Abstract
Purpose
The rate of embryonic aneuploidy increases with increasing female age and is the primary cause of lower pregnancy and live birth rates (LBR) in older reproductive age women. This retrospective cohort study evaluates single euploid embryo transfers to determine whether an age-related decline in reproductive efficiency persists.
Methods
A total of 8175 non-donor single embryo transfers (SET) after pre-implantation testing for aneuploidy (PGT-A) and cryopreservation were included. These were divided into five groups by patient age: < 35 years old (n = 3789 embryos transferred), 35–37 (n = 2200), 38–40 (n = 1624), 41–42 (n = 319), and > 42 (n = 243). Implantation rate (IR), clinical pregnancy rate (CPR), and LBR were calculated for each group as a percentage of embryos transferred and compared. CPR was also analyzed as a percentage of implanted pregnancies, and LBR as a percentage of clinical pregnancies, to determine when age has the greatest impact. These results were then adjusted for confounding variables via a multivariate logistic regression model.
Results
Implantation rates negatively correlated with age. After adjusting for confounders, women 38 years or older had a significantly lower IR than those under 35 (OR 0.85, 95%CI 0.73–0.99 for 38–40 years old; 0.69, 0.53–0.91 for 41–42, and 0.69, 0.51–0.94 for > 42). These differences are also apparent in CPR and LBR. The rates of progression to clinical pregnancy and live birth did not differ significantly by age group. Other factors observed to affect IR independently were anti-Müllerian hormone (AMH), day of embryo transfer, and embryo morphology.
Conclusion
While selection of euploid embryos may be effective in overcoming a significant proportion of the age-related decline in reproductive efficiency, a decrease in IR, CPR, and LBR persists even when analyzing only euploid embryo transfers. The observed impact of aging is, therefore, independent of ploidy, as well as of other variables that affect reproductive efficiency. These results indicate that factors other than aneuploidy contribute to reproductive senescence.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01739-0) contains supplementary material, which is available to authorized users.
Keywords: Reproductive aging, Aneuploidy, Single embryo transfer, Pregestational genetic testing
Introduction
Over the last century, women’s life expectancy in the USA has increased dramatically: females born in 1929 lived an average of 58.7 years, whereas those born in 2016 can expect to live to an average age of 81.1 years old [1]. This phenomenon, in addition to other social, professional, and cultural advances, has prompted many women to postpone childbearing. As a result, the average age of first-time mothers in the USA has continued to increase over the past 40 years [2, 3].
While these changes have obvious positive effects on society, the age-related decline in female fertility poses a significant challenge regarding the ability to conceive later in life [4]. A response to this societal need has been the increased utilization of assisted reproductive technologies (ART) in older reproductive age women. In 2003, the number of fresh, non-donor cycles performed in women older than 42 in the USA was 3203 [5]. This number increased by more than 300% to 9996 in 2016 [6]. Importantly, despite significant advances in ART, live birth rate (LBR) per retrieval in women older than 42 has not improved and remains at approximately 4%. These dynamics also contribute to the rapid increase in the number of IVF cycles performed using donor oocytes, from 11,627 in 2003 to 20,391 in 2016 [5–7].
The rapid rise in the number of older reproductive age women seeking fertility care is obvious. Meanwhile, molecular mechanisms responsible for aging in general and reproductive aging in particular remain incompletely understood [8]. A number of age-related molecular changes have been described, including shortening of telomeres [9] and predictable changes in DNA methylation [10–13]. However, whether these mechanisms play a substantial role in age-related decline in female fertility remains to be established [14].
What appears to be a unique aspect of reproductive aging is the role of oocyte and embryo aneuploidy. Indeed, embryo aneuploidy is lower at female aged 26 to 37 years and steeply increases thereafter until stabilizing at age 44 [15]. In recent years, pre-implantation genetic testing for aneuploidy (PGT-A) has emerged as a potential solution to diagnose aneuploidy, leading to high sustained implantation rates in older women transferred euploid embryos [16, 17]. However, whether or not selection of euploid embryos mitigates all aspects of age-related fertility decline remains controversial.
