Abstract
Purpose
To assess whether GnRH agonist trigger impacts the implantation potential of euploid embryos.
Methods
Retrospective cohort study done at an academic IVF center evaluating frozen-thawed embryo transfer (FET) cycles in which single-euploid blastocysts were transferred between 2014 and 2019. All embryos were generated in an IVF cycle which used GnRHa or hCG trigger and then were transferred in a programmed or natural FET cycle. Only the first FET cycle was included for each patient. Primary outcome was ongoing pregnancy rate or live birth rate (OPR/LBR). Secondary outcomes were implantation rate (IR), clinical pregnancy rate (CPR), clinical loss rate (CLR), and multiple pregnancy rate (MPR). Logistic regression was performed to control for confounding variables. A p value of < 0.05 was considered statistically significant.
Results
Two hundred sixty-three FET cycles were included for analysis (GnRHa = 145; hCG = 118). The GnRHa group was significantly younger (35.2 vs. 37.5 years) and had higher AMH values (4.50 ng/ml vs. 2.03 ng/ml) than the hCG group, respectively (p < 0.05). There was no significant difference in OPR/LBR (64.1% (93/145) vs. 65.3% (77/118); p = 0.90) between the GnRHa and hCG groups, respectively. There was also no significant difference in IR, CPR, CLR, or MPR between groups. After controlling for confounding variables, the adjusted odds ratio for OPR/LBR was 0.941 (95% CI, 0.534–1.658); p = 0.83) comparing GnRHa to hCG. Pregnancy outcomes did not significantly differ when groups were stratified by age (< 35 vs. > 35 years old).
Conclusions
Our findings confirm that euploid embryos created after hCG or GnRHa trigger have the same potential for pregnancy.
Keywords: Assisted Reproductive Technologies, In Vitro Fertilization, Preimplantation Genetic Testing, GnRH agonist trigger
Introduction
GnRH agonist (GnRHa) trigger for final oocyte maturation along with a shift towards an increasing number of “freeze-only” cycles has markedly increased the safety of in vitro fertilization (IVF) for all patients by nearly eliminating the risk of moderate and severe ovarian hyperstimulation syndrome (OHSS) [1, 2]. Despite this major breakthrough, there were initially concerns regarding its widespread usage due to early studies reporting significantly lower pregnancy rates following fresh embryo transfer compared to hCG trigger [3, 4]. However, it has now been well demonstrated that with use of adjuvant low dose hCG and appropriate luteal support, pregnancy outcomes with GnRHa trigger are favorable and comparable to hCG in fresh cycles [5–10].
Furthermore, in comparison to hCG, GnRHa trigger results in similar oocyte yield, proportion of mature oocytes and number of supernumerary embryos for cryopreservation in IVF cycles [1, 11]. Moreover, the implantation rate in embryos not screened with preimplantation genetic testing (PGT) derived after GnRHa trigger is comparable to hCG trigger after frozen-thawed embryo transfer (FET) cycles [12–14], as well as donor oocyte recipient cycles [11, 15, 16].
With the increasing utilization of PGT [17] and ‘freeze all’ cycles [18], it has become pertinent to determine whether the mode of trigger adversely impacts implantation rates of euploid embryos. A recent study showed that euploidy rates are comparable following GnRHa compared to hCG trigger, thereby confirming that GnRHa does not adversely impact embryo ploidy status and quality [19]. While this suggests that the reproductive potential does not differ according to trigger medication, it is also known that chromosome status is not the sole determining factor of whether an embryo results in pregnancy or live birth. Even with modern molecular techniques for identifying chromosomally normal embryos, a substantial proportion of euploid embryos fail to implant [20].
The potential reasons for failed implantation after transfer of euploid embryos are currently not well understood. It has been proposed that differential gene, protein, mRNA, mitochondrial DNA (mtDNA) and differential microRNA (miRNA) gene expression may play an important role in the implantation of euploid blastocysts [21–24]. It has been further suggested that the type of trigger medication may impact gene expression in the developing embryo as well [25]. As such, it is feasible that differences may exist in the ability of a blastocyst to implant after GnRHa compared to hCG trigger irrespective of ploidy. Thus, it is imperative to methodically scrutinize the IVF process to determine what modifiable variables may be contributing to the metabolic health of the embryo and correlate these findings with clinical outcomes.
