more arbitrary. It was, supposedly, based on two completely unverifiable assumptions: that a TEB always contains exactly 5 cells; and that those 5 cells always maintain their DNA content during the biopsy. Both assumptions are, however, not only unverifiable but also, likely, outright false since numbers of biopsied cells is, simply, impossible to accurately determine, and many cells, indeed, rupture and release their DNA during biopsy. What represents 100% DNA load is, therefore, never really known, automatically leading to the conclusion that accurate percentages of aneuploid DNA can never be determined. Therefore, to conclude that 80% aneuploid DNA load reflects 4/5 biopsied cells, as has been suggested as the argument behind the 80% threshold [22], is nonsensical. To base decisions, whether embryos are transferrable or must be disposed of, on such baseless criteria, is, therefore, scientifically not supportable and appears rather unprecedented in laboratory medicine. PGS 3.0, therefore, rather strengthened the criticism of PGS/PGT-A [23].
Accelerating hostilities between proponents and opponents of PGS/PGT-A
Opponents of PGS/PGT-A viewed introduction of PGS 3.0 as yet another attempt at diversion and, concomitantly, as also yet another acknowledgment that prior representations made by the laboratory community regarding the procedure had been factually false. The repetitiveness of these retreats, and immediate replacements with yet another itineration of PGS/PGT-A without proper prior validation studies by the laboratory industry, however, also led to increasing questions about the integrity of representations made by the industry for over 20 years of commercial utilization of PGS/PGT-A without evidence for any clinical utility in association with IVF.
Following Glasl’s escalation model [10], attacks between proponents and opponents of PGS/PGT-A became increasingly personal: Opponents of PGS accused supporters of the procedure of primarily economic motivations, while at least one leading proponent of PGS turned the argument around by accusing a prominent opponent of PGS and co-author of this manuscript of being in opposition only as a way of promoting his fertility center as being “different” [22].
A new peak in the conflict was reached with publication by Stephen Hall, one of the country’s premier medical writers, of a lead article in the September–October 2017 issue of the NEW YORK magazine, in which he presented the positions of proponents and opponents after interviewing most of the leading opinion makers on both sides of the argument worldwide [24]. Though demonstrably evenhanded, proponents of PGS/PGT-A perceived his article as one-sided, favoring the opposing position and, in some of the subsequent communications with the magazine’s editor, expressed uncomfortably aggressive opinions about Hall’s article.
The article galvanized the public into, for the first time, becoming more active participants in the conflict, with increasing numbers of patients demanding transfers of “abnormal” embryos. In the USA, this article, therefore, became a second major turning point. A recent study demonstrated that at least 400 chromosomal normal pregnancies have been established worldwide from transfers of embryos, by PGS/PGT-A reported to be “mosaic” and/or “aneuploid” [25]. Most of these deliveries occurred in the USA.
Considering these indisputable IVF outcomes after transfer of “abnormal” embryos, maintaining that PGS/PGT-A improved IVF outcomes became increasingly difficult. Proponents of PGS/PGT-A, therefore, started looking for other potential benefits PGS/PGT-A might bring to IVF. One suggestion at that point was that, within the mosaic range, percentages of aneuploid DNA load were predictive of pregnancy chances [19]. But since aneuploid DNA thresholds are unable to differentiate between normal-euploid, mosaic, and abnormal-aneuploid ranges, it appears unreasonable to assume that they may successfully differentiate embryos within the mosaic range into better and poorer prognosis embryos. Using one of the proponent’s own data sets, this suggestion was, indeed, quickly refuted when ROC curves at 10% DNA intervals demonstrated absolutely no differences in IVF outcomes [26]. Improved cost-effectiveness became another new argument in favor of PGS/PGT-A [27]. An accompanying editorial to the article by Paulson, however, immediately disarmed this argument [28].
Ethics of special considerations for human embryos
That the PGS/PGT-A procedure daily determines the fate of thousands of human embryos around the world makes this also a conflict of considerable ethical significance. There is worldwide consensus that human embryos are deserving of special care and attention [29]. Experimentation with human embryos, therefore, is carefully balanced and institutional review boards restrict research to projects where benefits to mankind are clearly apparent [29]. Using human embryos in clinical IVF of course enhanced scrutiny of how human embryos are treated in the process of IVF. Here, too, consensus existed, however, from the very beginning of clinical IVF that human embryos do have a special standing in society and must be treated appropriately.
