Abstract
Purpose
The preimplantation genetic testing for monogenic defects (PGT-M) is a beneficial strategy for the patients suffering from a Mendelian disease, which could protect their offspring from inheriting the disease. The purpose of this study is to report the effectiveness of PGT-M based on karyomapping for three cases of dynamic mutation diseases with trinucleotide repeat expansion.
Methods
PGT-M was carried out on three couples, whose family members were diagnosed with Huntington’s disease or spinocerebellar ataxias 2 or 12. The whole genome amplification was obtained using the multiple displacement amplification (MDA) method. Then, karyomapping was performed to detect the allele that is carrying the trinucleotide repeat expansion using single nucleotide polymorphism (SNP) linkage analyses, and the copy number variations (CNVs) of the embryos were also identified. Prenatal diagnosis was performed to validate the accuracy of PGT-M.
Results
PGT-M was successfully performed on the three couples, and they accepted the transfers of euploid blastocysts without the relevant pathogenic allele. The clinical pregnancies were acquired and the prenatal diagnosis of the three families confirmed the effectiveness of karyomapping. The three born babies were healthy and free of the pathogenic alleles HTT, ATXN2, or PPP2R2B corresponding to Huntington’s disease, spinocerebellar ataxias 2 or 12, respectively.
Conclusion
This study shows that karyomapping is a highly powerful and efficient approach for dynamic mutation detection in preimplantation embryos. In this work, we first report the birth of healthy babies that are free of the pathogenic gene for dynamic mutation diseases in patients receiving PGT-M by karyomapping.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01718-5) contains supplementary material, which is available to authorized users.
Keywords: Preimplantation genetic testing for monogenic defects (PGT-M), Dynamic mutation diseases, Trinucleotide repeat expansion, Karyomapping
Introduction
Dynamic mutation diseases are a series of severe, life-threatening neurological disorders caused by a nucleotide repeat expansion, which includes Huntington’s disease (HD), spinocerebellar ataxias (SCAs), fragile X syndrome, and myotonic dystrophy (DM), most of which have no effective clinical treatments until now. The dynamic nature of the nucleotide repeat expansion provided an explanation for the variable phenotypes across family members. With a prevalence of 0.4–14 cases per 100,000 in the diverse population all over the world [1], Huntington’s disease is the most common monogenic neurodegenerative disease that has the symptom of genetic dementia in an autosomal dominant pattern. This disease is associated with an unstable expansion of a CAG trinucleotide repeat in exon 1 of the HTT gene, while having 40 repeats or more is abnormal and completely penetrant [2, 3]. Surprisingly enough, the intrinsic instability of the CAG repeat during meiosis leads to the expansion of the disease gene in the repeat length inherited by successive generations [4]. Spinocerebellar ataxias (SCAs) are a class of neurodegenerative disorders that are characterized by a range of variable neurologic features in an autosomal dominant inherited fashion [5]. Spinocerebellar ataxia has currently more than 40 identified types, with a prevalence of 1–5:100,000 individuals [6]. Moreover, SCA1, SCA2, SCA3, SCA6, SCA7, SCA12, SCA17, and DRPLA are also caused by the trinucleotide CAG expansion in the responsible genes.
A longer trinucleotide repeat apparently correlates with an earlier age of onset and more severe clinical manifestations in many dynamic mutation diseases; thus, genetic anticipation will appear in successive generations of the affected families. However, there are still rare feasible clinical treatments to delay the disease progression of Mendelian diseases with dynamic mutations. Since they are dominant genetic disorders, most affected individuals inherit the pathogenic allele from one of their parents and have a 50% chance of passing the abnormal allele to each of their offspring. The preimplantation genetic testing for monogenic defects (PGT-M) can help couples to avoid having a fetus with the known genetic disease, and thus avoid the consequences of an inevitable termination of the pregnancy, a recurrent pregnancy loss or having an affected child. A variety of PGT methods have been so far reported to block the transmission of trinucleotide repeat pathogenic mutations to the offspring.
