Abstract
Purpose
The aim of this study is to describe the multidisciplinary approach and controlled ovarian hyperstimulation (COH) outcomes in adolescent and young adult (AYA) patients (ages 13–21) who underwent oocyte cryopreservation for fertility preservation (FP).
Methods
Multi-site retrospective cohort was performed from 2007 to 2018 at Northwestern University and Michigan University. Data were analyzed by chi-square test, t-test, and logistic regression.
Results
Forty-one patients began COH of which 38 patients successfully underwent oocyte retrieval, with mature oocytes obtained and cryopreserved without any adverse outcomes. To treat this group of patients, we use a multidisciplinary approach with a patient navigator. When dividing patients by ages 13–17 vs. 18–21, the median doses of FSH used were 2325 and 2038 IU, the median number of mature oocytes retrieved were 10 and 10, and median number frozen oocytes were 11 and 13, respectively. Median days of stimulation were 10 for both groups. There was no statistical difference in BMI, AMH, peak E2, FSH dosage, days stimulated, total oocytes retrieved, mature oocytes retrieved, and oocytes frozen between the two groups. Three patients were canceled for poor response.
Conclusion
COH with oocyte cryopreservation is a feasible FP option for AYAs who may not have other alternatives when appropriate precautions are taken, such as proper counseling and having a support team. These promising outcomes correspond to similar findings of recent small case series, providing hope for these patients to have genetically related offspring in the future.
Keywords: Fertility preservation, Adolescents, Oncofertility, IVF, Oocyte vitrification
Introduction
Infertility is predicted to affect up to 186 million individuals worldwide [1]. The adolescent and young adult (AYA) population can be affected by a number of gonadotoxic threatening diseases resulting in impaired fertility. Although cancer diagnoses and some subsequent therapies are commonly thought of as leading fertility insults, other nonmalignant diseases/conditions such as rheumatic or hematologic diseases leading to bone marrow transplantation, gender dysphoria, Turner’s syndrome, as well as others can also be detrimental to the gonads [2–5].
Many young cancer patients have reported that not having biological children of their own in the future is a significant concern [6–9] and future infertility has been documented as a source of stress, depression, and anxiety [10–13]. Though limited data is available, some studies have also shown data regarding transgender youth and the desire to have genetically related offspring [4, 14]. However, this population tends to have a higher interest in many types of family building options, but also studies revealed that when questioned, ~50% noted a possibility of desiring genetically related offspring in the future, although they currently did not have that desire [14, 15]. While exceedingly rare, declining chemotherapy out of fear of future infertility has been reported in young cancer patients [16]. Implementation of fertility preservation (FP) counseling as a key component of disease treatment has helped decrease decisional regret and anxiety over potential decline in fertility and supported by survivors and their parents/guardians [7, 10, 17–20]. When considering FP options, the age of the individual, pubertal status, underlying medical condition and/or comorbidities, cultural background, ethical concerns, and partner status should be factored into the decision [3].
Chemotherapy drugs and radiation therapy can often lead to undesirable side effects for younger patients such as subfertility and infertility. These therapies are not only utilized by AYAs with cancer diagnoses, but those with nonmalignant conditions, such as vasculitis, certain renal diseases, and systemic lupus erythematous, have also been exposed to gonadotoxic levels of alkylating agents [5, 21, 22]. Additionally those with severe hemoglobinopathies requiring stem cell transplantation are also at risk of future infertility [5, 23]. Other vulnerable populations impacted by fertility concerns include those with various congenital/genetic conditions (fragile X syndrome, Turner’s syndrome, cystic fibrosis, etc.) and those with gender dysphoria who may have been exposed to gender-affirming therapies [5, 24]. There has been an increase in the use of FP, which is the freezing of gametes or reproductive tissue, prior to gonadotoxic therapies for malignancies and other disorders that place patients at high risk for future infertility and premature ovarian failure [25–32]. Recent improvements in vitrification and thawing techniques have made preserving oocytes after controlled ovarian hyperstimulation (COH) an increasingly viable FP option for young patients or those without a partner [33–36], and experimental alternatives such as ovarian tissue cryopreservation (OTC) exist for prepubertal patients and patients uninterested in undergoing COH prior to disease treatment [37–44].
FP has been identified as a key issue for young survivors and other affected AYA by the American Society of Clinical Oncology (ASCO), the American Academy of Pediatrics (AAP), and the American Society of Reproductive Medicine (ASRM) [3, 45–47]. While multidisciplinary coordination and disease treatment strategies including FP have been developed [5, 48–51], there may be some ethical concerns regarding FP in pediatric patients [23, 52–54]. Among these concerns are efficacy of FP techniques in younger patients (specifically the prepubertal population), differences in comfort levels, and opinions of physicians implementing experimental techniques [55]. Additionally, there are ethical concerns surrounding disposition of cryopreserved oocytes or embryos resulting from FP procedures. While many reports regarding disposition outcomes in the adult population exist, they are very few regarding oocyte and/or embryo disposition in the AYA population. In light of vague medical society guidelines that do not delineate circumstances that are ethically permissible for adolescent and especially prepubertal FP, there is a tremendous amount of variety in physician attitudes even some restrictions set in hospitals themselves [54].
Physicians that find adolescent FP ethically permissible may still hesitate to initiate COH based on lack of large cohort studies detailing adolescent ovarian stimulation outcomes [56–59]. There are few studies that detail complications, delay in initiating time-sensitive disease treatment, and pregnancies and/or live births years after the completion of treatment. One study described comparable stimulation outcomes in eight 14–18-year-old sickle-cell anemia (SCA) patients that underwent hematopoietic stem cell transplantation (HSCT) but also noted that one patient was admitted to the hospital for moderate ovarian hyperstimulation [23]. Live birth from oocytes vitrified during teenage years has also been reported [33]. Additionally, discontinuation of testosterone treatment and increased estradiol (E2) levels from COH present an increased risk of gender dysphoria and incongruence for transgender persons [60]. The goal of this study is to describe the multidisciplinary approach and report stimulation outcomes as well as disposition outcomes in AYA patients who underwent oocyte cryopreservation for FP.