The principal goal of creating embryos in vitro is establishment of pregnancy for those patients who created them for the purpose of achieving pregnancy. “Wasting” embryos in this process is, therefore, not different from wasting embryos in inappropriate research projects. Previously described high false-positive diagnoses with PGS/PGT-A [17] leave no doubt that, due to PGS/PGT-A, large numbers of perfectly normal embryos with excellent pregnancy and live birth potential are being discarded daily for no good reason. Though this fact alone could be viewed as an ethical mandate to at least reduce utilization of PGS/PGT-A in association with IVF (any research project with similar unwarranted embryo loss rate would, unquestionably, be discontinued), it does not even consider the potential harm under the “do-no-harm” (per Latin translation from the original Greek, primum non nocere) [30] obligation of physicians that stems from the fact that the disposal of false-positive embryos may rob infertile women to significant degrees of their remaining pregnancy chances with IVF. Every unnecessarily disposed of false-positive embryo may have been the last chance of pregnancy for an infertile woman.
When appropriately informed of alleged advantages of PGS/PGT-A, the high false-positive rate of the procedure and its potential consequences for pregnancy and live birth chances, in informally interviewing a small number of infertile patients, we have found almost unanimity in rejecting the concept of PGS/PGT-A. These data may, however, be biased because they mostly relied on information obtained from patients who sought out CHR for the purpose of potentially transferring allegedly chromosomal “abnormal” embryos. An internationally renowned ethicist from another institution is, therefore, currently conducting an in-depth study involving patients, who faced the dilemma whether to transfer embryos, by PGS/PGT-A alleged to be chromosomal “abnormal,” or not.
Births of millions of offspring from IVF treatments all around the world, and the awarding of a Nobel Prize in Medicine and Physiology to Robert Edwards, Ph.D., have clearly established IVF’s benefits for mankind. Those benefits will only continue to expand, as percentages of IVF pregnancies among total births will continue to grow. In high IVF-utilization countries like Israel, IVF birth rates are almost approaching 10% of all live births. IVF has, however, never before faced a moment in time, when as many embryos with significant potential for healthy pregnancies and live births have been discarded without compensatory benefits, as PGS/PGT-A has been unable to demonstrate any clinical utility.
In this context, here analyzed PGS/PGT-A conflict also demonstrates how surprisingly hierarchical medical practice still is. The Social Dominance Theory, therefore, applies in all of its structural mechanisms when stating that, “decisions and behavior of individuals, formation of new social practices and operation of institutions are shaped by legitimizing myths” which, in turn, are “consensually held values, attitudes, beliefs stereotypes, and cultural ideologies [31]. Verlinsky’s PGS hypothesis has survived for over 20 years as exactly this kind of myth.
Summary and conclusions
The history of PGS/PGT-A has been a long series of legitimizing myths, promoted by a hierarchy of opinion leaders, considered experts in their field. In conflict theory, “experts” are considered least qualified to foresee the future in their areas of special expertise, as their life-long concentration on one area of expertise creates psychological conflicts that adversely affect their ability to reach unbiased conclusions [32].
Here presented history of PGS/PGT-A supports this theory, as “experts” over 20 years, apparently, have pursued a hypothesis, which biologically was nonsensical in the first place. Yet, psychologically dependent on the success of “their” hypothesis, they, until this day, have failed to acknowledge the shortcomings of the PGS hypothesis by repeatedly moving goal posts from PGS 1.0 to PGS 2.0 and, most recently, PGS 3.0. With increasing evidence for the failure of PGS 3.0, it is interesting to note that, through assessment of free DNA in spent media of embryos, “expert” proponents and the PGS/PGT-A industry have already started talking about PGS 4.0 [33], apparently still not comprehending (or wishing to comprehend) biological realities of preimplantation-stage embryos at blastocyst stage, which, simply, refute the logic of testing embryos at blastocyst stage that still have the ability to self-correct downstream [34].
By promoting hierarchy-enhancing legitimizing myths, those experts then provide moral and intellectual justification for fertility service providers in IVF centers with little or no knowledge about the biology of preimplantation-stage embryos, to offer a service, which, conveniently, also serves their financial interests. The construct of social dominance orientation can be well applied in this context to physicians and their patients [32], with physicians supporting a wide variety of legitimizing myths in treating their patients with PGS/PGT-A, even though increasing evidence has been demonstrating that PGS/PGT-A not only does not improve IVF outcomes but in many women actually reduces chances of successful conception and delivery of a healthy child. Interestingly, ASRM and its sister society SART just published another formal opinion on PGS/PGT-A which, 10 years removed from a first official policy statement in 2008 [11], once again concludes that PGS/PGT-A, still, has not proven any demonstrable clinical utility for IVF cycles [35]. This opinion, however, does not even do justice to here discussed conflict because by now hundreds of healthy pregnancies have been delivered after transfer of chromosomal “abnormal” embryos [15, 16, 20, 25, 36–38]. The potential harm form discarding of false-positive embryos is, therefore, no longer just theoretical.