A variety of polymerase chain reaction (PCR) methods for the dynamic mutation diseases of trinucleotide repeat expansion were consecutively reported until the year 1995, which could distinguish between the normal and pathogenic alleles by PGT. The work of Sermon et al. reported the fluorescent PCR using the Expand Long Template kit to detect the expanded allele in the patients with Huntington’s disease [7], fragile X syndrome [8], and myotonic dystrophy [9]. Moreover, triplet primed PCR was performed at the single cell level on couples who were not at all or only 50% informative [9]. All these reports have used multiplex PCR for identifying the pathogenic allele, which not only necessitates a specific preparation of the primers and optimization process for the multiplex PCR, but also has the risks of allele dropout (ADO) and the unavailability of long triplet repeat amplification products. Furthermore, the genome-wide embryo aneuploidy screening cannot be simultaneously performed. Recently, linkage analyses have gradually been applied in PGT for monogenic disease. Several short tandem repeat (STR) microsatellite markers that are adjacent to the dynamic mutation allele have been clinically applied in the diagnosis of trinucleotide repeat expansionary diseases, including the Huntington’s disease [10–12], fragile X syndrome [13], and spinocerebellar ataxias [14], for which live births are available. The requirement for couples is to have an informative family member for the specific disease STR marker. However, the available STRs near the dynamic mutation allele are limited, and the recombination events of STR and the pathogenic gene region will possibly lead to misdiagnosis.
In this study, we report a highly informative, sensitive, and convenient linkage analyses using karyomapping SNP array for PGT in three cases with dynamic mutation diseases, which can effectively identify the haplotypes with abnormal trinucleotide repeats, detect the copy number variations (CNVs) of the embryos, and screen the normal euploid embryos for transplantation. By performing the karyomapping linkage analyses on patients with Huntington’s disease or spinocerebellar ataxias, live births were obtained that are free of their parental pathogenic trinucleotide repeats.
Materials and methods
Patients
Three patients carrying a dynamic mutation allele received the assisted reproductive technology (ART) at the Center for Reproductive Medicine of The First Affiliated Hospital of Zhengzhou University. The subject was approved by the Internal Review Board of The First Affiliated Hospital of Zhengzhou University, and informed consent was obtained from all the patients.
ART procedure
Controlled ovarian stimulation of the patients was achieved using the standard agonist long protocol at a luteal period, then mature oocytes were fertilized using intracytoplasmic sperm injection (ICSI), and embryo culture was conducted following a standard protocol. Next, biopsy was performed for 3-5 trophectoderm (TE) cells at the blastocyst stage, and the blastocysts were vitrified and stored in liquid nitrogen.
Whole genome amplification and karyomapping array detection
Whole genome amplification (WGA) of the lysed TE cells was performed using the multiple displacement amplification (MDA) method [15] and following the standard protocol that is provided by the QIAGEN REPLI-g Single Cell kit. This procedure can evidently amplify the genomic DNA from the picogram level to the nanogram one for the chip experiment. The next amplification was operated according to the karyomapping array manual, which includes the steps of fragmentation, precipitation, resuspension, hybridization, washing, extending, and staining. Finally, we scanned the DNA on the HumanKaryomap-12 Bead Chips (Illumina) using an Illumina HiScan and analyzed the karyomapping array data using the BlueFuse Multi software (Illumina). Similarly, the DNA samples from the peripheral blood of the couple and affected family members were also subjected to analyses using the karyomapping SNP array.
Linkage analyses and CNVs detection with karyomapping
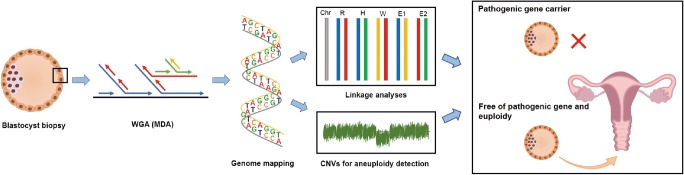
Covering over 300,000 SNPs inside karyomapping array, the parental origin of each chromosome and crossovers were analyzed using the BlueFuse Multi software. In order to identify the informative SNPs that were adjacent to the targeted trinucleotide repeat expansion region, we detected the genomes of the couple and a relative who was carrying the dynamic mutation allele; thus, the allele that was linked with the dynamic mutation site was identified, and the haplotype that was associated with the disease was established. According to the genome sequence of the biopsied blastocyst TE cells, the heterozygous or homozygous SNP readouts at 2 Mb region adjacent to the mutant gene were applied to identify the disease-carrying allele in the blastocyst (see Table 2, Supplemental Tables 1 and 2). Meanwhile, the genome-wide CNVs of the blastocysts were identified using the raw informative SNP data of the HumanKaryomap-12 Bead Chips using the BlueFuse Multi software (Illumina). The results of the B-allele and Log R charts were presented under strict criteria to define whether diploidy exists or not (see Fig. 2c and Supplemental Fig. 1). All the steps were double-checked by at least two experimental technicians. Figure 1 presents the workflow of PGT for dynamic mutation diseases by karyomapping.