Materials and methods
Study population
This was an IRB approved, multi-site retrospective study. Subjects were identified from oocyte retrieval patient logs. Specifically, we examined women aged between 13 and 21 years that froze oocytes after COH at Northwestern Medicine Fertility and Reproductive Medicine clinic and Michigan Medicine’s Center for Reproductive Medicine from January 2007 through January 2018. Subjects were excluded if they were older than 21 years at the time of presentation to each respective clinic or if they were being treated for something other than FP. For each patient, diagnosis prompting FP, age, BMI, and COH data were collected. Patients were stratified into two age groups for analysis: 13 to 17 years and 18 to 21 years.
Multidisciplinary coordination
The initial appointment with the reproductive endocrinology department was coordinated by the patient navigator. The role of the patient navigator is critical, as this individual assists in quickly maneuvering patients through a time-sensitive process. The patient navigator guides the patients and their families through the healthcare system, which at times can be complex, ensures efficient consult completion, ensures continued patient/family engagement, and assists in coordinating appointments and FP procedures. A patient navigator may also assist in identifying families that have financial need and connect them with resources to help cover costs associated with FP procedures [5, 54, 61]. All patients at one institution met with a psychologist or social worker to ensure that they understood the risks and benefits of undergoing COH, as well as the probability of future pregnancy from frozen oocytes after surviving disease treatment. Patients at the other institution are offered these services, but they are not mandated. At both institutions, patients <18 years gave assent in addition to written consent from the parents or guardians. Transabdominal ultrasound monitoring was available for those that could not tolerate vaginal exams. Anesthesiologists who were comfortable sedating minors were also part of the treatment team.
Controlled ovarian hyperstimulation
Our protocol has been documented in previous studies [62, 63]. Briefly, COH was started using recombinant follicle stimulating hormone (FSH) with or without urinary menotropins with dosage based on age and ovarian reserve measurements. Over time, our practice has evolved to include more random start (RS) protocols, meaning that patients who desire to begin stimulation immediately can do so. For a cycle-specific (CS) protocol, gonadotropins were initiated on the third day of menses, whereas for a RS protocol, gonadotropins were initiated at any point in the menstrual cycle. Response to medication was evaluated with regular ultrasounds (either transvaginal or transabdominal, depending on patient’s comfort level with vaginal exams) and E2 measurements, with gonadotropin dosage adjusted accordingly. For a CS protocol, once the leading follicle grew to at least 12 mm in diameter or E2 reached 300 pg/mL, the patient began a daily injection of gonadotropin-releasing hormone (GnRH) antagonist to prevent ovulation. For RS, antagonist was started once the new lead follicle grew to at least 12 mm. Starting gonadotropin dose was determined by anti-Müllerian hormone (AMH) and/or antral follicle count (AFC). According to the institutional IVF protocols for all patients, when at least three follicles measured 16 mm or greater in diameter, final follicular maturation was triggered by an injection of human chorionic gonadotropin, and oocyte retrieval was performed 36 h later. Oocyte retrieval was achieved with transvaginal ultrasound guidance in all patients. Slow cooling was used for oocyte cryopreservation until 2008, when vitrification became standard protocol.
Oocyte disposition
Prior to undergoing COH, each patient was asked to document their preferred disposition of their oocytes if they were to unfortunately pass away prior to use. The following options were offered: discard all oocytes, donate oocytes to research, or choose an individual to use oocytes to initiate pregnancy. The final decision was documented and signed on an intake form, usually during the patient’s initial visit.
Statistical methods
Data were analyzed using chi-square tests to compare categorical variables, t-tests to compare continuous variables, and one-way ANOVA tests for comparisons between multiple groups. Subsequently, linear and logistic regression analyses were performed to adjust for potentially confounding variables. Statistical analyses were performed with SPSS IBM Statistics 25.0 for Windows (SPSS, Chicago, IL). All p values were two-sided, and a p value of < 0.05 was considered statistically significant.