Therefore, we aimed to establish whether live birth and ongoing pregnancy rates differed in FET cycles with transfer of a single euploid blastocyst, which was generated from an IVF cycle in which GnRHa or hCG was used for trigger.
Materials and Methods
This was a retrospective cohort study performed at a single academic IVF center evaluating FET cycles in which a single euploid blastocyst was transferred between the time period of January 2013 and April 2019. Cycles were included if the euploid blastocyst was generated in a previous IVF cycle in which preimplantation genetic testing for aneuploidy (PGT-A) was done and hCG or GnRHa was used for final oocyte maturation. Since PGT-A was being performed, all embryos were cryopreserved while awaiting biopsy results and therefore no fresh embryo transfers were performed. Only the first FET cycle immediately following an IVF cycle (i.e. primary embryo transfers) was included. Therefore, only one cycle per patient was evaluated. Cycles were excluded if >1 embryo was transferred, donor oocyte was used to create the embryo, or if dual trigger with both hCG and GnRHa was used. This study was approved by our university’s institutional review board.
IVF stimulation protocols have been previously described [26, 27]. The IVF stimulation protocol was selected based on patient factors and physician preference. Gonadotropin dosing was adjusted according to patient response. Trigger for final oocyte maturation was administered when ≥3 follicles reached approximately 18 mm in diameter. Either hCG 3300 - 10000 IU SC (Pregynl, Merck; Novarel, Ferring Pharmaceuticals) or GnRHa 1 mg (leuprolide acetate, Abbott Laboratories) was used for trigger based on physician preference, protocol type and risk of OHSS. Transvaginal ultrasound-guided oocyte retrieval was performed 35 hours after trigger injection. Oocytes were fertilized with either intracytoplasmic sperm injection or conventional insemination. Trophectoderm biopsy was performed on good-quality blastocysts according to Gardner criteria (3BB or higher) [28]. PGT-A was done with either array comparative genomic hybridization (aCGH) or next generation sequencing (NGS).
Embryos were subsequently cryopreserved using vitrification. Vitrification was done with rapid exposure to a cryoprotectant solution consisting of 15% ethylene glycol, 15% dimethylsulphoxide, 20% dextran and 0.5 mmol/l sucrose in a Cryolock® device (Irvine Scientific, Santa Ana, CA, USA). Prior to vitrification, a laser pulse of 300 μs (constant 0.9 J) was applied in order to collapse the blastocoel. When ready for thaw, all blastocysts were rapidly warmed in solutions of 0.5 and 0.2 mmol/L sucrose and rinsed through HEPES-buffered human tubal fluid with 12 mg/mL human serum albumin. To allow time for re-expansion, embryos were thawed and equilibrated in culture 1–2 h before transfer.
FET was performed in either a natural or programmed cycle, as previously described [29]. The decision to do a programmed or natural FET cycle was based on the patient’s ovulatory status and physician preference. Programmed cycles consisted of downregulation with GnRHa in the luteal phase of the preceding cycle followed by increasing doses of oral or transdermal estradiol (E2) following menses. Intramuscular (IM) progesterone was started when endometrial thickness reached approximately 8 mm. FET was performed on the sixth day of IM progesterone. In a natural FET cycle, patients were monitored with daily bloodwork beginning on cycle day 10. FET was performed 6 days after detecting the LH surge and the luteal phase was supplemented with vaginal progesterone (Crinone, Merck; Endometrin, Ferring Pharmaceuticals) beginning 2 days after the LH surge.
The primary outcome was ongoing pregnancy rate or live birth rate (OPR/LBR). Secondary outcomes were implantation rate (IR), clinical pregnancy rate (CPR), clinical loss rate (CLR) and multiple pregnancy rate (MPR). Implantation rate was defined as the number of gestational sacs visualized on ultrasound divided by the number of embryos transferred. Clinical pregnancy was defined as at least one gestational sac with or without fetal pole visualized within the uterus on transvaginal ultrasound. Ongoing pregnancy was defined as fetal heart movement visualized on ultrasound that progressed beyond 10 weeks gestation. Clinical pregnancy loss was defined as a pregnancy loss prior to 20 weeks of gestation after confirming a clinical pregnancy on ultrasound. Pregnancy outcomes were compared by trigger medication type for all patients and also by groups stratified by age (<35 years old and ≥35 years old).