A careful and detailed conflict analysis of PGS/PGT-A, thus, at a variety of levels uncovered an ethical morass that makes it difficult to maintain objectivity in attempts at conflict resolution when one comes to the conclusion that one side in the conflict has, likely, behaved inappropriately. But conflict often involves unethical behavior and attempts at conflict resolution, still, must go on. The ethical dimensions of this conflict, indeed, require that an attempt at intervention be made that may facilitate a resolution which interrupts, or at least improves, continuous ethical transgressions through the clinical utilization of PGS/PGT-A. Building an evidence base for add-ons to IVF before their clinical utilization was recently suggested in an important editorial [38] and should be the basis for a compromising resolution of this conflict.
The FRM is, therefore, exploring the possibility of sponsoring in 2020 a public symposium of opinion leaders from among proponents and opponents of PGS/PGT-A, also inviting to attend representatives of other actors in the conflict (see Fig. 1, conflict map). The symposium will be charged with attempting to formulate a majority consensus statement. Beyond continuous conflict and resulting increasing confusion among medical providers and their patients, the only alternative to such a “peaceful” internal resolution within the field of reproductive medicine would be an invitation to the FDA to place PGS/PGT-A under the agency’s jurisdiction and order limitations to practice utilization.
If human embryos are able to self-correct their biological fate, reproductive medicine should be able to demonstrate similar abilities for self-correction by addressing and resolving the conflict over PGS/PGT-A without government intervention.
Glossary of in this manuscript encountered conflict resolution terminology
- Accelerant
Person or constellation that enhances a conflict.
- Actor
Participant in a conflict. In accordance with their respective importance to the conflict, they are designated as primary, secondary, and tertiary actors.
- Attractor
Attractors are constellations of conflicts that feed intractability.
- Clusters
Graphic depiction of cooperating units in a conflict map.
- Conflict analysis
Systematic study of causes, actors, and dynamics of a conflict.
- Conflict map
Graphic depiction between actors in a conflict in relationship to problem(s).
- Destructive phase of a conflict
According to Deutsch, destructive conflicts are characterized by a tendency to expand and escalate. Whether a conflict is destructive or constructive depends on the behavior of actors. Behaviors that escalate conflicts until they develop a life of their own are considered destructive (M. Deutsch. J Social Issues 1969; 25(1):11–12).
- Glasl’s escalation model stages
(1) Hardening; (2) debates and polemics; (3) actions not words; (4) images and coalitions; (5) loss of face; (6) strategies of threats; (7) limited destructive blows; (8) fragmentation of the enemy; and (9) together into the abyss (Jordan T. https://www.mediate.com/articles/jordan.cfm).
- Hubs and spokes
Parts of a conflict map that graphically demonstrate “how things work.”
- Networks
When the number of actors in a conflict is high, it lends itself to a so-called network analysis.
- Stakeholders
Individuals who may be impacted by a conflict’s outcome.
- Sustainable consensus
To reach sustainable consensus it is essential for reach sustainable conflict resolution.
Author contributions
This analysis was the product of a mandated externship by L.M. at the Foundation for Reproductive Medicine (FRM), a not-for-profit research foundation in New York City, as part of the Master of Science Program in Negotiations and Conflict Resolution at Columbia University, New York, NY.
L.M., therefore, conducted the research and wrote the first draft of the manuscript. Both authors revised the manuscript and agreed on its final content.
Funding information
This manuscript was supported by intramural funding from The Foundation for Reproductive Medicine, New York, NY.
Compliance with ethical standards
Competing interests
L.M. has no competing interests to report but has joined The CHR as a research fellow. N.G. is President of The Foundation for Reproductive Medicine in New York, NY, a not-for-profit research foundation that hosted the externship of L.M. and supported her research. N.G. is also the owner of a for-profit fertility center, The CHR in New York, NY. He is a co-inventor on several pending and already awarded US patents claiming therapeutic benefits from androgen supplementation in women with low functional ovarian reserve and relating to the FMR1 gene in diagnostic functions in female fertility. He receives royalties from Fertility Nutraceuticals, LLC, in which N.G. also holds shares. He is also a co-inventor on three pending AMH-related patent applications and in the past received research grants, travel funds, and/or speaker honoraria from Pharma and/or medical device companies, though none in any way related to hear presented materials. Neither author has commercial interests, relating to PGS/PGT-A.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Partnership for the Prevention of Armed Conflict (GPPAC). Conflict analysis framework: field guidelines and procedure. https://gppac.net/files/2018-11/GPPAC%20CAFGuide_Interactive%20version_febr2018_.pdf.
