Abstract
Purpose
To examine cycle blastocyst euploid rates among age subgroups of oocyte donors.
Methods
Retrospective cohort analysis of ova donation in vitro fertilization cycles (OD-IVF) for which trophectoderm biopsy for preimplantation genetic testing for aneuploidy (PGT-A) by array comparative genomic hybridization (aCGH) or next generation gene sequencing (NGS) was employed between January 2015 and December 2018 in a single high-volume fertility center.
Results
Compared to oocyte donors age 26–30, oocyte donors age ≤ 25 had similar cycle blastocyst euploid rates (80 [66.7, 87.5]%, vs. 75 [62.5, 87.5]%, median [IQR], p = 0.07), blastocyst formation rates (66.7 [50, 75]%, vs. 62.5 [52, 75]%, p = 0.55), and number of retrieved oocytes (29 [23, 37] vs. 27 [20, 35], p = 0.18). Age of oocyte donor from 18 to 34 was not correlated with cycle blastocyst euploid rate.
Conclusion
Oocyte donors age ≤ 25 had similar cycle blastocyst euploid rates, blastocyst formation rates, and number of retrieved oocytes compared to donors age 26–30. There was no correlation between cycle blastocyst euploid rates and age of the oocyte donor from 18 to 34 years. Given the lack of significant age-related change in cycle blastocyst euploid rates, our data support existing practices which do not favor a specific age subgroup of young oocyte donors.
Keywords: Egg donors, Oocyte donors, Age, Aneuploidy, Preimplantation genetic testing, IVF, Euploid
Introduction
Donor oocytes play a pivotal role in modern day reproductive medicine. In vitro fertilization with oocyte donation (OD-IVF) allows women of advanced reproductive age [1] or primary ovarian insufficiency to have high live birth rates. In the USA, the number of OD cycles significantly increased by approximately 125% over 16 years from 10,801 in the year 2000 to 24,300 in 2016 [2, 3].
Oocyte quantity and quality are known to decline with age [4], and therefore oocytes from younger women are typically preferred. The American Society for Reproductive Medicine (ASRM) recommends oocyte donors be between 21 and 34 years of age [5].
The role of preimplantation genetic testing for aneuploidy (PGT-A) in ART cycles is controversial with some evidence advocating its benefit solely among women of advanced reproductive age when oocyte-derived aneuploidy is more common [6]. The role for PGT-A in OD-IVF cycles is uncertain given the high presumed rate of euploidy with use of oocytes derived from young donors [6]. Nevertheless, a steady nationwide increase in PGT-A use during OD-IVF was reported from 2010 to 2013 [7].
Women age < 25 have been reported to have less favorable IVF treatment outcomes such as clinical pregnancy and live birth rates compared to women age 25–35 [8]. A bimodal distribution of aneuploidy was previously reported in blastocysts evaluated with trophectoderm biopsy and comprehensive chromosomal screening (CCS) [9]. The results of this particular study involving a general infertility population found the lowest prevalence of aneuploidy between the ages of 26 and 30. Surprisingly, there was a higher prevalence of aneuploidy among most age groups younger than 26 with women age 22, 23, 24, and 25 having aneuploidy rates of 44.4, 40.8, 27.8, and 44.4%, respectively, whereas women age 26, 27, 28, 29, and 30 had aneuploidy rates of 24.6, 27.1, 22.7, 20.7, and 23.2%, respectively. The higher aneuploidy rates reported among most age groups younger than 26 were only surpassed by women age ≥ 37. Interestingly, in that report, the no-euploid embryo rate was similar for women age 22 and 42.
If the euploid embryo rates in oocyte donors parallel those reported in the general IVF population, it is possible for some age subgroups of oocyte donors to yield lower rates of euploid embryos. The identification of specific subgroups of oocyte donors with lower rates of euploid embryos might have an impact on the way these donors are selected. Therefore, our primary objective is to examine the cycle blastocyst euploid rates by PGT-A among different age subgroups of oocyte donors.