Statistical analysis was done using SPSS. Student’s t-test or Mann-Whitney U was used to analyze continuous variables depending on whether the data is normally distributed, Chi-Square or Fischer’s exact test was used for categorical variables. Continuous variables were presented as mean ± SD and categorical variables presented as percentage and count. A binary logistic regression was performed to control for potential confounders including age, AMH and anovulation/PCOS and diminished ovarian reserve (DOR) diagnoses. All variables were entered using a forced entry method, where all the predictor variables were tested in one block to assess their predictive ability whilst controlling for other predictors in the model. A two-sided p-value of <0.05 was considered statistically significant.
Results
A total of 263 FET cycles were included for analysis. Of those, hCG trigger was used in 118 cycles and GnRHa trigger in 145 cycles. The baseline and demographic information for each trigger group of patients is presented in Table 1. The patients triggered with GnRHa were significantly younger (35.2 ± 4.4 years old vs. 37.5 ± 3.6 years old; p=0.02) and had significantly higher AMH levels (4.5 ± 3.76 ng/mL vs. 2.03 ± 1.92 ng/mL; p<0.01) compared to those triggered with hCG, respectively. There was no significant difference between groups in the other baseline characteristics evaluated including body mass index (BMI), history of prior live birth, use of preimplantation genetic testing for monogenic gene condition (PGT-M) or method of endometrial preparation for the FET cycle (natural or programmed). There were more patients with the diagnosis of PCOS/Anovulation and fewer with the diagnosis of decreased ovarian reserve/ovarian dysfunction in the GnRHa group compared to the hCG group, respectively.
Table 1.
Baseline and demographic characteristics
| GnRHa (n = 145) | hCG (n = 118) | p value | |
|---|---|---|---|
| Age (years) | 35.2 ± 4.4 | 37.5 ± 3.6 | 0.02 |
| BMI (kg/m2) | 25.3 ± 4.7 | 26.4 ± 5.8 | NS |
| AMH (ng/ml) | 4.50 ± 3.76 | 2.03 ± 1.92 | < 0.01 |
| Prior live birth, n (%) | 56 (38.6%) | 46 (39.0%) | NS |
| Use of PGT-M, n (%) | 36 (24.8%) | 28 (23.7%) | NS |
| Medicated FET protocol, n (%) | 48 (33.1%) | 40 (33.9%) | NS |
| Diagnosis, n (%) | < 0.01 | ||
| Unexplained | 40 (27.6%) | 45 (38.1%) | |
| Anovulation/PCOS | 20 (13.8%) | 3 (2.5%) | |
| Male factor | 15 (10.3%) | 18 (15.3%) | |
| Tubal factor | 5 (3.4%) | 6 (5.1%) | |
| Endometriosis | 3 (2.1%) | 6 (5.1%) | |
| Fibroids | 7 (4.8%) | 2 (1.7%) | |
| Recurrent pregnancy Loss | 27 (18.6%) | 18 (15.3%) | |
| Decreased ovarian reserve | 1 (0.7%) | 9 (7.6%) | |
| Other | 27 (18.6%) | 11 (9.3%) |
Table 2 displays pregnancy outcomes by trigger medication type for all patients. There was no statistically significant difference in the primary outcome of ongoing pregnancy rate/live birth rate (64.1% (93/145) vs. 65.3% (77/118); p=0.90) between GnRHa and hCG trigger groups, respectively. There was also no significant difference in IR (70.3% (100/145) vs. 72.0% (85/118); p=0.79) or CPR (69.0% (100/145) vs. 72.0% (85/118); p=0.68) between the GnRHa and hCG trigger groups respectively. Moreover, the CLR (7.0% (7/100) vs. 9.4% (8/85); p=0.38) did not differ when comparing the GnRHa to the hCG trigger group, respectively. Since all the cycles were single embryo transfers, the multiple pregnancy rates were low (4.0% (4/100) vs. 1.2% (1/85); p=0.60) in both the GnRHa and hCG trigger groups as expected, respectively.