Results
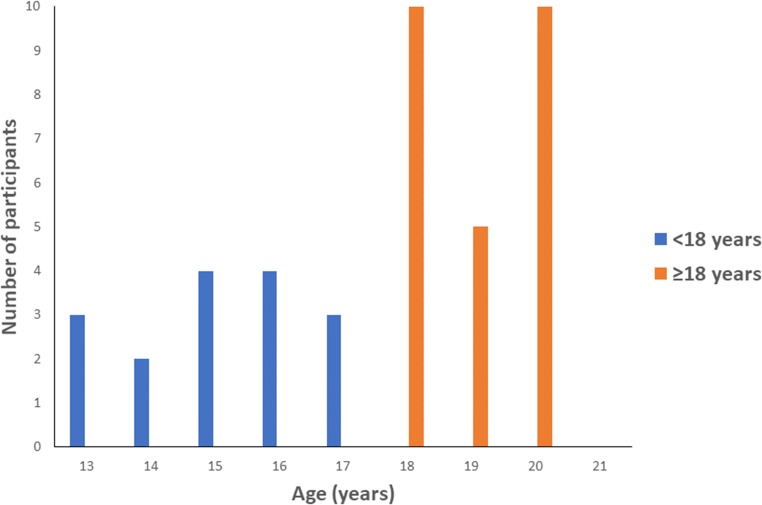
A total of 41 AYA patients began stimulation, and 38 completed a COH cycle, of which 36 froze oocytes and 2 froze embryos with a partner, for FP between the years 2007 and 2018, and were included in the analyses (Table 1). Out of the 38 who completed a cycle, 14 (36.68%) were ages 13 to < 18 years, and 24 (63.16%) were ages 18 to < 21 years. The frequency distribution of ages for each cohort is displayed in Fig. 1. The most common diagnosis for patients was cancer (total n = 22; n = 6 were recurrent cancer), followed by noncancerous disorders (total n = 16; n = 5 were hemoglobinopathies, specifically beta thalassemia n = 2, SCA n = 1, aplastic anemia n = 1, and paroxysmal nocturnal hemoglobinuria n = 1; remaining specific diagnoses were gender dysphoria (n = 5), Turner’s syndrome (n = 2), panhypopituitarism, NMDA autoimmune encephalitis, multiple sclerosis, and benign dermoid cyst, each with an n = 1). A total of 3 patients began ovarian stimulation but were canceled for poor response (primary malignancy n = 1, recurrent malignancy n = 1, nonmalignant disease n = 1 (diagnosis of primary ovarian insufficiency)). All remaining patients successfully completed COH and oocyte retrieval, with mature oocytes obtained and cryopreserved without any adverse outcomes.
Table 1.
Demographic characteristics of AYA patients who initiated an oocyte retrieval
| Demographic Measures (n=41) | <18 years old (n=16) | ≥18 years old (n=25) |
|---|---|---|
| Cancelled cycle (n) | 2 | 1 |
| Age | ||
| Median (Range) | 15.57 (13.00-17.81) | 19.27 (18.00-20.96) |
| BMI | ||
| Median (Range) | 23.31 (19.14-36.02) | 22.11 (15.72-47.50) |
| AMH | ||
| Median (Range) | 2.54 (0.08-6.50) | 1.95 (0.13-7.36) |
| Peak E2 | ||
| Median (Range) | 1446 (103-3469) | 1361 (25-4252) |
No significant difference observed between all measures except for age
AMH anti-Müllerian hormone; AYA adolescent and young adult; BMI body mass index; E2 estradiol
Fig. 1.
AYA study subject demographics by age group
When dividing the groups by ages < 18 years old and ≥ 18 years old, the median doses of FSH used were 2325 and 2038 IU (NS), the median number of mature oocytes retrieved were 10 (NS), and median number frozen oocytes were 11 and 13 (NS), respectively (Table 2A). The median days of stimulation were 10, and median number of total oocytes retrieved was 13 for both groups (NS, Table 2A). There was no statistical difference in BMI, AMH, peak E2, total FSH dosage, total HMG dosage, days stimulated, total oocytes retrieved, mature oocytes retrieved, and oocytes frozen between the two groups (Table 2A). When separating the groups by diagnoses of malignancy, recurrent malignancy, and nonmalignant disease, there was no statistically significant difference in stimulation outcomes among the measured parameters, although among certain diagnoses, there was a trend toward significance (Table 2B). Of note, AMH was lower in the recurrent cancer group (Table 2B). When dividing the groups by history of chemotherapy prior to stimulation, subjects that had previously undergone chemotherapy had significantly lower AMH (0.60 vs. 2.32; p = 0.032), lower total oocytes retrieved (6 vs. 13; p = 0.015), lower mature oocytes retrieved (4 vs. 12; p = 0.003), and lower oocytes cryopreserved (4 vs. 13; p = 0.049) (Table 2C). There were no cases of ovarian hyperstimulation syndrome (OHSS), and no complications from the stimulation or retrieval were noted.
Table 2A.
COH cycle characteristics of AYA patients who completed an oocyte retrieval by age groups
| Stimulation Measures (n=38a) | <18 years old | ≥18 years old | p-value |
|---|---|---|---|
| Number of Subjects (n) | 14 | 24 | |
| Subjects with history of chemotherapy (n) | 2 | 7 | |
| AMH (ng/ml) | |||
| Median (Range) | 2.72 (0.25-6.50) | 2.00 (0.13-7.36) | 0.505 |
| Peak E2 (pg/ml) | |||
| Median (Range) | 1465 (302-3469) | 1422 (363-4252) | 0.855 |
| Number of days of stimulation | |||
| Median (Range) | 10 (8-15) | 10 (8-15) | 0.749 |
| Total FSH Dosage (IU) | |||
| Median (Range) | 2325 (0-3375) | 2038 (525-5850) | 0.889 |
| Total HMG Dosage (IU) | |||
| Median (Range) | 750 (0-3375) | 750 (0-2700) | 0.930 |
| Total number of oocytes retrieved | |||
| Median (Range) | 13 (4-31) | 13 (2-37) | 0.872 |
| Number of mature oocytes | |||
| Median (Range) | 10 (0-25) | 10 (2-22) | 0.981 |
| Number of oocytes cryopreserved | n=14 | n=22 | |
| Median (Range) | 11 (1-28) | 13 (2-27) | 0.949 |
| Number of embryos cryopreserved | n=0 | n=2 | |
| Median (Range) | 3.5 (3-4) | ||
No significant differences were observed between groups
a: n = 3 low-response cancelations, of which n = 2 were < 18 years old
AMH anti-Müllerian hormone; AYA adolescent and young adult; COH controlled ovarian hyperstimulation; E2 estradiol; HMG human menopausal gonadotropin
p values determined by independent samples t-test
Table 2B.