Methods
The institutional review board of the University of California, Los Angeles, reviewed and approved the research protocol. This is a retrospective cohort analysis of OD-IVF cycles with trophectoderm biopsy for PGT-A between January 2015 and December 2018 at a single high-volume fertility center. For PGT-A, both high-density oligonucleotide array comparative genomic hybridization (aCGH) and next generation gene sequencing (NGS) were used since our laboratory transitioned from the former to the latter in the end of 2016. Results after PGT-A were divided into euploid and aneuploid since mosaic results were not reported before 2017. After 2017, mosaic results were counted as aneuploid and those cycles included in the analysis, whereas cycles where any embryo resulted in a no-result call after PGT-A were excluded. Cycles where the diagnosis included male factor were also excluded, and only normal sperm samples according to the World Health Organization reference values were used for fertilization [10]. Potential oocyte donors had a medical evaluation, and only those without any significant medical, psychological, or gynecologic abnormalities and with an antral follicle count of at least ten were selected. For the primary analysis, only first cycles from donors age ≤ 30 were included for comparison and divided in subgroups comprising Group 1: donors age ≤ 25 and Group 2: donors age 26–30. Cycles from oocyte donors age 31–34 were not included because of the low encountered numbers (n = 12); however, to evaluate the effect of donor age as a continuous variable on cycle blastocyst euploid rates, all cycles were analyzed.
Ovarian stimulation, embryo culture, and biopsy
Controlled ovarian hyperstimulation was performed using standard long gonadotropin releasing hormone (GnRH) agonist or GnRH antagonist protocols utilizing a combination of recombinant follicle stimulating hormone (FSH) 75–150 IU/night (Follistim: Merck, Keniworth, NJ, USA; or Gonal-F: EMD Serono Inc., Rockland, MD, USA) and human menopausal gonadotropin (HMG) 75–150 IU/night (Menopur; Ferring Pharmaceuticals, Parsippany, NJ, USA). Final oocyte maturation was triggered with either subcutaneous human chorionic gonadotropin (hCG) (5,000–10,000 units) or a combination of subcutaneous GnRH agonist (leuprolide acetate 1 mg) and hCG 1,000 IU when two lead follicles reached ≥ 18 mm in mean diameter. The oocyte retrieval was performed 35.5 h after the trigger injection.
Mature oocytes were fertilized either with conventional insemination or intracytoplasmic sperm injection (ICSI). For oocytes undergoing conventional insemination, motile sperm at a concentration of 150,000–200,000/mL were co-incubated overnight in Quinn’s Fertilization media (CooperSurgical Inc. Trumbull, CT) with 5% human serum albumin (CooperSurgical Inc. Trumbull, CT). For oocytes undergoing ICSI, mature oocytes were injected 3–4 h post retrieval and subsequently cultured in Quinn’s Advantage Plus Cleavage media (CooperSurgical Inc. Trumbull, CT). Fertilization confirmation was done 16–18 h after the insemination or ICSI. All embryos with two pronuclei were then continuously cultured in Quinn’s Advantage Plus Cleavage media.
At day 3 post retrieval, all cleavage-stage embryos were transferred to Quinn’s Advantage Plus Blastocyst media for group culture. All embryos were cultured in the MINC incubators (Cook Medical, Bloomington, IN) supplied with premixed gas (6% CO2, 5% O2 and 89% N2) and routinely incubated up to 7 days. Cultured embryos were graded on days 5, 6, or 7 and determined to be ready for biopsy at the blastocyst stage when a clear distinction between the inner cell mass (ICM) and the trophectoderm (TE) could be observed. Based upon the Gardner grading system [11], only blastocysts with expansion score higher or equal to 2 and the quality of ICM and TE higher or equal to C were biopsied. Blastocysts were stabilized with a holding pipette, and a 20 um biopsy pipette was then used to remove approximately 3–5 TE cells for biopsy with assisted cutting by the Lykos laser (Hamilton Thorne, Beverly, MA, USA). Biopsied cells were washed and placed in tubes according to the testing lab protocol and kept frozen at −20 °C before sending for PGT-A.
Preimplantation genetic testing for aneuploidy (PGT-A)
Biopsied TE cells were analyzed for all 24 chromosomes using either high-density oligonucleotide aCGH by the testing laboratory Pacgenomics, Augora Hill, CA, or Next Generation Gene Sequencing (NGS) by the testing laboratories Pacgenomics, Augora Hill, CA, GoodStart Genetics (Invitae), San Francisco, CA, or CombiMatrix (Invitae), Irvine, CA.
Statistics
Stimulation parameters and cycle laboratory outcomes including PGT-A results were compared between oocyte donor age groups using a Wilcoxon rank-sum test. Cycle blastocyst euploid rates including all oocyte donors age 18–34 were also investigated with a Spearman correlation and a logistic regression analysis for the lowest quartile of euploid rate (< 65%). Analyses were performed with SAS 9.4 (Cary, NC).