Table 2.
Pregnancy outcomes, all patients
| GnRHa | hCG | p value | |
|---|---|---|---|
| IR (#sacs/ET) | 100/145 (70.3%) | 85/118 (72.0%) | 0.79 |
| CPR, n (%) | 100/145 (69.0%) | 85/118 (72.0%) | 0.68 |
| OPR/LBR, n (%) | 93/145 (64.1%) | 77/118 (65.3%) | 0.90 |
| CLR, n (%) | 7/100 (7.0%) | 8/85 (9.4%) | 0.38 |
| MPR, n (%) | 4/100 (4.0%) | 1/85 (1.2%) | 0.60 |
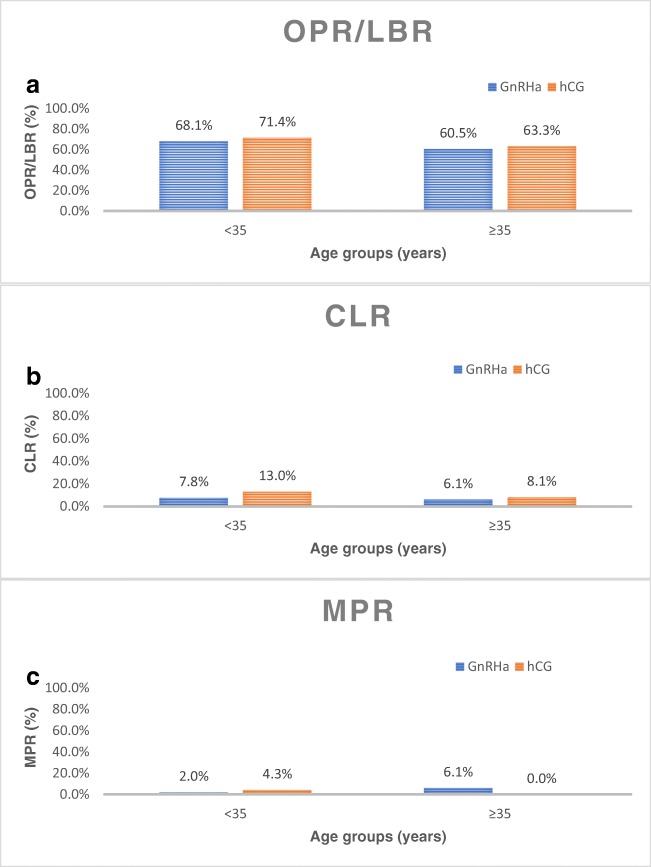
When the groups were stratified by age, the OPR/LBR in the <35 year old group was not significantly different (68.1% (47/69) vs. 71.4% (20/28); p=0.81) between GnRHa and hCG groups, respectively (Figure 1A). Similarly, in the ≥35 year old group, OPR/LBR also did not differ significantly (60.5% (46/76) vs. 63.3% (57/90); p=0.75) between GnRHa and hCG groups, respectively (Figure 1A). There was also no significant difference between groups, when stratified by age, in CLR and MPR (Figure 1B, 1C). In the <35 year old group, IR did not differ according to trigger type (GnRHa = 73.9% (51/69) vs. hCG = 85.7% (24/28); p=0.29) nor did CPR (GnRHa = 73.9% (51/69) vs. hCG = 82.1% (23/28); p=0.44). In the ≥35 year old group, similarly, IR did not differ between trigger groups (GnRHa = 67.1% (51/76) vs. hCG = 67.8% (61/90); p=1.0) nor did CPR (GnRHa = 64.5% (49/76) vs. hCG = 68.9% (62/90); p=0.62).
Fig. 1.