COH cycle characteristics of AYA patients who completed an oocyte retrieval grouped by diagnosis
| Stimulation Measures (n=38a) | Primary Malignancy | Recurrent Malignancy | Other Reason | p-value |
|---|---|---|---|---|
| Number of Subjects (n) | 16 | 6 | 16 | |
| Subjects with history of chemotherapy (n) | 3 | 5 | 1 | |
| AMH (ng/ml) | ||||
| Median (Range) | 2.10 (0.20-6.63) | 0.90 (0.13-2.10) | 3.41 (0.25-7.36) | 0.137 |
| Peak E2 (pg/ml) | ||||
| Median (Range) | 1450 (363-4252) | 1295 (669-1484) | 1763 (302-3769) | 0.534 |
| Number of days of stimulation | ||||
| Median (Range) | 10 (8-15) | 12 (9-13) | 10 (8-15) | 0.144 |
| Total FSH Dosage (IU) | ||||
| Median (Range) | 2212 (0-5850) | 2813 (1650-3075) | 2150 (1050-3375) | 0.298 |
| Total HMG Dosage (IU) | ||||
| Median (Range) | 1088 (0-2700) | 1013 (0-1800) | 675 (0-3375) | 0.192 |
| Total number of oocytes retrieved | ||||
| Median (Range) | 14 (2-37) | 8 (3-18) | 13 (4-30) | 0.129 |
| Number of mature oocytes | ||||
| Median (Range) | 11 (2-25) | 6 (2-10) | 12 (0-23) | 0.067 |
| Number of oocytes cryopreserved | n=15 | n=5 | n=16 | |
| Median (Range) | 12 (2-27) | 6 (3-18) | 13 (7-28) | 0.194 |
| Number of embryos cryopreserved | n=1 | n=1 | n=0 | |
| Median (Range) | 4 | 3 | ||
No significant differences were observed between groups
a: n = 3 low-response cancelation, n = 1 from each group
AMH anti-Müllerian hormone; AYA adolescent and young adult; COH controlled ovarian hyperstimulation; E2 estradiol; HMG human menopausal gonadotropin
p values determined by Kruskal-Wallis H test
Table 2C.
COH cycle characteristics of AYA patients who completed an oocyte retrieval by history of chemotherapy
| Stimulation Measures (n=38a) | No history of chemotherapy | History of chemotherapy | p-value |
|---|---|---|---|
| Number of Subjects (n) | 29 | 9 | |
| Age | |||
| Median (Range) | 18.30 (13.00-20.76) | 18.78 (15.00-21.00) | 0.638 |
| AMH (ng/ml) | |||
| Median (Range) | 2.32 (0.20-7.36) | 0.60 (0.13-3.50) | 0.032 |
| Peak E2 (pg/ml) | |||
| Median (Range) | 1539 (302-3769) | 1256 (363-4252) | 0.186 |
| Number of days of stimulation | |||
| Median (Range) | 10 (8-15) | 12 (8-15) | 0.210 |
| Total FSH Dosage (IU) | |||
| Median (Range) | 2175 (0-3300) | 2925 (525-5850) | 0.114 |
| Total HMG Dosage (IU) | |||
| Median (Range) | 750 (0-2700) | 1275 (0-3375) | 0.741 |
| Total number of oocytes retrieved | |||
| Median (Range) | 13 (5-31) | 6 (2-37) | 0.015 |
| Number of mature oocytes | |||
| Median (Range) | 12 (4-25) | 4 (0-22) | 0.003 |
| Number of oocytes cryopreserved | n=29 | n=7 | |
| Median (Range) | 13 (5-28) | 4 (1-27) | 0.049 |
| Number of embryos cryopreserved | n=0 | n=2 | |
| Median (Range) | 3.5 (3-4) | ||
a: n = 3 low-response cancelations, of which n = 2 had history of chemotherapy prior to stimulation
AMH anti-Müllerian hormone; AYA adolescent and young adult; COH controlled ovarian hyperstimulation; E2 estradiol; HMG human menopausal gonadotropin
p values determined by independent samples t-test
For oocyte/embryo disposition elected at the time of cryopreservation, 63.4% (n = 26) of patients chose to donate to research, 26.8% (n = 11) to donate to a person of their choosing, and 4.9% (n = 2) to discard (n = 2 unavailable) (Table 3).
Table 3.
Disposition selection of oocytes
| <18 years old (n=16) | ≥18 years old (n=25) | |
|---|---|---|
| Discard all oocytes | 1 (6.30%) | 1 (4.00%) |
| Donate to research | 11 (68.80%) | 15 (60.00%) |
| Selected individual | 3 (18.80%) | 8 (32.00%) |
| Not noted | 1 (6.30%) | 1 (4.00%) |
Discussion
An ideal FP option for young female adults is one that can be applied to those who are single or not in a committed relationship. Therefore, COH with oocyte cryopreservation is a reasonable option to consider in this population. To our knowledge, this is the largest multicenter cohort demonstrating the safety profile of COH in the AYA population desiring FP for both oncology-based and non-oncology-based diagnoses. In our study, we divided age groups based on the suspected maturity of the hypothalamic-pituitary-ovarian (HPO) axis, as it is anticipated that many females < 18 years of age may have an immature HPO axis. Therefore, our two cohorts compared were ages 13 to < 18 years and ≥ 18 to 21 years old. Most patients in this study successfully underwent COH and oocyte retrieval, resulting in the collection and cryopreservation of mature oocytes without the occurrence of any adverse outcomes. Our younger patients (< 18 years old) did require higher doses of gonadotropins and froze fewer oocytes, likely attributed to the immaturity of the HPO axis, but these differences were not statistically significant. There were no statistically significant differences in the other parameters measured, which included BMI, AMH, peak E2, and days stimulated.
