Abstract
Preimplantation genetic testing for aneuploidy (PGT-A) does not create normal embryos, but selecting a viable embryo for a fresh transfer has the potential to deliver an extra effect for live birth from a stimulated cycle by evading the attrition associated with embryo cryopreservation. Improved genetic tests are now available for selecting viable embryos; however, current embryo cryopreservation techniques also have a superior survival rate, which means it is now possible to transfer most morphologically suitable embryos from a stimulated cycle one at a time. The cumulative live birth rate from a stimulated cycle is now unlikely to be superior compared with morphological assessment alone, with any benefit likely to be associated with a reduction in the risk of miscarriage and the time to pregnancy. This communication offers a perspective on the likely benefit and disbenefit of PGT-A based on the outcome of modern-day clinical studies. Caution should be advised regarding offering PGT-A to every woman. Quantifying the likely miscarriage benefit and live birth disbenefit for an appropriate patient group may help to better inform couples who might be considering adding aneuploidy screening to their treatment cycle.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01712-x) contains supplementary material, which is available to authorized users.
Keywords: Preimplantation genetic testing, Aneuploidy screening, Single-embryo transfer
The primary unit of benefit for assisted conception is a healthy live born baby. Effective selection techniques to identify viable embryos should in principle help to optimise the process. Preimplantation genetic testing for aneuploidy (PGT-A) does not create normal embryos, but selecting a viable embryo for a fresh transfer has the potential to deliver a superior cumulative live birth rate (an extra effect for live birth) from a stimulated cycle by evading the attrition associated with embryo cryopreservation [1]. However, hypothetical studies have argued that not-testing is likely to be superior to testing [2, 3], that the predictive value of genetic testing, even at the blastocyst stage, is too low for clinical use [4], and that PGT-A results in a substantial loss rate of potential live births [5].
In the past, embryo transfer typically occurred on day 2 and slow-freezing of surplus embryos was inefficient, occasioning the transfer of several embryos when available, and a risk of multiple pregnancy with clinical complications. This was the best time for PGT-A to be effective, where aneuploidy of maternal origin could be detected by testing the polar bodies. Unfortunately the fluorescence in situ hybridisation (FISH) technique available at the time was typically restricted to detecting the copy number of only a few chromosome pairs [6]. Advances in assisted reproductive technology (ART) included reliable in vitro culture to the blastocyst stage. Testing on day 3 became common using FISH for 5–8 chromosome pairs [7] but was likely to be complicated by the nature of the cleavage-stage embryo [8] and the suboptimal diagnostic accuracy of the FISH technique [9]; this approach was eventually shown in randomised control trials to be ineffective in most people’s hands [10]. Trophectoderm sampling on day 5/6 is currently the moment of choice and facilitates the use of new advanced genetic testing techniques for all 23 pairs of chromosomes, to exclude embryos with aneuploidy prior to transfer. However, current embryo cryopreservation techniques have a superior survival rate [11], which means it is now possible to transfer most morphologically suitable embryo from a stimulated cycle one at a time. Using a current selection technique, the cumulative live birth rate from a stimulated cycle is now unlikely to be superior compared with morphological assessment alone, with any benefit likely to be associated with a reduction in the risk of miscarriage and the time to pregnancy [1]. In a hypothetical trial for a first stimulated cycle [3, 12], using an ideal test (100% diagnostic accuracy and no chance of miscarriage following testing), it was estimated that only around 1 in 19 (54/1000) women were likely to benefit by avoiding a clinical miscarriage without reducing their chance of a healthy baby (and in a shorter time period). Around 1 in 4 (273/1000) women were likely to pay more for no benefit, which increased to 1 in 2 (449/1000) when the diagnostic accuracy was reduced to that typically expected in practice [3, 12].