Results
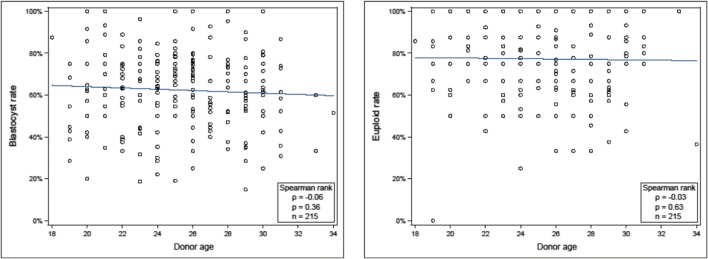
Between January 2015 and December 2018, PGT-A was performed on embryos derived from 215 oocyte donors between the ages of 18 and 34. There were 111 cycles where the donor’s age was 18–25 and 92 cycles where the donor’s age was 26–30 (mean donor ages ± SD were 22.6 ± 1.9 and 27.9 ± 1.5 years, respectively). There were no significant differences in the amount of gonadotropins administered, peak estradiol, number of oocytes retrieved, or cycle blastocyst formation rates between the groups (Table 1). In addition, there was no significant difference in the cycle blastocyst euploid rate of Group 1 compared to Group 2 (80 [66.7, 87.5]%, median [IQR] vs. 75 [62.5, 87.5]%, p = 0.07). Cycle blastocyst euploid rates were also compared excluding oocyte donors age < 22 (as there were no women in this group in the study by Franasiak et al. [9]). Again, no difference was seen in cycle blastocyst euploid rates between the groups (age 22–25: 77.8 [66.7, 87.5]%, median [IQR] vs. age 26–30: 75 [62.5, 87.5]%, p = 0.16). A logistic regression for the lowest quartile of cycle blastocyst euploid rate (< 65%) was done including the 12 cycles from donors age 31–34 for a total of 215 cycles. Age of the oocyte donor was not found to be associated with cycle blastocyst euploid rate (1.04 [0.94, 1.14], OR [95% CI], p = 0.45). Spearman correlation of cycle blastocyst formation and blastocyst euploid rates using all oocyte donors age 18–34 failed to show a correlation (p = 0.36 and p = 0.63, respectively) (Fig. 1). Individual IVF cycle outcomes by age of the oocyte donor can be seen in Table 2.
Table 1.
Cycle characteristics between donor age subgroups (n = 203)
| Donor age group 1 | Donor age group 2 | P valueb | |
|---|---|---|---|
| ≤25 years (n = 111) | 26–30 years (n = 92) | ||
| Median (IQR)a | |||
| Gonadotropins administered (IU) | 3075 (2700, 3750) | 3000 (2375, 3600) | 0.18 |
| Peak E2 (pg/ml) | 3544 (2437, 4956) | 3431 (2569, 4856) | 0.95 |
| Number of oocytes retrieved | 29 (23, 37) | 27 (20, 35) | 0.18 |
| Cycle blastocyst formation rate (%) | 66.7 (50, 75) | 62.5 (52, 75) | 0.55 |
| Cycle blastocyst euploid rate (%) | 80 (66.7, 87.5) | 75 (62.5, 87.5) | 0.07 |
aInterquartile ranges
bWilcoxon rank-sum test
Fig. 1.
Correlation of oocyte donor age with cycle blastocyst and euploid rate (n = 215)
Table 2.