A prior study evaluating the effect of maternal age at the time of oocyte retrieval on implantation potential of euploid blastocysts concluded that age had no impact on the said potential after adjusting for embryo morphology [18]. Of note, of the 785 transfers included in the analysis, approximately 10% were double embryo transfers. This was appropriately controlled for in the statistical analysis, but it is important to note that IR was defined as percentage of embryos transferred, and not of transfers performed. This publication contributed very valuable insight, particularly regarding the weight of morphology on implantation potential of euploid embryos.
In this study, we aimed to determine if increasing female age is associated with a decline in fertility that is independent of aneuploidy. As such, we evaluated the outcome of 8175 single euploid embryo transfers to eliminate the impact of aneuploidy on reproductive efficiency. We then sought to determine if the age-related decline in reproductive efficiency persists in this population after adjusting for identifiable confounding variables.
Materials and methods
Inclusion and exclusion criteria
This study aimed to limit confounding factors that could affect ART outcomes to better assess the effect of aging alone. In order to eliminate the known association between increasing age and aneuploidy, only transfers of embryos that had previously undergone PGT-A and found to be euploid were included in the study. At RMA New Jersey (Basking Ridge, NJ, USA), where the study was conducted, PGT-A is offered routinely to women of all ages as a method of improving implantation rates and decreasing miscarriage rates. Similarly, only single embryo transfers (SET) were considered, as multiple embryo transfers are more common in older women and can also affect IR, CPR, and LBR. In addition, all donor oocyte cycles were excluded from this analysis, as said oocytes are generally harvested from significantly younger patients and represent potential confounders. Due to the potential difference between cryopreserved and fresh embryos’ implantation potential [19], we selected only single embryo transfers that had undergone PGT-A and vitrification. All patients underwent uterine cavity evaluation prior to undergoing embryo transfer, to exclude the presence of anatomical abnormalities.
IVF cycle management
Standard regimens for controlled ovarian hyperstimulation were employed using purified urinary follicle-stimulating hormone (FSH) or recombinant FSH and LH activity in the form of low-dose hCG or human menopausal gonadotropins (hMG) along with GnRH agonist (long downregulation or microdose flare) or GnRH antagonist to prevent a premature LH surge. Importantly, while some patient-to-patient differences exist in the protocols of controlled ovarian stimulation and oocyte maturation, none of these were deemed reasons for exclusion, as several publications have concluded that there is no difference in outcomes [20, 21] or aneuploidy [22] with the employed protocols.
Monitoring of IVF cycles was per practice routine. Oocyte maturation was induced with recombinant hCG, purified urinary hCG, or with GnRH agonist. This was performed when at least two follicles reached 17–18 mm or when the follicular cohort was deemed to be mature by the patient’s primary physician. Transvaginal oocyte aspiration was performed approximately 36 h later. Cumulus stripping occurred after retrieval. Insemination of mature oocytes was performed by intracytoplasmic sperm injection (ICSI). Expanded blastocysts underwent trophectoderm biopsy, which was sent for PGT-A analysis. Only euploid embryos were included in this study. All cycles included were from cryopreserved embryos transferred in a subsequent thaw cycle.
PGT-A analysis in trophectoderm biopsies
PGT-A was carried out from trophectoderm biopsies obtained on day 5 or 6 of in vitro culture, when the embryo reached a developmental stage determined to be adequate for biopsy. An expansion score was given to each of these embryos on day 5 based on the previously published criteria [23]. Trophectoderm biopsies (from day 5 and day 6) were loaded into PCR tubes and then processed by alkaline lysis as previously described [24]. To perform PGT-A using 24 chromosome PCR, multiplex amplification of 96 loci (4 for each chromosome) was performed using TaqMan Copy Number Assays (Thermo Fisher), using a 2720 thermocycler (Thermo Fisher). Real-time PCR was performed in quadruplicate for each of the individual 96 loci using TaqMan Gene Expression Master Mix (Thermo Fisher), as recommended by the supplier (Thermo Fisher). To perform PGT-A using next-generation sequencing (NGS), lysates were amplified and then quantified with D1k ScreenTape (Agilent Technologies Inc.) [25]. Pooled libraries with up to 48 samples were purified utilizing the Agencourt Ampure XP Systems (Beckman Coulter) as per manufacturer recommendations. Ion Sphere particles containing clonally amplified libraries were prepared, enriched, and then sequenced using the Ion Proton instrument (Thermo Fisher) following manufacturer’s instructions. The reads were filtered for quality and aligned to the human genome, and the copy number of each chromosome was determined [25].