The debate is ongoing about offering PGT-A as an add-on to IVF/ICSI [13]. The purpose of this communication is to offer a perspective on the likely benefit and disbenefit of PGT-A based on the outcome of modern-day clinical studies. A model published previously [1] is used to extrapolate what the outcome might be if embryos are transferred one at a time until one ongoing clinical pregnancy or live birth occurs, or until all the embryos are used (a full cycle). The model estimates the numbers of ongoing pregnancies or live births, clinical miscarriages, and embryo transfer procedures when the diagnostic accuracy and vitrified-warmed survival rates are varied from 100% for different numbers of available morphologically transferable embryos without testing. The probabilities transferring one embryo at a time were estimated for 6 study reports using the approach detailed in the Electronic supplementary material. The studies encompass microarray comparative genomic hybridisation (aCGH) testing of polar bodies [14] and cleavage-stage blastomeres [15–17], and blastocyst-stage trophectoderm samples using aCGH [18, 19] and next-generation sequencing (NGS) [20] (Table 1). Two of these studies [19, 20] are presented below and the findings discussed.
Table 1.
Summary of PGT-A studies assessed and outcome measures
| Clinical study | Maternal age (years) | Type of study and primary outcome | Biopsy moment | Test | Prev. (%) | PPV (%) | NPV (%) | Excl. (%) | Benefit* (CM) | Disbenefit (LB/OP) |
|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [18] | < 35 | Prospective pilot OP | Day 5 | aCGH | 58.3 | 91.9 | 69.1 | 8.7 | 1 in 9 | 1 in 126 |
| Rubio et al. [15] | 38–41 | RCT LB | Day 3 | aCGH | 82.8 | 91.8 | 50.0 | 37.6 | 1 in 3 | 1 in 4 |
| Verpoest et al. [14] | 36–40 | RCT LB | PB | aCGH | 87.0 | 92.5 | 22.9 | 37.7 | 1 in 6 | 1 in 3 |
| Munné et al. [20] | 25–40 | RCT OP | Day 5/6 | NGS | 54.3 | 57.6 | 50.0 | 52.6 | 1 in 21 | 1 in 5 |
| Munné et al. [20] | 35–40 | Post hoc OP | Day 5/6 | NGS | 62.8 | 70.6 | 50.8 | 50.2 | 1 in 6 | 1 in 3 |
| Lee et al. [16, 17] | 37–45 | Retrospective LB | Day 3 | aCGH | 90.9 | 95.6 | 29.1 | 39.1 | 1 in 12 | 1 in 3 |
| Sato et al. [19] | 35–42 | Prospective pilot LB | Day 5/6 | aCGH | 78.4 | 91.1 | 52.4 | 29.3 | 1 in 18 | 1 in 6 |
RCT, randomised controlled trial; LB, live birth; OP, ongoing pregnancy; PPV, positive predictive value—the likelihood that an abnormal test result correctly predicts non-viability; NPV, negative predictive value—the likelihood that a normal test result correctly predicts viability; CM, clinical miscarriage; Prev., prevalence of non-viable morphologically transferable embryos; Excl., proportion of viable embryos excluded due to false abnormal test results; Benefit, the number of women likely to avoid a clinical miscarriage; Disbenefit, the likely reduction in the number of women with a live birth/ongoing pregnancy
*An overestimate; there is a bias in favour of testing caused by more women with more than 1 miscarriage without testing
Salient features of the STAR trial [20] are explained in detail in the Electronic supplementary material, and the model outcomes are presented in Fig. 1. With a prevalence of non-viable morphologically transferable embryos of 54.3%, the likelihood of an abnormal test result correctly predicting no ongoing pregnancy was estimated to be 57.6% and the likelihood of a normal test result correctly predicting an ongoing pregnancy was 50.0%. The PGT-A ongoing pregnancy rate per embryo transferred was superior (50.0% vs 45.7%) indicating that testing was more effective than a conventional morphological assessment alone to select a viable embryo, however, with 52.6% fewer ongoing pregnancies overall due to the exclusion of viable embryos with false positive test results.
Fig. 1.