- 2.Verlinsky Y, Cieslak J, Ivakhnenko V, Evsikov S, Wolf G, White M, Lifchez A, Kaplan B, Moise J, Valle J, Ginsberg N, Strom C, Kuliev A. Preimplantation diagnosis of common aneuploidies by the first and second polar body FISG analysis. J Assist Reprod Genet. 1998;15:285–289. doi: 10.1023/A:1022592427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staessen C, Platteau P, Van Assche E, Miciels A, Tournaye H, Camus M, Devroey P, Liebaers I, van Steirteghem A. Comparison of blastocyst transfer with and without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19:2849–2858. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 4.Platteau P, Staessen C, Michiels A, Van Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in women older than 37 years. Fertil Steril. 2005;84:319–324. doi: 10.1016/j.fertnstert.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Platteau P, Staessen C, Michiels A, van Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic screening for aneuploidy screening in patients with unexplained recurrent miscarriages. Fertil Steril. 2005;83:393–397. doi: 10.1016/j.fertnstert.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Weghofer A, Barad D. Preimplantation genetic screening: “established” and ready for prime time? Fertil Steril. 2008;89(4):780–788. doi: 10.1016/j.fertnstert.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM, Buys CH, Heineman MJ, Repping S, van der Veen F. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 8.FDA, 2017. [Laboratory developed tests, U.S. Food & Drug Administration, U.S. Department of Health and Human Services, January 13, 2017, https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/LaboratoryDevelopedTests/default.htm.
- 9.Deutsch M. Conflicts: productive and destructive. J Social Issues. 1969;25:7–41. doi: 10.1111/j.1540-4560.1969.tb02576.x. [DOI] [Google Scholar]
- 10.Glasl F, et al. The process of conflict escalation and roles of third parties. In: Bomers GBJ, et al., editors. Conflict Managment and Industrial Relations. Dordercht: Springer Science Business Media; 1982. pp. 119–140. [Google Scholar]
- 11.Practice Committee of the American Society for Reproductive Medicine Preimplantation genetic testing: a Practice Committee opinion. Feril Steril. 2008;90:S136–S143. doi: 10.1016/j.fertnstert.2008.08.062. [DOI] [PubMed] [Google Scholar]
- 12.Gleicher N, Metzger J, Croft G, Kushnir VA, Albertini DF, Barad DH. A single trophectoderm biopsy at blastocyst stage is mathematically unable to determine embryo ploidy accurately enough for clinical use. Reprod Biol Endocrinol. 2017;15(1):23. doi: 10.1186/s12958-017-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton H, Graham SJ, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, Voet T, Zernicka-Goetz M. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165. doi: 10.1038/ncomms11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rito T, Naftaly J, Gleicher N, Brivanlou A. Self-correction of aneuploidy in human blastocystsmand self-organizing gastruloids. Fertil Steril, 2019; 112 (suppl 3), e127;P49
- 15.Gleicher N, Vidali A, Braverman J, Kushnir VA, Albertini DF, Barad DH. Further evidence against use of PGS in poor prognosis patients: report of normal births after transfer of embryos reported as aneuploid. Fertil Steril 2015;104(Suppl).
- 16.Greco E, Giulia Minasi M, Florentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2989–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- 17.Paulson RJ. Preimplantation genetic screening: what is the clinical efficiency? Fertil Steril. 2017;108(2):228–230. doi: 10.1016/j.fertnstert.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 18.PGDIS. Preimplantation Genetic Diagnosis Society (PGDIS) position statement on chromosome mosaicism and preimplantation aneuploidy testing at the blastocyst stage, Chicago, Illinois; July 19, 2016 http://pgdis.org/docs/newsletter_071816.html.