Age groups
A total of 8175 embryo transfers—all between the years 2011 and 2018—met the inclusion criteria. These were then divided into five subgroups according to the age of the patient at the time of oocyte retrieval. The cutoffs were determined by the Society for Assisted Reproductive Technology age groups: under 35 years old, 35 to 37 years old, 38 to 40 years old, 41–42 years old, and older than 42 years old.
Implantation, clinical pregnancy, and live birth rates
For each of the aforementioned groups, the IR, CPR, and LBR were calculated. For the purpose of this study, implantation was defined as a positive serum β-hCG level 9–10 days after embryo transfer, and clinical pregnancy was identified as a visualized gestational sac on ultrasound, regardless of whether they progressed any further.
In order to evaluate the effect of aging at different stages of the transfer-to-birth period, CPR was also calculated as a percentage of implanted pregnancies, and LBR as a percentage of clinical pregnancies. This allowed to distinguish the impact of aging at different phases of development.
Potential confounders
Several parameters were identified as potential confounders, as they, too, may have an impact on reproductive efficiency and change with age. The variables evaluated were body mass index (BMI), paternal age, antral follicle count, AMH, cycle day 3 FSH, cycle number, day of embryo transfer, and embryo morphology. A significant impact of these on IR was used as the criterion for inclusion into a multivariate logistic regression model to adjust for the aforementioned potential confounding effect.
Statistical analysis
Comparisons among patients in different age groups were conducted using Fisher’s exact tests for categorical variables and one-way ANOVA or Kruskal-Wallis tests for normally distributed and non-normally distributed continuous variables, respectively. A multivariate logistic regression model was used to estimate the OR of pregnancy outcomes after adjusting for age groups, transfer number, anti-Müllerian hormone, day of embryo transfer, and embryo morphology. All statistical analyses were performed by using SAS Survey Procedures (SAS 9.4, SAS Institute Inc., Cary, NC, USA). Statistical significance was defined by a two-sided test with a p value of < 0.05.
Results
Group distribution and demographics
Of the 8175 embryo transfers, 3789 (46%) used oocytes from patients younger than 35 years of age, 2200 (27%) were in the 35- to 37-year-old group, 1624 (20%) in the 38- to 40-year-old group, 319 (4%) from patients aged 41 to 42, and 243 (3%) from women older than 42 years of age.
All the potentially confounding parameters analyzed were significantly different between age groups: BMI, paternal age, antral follicle count (AFC), AMH, day 3 FSH, cycle number, day of transfer, and embryo morphology (Table 1). Therefore, a logistic regression model was developed to isolate which of them had an impact on IR. The results revealed that AMH positively correlated with implantation, whereas day of transfer did so negatively. Embryo morphology was also observed to have an association with implantation (supplemental Table 1). These three parameters were then adjusted for in a subsequent multivariate logistic regression to isolate the effect of aging on reproductive outcomes.
Table 1.