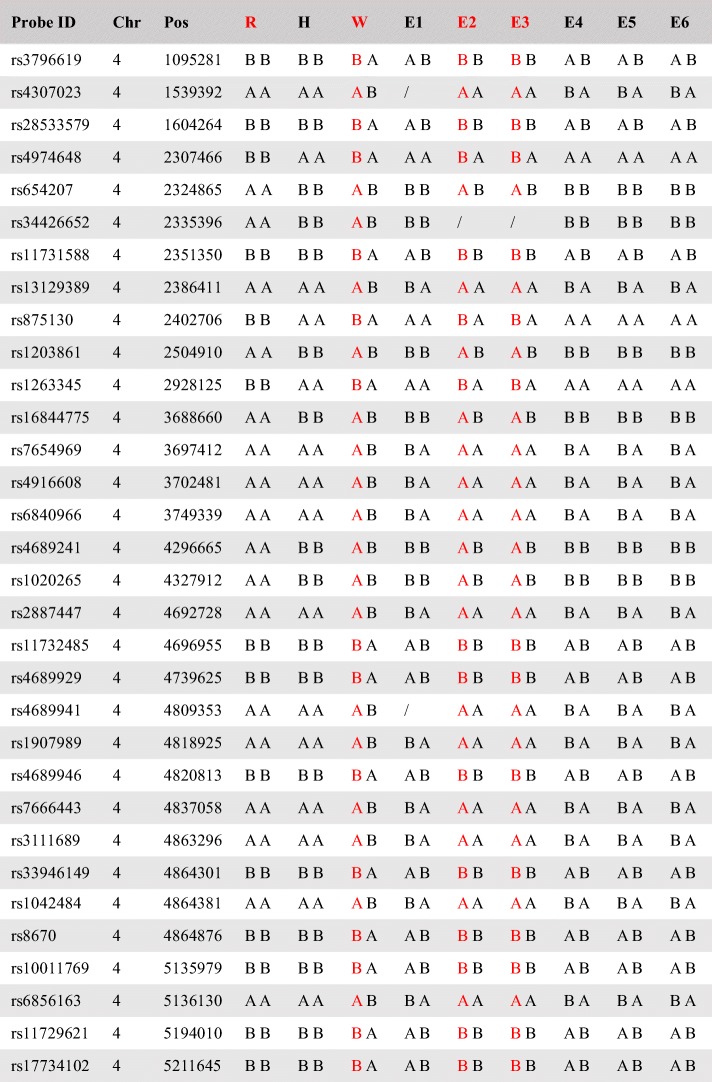
Table 2.
Informative SNPs flanking HTT gene of Huntington’s disease in case 1
There are 32 informative SNPs for linkage analyses
Probe ID the sole label of SNP in dsSNP; Chr chromosome; Pos position; R reference; H husband; W wife; E embryo; and “/” not available
Red indicates SNPs associated with pathogenic triplet expansion
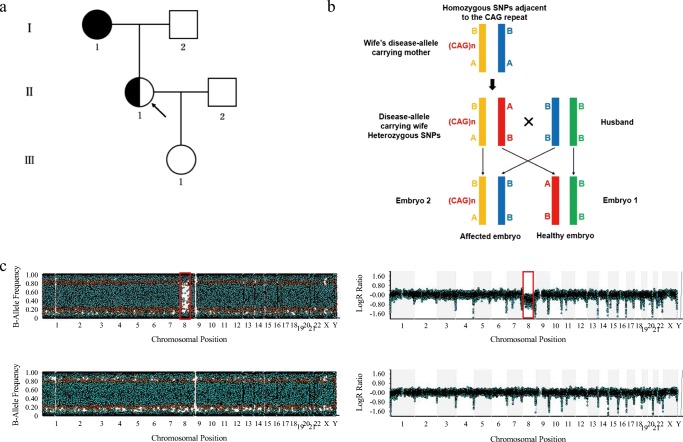
Fig. 2.