IVF cycle outcomes relative to the age of the oocyte donor
| Age (y) | Cohort of oocyte donors evaluated (n) | Oocytes retrieved (mean ± SD) | Cycle blastocyst rate (mean ± SD) | Cycle blastocyst euploid rate (mean ± SD) |
|---|---|---|---|---|
| 18 | 1 | 29 | 87.5% | 85.7% |
| 19 | 7 | 26.7 ± 10.2 | 50.4 ± 16.6% | 67.6 ± 32.3% |
| 20 | 12 | 27.7 ± 13.9 | 62.6 ± 23.1% | 83.1 ± 17.4% |
| 21 | 12 | 29.8 ± 12.6 | 70.7 ± 19.1% | 83.5 ± 10% |
| 22 | 16 | 36.7 ± 13 | 61.1 ± 16.8% | 79.4 ± 16.5% |
| 23 | 17 | 31.8 ± 12.3 | 62 ± 20.4% | 75.1 ± 14.9% |
| 24 | 25 | 28.9 ± 8.2 | 59.4 ± 16.8% | 76.3 ± 19.5% |
| 25 | 21 | 35.2 ± 16.9 | 69.5 ± 16.2% | 82.5 ± 14.6% |
| 26 | 27 | 29.7 ± 11.3 | 66.2 ± 18.1% | 73.6 ± 17.1% |
| 27 | 14 | 26.4 ± 14.3 | 58.7 ± 16.7% | 73 ± 19.6% |
| 28 | 14 | 32.3 ± 13.7 | 65.5 ± 19.5% | 69.5 ± 21.2% |
| 29 | 19 | 33.6 ± 18.6 | 56.1 ± 19.6% | 72.2 ± 15.5% |
| 30 | 18 | 26.2 ± 9.9 | 64.4 ± 17.4% | 84 ± 15.7% |
| 31 | 9 | 29.6 ± 8.3 | 57.2 ± 18.7% | 84.8 ± 9.9% |
| 32 | 0 | N/A | N/A | N/A |
| 33 | 2 | 10.5 ± 5 | 46.7 ± 18.9% | 100% |
| 34 | 1 | 36 | 51.5% | 36.4% |
Discussion
In this cohort analysis evaluating OD-IVF cycles, we did not identify differing median cycle blastocyst euploid rates by PGT-A among age subgroups of young oocyte donors. Women age ≤ 25 had similar blastocyst euploid rates compared with women age 26–30. Even if we were to make the argument that there was a statistical trend between the groups, the 5% difference in median cycle blastocyst euploid rates is probably not clinically significant. We had 80% power to detect more clinically meaningful differences in median cycle blastocyst euploid rates of at least 7.5% between the groups. The overall high euploid rate in both age subgroups of young oocyte donors support previous retrospective studies that failed to demonstrate a benefit in pregnancy outcomes from embryos that underwent PGT-A during OD-IVF cycles [12, 13].
In addition, there was no significant association between cycle blastocyst euploid rate and the age of the donor from 18 to 34 years. The latter is consistent with a recent report of statistically similar live birth rates among subgroups of oocyte donors with similar age as ours and also in accordance with ASRM guidelines where it is implied that donors age 21–34 may be equally preferable for oocyte donation [5, 14]. However, it is important to note that out of the 12 cycles from 31 to 34 years of age included in our study, 9 were from oocyte donors age 31, there were no cycles from donors age 32, and only 3 cycles were from donors age 33 and 34. The latter could have implications in the external validity of our findings when addressing this particular oocyte donor age range.
The results from this study also allow for speculation in the topic of naturally occurring embryo euploid rates according to age. Yang et al. [15] reported a 40% rate of aneuploidy among women younger than 33 years of age. Franasiak et al. [9], in the largest systematic report of CCS in the general IVF population to date, using quantitative polymerase chain reaction (qPCR) reported a bimodal distribution in embryo aneuploidy rates according to age which included a peak involving most women younger than age 26. As was previously discussed regarding this latter study, the rates of aneuploidy reached > 40% at age 25, and the no-euploid embryo rate was close to one-third at age 22 which was similar to women age 42. In this population, 61% of the cases originated from routine care in an effort to optimize transfer order, increase pregnancy rates, and decrease pregnancy loss rates, whereas 36% of them were single-gene cases. There is no mention of the percentage of cases that were OD-IVF cycles.
The physiologic mechanisms behind the J-shaped distribution of these findings remain elusive but could be related to the population make-up of the cases presented by Franasiak et al. [9] which mainly originated from “routine care” patients. This subset of young women may have more diminished ovarian reserve, partners with severe male factor or an intrinsic higher than average oocyte aneuploidy rate significantly contributing to their infertility. Nevertheless, diminished ovarian reserve in younger women has not been associated with higher aneuploidy rates compared to age-matched controls, and even though previous studies have suggested an increased aneuploidy rate with higher gonadotropin doses, this is unlikely to be the cause in most younger women with an age-appropriate ovarian reserve. Besides, this has recently been controverted by a study comparing aneuploidy rates in natural and gonadotropin-stimulated cycles [16–19]. Furthermore, severe male factor has not been found to be associated with embryo aneuploidy rates [20].