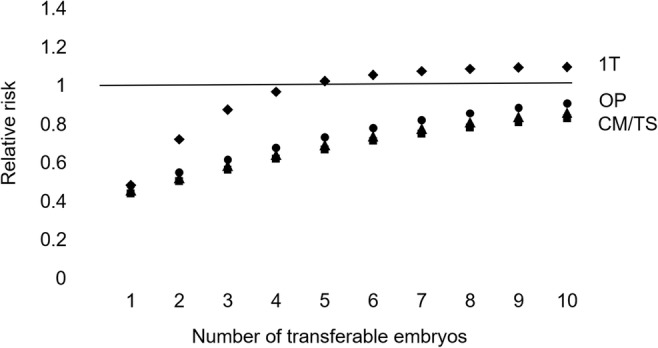
The STAR trial [20]. The theoretical relative risk for PGT-A compared with a conventional embryo morphology assessment with a single-embryo transfer. Ongoing pregnancy considering only up to 1 transfer attempt (1T). Ongoing pregnancies (OP), clinical miscarriages (CM), and transfer procedures (TS) for a full cycle
Testing was more effective than a morphological assessment alone for an ongoing clinical pregnancy when 5 or more embryos were available for transfer or testing. For 100,000 women with 7 morphologically transferable embryos (the average from the study report) and considering only up to 1 transfer attempt, the numbers of women with an ongoing pregnancy are 48,717 with PGT-A and 45,687 without testing (difference 6.6%). Following a full cycle for 100,000 women, there are 79,694 (79.7%) vs 98,032 (98.0%) ongoing pregnancies (difference − 18.7%). There are 15,593 vs 20,283 miscarriages (difference − 23.1%) and 159,385 vs 214,573 transfers (difference − 25.7%). Approximately 1 in 21 women are likely to benefit by avoiding a clinical miscarriage, with the disbenefit of a reduction in the number of women with an ongoing pregnancy of around 1 in 5.
The STAR trial evaluated if PGT-A could increase the chance of having an ongoing pregnancy for a single embryo transferred but did not achieve statistical significance. The majority of women included were aged less than 35 years (55%); with a prevalence of non-viable morphologically transferable embryos estimated to be 54.3%, the likelihood of an abnormal test result correctly predicting no ongoing pregnancy (PPV) was 57.6%, compared with 58.3% and 91.9% (ongoing pregnancy) for the Yang et al. study [18], 87.0% and 92.5% (live birth) for the ESTEEM trial [14, 21], and 82.8% and 91.8% (live birth) for the Rubio et al. trial [15] (Table 1).
The post hoc analysis of older women aged 35 to 40 years found a significant increase in the OPR per embryo transfer (50.8% vs 37.2%). With a prevalence of non-viable morphologically transferable embryos of 62.8% (greater), the likelihood of an abnormal test result correctly predicting no ongoing pregnancy was estimated to be 70.6% (higher) and the likelihood of a normal test result correctly predicting an ongoing pregnancy was 50.8% (similar). The PGT-A ongoing pregnancy rate per embryo transferred was superior (50.8% vs 37.2%) indicating that testing was more effective than a conventional morphological assessment alone to select a viable embryo, with 50.2% fewer ongoing pregnancies overall due to the exclusion of viable embryos with false positive test results.
Testing was more effective than a morphological assessment alone for an ongoing clinical pregnancy when 4 or more embryos were available for transfer or testing. For 100,000 women with 5 morphologically transferable embryos and considering only up to 1 transfer attempt, the numbers of women with an ongoing pregnancy are 44,587 with PGT-A and 37,241 without testing (difference 19.7%). Following a full cycle for 100,000 women, there are 61,592 (61.6%) vs 88,403 (88.4%) ongoing pregnancies (difference − 30.3%). There are 9777 vs 26,193 miscarriages (difference − 62.7%) and 121,196 vs 237,381 transfers (difference − 48.9%). Approximately 1 in 6 women are likely to benefit by avoiding a clinical miscarriage, with the disbenefit of a reduction in the number of women with an ongoing pregnancy of around 1 in 3. Although PGT-A is a better test for this older age group and more effective to avoid miscarriage, including the transfer of all available cryopreserved embryos, the cumulative OPR is inferior.
The prospective study of Sato et al. [19] included women aged 35 to 42 years with no previous live birth and 2 or more previous IVF-ET clinical miscarriages with at least 1 with aneuploidy (recurrent pregnancy loss (RPL)). Testing for every chromosome was done at the blastocyst stage by trophectoderm biopsy and using aCGH, and the model outcomes (see Electronic supplementary material) are presented in Fig. 2. With 78.4% prevalence of non-viable morphologically transferable embryos, the predictive value is estimated to be 91.1% for an abnormal test result and 52.4% for a normal test result. The PGT-A live birth rate per transfer is superior indicating that testing is more effective than a conventional morphological assessment alone to select a viable embryo, with fewer live births (29.3%) overall due to the exclusion from transfer of viable embryos with false positive test results.