Karyomapping strategy for case 1 in which the wife was a Huntington’s disease carrier. a Pedigree diagram. Filled symbols represent the affected individuals, half-filled symbols represent the disease allele carriers without onset yet, and open symbols represent the unaffected individuals. Circles and squares indicate the females and males, respectively. The arrow indicates the disease allele carrier receiving PGT-M. b Schematic representation of the linkage identified for the disease-carrying allele. We sequenced the amplified genomes from each embryo, couple, and the wife’s mother. The heterozygous SNPs (AB) for the wife and homozygous ones (AA or BB) for the wife’s mother and the husband were applied in the linkage analyses within a range of 2 Mb upstream or downstream flanking the HTT gene. Yellow indicates the allele carrying the pathogenic CAG expansion, which is inherited from the wife’s mother to embryo E2. c. Upper: The CNVs of embryo E1 using the raw data of the karyomapping microarray. A significant chromosomal abnormality is identified. The AA and BB alleles of the 8th chromosome are observed with no AB alleles represented in the B-allele chart. A significant shift in the smooth Log R ratio also indicates monosomy 8. Bottom: Embryo E6 is identified to be euploid by the B-allele and Log R charts
Fig. 1.
Workflow of PGT for dynamic mutation diseases by karyomapping
Results
Case 1: Huntington’s disease
The first case was a couple, both aged 29. As shown in Table 1, the wife was an autosomal dominant Huntington’s disease carrier without an associated symptoms onset. The genetic diagnosis showed that one of her HTT alleles contained a sequence of 41 CAG trinucleotide repeats, which was inherited from her onset mother (Fig. 2a). The couple underwent ART treatment in our center. Eight metaphase II oocytes were collected and fertilized, and the TE cells of each blastocyst were biopsied for the genetic diagnosis. All of the DNA samples that were amplified by MDA were considered to have sufficient SNP call rates for successful karyomapping, with an average of 0.95. We focused on the SNP that was heterozygous (AB) in the wife and homozygous (AA or BB) in the husband and the wife’s disease-carrying mother in the Huntington’s disease family. Hence, if one SNP from the wife’s allele is identical to the SNP from her mother’s allele at the same point, this allele is then a disease causing one (Fig. 2b). The same strategy was used to deduce the inherited allele of the embryos under screening by the karyomapping SNP microarray, and 32 informative SNPs were available in the first case. For example, at the first listed SNP position in Table 2, the alleles of the wife are heterozygous A/B, while those of her disease onset mother are homozygous B/B. Therefore, the allele B of the wife must be inherited from her mother and associated with the abnormal trinucleotide repeats. Similarly, embryo E2 is identified as homozygous B/B, since one allele B is derived from the husband, then the other B must be derived from the wife and associated with the abnormal trinucleotide repeats. Combining other informative SNPs, the linkage analyses were applied in embryo E2 to make sure to get an accurate diagnosis; hence, embryo E2 would not be transferred. As shown in Fig. 2c, embryo E1 presented the abnormal molecular karyotype with a monosomy of the 8th chromosome, where the AA and BB alleles were observed without any AB alleles represented in the B-allele chart. Simultaneously, the Log R chart revealed a significant shift of the 8th chromosome.
Table 1.