Demographics and baseline characteristics. Day of embryo transfer and embryo morphology are shown as n (% of total); all others are shown as mean (SD)
| < 35 (n = 3789) | 35–37 (n = 2200) | 38–40 (n = 1624) | 41–41 (n = 319) | > 42 (n = 243) | p value | |
|---|---|---|---|---|---|---|
| Patient age (years) | 31.56 (2.69) | 36.47 (0.87) | 39.41 (0.87) | 41.44 (0.29) | 43.02 (0.90) | < 0.0001 |
| BMI (kg/m2) | 24.86 (5.12) | 25.99 (5.42) | 26.41 (5.90) | 26.81 (5.69) | 25.88 (5.34) | < 0.0001 |
| Paternal age (years) | 34.01 (4.58) | 38.46 (4.35) | 41.13 (5.04) | 42.71 (5.93) | 43.83 (5.10) | < 0.0001 |
| Antral follicle count | 19.78 (10.33) | 16.32 (8.86) | 13.90 (7.31) | 12.47 (5.99) | 12.77 (6.51) | < 0.0001 |
| Anti-Müllerian hormone (ng/mL) | 3.80 (4.01) | 2.93 (4.65) | 2.20 (2.27) | 1.73 (1.94) | 1.73 (2.24) | < 0.0001 |
| Day 3 follicle-stimulating hormone (IU/L) | 6.90 (2.74) | 7.43 (2.96) | 7.68 (2.97) | 8.34 (3.81) | 7.75 (3.66) | < 0.0001 |
| Day of embryo transfer | ||||||
| 5 | 1212 (31.99%) | 618 (28.09%) | 386 (23.77%) | 69 (21.63%) | 44 (18.11%) | < 0.0001 |
| 6 | 2564 (67.67%) | 1568 (71.27%) | 1226 (75.49%) | 247 (77.43%) | 198 (81.48%) | |
| 7 | 13 (0.34%) | 14 (0.64%) | 12 (0.74%) | 3 (0.94%) | 1 (0.41%) | |
| Embryo morphology | ||||||
| Excellent | 1057 (27.90%) | 513 (23.32%) | 302 (18.60%) | 48 (15.05%) | 38 (15.64%) | < 0.0001 |
| Good | 2061 (54.39%) | 1200 (54.55%) | 919 (56.59%) | 183 (57.37%) | 126 (51.85%) | |
| Poor | 671 (17.71%) | 487 (22.14%) | 403 (24.82%) | 88 (27.59%) | 79 (32.51%) | |
Implantation, clinical pregnancy, and live birth rates
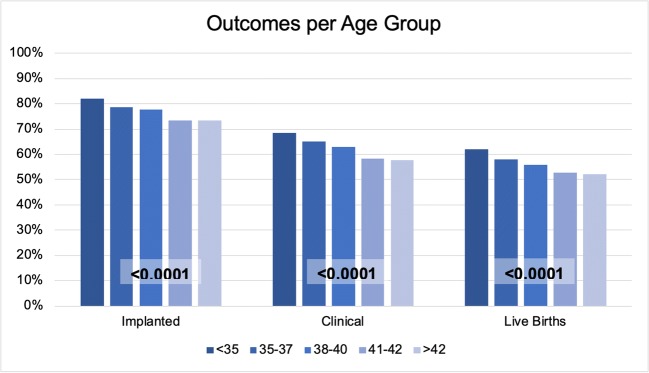
The percentage of embryo transfers resulting in implantation negatively correlated with oocyte age (Figs. 1 and 2): 82.0% in the group under 35, 78.7% in those 35–37, 77.6% in 38–40, 73.4% in 41–42, and 73.3% in patients above 42 years old (p < 0.0001). This difference was observed to continue throughout clinical pregnancy rates (68.4%, 65.1%, 62.8%, 58.3%, and 57.6%; p < 0.0001) and live birth rates (62.5%, 58.3%, 56.0%, 52.2%, 52.9%; p < 0.0001) (supplemental Table 2).
Fig. 1.
Pregnancy rates per embryo transfer in each age group. These are further subdivided into 3 stages: implanted (positive β-hCG), clinical (fetal heart rate observed), and live birth rates. p values are shown in boxes
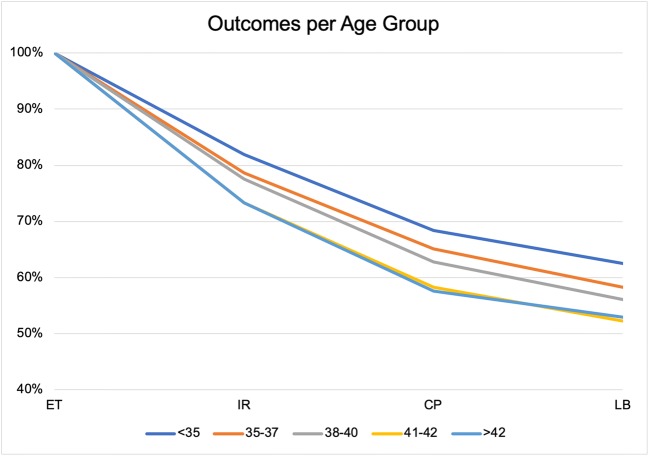
Fig. 2.