Fig. 2.
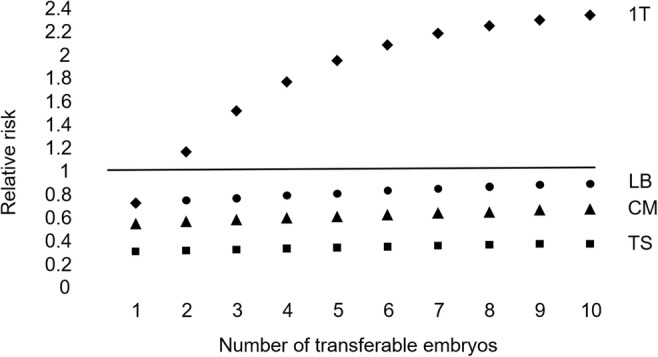
Based on Sato et al. [19]. The theoretical relative risk for PGT-A compared with a conventional embryo morphology assessment with a single-embryo transfer. Live birth event considering only up to 1 transfer attempt (1T). Live birth events (LB), clinical miscarriages (CM), and transfer procedures (TS) for a full cycle
For 100,000 women with 4 morphologically transferable embryos (the likely average for the study) and considering only up to one transfer attempt, the numbers of women with a live birth event are 37,862 with PGT-A and 21,621 without testing (difference 75.1%). Following a full cycle for 100,000 women, there are 46,245 (46.2%) vs 59,699 (59.7%) live births (difference − 22.5%). There are 7708 vs 13,267 miscarriages (difference − 41.9%) and 88,285 vs 276,116 transfers (difference − 68.0%). Approximately 1 in 18 women are likely to benefit by avoiding a clinical miscarriage, with the disbenefit of a reduction in the number of women with a live birth of around 1 in 6.
Investigations prior to the study included parental karyotyping, a cavity examination, and antiphospholipid syndrome. The likely miscarriage benefit is modest and may be because the study included women who have had an aneuploid miscarriage and who (counterintuitively) have a good prognosis. Detection of aneuploidy in products of conception explains the pregnancy loss and is likely to be sporadic; recurrent pregnancy losses which are chromosomally normal may be due to other factors which may persist next time. It is noted that the Japan Society of Obstetrics and Gynecology decided not to continue the pilot study and not to conduct a randomised controlled trial, and did not change the ruling that PGT-A is prohibited.
In conclusion, caution should be advised regarding offering PGT-A to every woman, and it should not be expected to improve the chance of having a baby when taking into account every morphologically transferable embryo from a stimulated cycle. The psychological burden of repeated implantation failure and spontaneous miscarriage of a much wanted clinical pregnancy can be severe, and it should be recognised that a reduction in the chance of a live birth might be of secondary concern to some women with a history of recurrent pregnancy loss. Gauging whether or not PGT-A is appropriate in an individual case is likely to be complex, and to depend on who is making the decision and how each couple is counselled. Quantifying the likely miscarriage benefit and live birth disbenefit for an appropriate patient group may help to better inform couples who might be considering adding aneuploidy screening to their treatment cycle.
Electronic supplementary material
(PDF 338 kb)
Authors’ contributions
The author is responsible for the content and writing of the paper.
Data availability
Supplementary file is provided.