There are many concerns about FP among an adolescent population. Some issues that may arise include multiple parental concerns regarding the safety and complexity of the procedure, parent decision-making on behalf of the patient, adolescent decision capacity, the use of experimental options in certain populations, and the decision on what to do with the gametes if there is an untimely death of the patient [5]. For these reasons, we think that having a psychologist and or social worker as part of the team is crucial. At times, this decision could be difficult for the parents/guardians due to religious and cultural views on sexuality and concerns for loss of virginity due to the transvaginal approach during oocyte retrieval [23]. Although these concerns can be challenging to address, it should not inhibit or preclude offering COH as an FP option to the AYA population. It also provides an opportunity for patient and parental education.
Since FP in adolescent patients introduces complex challenges, a multidisciplinary approach should be taken when caring for this population. This includes performing appropriate pre-procedure counseling and having a support team in place. In our study, an integrative team was involved in providing care and support to our young adult patients. Our group consisted of a patient navigator, REI clinicians comfortable with the use of COH protocols in adolescents, psychologists or social workers, nursing staff, and anesthesiologists that were comfortable sedating minors. For those patients not comfortable with vaginal exams, a transabdominal ultrasound was performed to assess AFC as well as to monitor response to gonadotropins. Although studies have suggested that transabdominal ultrasound may decrease the accuracy of AFC, overall this did not pose a significant issue in our patient population and has previously been successfully used for monitoring in similar populations [23, 64–66]. Even in patients who had monitoring performed transabdominally, the oocyte retrieval was accomplished transvaginally without any issues.
There have been several previously published studies evaluating outcomes with COH in the AYA population; however, the majority of them tend to be small case reports or case series [23, 64, 65, 67]. Larger studies do exist, but there are still conflicting reports regarding safety and outcomes. The largest published study to date is a recent study by Hipp et al. using data from the SART CORS database [68]. In this study, a de-identified file released from SART CORS was analyzed; thus, there could be omitted or missing data and other details or minor complications that would not necessarily be reported. Additionally, more specific details of the patients included would not be available (i.e., type of cancer or reason for FP, etc.) [68].
We found that overall ovarian stimulation was well tolerated with no adverse side effects. Similar to our study, one recently published prospective study in Sweden consisting of 24 adolescent females (ages 14–17 years) demonstrated safe and successful oocyte cryopreservation in this population [69]. Live birth rates of 54% and 46% were demonstrated with the use of cryopreserved embryos and cryopreserved oocytes, respectively. Utilization rates among those individuals of childbearing age who were still alive at time of follow-up were 29% and 8% for embryos and oocytes, respectively [69]. In our patient cohort, none of the patients have returned to use their gametes.
Although several reports exist regarding the oocyte/embryo disposition selection in the adult population, the data regarding this in the adolescent population is limited. In our study, the selected options for oocyte disposition included discarding all oocytes, donating to research, and selecting an individual to allow to use and to initiate pregnancy or were not noted/unavailable in the patient record (Table 3). There was only a small difference in the selected disposition preference when comparing the younger cohort to the older cohort. The most notable difference was that fewer subjects from the < 18-year-old group chose to select an individual to use their oocytes in the case of death of the patient.
There have been concerns regarding safety of COH in the adolescent population. Pecker et al. demonstrated serious complications related to SCA in young women (ages 15–32 years) who underwent various FP strategies, which included COH using the antagonist protocol [70]. Some of the complications experienced were pre-procedural, requiring the stimulation cycle to be postponed, while others experienced postharvest painful crisis [70]. Another study reported one patient who experienced a moderate case of OHSS, requiring hospital admission and supportive care [23]. We did not experience any of these adverse outcomes in our patient population. One option to limit the risk of OHSS may be to have a low threshold for considering use of a GnRH agonist trigger instead of hCG. However, there are concerns using GnRH agonists in the adolescent population due to lack of the maturity of the HPO axis [65]. Therefore, it is imperative to check serum progesterone and luteinizing hormone (LH) levels ~ 8–14 h following a GnRH agonist trigger to ensure that the GnRH trigger was effective [71, 72] (typically an inadequate response is defined as a progesterone < 3 ng/ml and an LH < 15 IU/L [72].) To determine the true efficacy of the use of a GnRH trigger in this population, more large studies would need to be completed. Several years ago, a novel concept of a double trigger, combining a GnRH agonist and hCG administration 40 and 34 h, respectively, prior to oocyte retrieval in those women with a high proportion of immature oocytes was found to improve outcomes [73]. However, there is lack of evidence of use of this protocol in the AYA population; thus, further studies are needed.
The main strength of this study is that it is one of the few larger studies demonstrating the efficacy and safety of COH as an FP option in the AYA population, thus providing a resource to other providers when considering FP options in this patient population. However, the main limitation is that it is a retrospective investigation, which poses inherent issues. It is possible that outcome data may be lacking. Additionally, selection bias may exist when comparing patients who pursue FP with those who do not, especially within the oncology population as those who are healthier may be more willing to delay treatment for the cancer and purse fertility options. There were also patients in our cohort who had undergone chemotherapy prior to COH, which resulted in statistically significant differences in AMH and oocytes retrieved (Table 2C). Finally, because cryopreserved gametes are stored at a long-term storage facility and we are studying a population that is remote from the age in which they will utilize them, it is difficult to plan for longer-term follow-up studies to determine use of gametes and pregnancy outcomes.
Taken together, the results of this study demonstrate that COH and oocyte cryopreservation are a safe and feasible FP option for AYA patients who may not have other alternatives if a multidisciplinary team approach and appropriate precautions are taken. Future studies should focus on developing multicenter nationwide databases that are compliant with HIPAA and research safety guidelines but could allow for prospectively following these patients, thus providing the most definitive answers regarding long-term reproductive outcomes. Our study agrees with several other small studies and case reports and offers a safe option for this patient population to have genetically related offspring in the future.