Outcomes per group, shown as progression from embryo transfer to live birth. Of note, there is virtually no difference in outcomes of patients 41–42 years old compared with those older than 42. ET, embryo transfers; IR, implanted; CP, clinical pregnancy; LB, live birth
Pregnancy development phases
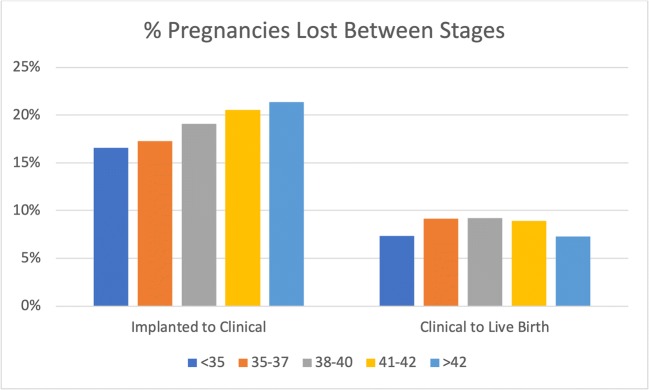
When comparing the progression through the phases of pregnancy, there was no significant difference between age groups. Thus, while there was an up to 8.7% decrease in how many embryo transfers implanted as age increased (82.0% in < 35 years old vs. 73.3% in > 42 years old), the difference in the number of implanted pregnancies progressing to clinical ones was non-significant: 83.5% in those under 35, 82.7% in those 35–37, 81.0% in 38–40, 79.5% in 41–42, and 78.7% in patients above 42 years old (p = 0.11). This difference was also non-significant when evaluating the rate of clinical pregnancies resulting in live births: 92.7%, 90.8%, 90.8%, 91.1%, and 91.8%, respectively (p = 0.22) (supplemental Table 3). The loss rate between each stage for each age group is presented in Fig. 3. A clear trend by age group is seen in the percentage of pregnancies lost between implantation and ultrasonographic confirmation. However, this difference was non-significant after adjusting for other parameters.
Fig. 3.
Percentage of pregnancies lost between implantation and clinical pregnancy stages, and between clinical pregnancy and live birth, by age group
Isolating the impact of age
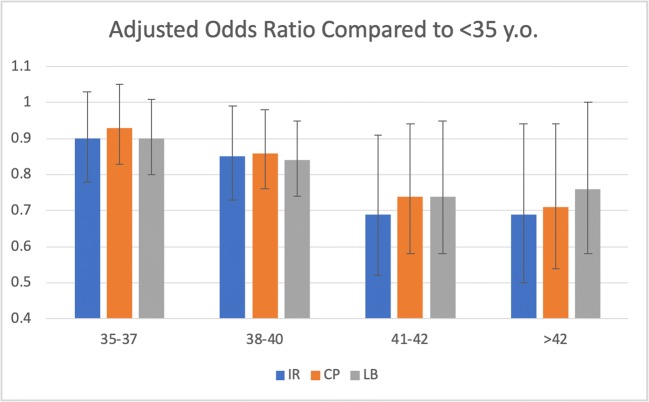
A multivariate logistic regression model adjusting for AMH, day of transfer, and embryo morphology was conducted to elucidate the independent impact of age on IR, CPR, and LBR. When compared with the youngest cohort, the group aged 35 to 37 showed no significant difference in IR (AOR 0.89, 95%CI 0.78–1.03). However, significantly decreased implantation rates were observed in the 38- to 40-year-old group (AOR 0.85, 95%CI 0.73–0.99), those between the ages of 41 and 42 (AOR 0.69, 0.53–0.91), and the group above 42 (AOR 0.69, 0.51–0.94) (Table 2, Fig. 4). Of note, the outcomes of the two oldest groups were comparable. Interestingly, while these effects prevailed through CPR and LBR, the subsequent AOR of progressing from implantation to clinical pregnancy and then to live birth demonstrated no significant differences between age groups (Table 2, Fig. 4).