Overview of the family information of dynamic mutation diseases and characteristics of oocytes, zygotes, and blastocysts
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Disease | Huntington’s disease | Spinocerebellar ataxia 2 | Spinocerebellar ataxia 12 |
| Gene | HTT | ATXN2 | PPP2R2B |
| Mutation assessed | CAG repeat 17/41 | CAG repeat 16/35 | CAG repeat 16/53 |
| Affected partner | Wife | Husband | Husband |
| Reference | Wife’s mother | Husband’s mother | Husband’s mother |
| No. of retrieved oocytes | 13 | 15 | 27 |
| No. of MII oocytes | 8 | 13 | 25 |
| No. of 2PN zygotes | 6 | 11 | 22 |
| No. of biopsied blastocysts | 6 | 9 | 13 |
MII metaphase II and 2PN two pronucleus
Accordingly, the karyomapping strategy was performed for all the embryos. Embryo E1 was a monosomy of the 8th chromosome, embryo E2 and E3 were considered to have the pathogenic allele carrier status, and the remaining three embryos were unaffected and suitable for transfer. All the testing results are detailed in Table 3. Among the embryos that were neither affected nor aneuploid, the euploid embryo E6 with a higher morphology grade (5BB) was transferred, its B-allele and Log R charts are displayed in Fig. 2c. The ultrasound examination, performed 35 days after the embryo transfer, showed the single intrauterine gestational sac with a normal fetal heartbeat, which was detectable for the patient. Moreover, in order to verify the CAG trinucleotide repeats of the HTT allele and chromosomal euploidy, a prenatal diagnosis was conducted with the multiplex ligation dependent probe amplification (MLPA), SNP microarray and karyotype analyses using the amniotic fluid cells at 19-week gestation. The genetic status was confirmed to have a normal chromosomal copy number with a very low risk for developing Huntington’s disease. The born of a healthy baby girl on September 13, 2016 was gratifying for us.
Table 3.
Pathogenic carrier status and genome-wide copy number variations (CNVs) of the embryos
| Embryo | Pathogenic carrier status | CNVs status | Recommendation | |
|---|---|---|---|---|
| Case 1 | 1 | Normal | Monosomy 8 | Not transferred |
| 2 | Carrier | Euploid | Not transferred | |
| 3 | Carrier | Euploid | Not transferred | |
| 4 | Normal | Euploid | Transferred | |
| 5 | Normal | Euploid | Transferred | |
| 6 | Normal | Euploid | Transferred | |
| Case 2 | 1 | Carrier | Euploid | Not transferred |
| 2 | Normal | Euploid | Transferred | |
| 3 | Normal | Euploid | Transferred | |
| 4 | Carrier | Euploid | Not transferred | |
| 5 | Carrier | Euploid | Not transferred | |
| 6 | Normal | Euploid | Transferred | |
| 7 | Carrier | Partial monosomy 5 | Not transferred | |
| 8 | Normal | Euploid | Transferred | |
| 9 | Normal | Euploid | Transferred | |
| Case 3 | 1 | Normal | Partial monosomy 22 | Not transferred |
| 2 | Carrier | Trisomy sex chromosome | Not transferred | |
| 3 | Normal | Euploid | Transferred | |
| 4 | Carrier | Monosomy 14 | Not transferred | |
| 5 | Normal | Multiple trisomy | Not transferred | |
| 6 | Carrier | Euploid | Not transferred | |
| 7 | Carrier | Euploid | Not transferred | |
| 8 | Normal | Euploid | Transferred | |
| 9 | Carrier | Euploid | Not transferred | |
| 10 | Normal | Euploid | Transferred | |
| 11 | Normal | Euploid | Transferred | |
| 12 | Carrier | Euploid | Not transferred | |
| 13 | Carrier | Euploid | Not transferred |
Case 2: spinocerebellar ataxia 2
The second patient was an autosomal dominant spinocerebellar ataxia 2 (SCA2) male carrier, whose pathogenic allele was inherited from his diagnosed mother, and his maternal uncle had the same symptoms. The husband had an allele with 35 CAG abnormal repeats, which were located in the 5′ end of the coding region of the ATXN2 gene, and he had not yet shown any symptoms. The couple, both aged 29, already had two affected fetuses with a detected CAG abnormal expansion by the chorionic villi sampling and had to subsequently suffer pregnancy losses (Fig. 3a). They underwent one clinical cycle in our center. The conditions of the oocytes, fertilizations, and blastocysts are shown in Table 1. The linkage analyses were performed, and 19 informative SNPs were available within a 2 Mb region flanking the mutant gene. The haplotyping was established and CNV was detected, the results for all the blastocysts are summarized in Table 3. After excluding all the affected and aneuploid embryos, we selected embryo E3 for transfer. On day 35 after the embryo transfer, ultrasound examination confirmed the clinical pregnancy. A prenatal diagnosis was conducted with MLPA and SNP microarray using the amniotic fluid cells at 22-week gestation. The results of the genetic testing were in accordance with those obtained from the karyomapping analyses. A healthy baby boy was born on February 1, 2018.