Funding Information
This study is supported by the Northwestern Memorial Foundation Evergreen Grant (to MEP) and P50 HD076188 (MEP, PI: T. Woodruff).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Hirshfeld-Cytron J, Gracia C, Woodruff TK. Nonmalignant diseases and treatments associated with primary ovarian failure: an expanded role for fertility preservation. J Women's Health (Larchmt) 2011;20(10):1467–1477. doi: 10.1089/jwh.2010.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCracken K, Nahata L. Fertility preservation in children and adolescents: current options and considerations. Curr Opin Obstet Gynecol. 2017;29(5):283–288. doi: 10.1097/gco.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Matson M, Macapagal K, Johnson EK, Rosoklija I, Finlayson C, et al. Attitudes toward fertility and reproductive health among transgender and gender-nonconforming adolescents. J Adolesc Health. 2018;63(1):62–8. 10.1016/j.jadohealth.2017.11.306. [DOI] [PMC free article] [PubMed]
- 5.Moravek MB, Appiah LC, Anazodo A, Burns KC, Gomez-Lobo V, Hoefgen HR, et al. Development of a pediatric fertility preservation program: a report from the pediatric initiative network of the Oncofertility Consortium. J Adolesc Health. 2019;64(5):563–73. 10.1016/j.jadohealth.2018.10.297. [DOI] [PMC free article] [PubMed]
- 6.Reh AE, Lu L, Weinerman R, Grifo J, Krey L, Noyes N. Treatment outcomes and quality-of-life assessment in a university-based fertility preservation program: results of a registry of female cancer patients at 2 years. J Assist Reprod Genet. 2011;28(7):635–641. doi: 10.1007/s10815-011-9559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrbar V, Urech C, Alder J, Harringer K, Zanetti Dallenbach R, Rochlitz C, et al. Decision-making about fertility preservation-qualitative data on young cancer patients' attitudes and needs. Archives of Women's Mental Health. 2016;19(4):695–699. doi: 10.1007/s00737-016-0604-x. [DOI] [PubMed] [Google Scholar]
- 8.Angarita AM, Johnson CA, Fader AN, Christianson MS. Fertility preservation: a key survivorship issue for young women with cancer. Front Oncol. 2016;6:102. doi: 10.3389/fonc.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis EI, Missmer SA, Farland LV, Ginsburg ES. Public support in the United States for elective oocyte cryopreservation. Fertil Steril. 2016;106(5):1183–1189. doi: 10.1016/j.fertnstert.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Stein DM, Victorson DE, Choy JT, Waimey KE, Pearman TP, Smith K, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol. 2014;3(2):75–82. doi: 10.1089/jayao.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson J, Jervaeus A, Lampic C, Eriksson LE, Widmark C, Armuand GM, et al. ‘Will I be able to have a baby?’ Results from online focus group discussions with childhood cancer survivors in Sweden. Hum Reprod. 2014;29(12):2704–11. 10.1093/humrep/deu280. [DOI] [PMC free article] [PubMed]
- 12.Benedict C, Shuk E, Ford JS. Fertility issues in adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(1):48–57. doi: 10.1089/jayao.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakefield CE, McLoone JK, Robertson EG, Cohn RJ. Fertility concerns among child and adolescent cancer survivors and their parents: a qualitative analysis. J Psychosoc Oncol. 2016;34(5):347–362. doi: 10.1080/07347332.2016.1196806. [DOI] [PubMed] [Google Scholar]
- 14.Barnard EP, Dhar CP, Rothenberg SS, Menke MN, Witchel SF, Montano GT, et al. Fertility preservation outcomes in adolescent and young adult feminizing transgender patients. Pediatrics. 2019;144(3):e20183943. doi: 10.1542/peds.2018-3943. [DOI] [PubMed] [Google Scholar]
- 15.Strang JF, Jarin J, Call D, Clark B, Wallace GL, Anthony LG, et al. Transgender youth fertility attitudes questionnaire: measure development in nonautistic and autistic transgender youth and their parents. J Adolesc Health. 2018;62(2):128–35. 10.1016/j.jadohealth.2017.07.022. [DOI] [PubMed]
- 16.Ruddy KJ, Gelber SI, Tamimi RM, Ginsburg ES, Schapira L, Come SE, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–1156. doi: 10.1200/jco.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28(31):4683–4686. doi: 10.1200/jco.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemasik EE, Letourneau JM, Katz A, Belkora J, Cedars M, Rosen MP. Fertility preservation counseling at the time of cancer diagnosis reduces distress and anxiety. Fertil Steril. 2012;98(3 Supplement):S46. doi: 10.1016/j.fertnstert.2012.07.1067. [DOI] [Google Scholar]
- 19.Lawson AK, Klock SC, Pavone ME, Hirshfeld-Cytron J, Smith KN, Kazer RR. Psychological counseling of female fertility preservation patients. J Psychosoc Oncol. 2015;33(4):333–353. doi: 10.1080/07347332.2015.1045677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande NA, Braun IM, Meyer FL. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: a systematic review. Cancer. 2015;121(22):3938–3947. doi: 10.1002/cncr.29637. [DOI] [PubMed] [Google Scholar]
- 21.Gajjar R, Miller SD, Meyers KE, Ginsberg JP. Fertility preservation in patients receiving cyclophosphamide therapy for renal disease. Pediatr Nephrol. 2015;30(7):1099–1106. doi: 10.1007/s00467-014-2897-1. [DOI] [PubMed] [Google Scholar]
- 22.Nahata L, Sivaraman V, Quinn GP. Fertility counseling and preservation practices in youth with lupus and vasculitis undergoing gonadotoxic therapy. Fertil Steril. 2016;106(6):1470–1474. doi: 10.1016/j.fertnstert.2016.07.1102. [DOI] [PubMed] [Google Scholar]
- 23.Lavery SA, Islam R, Hunt J, Carby A, Anderson RA. The medical and ethical challenges of fertility preservation in teenage girls: a case series of sickle cell anaemia patients prior to bone marrow transplant. Hum Reprod. 2016;31(7):1501–1507. doi: 10.1093/humrep/dew084. [DOI] [PubMed] [Google Scholar]