- 19.Kredo T, Bernhardsson S, Machingaidize S, Yung T, Louw Q, Ochodo E, Grimmer K. Guide to clinical practice guidelines: the current state of play. Int J Quality in Health Care. 2016;28(1):122–128. doi: 10.1093/intqhc/mzv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munné S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, Tarozzi N, Borini A, Becker A, Zhang J, Maxwell S, Grifo J, Barbariya D, Wells D, Fragouli E. Detailed investigation into the cytogenic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108(1):62–71. doi: 10.1016/j.fertnstert.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Scott RT, Jr, Galliano D. The challenge of embryonic mosaicism in preimplantation genetic screening. Fertil Steril. 2016;105(5):1150–1152. doi: 10.1016/j.fertnstert.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Munne S. Origins of mosaicism and criteria for transfer of mosaic embryos. Reprod BioMed Online. 2018;35(4):369–370. doi: 10.1016/j.rbmo.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Rosenwaks Z, Handyside AH, Fiorentino F, Gleicher N, Paulson RJ, Schattman GL, Scott RT, Jr, Summers MC, Treff NR, Xu K. The pros and cons of preimplantation genetic testing for aneuploidy: clinical and laboratory perspectives. Fertil Steril. 2018;110(3):353–361. doi: 10.1016/j.fertnstert.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Hall S. Tens of thousands of women thought they couldn’t have babies. But what if they could? The abnormal embryos that aren’t. New York, 2017, p32.
- 25.Patrizio P, Shoham G, Shoham Z, Leong M, Barad DH, Gleicher N. Worldwide live births following transfer of chromosomally “abnormal” embryos after PGT/A” results of a worldwide web-based survey. 2019; doi: 10.1007/s10815-019-01510-0; In press [DOI] [PMC free article] [PubMed]
- 26.Kushnir VA, Darmon S, Albertini DF, Barad DH, Gleicher N. Degree of mosaicism in trophectoderm does not predict pregnancy potential: a corrected analysis of pregnancy outcomes following transfer of mosaic embryos. Reprod Biol Endocrinol. 2018;16(1):6. doi: 10.1186/s12958-018-0322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somigliana E, Busnelli A, Paffoni A, Vigano P, Riccaboni A, Rubio C, Capalbo A. Cost-effectiveness of preimplantation genetic testing for aneuploidy. Fertil Steril. 2019;111(6):1169–1175. doi: 10.1016/j.fertnstert.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Paulson RJ. Mathematics should clarify, not obfuscate: an inaccurate and misleading calculation of the cost-effectiveness of preimplantation genetic testing for aneuploidy. Fertil Steril. 2019;111(6):1113–1114. doi: 10.1016/j.fertnstert.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Gleicher N, Caplan AL. An alternative proposal to the destruction of abandoned human embryos. Nat Biotechnol. 2018;36(2):139–141. doi: 10.1038/nbt.4070. [DOI] [PubMed] [Google Scholar]
- 30.Shmerling RH. First, do no harm. Harvard health Publsihing, Harvard Medical School https://www.health.harvard.edu/blog/first-do-no-harm-201510138421.
- 31.Pratto F, Sidanius J, Levin S. Social dominance theory and the dynamics of intergroup relations: taking stock and looking forward. European Rev of Social Psychology. 2006;17:271–320. doi: 10.1080/10463280601055772. [DOI] [Google Scholar]
- 32.Tetlock PE. Expert political judgment. Princenton, NJ: Princeton University Press; 2005. [Google Scholar]
- 33.Xu J, Fang R, Chen L, Chen D, Xiao JP, Yang W, Wang H, Song X, Ma T, Bo S, Shi C, Ren J, Huang L, Cai LY, Yao B, Xie XS, Lu S. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci U S A. 2016;113(42):11907–11912. doi: 10.1073/pnas.1613294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleicher N, Barad DH. Not even noninvasive cell-free DNA can rescue preimplantation genetic testing. Proc Natl Acad Sci U S A. 2019. 10.1073/pnas.1911710116. [DOI] [PMC free article] [PubMed]
- 35.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429–436. doi: 10.1016/j.fertnstert.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Morales R, Lledó B, Ortiz JA, Ten J, Lláce J, Bernabeu R. Embryos showing mosaicism in trophectoderm cells can achieve good pregnancy rates. Hum Reprod. 2016;31(Supl 1):i14, O–i14030. [Google Scholar]
- 37.Victor A, Tyndall JC, Brake AJ, Lepkowsky LT, Murphy AE, Griffin DK, McCoy RC, Barnes FL, Zouves CG, Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Feril Steril. 2019;111(2):280–293. doi: 10.1016/j.fertnstert.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Macklon NS, Ahuja KK, Fauser BMJM. Building and evidence base for IVF “add-ons”. Reprod BioMed Online. 2019;38(2):853–856. doi: 10.1016/j.rbmo.2019.04.005. [DOI] [PubMed] [Google Scholar]