A OPR/LBR stratified by age group (< 35: p = 0.81; ≥ 35: p = 0.75). B CLR stratified by age group (< 35: p = 0.67; ≥ 35: p = 1.0). C MPR stratified by age group (< 35: p = 0.53); ≥ 35: p = 0.08)
A binary logistic regression analysis was performed and the full model containing all the covariates including age, AMH, anovulation/PCOS and DOR diagnoses, was not statistically significant, χ2(5, N=263)=2.45, p=0.780, indicating that the model did not predict livebirth. Therefore, after controlling for all the confounding variables in the model, the OPR/LBR did not differ between the GnRHa or hCG trigger groups (Adjusted odds ratio 0.941 (95% CI: 0.534-1.658); p=0.83).
Discussion
This study demonstrates no difference in the reproductive potential of euploid blastocysts generated after GnRHa or hCG trigger. In this analysis, we showed that ongoing pregnancy and live birth rates did not significantly differ between the GnRHa and hCG trigger groups in FET cycles in which a single euploid blastocyst was transferred. Furthermore, the implantation and clinical pregnancy rates were comparable in the two groups. Since only single euploid embryos were transferred, the multiple pregnancy rates and clinical loss rates were low in both groups. These findings further support the efficacy of GnRHa as an alternative to hCG for final oocyte maturation, particularly in patients who are not having a fresh transfer and cryopreserving all embryos.
To examine the impact of trigger medication on pregnancy outcomes, other groups have reported no adverse impact on pregnancy rates in FET cycles with non-PGT tested embryos when the preceding fresh IVF cycles were triggered with either hCG or GnRHa, to eliminate the effect of abnormal endometrial receptivity after fresh cycles. Griesinger et al. (2007) reported on LBR following FET cycles in patients who received hCG or GnRHa trigger in a previous IVF cycle. This was a secondary analysis of data from a randomized control trial comparing oocyte maturity in normal responder patients who were randomized to either hCG or GnRHa trigger. They observed that the LBR was not impaired and in fact both LBR per embryo transfer and cumulative LBR was higher in the GnRHa triggered group compared to the hCG group [13].
Another strategy to assess the impact of trigger medication on pregnancy outcomes has been to analyze donor oocyte cycles. This method controls for oocyte quality and baseline patient characteristics as oocyte donors tend come from a young and healthy pool of patients with favorable ovarian reserve. It also spares the endometrium that will ultimately receive an embryo the potentially detrimental effects of high dose gonadotropin stimulation and adverse impact of GnRHa trigger on the endometrium. Melo et al. (2009) performed a prospective randomized control trial in which 100 oocyte donors underwent IVF stimulation with antagonist protocols and were randomized to either GnRHa or recombinant hCG trigger. They found no significant difference in CPR [15]. This finding has been confirmed by other investigators [16].
Evaluation of PGT-A screened euploid embryos allows for a better assessment of an embryo’s true reproductive potential regardless of maternal baseline characteristics. It was recently reported that euploidy rates in biopsied blastocysts was comparable following GnRHa and hCG trigger [19]. Clearly, additional factors are at play as nearly 30-40% chromosomally normal embryos, even those screened with highly accurate modern molecular screening techniques such as aCGH and NGS, fail to implant [20]. Even among euploid embryos, it has been shown that implantation rate differs according to their morphologic grading and rate of development to the blastocyst stage [30].
The role of molecular factors other than aneuploidy including differential gene, protein, mRNA, mtDNA and miRNA expression in predicting pregnancy have been the focus of multiple investigations. Fragouli et al. (2017) showed significantly higher levels of mtDNA in blastocysts that failed to implant compared to those that did [21]. Similarly, multiple groups have demonstrated differential expression of miRNAs in bastocysts that implant compared to those that did not [22, 23]. While these studies have identified potential biomarkers of embryo viability, it remains unclear what factors lead to these alterations in gene, mRNA and protein expression. It is plausible that variables in the IVF process such as trigger medication may be responsible. In fact, one study showed differential expression of messenger RNA in cumulus cells and granulosa cells from patients who underwent IVF and were randomized to hCG or GnRHa trigger [25]. Nevertheless, in this study, we showed no differences in pregnancy rates between the two trigger modalities, confirming that GnRHa trigger does not adversely impact the implantation potential of an euploid embryo.