Most likely, this population of patients biologically differs from fertile women and oocyte donors. This topic has been explored by Kort et al. [21] who compared aneuploidy rates among a fertile group composed of patients undergoing PGT-A for sex selection vs. a group of patients with recurrent pregnancy loss, male factor infertility, unexplained infertility, prior failed IVF, or previous aneuploid conceptions. They found a significantly higher age-independent aneuploidy rate in the infertile group when compared to patients without infertility undergoing sex selection. What is particularly notable from their findings is that the group with recurrent pregnancy loss, previous IVF failure, and previous aneuploid losses also demonstrated a J-shaped distribution of their aneuploidy rates, whereas the group that did IVF for sex selection did not. In the latter group, aneuploidy rates were directly proportional to age. These findings could be extrapolated to our findings in the donor population, where an aneuploid peak was also not found in the younger patients, presumably fertile donors, thus supporting findings in previous studies on embryo aneuploidy rates in fertile women differing from that of an infertile young population. This disparity in aneuploidy rates could potentially explain some cases of infertility among younger women.
The strengths of our study lie in the consistency of methods used during cycles, and the relatively large sample size of OD-IVF embryos obtained and tested using data from a single center. Weaknesses include possible limitations on the external validity of our findings since aneuploidy rates in OD-IVF cycles have been found to differ between fertility centers and because our results were evaluated as euploid rates per cycle and not per total number of embryos like in previous studies [9, 21, 22]. In addition, the evaluation of embryonic ploidy was performed with respect to oocyte donor age and does not account for paternal age. However, aneuploidies in the embryo typically originate from chromosome segregation errors in meiosis I of the oocyte, rather than from the sperm [23]. Furthermore, neither trigger type or fertilization technique (i.e., IVF vs. ICSI) were included in our study, but these have not been found to affect euploid rates in patients undergoing ART [24–26]. Finally, even though cycles analyzed via NGS or aCGH were equally included, both PGT-A platforms have been found to provide highly consistent chromosomal results with the only difference being that NGS can more precisely identify embryos with chromosomal mosaicism and segmental aneuploidies [27–29]. These abnormalities were counted as aneuploid in our study; therefore, there is the possibility that the aneuploidy rate could have been artificially augmented in the cases evaluated with NGS. However, since in the literature the overall rate of mosaicism appears to be low and not affected by female age, we would not anticipate a significant effect on our findings [30].
In conclusion, given the lack of significant age-related change in cycle blastocyst euploid rates, our data support existing practices which do not favor a specific age subgroup of young oocyte donors. Our findings also suggest that young fertile women may display an aneuploidy rate pattern distinct from that previously reported for the general IVF population of the same age range.
Acknowledgments
The authors thank Lorna Herbert, M.P.H, for her assistance in statistical analysis.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prevention, C.f.D.C.a. 2014 ART fertility clinic success rates. Centers for Disease Control and Prevention 2016 [cited Centers for Disease Control and Prevention; Centers for Disease Control and Prevention]. Available from: http://www.cdc.gov/art/reports/index.html.
- 2.Kawwass JF, Monsour M, Crawford S, Kissin DM, Session DR, Kulkarni AD, Jamieson DJ, National ART Surveillance System (NASS) Group Trends and outcomes for donor oocyte cycles in the United States, 2000-2010. JAMA. 2013;310(22):2426–2434. doi: 10.1001/jama.2013.280924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, A.S.f.R.M., Society for Assisted Reproductive Technology. Assisted Reproductive Technology National Summary Report. 2016. Available from: https://www.cdc.gov/art/pdf/2016-report/ART-2016-National-Summary-Report.pdf.
- 4.American College of, O., P. Gynecologists Committee on Gynecologic, and C. Practice. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–4. [DOI] [PubMed]
- 5.Practice Committee of American Society for Reproductive, M. and T. Practice Committee of Society for assisted reproductive, Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril. 2013;99(1):47–62. [DOI] [PubMed]
- 6.Practice Committees of the American Society for Reproductive, M. et al. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429–436. [DOI] [PubMed]
- 7.Barad DH, et al. Impact of preimplantation genetic screening on donor oocyte-recipient cycles in the United States. Am J Obstet Gynecol. 2017;217(5):576 e1–576 e8. doi: 10.1016/j.ajog.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Wu LH, et al. Ivf outcomes are paradoxically poorer under age 25. Fertil Steril. 2012;98(3):S264. doi: 10.1016/j.fertnstert.2012.07.962. [DOI] [Google Scholar]
- 9.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 11.Gardner, D.K.a.S., WB. In vitro culture of human blastocysts, in Towards reproductive certainty. In: Jansen DMR, Editor. Parthenon publishing: Carnforth; 1999. p. 378–388.