Table 2.
Multivariate logistic regression model adjusting for age, AMH, day of transfer, and morphology, with AOR of IR, CPR, and LBR, as well as progression to clinical pregnancy and live birth. Statistically significant findings italicized
| Implantation | Clinical pregnancy | Live birth | Implanted pregnancies progressing to clinical pregnancy | Clinical pregnancies progressing to live birth | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Oocyte age groups | ||||||||||
| < 35 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 35–37 | 0.9 (0.78, 1.03) | 0.11 | 0.93 (0.83, 1.05) | 0.25 | 0.9 (0.8, 1.01) | 0.069 | 1.01 (0.85, 1.18) | 0.94 | 0.81 (0.64, 1.04) | 0.095 |
| 38–40 | 0.85 (0.73, 0.99) | 0.035 | 0.86 (0.76, 0.98) | 0.026 | 0.84 (0.74, 0.95) | 0.0073 | 0.92 (0.77, 1.1) | 0.34 | 0.84 (0.63, 1.1) | 0.2 |
| 41–41 | 0.69 (0.52, 0.91) | 0.008 | 0.74 (0.58, 0.94) | 0.015 | 0.74 (0.58, 0.95) | 0.018 | 0.85 (0.6, 1.2) | 0.35 | 0.87 (0.5, 1.5) | 0.62 |
| ≥42 | 0.69 (0.5, 0.94) | 0.016 | 0.71 (0.54, 0.94) | 0.016 | 0.76 (0.58, 1) | 0.046 | 0.81 (0.55, 1.2) | 0.29 | 1.05 (0.54, 2.05) | 0.89 |
| Anti-Müllerian hormone | ||||||||||
| First quartile | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Second quartile | 1.07 (0.92, 1.25) | 0.37 | 1.08 (0.95, 1.23) | 0.24 | 1.14 (1, 1.3) | 0.052 | 1.09 (0.9, 1.31) | 0.37 | 1.46 (1.1, 1.94) | 0.0084 |
| Third quartile | 1.17 (1, 1.37) | 0.049 | 1.12 (0.98, 1.28) | 0.098 | 1.19 (1.04, 1.36) | 0.011 | 1.04 (0.86, 1.26) | 0.67 | 1.46 (1.1, 1.95) | 0.0084 |
| Fourth quartile | 1.3 (1.11, 1.54) | 0.0016 | 1.22 (1.06, 1.41) | 0.0048 | 1.28 (1.11, 1.46) | 0.00046 | 1.09 (0.9, 1.33) | 0.36 | 1.45 (1.08, 1.93) | 0.012 |
| Day of rmbryo transfer | ||||||||||
| 5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 6 | 0.83 (0.72, 0.96) | 0.012 | 0.86 (0.76, 0.97) | 0.011 | 0.92 (0.82, 1.04) | 0.17 | 0.91 (0.77, 1.07) | 0.26 | 1.19 (0.94, 1.52) | 0.14 |
| 7 | 0.75 (0.37, 1.53) | 0.43 | 0.88 (0.46, 1.66) | 0.68 | 0.81 (0.43, 1.51) | 0.5 | 1.1 (0.44, 2.76) | 0.83 | 0.59 (0.19, 1.78) | 0.34 |
| Embryo morphology | ||||||||||
| Poor | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Excellent | 1.69 (1.41, 2.03) | <0.0001 | 2.05 (1.75, 2.39) | <0.0001 | 2.01 (1.72, 2.35) | <0.0001 | 2.16 (1.74, 2.68) | <0.0001 | 1.4 (0.98, 1.98) | 0.061 |
| Good | 1.59 (1.39, 1.82) | <0.0001 | 1.87 (1.66, 2.1) | <0.0001 | 1.78 (1.58, 2) | <0.0001 | 1.92 (1.63, 2.26) | <0.0001 | 1.12 (0.85, 1.47) | 0.42 |
Fig. 4.