- 24.Nahata L, Quinn GP, Tishelman AC. Counseling in pediatric populations at risk for infertility and/or sexual function concerns. Pediatrics. 2018;142(2):e20181435. doi: 10.1542/peds.2018-1435. [DOI] [PubMed] [Google Scholar]
- 25.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358(9278):271–276. doi: 10.1016/S0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 26.Bath LE, Wallace WHB, Critchley HOD. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG. 2002;109(2):107–114. doi: 10.1111/j.1471-0528.2002.t01-1-01007.x. [DOI] [PubMed] [Google Scholar]
- 27.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10(3):251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 28.Huang JYJ, Tulandi T, Holzer H, Tan SL, Chian R-C. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89(3):567–572. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 29.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy--a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45(9):1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Wallberg KA, Keros V, Hovatta O. Clinical aspects of fertility preservation in female patients. Pediatr Blood Cancer. 2009;53(2):254–260. doi: 10.1002/pbc.21995. [DOI] [PubMed] [Google Scholar]
- 31.Salem WH, Letourneau JM, Chan J, Chan S-W, Cedars M, Rosen MP. Cancer survivors of gynecologic malignancies are at risk for decreased opportunity for fertility preservation. Contraception and Reproductive Medicine. 2017;2(1):12. doi: 10.1186/s40834-017-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shliakhtsitsava K, Romero SAD, Dewald SR, Su HI. Pregnancy and child health outcomes in pediatric and young adult leukemia and lymphoma survivors: a systematic review. Leukemia & Lymphoma. 2018;59(2):381–397. doi: 10.1080/10428194.2017.1352097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TJ, Hong SW. Successful live birth from vitrified oocytes after 5 years of cryopreservation. J Assist Reprod Genet. 2011;28(1):73–76. doi: 10.1007/s10815-010-9487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril 2013;99(1):37–43. doi:10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed]
- 35.Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol. 2016;127(3):474–480. doi: 10.1097/AOG.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 36.Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016;105(2):459–66 e2. 10.1016/j.fertnstert.2015.10.026 S0015-0282(15)02037-3 [pii]. [DOI] [PubMed]
- 37.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97(2):387–90. doi:S0015–0282(11)02823–8 [pii]]]><![CDATA[10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed]
- 38.Chian R-C, Uzelac PS, Nargund G. In vitro maturation of human immature oocytes for fertility preservation. Fertil Steril. 2013;99(5):1173–81. doi:10.1016/j.fertnstert.2013.01.141. [DOI] [PubMed]
- 39.Prasath EB, Chan MLH, Wong WHW, Lim CJW, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 40.Uzelac PS, Delaney AA, Christensen GL, Bohler HCL, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104(5):1258–60. doi:10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed]
- 41.Duncan FE, Pavone ME, Gunn AH, Badawy S, Gracia C, Ginsberg JP, et al. Pediatric and teen ovarian tissue removed for cryopreservation contains follicles irrespective of age, disease diagnosis, treatment history, and specimen processing methods. J Adolesc Young Adult Oncol. 2015;4(4):174–83. 10.1089/jayao.2015.0032. [DOI] [PMC free article] [PubMed]
- 42.Donnez J, Dolmans M-M. Ovarian tissue freezing: current status. Curr Opin Obstet Gynecol. 2015;27(3):222–230. doi: 10.1097/GCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 43.Fasano G, Dechène J, Antonacci R, Biramane J, Vannin A-S, Van Langendonckt A, et al. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod BioMed Online. 2017;34(6):575–582. doi: 10.1016/j.rbmo.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong AG, Kimler BF, Smith BM, Woodruff TK, Pavone ME, Duncan FE. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium's National Physicians Cooperative. Future Oncol. 2018;14(4):363–378. doi: 10.2217/fon-2017-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 46.Fallat ME, Hutter J. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121(5):e1461–e14e9. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 47.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10. 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed]
- 48.Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94(5):1652–1656. doi: 10.1016/j.fertnstert.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Gracia CR, Chang J, Kondapalli L, Prewitt M, Carlson CA, Mattei P, et al. Ovarian tissue cryopreservation for fertility preservation in cancer patients: successful establishment and feasibility of a multidisciplinary collaboration. J Assist Reprod Genet. 2012;29(6):495–502. 10.1007/s10815-012-9753-7. [DOI] [PMC free article] [PubMed]
- 50.Estes SJ. Fertility preservation in children and adolescents. Endocrinol Metab Clin. 2015;44(4):799–820. doi: 10.1016/j.ecl.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. 10.1200/JCO.2018.78.1914. [DOI] [PubMed]
- 52.Cohen CB. Ethical issues regarding fertility preservation in adolescents and children. Pediatr Blood Cancer. 2009;53(2):249–253. doi: 10.1002/pbc.21996. [DOI] [PubMed] [Google Scholar]
- 53.McQuillan SK, Malenfant D, Jayasinghe YL, Orme LM, Grover SR. Audit of current fertility preservation strategies used by individual pediatric oncologists throughout Australia and New Zealand. Journal of Pediatric Oncology. 2013;1(2):112–8. doi:10.14205/2309-3021.2013.01.02.6.