- 12.Haddad G, Deng M, Wang CT, Witz C, Williams D, Griffith J, Skorupski J, Gill J, Wang WH. Assessment of aneuploidy formation in human blastocysts resulting from donated eggs and the necessity of the embryos for aneuploidy screening. J Assist Reprod Genet. 2015;32(6):999–1006. doi: 10.1007/s10815-015-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masbou AK, et al. A Comparison of Pregnancy Outcomes in Patients Undergoing Donor Egg Single Embryo Transfers With and Without Preimplantation Genetic Testing. Reprod Sci. 2018:1933719118820474. [DOI] [PubMed]
- 14.Humphries LA, Dodge LE, Kennedy EB, Humm KC, Hacker MR, Sakkas D. Is younger better? Donor age less than 25 does not predict more favorable outcomes after in vitro fertilization. J Assist Reprod Genet. 2019;36(8):1631–1637. doi: 10.1007/s10815-019-01494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24. doi: 10.1186/1755-8166-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio C, Mercader A, Alamá P, Lizán C, Rodrigo L, Labarta E, Melo M, Pellicer A, Remohí J. Prospective cohort study in high responder oocyte donors using two hormonal stimulation protocols: impact on embryo aneuploidy and development. Hum Reprod. 2010;25(9):2290–2297. doi: 10.1093/humrep/deq174. [DOI] [PubMed] [Google Scholar]
- 17.Baart EB, Martini E, Eijkemans MJ, van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22(4):980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 18.Hong KH, Franasiak JM, Werner MM, Patounakis G, Juneau CR, Forman EJ, Scott RT Jr Embryonic aneuploidy rates are equivalent in natural cycles and gonadotropin-stimulated cycles. Fertil Steril. 2019;112(4):670–676. doi: 10.1016/j.fertnstert.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Morin SJ, et al. Diminished ovarian reserve and poor response to stimulation in patients <38 years old: a quantitative but not qualitative reduction in performance. Hum Reprod. 2018. [DOI] [PubMed]
- 20.Mazzilli R, et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017;108(6):961–972. doi: 10.1016/j.fertnstert.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Kort JD, McCoy R, Demko Z, Lathi RB. Are blastocyst aneuploidy rates different between fertile and infertile populations? J Assist Reprod Genet. 2018;35(3):403–408. doi: 10.1007/s10815-017-1060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munne S, et al. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32(4):743–749. doi: 10.1093/humrep/dex031. [DOI] [PubMed] [Google Scholar]
- 23.Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16 Spec No. 2:R203–8. [DOI] [PubMed]
- 24.Bishop LA, et al. IVF trigger type does not impact aneuploidy rates in PGS embryos. Fertil Steril. 2017;108(3):e332–e333. doi: 10.1016/j.fertnstert.2017.07.977. [DOI] [Google Scholar]
- 25.Palmerola KL, Vitez SF, Amrane S, Fischer CP, Forman EJ. Minimizing mosaicism: assessing the impact of fertilization method on rate of mosaicism after next-generation sequencing (NGS) preimplantation genetic testing for aneuploidy (PGT-A) J Assist Reprod Genet. 2019;36(1):153–157. doi: 10.1007/s10815-018-1347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Nieto CA, et al. Aneuploidy rates are unaffected by choice of trigger medication in human IVF-ET cycles. Fertil Steril. 2017;108(3):e223–e224. [Google Scholar]
- 27.Huang J, Yan L, Lu S, Zhao N, Xie XS, Qiao J. Validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of blastocysts. Fertil Steril. 2016;105(6):1532–6. doi: 10.1016/j.fertnstert.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, Kokocinski F, Michel CE. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil Steril. 2014;101(5):1375–1382. doi: 10.1016/j.fertnstert.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 29.Lai HH, et al. Identification of mosaic and segmental aneuploidies by next-generation sequencing in preimplantation genetic screening can improve clinical outcomes compared to array-comparative genomic hybridization. Mol Cytogenet. 2017;10:14. doi: 10.1186/s13039-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubio C, et al. Clinical application of embryo aneuploidy testing by NGS. Biol Reprod. 2019. [DOI] [PubMed]