Adjusted odds ratio of implantation (IR), clinical pregnancy (CP), and live birth (LB) per age group, compared with patients under 35 years old. Adjusted for embryo morphology, day of embryo transfer, and AMH. Error bars represent 95% confidence interval
Discussion
Increasing age is associated with diminished implantation rates despite controlling for aneuploidy. This decrease became apparent when comparing patients under 35 years old with those aged 38 and above. Therefore, although aneuploidy is the most significant determinant of cycle outcome in the older population, an age-related decline in implantation occurs in euploid embryos supporting the view that factors other than chromosome segregation errors play a role in age-related fertility decline. These results contradict the previous findings, in which the differences in outcomes based on age were non-significant after adjusting for embryo morphology [18]. This may be due to the different sample size of our cohorts, perhaps allowing for detection of smaller differences, our inclusion of only single embryo transfers, or other factors.
It is noteworthy that while there is some controversy regarding the validity and universal use of PGT-A, we believe that analyzing a dataset of only euploid embryo transfers that had been confirmed by PGT-A allows these results to be largely devoid of the confounding effect of increasing aneuploidy in the older population.
Interestingly, while the decreased implantation rate resulted in lower clinical and live birth rates, the subsequent progress of those implanted pregnancies to clinical ones and then to live birth was not impacted by age. This suggests that—after aneuploidy—the main age-related obstacle to a successful pregnancy is implantation failure, as the differences between age groups in the rates of implanted pregnancies progressing to live births are not significant.
The cause of the increasing implantation failure with age, however, remains unclear. Performing a multivariate logistic regression to account for some of the more obvious confounders—including variables such as AMH, paternal age, or embryo morphology—allowed us to identify that the effect of aging is independent not only of ploidy but also of other parameters that are detrimental to implantation rates: AMH, embryo morphology, and day of transfer. Conversely, this process identified paternal age, BMI, AFC, and cycle day 3 FSH to have no independent association with reproductive efficiency in the setting of embryonic euploidy. Interestingly, normal AMH levels were observed to have a positive correlation with progression from clinical pregnancy to live birth, but not so in the progression of implanted pregnancies to become clinical ones.
This study was conducted on the dataset of a single IVF center. While this provides a robust set of data with a constant and known ART process and laboratory, it also limits its generalizability. In addition, the results of this analysis are limited by its retrospective nature and the wide array of indications for the included IVF cycles. In addition, although we were not able to analyze the uterine factor, the uterus and endometrium may well play an important role in the development of the pre-implanted embryo. Although age is widely considered to have no effect on the ability of a uterus to sustain embryo implantation [26], the effect of the aging uterus on implantation rates may have long been obscured by the likely elevated rates of aneuploidy in the studied populations. While uterine factors such as adenomyosis, fibroids, or prior uterine surgeries may also impact implantation rate, this information was not available from the analyzed dataset, therefore representing a potential limitation of our study. Newer studies are now unveiling the extent of the effect of uterine aging on reproductive outcomes [27], and the growing populations of gestational carriers and PGT-A tested embryos present an unprecedented opportunity to better isolate and understand the uterine factor.
Our research demonstrates a declining implantation potential with age after controlling for aneuploidy, as well as several other associated factors. Further research is needed to clarify the reasons behind the age-related decline in implantation of euploid embryos. Similarly, studies analyzing the potential effect of age on the ability of a uterus to carry a pregnancy could be beneficial, but should be undertaken in the setting of known euploid embryo transfers.
Electronic supplementary material
(DOCX 29 kb)
Compliance with ethical standards
Conflict of interest
Emre Seli is a consultant for and receives research funding from Foundation for Embryonic Competence.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arias E, Xu J, Kochanek KD. United States life tables, 2016. Natl Vital Stat Rep. 2019;68(4):1–66. [PubMed] [Google Scholar]
- 2.Mathews TJ, Hamilton BE. Mean age of mother, 1970-2000. Natl Vital Stat Rep. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 3.Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000-2014. NCHS Data Brief. 2016;232:1–8. [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–634. doi: 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Society for Assisted Reproductive Technology. 2003 Assisted Reproductive Technology National Summary Report.