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scriven PN. Towards a better understanding of preimplantation genetic screening and cumulative reproductive outcome: transfer strategy, diagnostic accuracy and cost-effectiveness. AIMS Genetics. 2016;3:177–195. doi: 10.3934/genet.2016.3.177. [DOI] [Google Scholar]
- 2.Orvieto R. Preimplantation genetic screening- the required RCT that has not yet been carried out. Reprod Biol Endocrinol. 2016;14:35. doi: 10.1186/s12958-016-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scriven PN. Towards a better understanding of preimplantation genetic screening for aneuploidy: insights from a virtual trial for women under the age of 40 when transferring embryos one at a time. Reprod Biol Endocrinol. 2017;15:49. doi: 10.1186/s12958-017-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleicher N, Metzger J, Croft G, Kushnir VA, Albertini DF, Barad DH. A single trophectoderm biopsy at blastocyst stage is mathematically unable to determine embryo ploidy accurately enough for clinical use. Reprod Biol Endocrinol. 2017;15:33. doi: 10.1186/s12958-017-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulson RJ. Preimplantation genetic screening: what is the clinical efficiency? Fertil Steril. 2017;108:228–230. doi: 10.1016/j.fertnstert.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Verlinsky Y, Cieslak J, Ivakhnenko V, Evsikov S, Wolf G, White M, Lifchez A, Kaplan B, Moise J, Valle J, Ginsberg N, Strom C, Kuliev A. Preimplantation diagnosis of common aneuploidies by the first- and second-polar body FISH analysis. J Assist Reprod Genet. 1998;15:285–289. doi: 10.1023/A:1022592427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munné S, Magli C, Bahçe M, Fung J, Legator M, Morrison L, Cohert J, Gianaroli L. Preimplantation diagnosis of the aneuploidies most commonly found in spontaneous abortions and live births: XY, 13, 14, 15, 16, 18, 21, 22. Prenat Diagn. 1998;18:1459–1466. doi: 10.1002/(SICI)1097-0223(199812)18:13<1459::AID-PD514>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Munné S, Cohen J. Chromosome abnormalities in human embryos. Hum Reprod Update. 1998;4:842–855. doi: 10.1093/humupd/4.6.842. [DOI] [PubMed] [Google Scholar]
- 9.Scriven PN, Bossuyt PM. Diagnostic accuracy: theoretical models for preimplantation genetic testing of a single nucleus using the fluorescence in situ hybridization technique. Hum Reprod. 2010;25:2622–2628. doi: 10.1093/humrep/deq196. [DOI] [PubMed] [Google Scholar]
- 10.Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–466. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 11.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scriven PN. Active selection and single embryo transfer: insights from virtual trials. EMJ Repro Health. 2018;4:108–115. [Google Scholar]
- 13.Schattman GL. Preimplantation genetic testing for aneuploidy: it’s déjà vu all over again! Fertil Steril. 2019;112:1046–1047. doi: 10.1016/j.fertnstert.2019.08.102. [DOI] [PubMed] [Google Scholar]
- 14.Verpoest W, Staessen C, Bossuyt PM, Goossens V, Altarescu G, Bonduelle M, et al. Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: a randomized clinical trial. Hum Reprod. 2018;33:1767–1776. doi: 10.1093/humrep/dey262. [DOI] [PubMed] [Google Scholar]
- 15.Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J, Pellicer A, Simón C. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107:1122–1129. doi: 10.1016/j.fertnstert.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Lee E, Chambers GM, Hale L, Illingworth P, Wilton L. Assisted reproductive technology (ART) cumulative live birth rates following preimplantation genetic diagnosis for aneuploidy (PGD-A) or morphological assessment of embryos: a cohort analysis. Aust N Z J Obstet Gynaecol. 2017;58:525–532. doi: 10.1111/ajo.12756. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Costello MF, Botha WC, Illingworth P, Chambers GM. A cost-effectiveness analysis of preimplantation genetic testing for aneuploidy (PGT-A) for up to three complete assisted reproductive technology cycles in women of advanced maternal age. Aust N Z J Obstet Gynaecol. 2019;59:573–579. doi: 10.1080/01443615.2018.1534813. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24. doi: 10.1186/1755-8166-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Sugiura-Ogasawara M, Ozawa F, Yamamoto T, Kato T, Kurahashi H, et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. 2019;34:2340–2348. doi: 10.1093/humrep/dez229. [DOI] [PubMed] [Google Scholar]
- 20.Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, STAR Study Group et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–1079. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 21.Scriven PN. The usefulness of preimplantation genetic testing for chromosome aneuploidy informed by a randomised controlled trial. OBM Genetics. 2019;3:6. doi: 10.21926/obm.genet.1901061. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 338 kb)
Data Availability Statement
Supplementary file is provided.