In our investigation, only one FET cycle that immediately followed an IVF cycle per patient was included thus eliminating any impact of multiple cycles per patient on outcome. Moreover, only single euploid blastocyst transfers were included resulting in high ongoing and live birth rates while maintaining significantly low multiple pregnancy and loss rates in both groups. Even so, this study was conducted retrospectively, thus the evidence presented remains subject to confounding and bias. As highlighted previously, the patients who received GnRHa compared to hCG trigger differed at baseline, with the GnRHa group reflecting a high responder younger population. Unsurprisingly, the GnRHa group had a larger proportion of patients who were anovulatory and the hCG group had a higher proportion of patients with decreased ovarian reserve. However, previous studies have shown that once a euploid embryo is available for transfer, the impact of maternal age on pregnancy rates significantly declines and pregnancy rates are comparable across age groups [31–33]. Nevertheless, we did perform logistic regression analysis to control for potential confounding variables including age, AMH, diagnosis and also stratified by age group. We still found that the odds of ongoing pregnancy or live birth did not differ according to trigger medication in FET cycles.
While there were no differences in OPR/LBR between the two groups, this study may be underpowered and therefore limited by a type 2 error. However, we performed a post-hoc sample size calculation which showed that a sample size of 24,897 would be required in each group to show a significant difference in OPR/LBR between the GnRHa trigger group proportion of 0.641 and hCG trigger proportion of 0.653 at the 80% power and 5% level of significance. Despite these limitations, this study clearly provides further evidence that GnRHa trigger does not adversely affect the embryo implantation potential. While a prospective randomized controlled trial would be the optimal method to validate these findings, the large sample size required to show the difference in live birth rate noted in this study would be too impractical to implement.
In conclusion, our findings indicate that GnRHa trigger for final oocyte maturation does not significantly impact the reproductive potential of euploid blastocysts. This adds to the growing compendium of studies that have demonstrated the safety and effectiveness of GnRHa trigger. GnRHa trigger should be considered as a viable option not only in high responder patients, but also for those cryopreserving all embryos either electively or for other indications such as PGT and fertility preservation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict(s) of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alyasin A, Mehdinejadiani S. GnRH Agonist Trigger versus HCG Trigger in GnRH Antagonist in IVF/ICSI Cycles: A Review Article. Int J Reprod BioMed. 2016;14:557–566. doi: 10.29252/ijrm.14.9.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The Use of Gonadotropin-Releasing Hormone (GnRH) Agonist to Induce Oocyte Maturation after Cotreatment with GnRH Antagonist in High-Risk Patients Undergoing in Vitro Fertilization Prevents the Risk of Ovarian Hyperstimulation Syndrome: A Prospective Randomized Controlled Trial. Fertil Steril. 2008;89:84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grondahl ML, Westergaard L, Andersen CY. GnRH Agonist (Buserelin) or HCG for Ovulation Induction in GnRH Antagonist IVF / ICSI Cycles : A Prospective Randomized Study. Hum Reprod. 2005;20:1213–1220. doi: 10.1093/humrep/deh765. [DOI] [PubMed] [Google Scholar]
- 4.Youssef, M., Van der Veen, F., Al-Inany, H., Griesinger, G., Mochtar, M., Aboulfoutouh, I., Khattab, S., Van Wely, M. Gonadotropin-Releasing Hormone Agonist versus HCG for Oocyte Triggering in Antagonist Assisted Reproductive Technology Cycles. Cochrane Database of Syst Rev 2009; 4. [DOI] [PubMed]