- 54.Bortoletto P, Confino R, Smith BM, Woodruff TK, Pavone ME. Practices and attitudes regarding women undergoing fertility preservation: a survey of the National Physicians Cooperative. J Adolesc Young Adult Oncol. 2017;6(3):444–449. doi: 10.1089/jayao.2017.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDougall RJ, Gillam L, Delany C, Jayasinghe Y. Ethics of fertility preservation for prepubertal children: should clinicians offer procedures where efficacy is largely unproven? J Med Ethics. 2018;44(1):27–31. doi: 10.1136/medethics-2016-104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resetkova N, Hayashi M, Kolp LA, Christianson MS. Fertility preservation for prepubertal girls: update and current challenges. Current Obstetrics and Gynecology Reports. 2013;2(4):218–225. doi: 10.1007/s13669-013-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shnorhavorian M, Harlan LC, Smith AW, Keegan THM, Lynch CF, Prasad PK, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: a population-based study. Cancer. 2015;121(19):3499–506. [DOI] [PMC free article] [PubMed]
- 58.Moss JL, Choi AW, Fitzgerald Keeter MK, Brannigan RE. Male adolescent fertility preservation. Fertil Steril. 2015;105(2):267–273. doi: 10.1016/j.fertnstert.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Johnson EK, Finlayson C, Rowell EE, Gosiengfiao Y, Pavone ME, Lockart B, et al. Fertility preservation for pediatric patients: current state and future possibilities. J Urol. 2017;198(1):186–94. 10.1016/j.juro.2016.09.159. [DOI] [PubMed]
- 60.Armuand G, Dhejne C, Olofsson JI, Rodriguez-Wallberg KA. Transgender men's experiences of fertility preservation: a qualitative study. Hum Reprod. 2017;32(2):383–390. doi: 10.1093/humrep/dew323. [DOI] [PubMed] [Google Scholar]
- 61.Moravek MB, Confino R, Smith KN, Kazer RR, Klock SC, Lawson AK, et al. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil Steril. 2018;109(2):349–55. 10.1016/j.fertnstert.2017.10.029. [DOI] [PMC free article] [PubMed]
- 62.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril. 2010;94(1):149–55. doi:10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed]
- 63.Pavone ME, Innes J, Hirshfeld-Cytron JE, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Hum Reprod Sci. 2011;4(1):23–28. doi: 10.4103/0974-1208.82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oktay K, Rodriguez-Wallberg KA, Sahin G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril. 2010;94(2):753.e15-.e19. doi:10.1016/j.fertnstert.2010.01.044. [DOI] [PubMed]
- 65.Reichman DE, Davis OK, Zaninovic N, Rosenwaks Z, Goldschlag DE. Fertility preservation using controlled ovarian hyperstimulation and oocyte cryopreservation in a premenarcheal female with myelodysplastic syndrome. Fertil Steril. 2012;98(5):1225–1228. doi: 10.1016/j.fertnstert.2012.07.1056. [DOI] [PubMed] [Google Scholar]
- 66.Peddie VL, Maheshwari A. Successful controlled ovarian stimulation and vitrification of oocytes in an adolescent diagnosed with myelodysplastic/pre-malignant clone with monosomy 7. Hum Fertil (Camb) 2018;21(1):39–44. doi: 10.1080/14647273.2017.1347288. [DOI] [PubMed] [Google Scholar]
- 67.Assouline E, Crocchiolo R, Prebet T, Broussais F, Coso D, Gamerre M, et al. Impact of reduced-intensity conditioning allogeneic stem cell transplantation on women's fertility. Clin Lymphoma Myeloma Leuk. 2013;13(6):704–710. doi: 10.1016/j.clml.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Hipp HS, Shandley LM, Schirmer DA, McKenzie L, Kawwass JF. Oocyte cryopreservation in adolescent women. J Pediatr Adolesc Gynecol. 2019;32(4):377–382. doi: 10.1016/j.jpag.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Wallberg KA, Marklund A, Lundberg F, Wikander I, Milenkovic M, Anastacio A, et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non-oncologic indications in Sweden–trends in patients’ choices and benefit of the chosen methods after long-term follow-up. Acta Obstet Gynecol Scand. 2019. 10.1111/aogs.13559. [DOI] [PubMed]
- 70.Pecker LH, Maher JY, Law JY, Beach MC, Lanzkron S, Christianson MS. Risks associated with fertility preservation for women with sickle cell anemia. Fertil Steril. 2018;110(4):720–731. doi: 10.1016/j.fertnstert.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 71.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Efficacy of induced luteinizing hormone surge after “trigger” with gonadotropin-releasing hormone agonist. Fertil Steril. 2011;95(2):826–828. doi: 10.1016/j.fertnstert.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Beall S, Moon K, Widra E, Segars J, Chang F, Richter K. Human chorionic gonadotropin (hCG) re-trigger following a poor response to leuprolide acetate (LA) trigger is not associated with poor in vitro fertilization (IVF) treatment outcomes. Fertil Steril. 2012;98(3, Supplement):S52. doi:10.1016/j.fertnstert.2012.07.187.
- 73.Zilberberg E, Haas J, Dar S, Kedem A, Machtinger R, Orvieto R. Co-administration of GnRH-agonist and hCG, for final oocyte maturation (double trigger), in patients with low proportion of mature oocytes. Gynecol Endocrinol. 2015;31(2):145–147. doi: 10.3109/09513590.2014.978850. [DOI] [PubMed] [Google Scholar]