Fig. 3.
Pedigree of case 2 (spinocerebellar ataxia 2) and case 3 (spinocerebellar ataxia 12). Pedigrees of case 2 and case 3 are shown in a and b, respectively. Filled symbols represent the affected individuals, half-filled symbols represent the disease allele carriers without onset yet and open symbols represent the unaffected individuals. Circles and squares indicate the females and males, respectively. The arrow indicates the disease allele carrier receiving PGT-M.
Case 3: spinocerebellar ataxia 12
The third case involved a couple of a maternal age of 26 and a paternal age of 27. The husband carried an autosomal dominant inherited spinocerebellar ataxia 12 (SCA12) and had not shown abnormal clinical manifestations yet. His mother, three maternal uncles, and one maternal aunt have gradually developed symptoms of spinocerebellar ataxia after the age of thirty (Fig. 3b). The genetic diagnosis of the husband and his mother showed a 53 CAG repeats expansion, which was located at the upstream of the transcription start site of the PPP2R2B gene, already known to cause this disease. As shown in Table 1, thirteen embryos of the couple developed to the blastocyst stage, and the biopsy and amplification of the TE cells were successful. In order to carry out the linkage analyses, we conducted the karyomapping SNP array for the family members and embryos. Ultimately, 27 informative SNPs were available within a 2 Mb region flanking the mutant gene, with an average SNP call rate of 0.95. The pathogenic carrier status and CNVs for all the embryos are summarized in Table 3. The couple underwent two cycles of frozen embryo transfer with two normal embryos: E10 and E11; after the second cycle, the couple fortunately received a successful clinical pregnancy. The genetic status was confirmed by prenatal diagnosis at 18-week gestation to include 10/16 CAG repeats located on the 5′ UTR of the PPP2R2B gene, which have a very low risk for developing spinocerebellar ataxia 12. The SNP microarray of the amniotic fluid cells confirmed the euploidy of the fetus. A healthy baby boy was born on July 31, 2019.
Discussion
Genetic diseases that are caused by unstable repeated DNA expansions are rare, but they still represent a substantial cause of morbidity. The dynamic mutation diseases are usually life-threatening neurological disorders with variable expressivity and severity according to the disease phenotype caused by a nucleotide repeat expansion, and the longer repeat expansion is linked to genetic anticipation. Currently, no effective treatments exist for those carriers [2, 16]. The prenatal diagnosis can recognize any abnormal nucleotide repeats in the fetus using the amniotic fluid cells or chorionic villi sampling. On the other hand, PGT-M is an alternative method, which provides an informative route for disease carriers to prevent the transmission of the disturbing state to the offspring. Through processing PGT-M for three dynamic mutant gene carrier families by karyomapping, we successfully obtained three healthy babies, which did not have the carrier state of the pathogenic genes HTT, ATXN2, and PPP2R2B of the Huntington’s disease and spinocerebellar ataxia 2 and 12, respectively.
Karyomapping is an ideal strategy to analyze single or small numbers of cells that are biopsied from each embryo for PGT. Besides, the feasible WGA is now available by MDA. The only requirement is to have DNA available from both members of the couple and a family member with a known disease status. It is possible to identify the informative SNPs of each parental haplotype and map the inheritance of these haplotypes across each chromosome. As a result, performing karyomapping by linkage analyses can identify the parental and grandparental origin across each chromosome and segment, including the recombination events that are unique for every individual [17]. In this work, we mapped adequate informative SNPs flanking the trinucleotide repeat region for three dynamic mutation families to ensure the responsible haplotype linkage analyses of the couples and embryos. Interestingly, a recombination was observed at the upstream CAG repeats of the PPP2R2B gene in embryo E13 of family 3, corresponding SNPs are shown in Supplemental Table 2. Considering the recombination position, which was unlocated at the targeted gene region, we speculated that the CAG repeat region is well associated with the downstream flanking region without the disturbance of recombination; hence, efficient linkage analyses are available and embryo E13 is diagnosed to be free of the pathogenic allele.