- 5.Engmann L, Benadiva C, Humaidan P. GnRH Agonist Trigger for the Induction of Oocyte Maturation in GnRH Antagonist IVF Cycles: A SWOT Analysis. Reprod BioMed Online. 2016;32:274–285. doi: 10.1016/j.rbmo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Haahr T, Roque M, Esteves S, Humaidan P. GnRH Agonist Trigger and LH Activity Luteal Phase Support versus Hcg Trigger and Conventional Luteal Phase Support in Fresh Embryo Transfer IVF/ICSI Cycles-a Systematic PRISMA Review and Meta-Analysis. Front Endocrinol. 2017;8:1–10. doi: 10.3389/fendo.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humaidan P, Ejdrup-Bredkjaer H, Westergaard LG, Yding Anderson C. 1,500 IU Human Chorionic Gonadotropin Administered at Oocyte Retrieval Rescues the Luteal Phase When Gonadotropin-Releasing Hormone Agonist Is Used for Ovulation Induction: A Prospective, Randomized, Controlled Study. Fertil Steril. 2010;93:847–854. doi: 10.1016/j.fertnstert.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Iliodromiti S, Blockeel C, Tremellen KP, Fleming R, Torunaye H, Humaidan P, Nelson SM. Consistent High Clinical Pregnancy Rates and Low Ovarian Hyperstimulation Syndrome Rates in High-Risk Patients after GnRH Agonist Triggering and Modified Luteal Support: A Retrospective Multicentre Study. Hum Reprod. 2013;28:2529–2536. doi: 10.1093/humrep/det304. [DOI] [PubMed] [Google Scholar]
- 9.Radesic B, Tremellen K. Oocyte Maturation Employing a GnRH Agonist in Combination with Low-Dose HCG Luteal Rescue Minimizes the Severity of Ovarian Hyperstimulation Syndrome While Maintaining Excellent Pregnancy Rates. Hum Reprod. 2011;26:3437–3442. doi: 10.1093/humrep/der333. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro B, Said T, Garner F, Aguirre M, Hudson S. Comparison of "triggers" using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril. 2011;95:2715–2717. doi: 10.1016/j.fertnstert.2011.03.109. [DOI] [PubMed] [Google Scholar]
- 11.Galindo A, Bodri D, Guillen JJ, Colodron M, Vernaeve VS, Coll O. Triggering with HCG or GnRH Agonist in GnRH Antagonist Treated Oocyte Donation Cycles : A Randomised Clinical Trial. Gynecol Endocrinol. 2009;25:60–66. doi: 10.1080/09513590802404013. [DOI] [PubMed] [Google Scholar]
- 12.Eldar-geva T, Zylber-Haran E, Babayof R, Halevy-Shalem T, Ben-Chetrit A, Tsafrir A, Varshaver I, Brooks B, Margalioth EJ. Similar Outcome for Cryopreserved Embryo Transfer Following GnRH-Antagonist / GnRH- Agonist , GnRH-Antagonist/HCG or Long Protocol Ovarian Stimulation. Reprod BioMed Online. 2007;14:148–154. doi: 10.1016/S1472-6483(10)60781-X. [DOI] [PubMed] [Google Scholar]
- 13.Griesinger G, Kolibianakis EM, Papanikolaou EG, Diedrich K, Van Steirteghem A, Devroey P, Ejdrup-Bredkjaer H, Humaidan P. Triggering of Final Oocyte Maturation with Gonadotropin-Releasing Hormone Agonist or Human Chorionic Gonadotropin. Live Birth after Frozen-Thawed Embryo Replacement Cycles. Fertil Steril. 2007;88:616–621. doi: 10.1016/j.fertnstert.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Griesinger G, Berndt H, Schultz L, Depenbusch M, Schultze-Mosgau A. Cumulative Live Birth Rates after GnRH-Agonist Triggering of Final Oocyte Maturation in Patients at Risk of OHSS : A Prospective , Clinical Cohort Study. Eur J Obstet Gynecol. 2010;149:190–194. doi: 10.1016/j.ejogrb.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Melo M, Busso CE, Beliver J, Alama P, Garrido N, Meseguer M, Pellicer A, Remohl K. GnRH Agonist versus Recombinant HCG in an Oocyte Donation Programme: A Randomized, Prospective, Controlled, Assessor-Blind Study. Reprod BioMed Online. 2009;19:486–492. doi: 10.1016/j.rbmo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Sismanoglu A, Tekin H, Erden HF, Ciray NH, Ulug U, Bahceci M. Ovulation Triggering with GnRH Agonist vs. HCG in the Same Egg Donor Population Undergoing Donor Oocyte Cycles with GnRH Antagonist: A Prospective Randomized Cross-over Trial. J Assist Reprod Genet. 2009;26:251–256. doi: 10.1007/s10815-009-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society for Assisted Reproductive Technology. SART National Summary Report. 2015.