- 6.Society for Assisted Reproductive Technology. 2016 Assisted Reproductive Technology National Summary Report.
- 7.Kawwass JF, Monsour M, Crawford S, Kissin DM, Session DR, Kulkarni AD, Jamieson DJ, National ART Surveillance System (NASS) Group Trends and outcomes for donor oocyte cycles in the United States, 2000-2010. JAMA. 2013;310(22):2426–2434. doi: 10.1001/jama.2013.280924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seli E. Ovarian aging. Semin Reprod Med. 2015;33(6):375–376. doi: 10.1055/s-0035-1567817. [DOI] [PubMed] [Google Scholar]
- 9.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 10.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1016/j.contraception.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10(4):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, McGue M, Christensen K. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15(1):149–154. doi: 10.1111/acel.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8:64. doi: 10.1186/s13148-016-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morin SJ, Tao X, Marin D, Zhan Y, Landis J, Bedard J, Scott RT, Seli E. DNA methylation-based age prediction and telomere length in white blood cells and cumulus cells of infertile women with normal or poor response to ovarian stimulation. Aging (Albany NY) 2018;10(12):3761–3773. doi: 10.18632/aging.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT., Jr The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663.e1. doi: 10.1016/j.fertnstert.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Forman EJ, Hong KH, Franasiak JM, Scott RT., Jr Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 2014;210(2):157.e1–157.e6. doi: 10.1038/s41598-020-61117-9. [DOI] [PubMed] [Google Scholar]
- 17.Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT., Jr Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 2018;110(5):896–904. doi: 10.1016/j.fertnstert.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Irani M, Zaninovic N, Rosenwaks Z, Xu K. Does maternal age at retrieval influence the implantation potential of euploid blastocysts? Am J Obstet Gynecol. 2019;220:379.e1–379.e7. doi: 10.1097/NRL.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 19.Franasiak JM, Forman EJ, Patounakis G, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT., Jr Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open. 2018;2018(4):hoy022. doi: 10.1016/j.fertnstert.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broekmans FJ. Individualization of FSH doses in assisted reproduction: facts and fiction. Front Endocrinol (Lausanne) 2019;10:181. doi: 10.3389/fendo.2019.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J, Torrance H, Broekmans FJ. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI) Cochrane Database Syst Rev. 2018;2:CD012693. doi: 10.1002/14651858.CD012693.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labarta E, Bosch E, Alamá P, Rubio C, Rodrigo L, Pellicer A. Moderate ovarian stimulation does not increase the incidence of human embryo chromosomal abnormalities in in vitro fertilization cycles. J Clin Endocrinol Metab. 2012;97(10):E1987–E1994. doi: 10.1210/jc.2012-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Treff NR, Su J, Tao X, Levy B, Scott RT., Jr Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil Steril. 2010;94(6):2017–2021. doi: 10.1016/j.fertnstert.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman RS, Tao X, Marin D, Werner MD, Hong KH, Lonczak A, Landis J, Taylor D, Zhan Y, Scott RT, Jr, Treff NR. Preclinical validation of a targeted next generation sequencing-based comprehensive chromosome screening methodology in human blastocysts. Mol Hum Reprod. 2018;24(1):37–45. doi: 10.1093/molehr/gax060. [DOI] [PubMed] [Google Scholar]
- 26.Navot D, Drews MR, Bergh PA, Guzman I, Karstaedt A, Scott RT, Jr, Garrisi GJ, Hofmann GE. Age-related decline in female fertility is not due to diminished capacity of the uterus to sustain embryo implantation. Fertil Steril. 1994;61(1):97–101. doi: 10.1016/S0015-0282(16)56459-0. [DOI] [PubMed] [Google Scholar]
- 27.Segal TR, Kim K, Mumford SL, Goldfarb JM, Weinerman RS. How much does the uterus matter? Perinatal outcomes are improved when donor oocyte embryos are transferred to gestational carriers compared to intended parent recipients. Fertil Steril. 2018;110(5):888–895. doi: 10.1016/j.fertnstert.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 29 kb)