Another feature of karyomapping is that the analyses of the SNPs across the genome are not only able to identify the parental origin of each chromosome but also can detect the chromosome aneuploidy. By mapping the SNP genotypes across the whole genome, the trisomy and monosomy of any chromosome will be identified, as well as the deletion and duplication of small fragments [17]. It is particularly important for PGT-M to combine the genome-wide linkage analyses with the detection of the chromosome aneuploidy, as a result of a high incidence of the chromosomal abnormalities before and after the fertilization process following in vitro fertilization (IVF) [18]. Additionally, it is estimated that the IVF failure and miscarriage are correlated with the aneuploidy of the embryo chromosome [19]. In this study, genome-wide CNVs analyses were performed for all the embryos using the raw data of the HumanKaryomap-12 Bead Chips with the BlueFuse Multi software. As a result, multiple chromosomal abnormalities of the embryos were identified, including monosomy, trisomy, multiple trisomy of the chromosome, and deletion of partial fragments (Fig. 2c and Supplemental Fig. 1). The euploid embryo transfers for patients can mitigate the risk of spontaneous miscarriage that is caused by chromosome aneuploidy.
Compared with the traditional PGT technologies, which require complicated and individualized procedure for the multiplexed STR analyses, karyomapping provides a universally applicable method for all kinds of patients who have dynamic mutation diseases with an equal cost for each sample. No specific testing primers are required, which is time-saving. Another advantage is the WGA technology of MDA, which offers a reliable single cell amplification starting with a small quantity of the DNA material to a high quantity with many advantages including the low error rate, good reproducibility, and unbiased amplification [15, 20]; thus, it can maintain the accuracy of the SNPs linkage analyses, especially for dynamic mutation diseases. The prenatal diagnosis of the fetal amniotic fluid cells confirmed no pathogenic trinucleotide expansion, further supporting the reliability of karyomapping.
It would be difficult for the previously available diagnostic methods, such as the multiple PCR for the targeted gene region [7–9], to directly detect the pathogenic repeat sequences due to the difficulty of acquiring the amplification of long identical sequences. The STR markers that are adjacent to the dynamic mutation alleles could provide the clinical diagnosis of the trinucleotide repeat expansion disease [12–14], but the limited number of available STRs and recombination events may not facilitate using it as a routine clinical application. It was previously reported that the next generation sequencing based PGT procedure, mutated allele revealed by sequencing with aneuploidy and linkage analyses (MARSALA), can simultaneously detect single gene disorders and aneuploidy, where the PCR amplification of the WGA product can identify the single nucleotide variants that are associated with the monogenic diseases [21]. However, the testing strategy of the long trinucleotide repeat sequence was not described by this method as yet. Meanwhile, genome-wide karyomapping is highly feasible and provides accurate analyses for the inheritance of almost any monogenic disease at the single cell level. Several studies have illustrated the effectiveness of this method [22–25], which also contained the PGT of dynamic mutation diseases such as the Huntington’ disease and fragile X syndrome. However, to the best of our knowledge, no live births have been reported considering the triplet repeat diseases with this method as yet.
Next generation sequencing (NGS) is a massively parallel sequencing, which greatly reduces the cost of human genome sequencing. The NGS-based preimplantation genetic aneuploidy testing has been validated in multiple centers [26]. However, enough resolution is hardly available to directly detect genetic mutations, due to the high required sequencing depth [27], possible sequencing bias and ADO. Moreover, the major limitation of the NGS technology is the relatively short reads, which may lead to misassembles and gaps of repeat sequences. Fortunately, third generation sequencing technologies, such as the nanopore sequencing, Illumina synthetic long-read technology (SLRs), and single molecule real time (SMRT) sequencing, are developing due to various advantages [28]. Representing an enormous technological progress, the long-read sequencing can generate 10 kb to 1 Mb reads, leading to a superior performance in analyzing the repeat expansion regions and complex structural variations. However, the cost is high. In addition, no requirement of the PCR amplification allows less bias and more homogeneous genome coverage. Long-read sequencing may be an efficient application in PGT of dynamic mutation diseases in the future.