- 18.Shapiro B, Daneshmand S, Garner F, Aguirre M, Hudson C. Freeze-all can be a suoerior therapy to fresh cycle in patients with prior fresh blastocyst implantation failure. Reprod Biomed Online. 2014;29:286–290. doi: 10.1016/j.rbmo.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Thorne J, Loza A, Kaye L, Nulsen J, Benadiva C, Grow D, Engmann L. Euploidy rates between cycles triggered with gonadotropin-releasing hormone agonist and human chorionic gonadotropin. Fertil Steril. 2019;112:258–265. doi: 10.1016/j.fertnstert.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Friedenthal J, Maxwell S, Munne S, Kramer Y, McCulloh D, McCaffrey C, Grifo J. Next generation sequencing for preimplantation genetic screening improves pregnancy outcomes compared with array comparitive genomic hybridization in single thawed euploid embryo transfer cycles. Fertil Steril. 2018;104:627–632. doi: 10.1016/j.fertnstert.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Fragouli E, McCaffrey C, Ravichandran K, Spath K, Grifo JA, Munne S, Wells D. Clinical implications of mitrochondrial DNA quantification on pregnancy outcomes: a blinded prospective non-selection study. Hum Reprod. 2017;32:2340–2347. doi: 10.1093/humrep/dex292. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbluth E, Shelton D, Wells L, Sparks A, Van Voorhis B. Human embryos secrete microRNAs into culture media--a potential biomarker for implantation. Fertil Steril. 2014;101:1493–1500. doi: 10.1016/j.fertnstert.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 23.Capalbo A, Ubaldi F, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, Ilic D, Rienzi L. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105:225–235. doi: 10.1016/j.fertnstert.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Krisher R, Schoolcraft W, Katz-Jaffe M. Omics as a window to view embryo viability. Fertil Steril. 2015;103:333–341. doi: 10.1016/j.fertnstert.2014.12.116. [DOI] [PubMed] [Google Scholar]
- 25.Borgbo T, Povlsen B, Andersen C, Borup R, Humaidan P, Grondalh M. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2013;100:994–1001. doi: 10.1016/j.fertnstert.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Diluigi AJ, Engmann L, Schmidt DW, Benadiva CA, Nulsen JC. A Randomized Trial of Microdose Leuprolide Acetate Protocol versus Luteal Phase Ganirelix Protocol in Predicted Poor Responders. Fertil Steril. 2011;95:2531–2533. doi: 10.1016/j.fertnstert.2011.01.134. [DOI] [PubMed] [Google Scholar]
- 27.Johnston-MacAnanny EB, DiLuigi A, Engmann L, Maier D, Benadiva C, Nulsen J. Selection of first in vitro fertilization (IVF) cycle stimulation protocol for good prognosis patients: GnRH Antagonist vs. Agonist Protocols. J Reprod Med. 2011;56:12–16. [PubMed] [Google Scholar]
- 28.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Kaye L, Marsidi A, Rai P, Thorne J, Nulsen J, Engmann L, Benadiva C. Frozen Blastocyst Transfer Outcomes in Immediate versus Delayed Subsequent Cycles Following GnRH Agonist or HCG Triggers. J Assist Reprod Genet. 2018;35:669–675. doi: 10.1007/s10815-017-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irani M, O'Neill C, Palermo G, Xu K, Zhang C, Qin X, Zhan Q, Clarke R, Ye Z, Zaninovic N, Rosenwaks Z. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110:95–102. doi: 10.1016/j.fertnstert.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, Griffin DK, Wells D. Diminished Effect of Maternal Age on Implantation after Preimplantation Genetic Diagnosis with Array Comparative Genomic Hybridization. Fertil Steril. 2013;100:1695–1703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 32.Lee HL, McCulloh DH, Hodes-Wertz B, Adler A, McCaffrey C, Grifo JA. In Vitro Fertilization with Preimplantation Genetic Screening Improves Implantation and Live Birth in Women Age 40 through 43. J Assist Reprod Genet. 2015;32:435–444. doi: 10.1007/s10815-014-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106:597–602. doi: 10.1016/j.fertnstert.2016.04.027. [DOI] [PubMed] [Google Scholar]