The karyomapping strategy for dynamic mutation diseases has some limitations. First, there is the requirement of an available affected relative who is associated with the disorder. In fact, the genome sequences of the couple are prerequisites for the haplotyping analyses. Second, it cannot identify de novo mutations within the embryos. Recombination events across the genome are inevitable, and chromosome recombination that is adjacent to the mutant gene might disturb the SNPs linkage analyses. Therefore, the availability of sufficient informative SNPs will improve the accuracy of karyomapping. ADO, which is caused by the amplification, can potentially lead to a heterozygous locus being identified as homozygous, particularly when the starting material is a single cell. All of the biopsied embryos received an extremely low rate of ADO except for embryo E8 of case 2, which had a rate of 0.59. This result might be due to the poor quality of the biopsied cells or poor amplification. Nevertheless, eight informative SNPs were available for embryo E8, ensuring the dependability of the linkage analyses.
In conclusion, our study further confirmed that karyomapping is a highly powerful and feasible approach for dynamic mutation detection in the preimplantation embryos, which may be hard and complicated to be achieved via conventional PGT methods. The karyomapping linkage analyses successfully resulted in the born of live births, which were free of their parental pathogenic trinucleotide repeats, for families with Huntington’s disease and spinocerebellar ataxias. The advancement of technology is significantly improving its clinical applicability, and it could potentially be successfully applied to all the patients with dynamic mutation diseases to give birth to unaffected healthy babies, thus avoiding the vertical transmission of dynamic mutation diseases.
Electronic supplementary material
Copy number variations (CNVs) of case 2 and case 3. The CNVs of case 2 are shown by the B-allele and Log R charts in a and b: a. Del(5)(pter-p13.1) in embryo E7; b. Transferred euploid embryo E3. The CNVs of case 3 are displayed in c-g: c. Del(22)(q13.1-q13.2) in embryo E1; d. 47, XXY in embryo E2; e. Monosomy of the 14th chromosome in embryo E4; f. Multiple trisomy of the chromosomes 2, 5, 10, 11, 14, 15 and 16 in embryo E5; g. Transferred euploid embryo E11. (PNG 3305 kb)
(DOCX 34 kb)
Acknowledgments
We thank the families for taking part and everybody involved in the research for their contributions.
Author contributions
Y.S. and J.X designed the study; G.Y., S.S., W.S., and H.J. performed embryo culture and biopsy; H.S. and G.L. collected the data; W.N., Y.L., and D.S. analyzed the data; D.S. drafted the manuscript; and J.X. revised it.
Funding information
This work was supported by National Key R&D Program of China (2019YFA0110900 to Yingpu Sun and Jiawei Xu, and 2019YFA0802200 to Jiawei Xu), National Natural Science Foundation of China (31870817 to Jiawei Xu.), Scientific and Technological Innovation Talent Project of Universities of Henan Province (20HASTIT045 to Jiawei Xu), and Henan Provincial Obstetrical and Gynecological Diseases (Reproductive Medicine) Clinical Research Center (to Yingpu Sun and Jiawei Xu).
Compliance with ethical standards
All couples signed informed consent forms for ICSI treatment, PGT, cryopreservation, thawing, and transfer of embryos. The protocols for this study were evaluated and approved by the Internal Review Board of The First Affiliated Hospital of Zhengzhou University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dayuan Shi and Jiawei Xu contributed equally to this work.
Contributor Information
Jiawei Xu, Email: jiawxu@foxmail.com.
Yingpu Sun, Email: syp2008@vip.sina.com.
References
- 1.Kay C, Hayden MR, Leavitt BR. Epidemiology of Huntington disease. Handb Clin Neurol. 2017;144:31–46. doi: 10.1016/B978-0-12-801893-4.00003-1. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh R, Tabrizi SJ. Clinical features of Huntington’s disease. Adv Exp Med Biol. 2018;1049:1–28. doi: 10.1007/978-3-319-71779-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Group THsDCR A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 4.Ziihlke C, Rless O, Bockel B, Lange H, Thies U. Mitotic stability and meiotic variability of the (CAG)n repeat in the Huntington disease gene. Hum Mol Genet. 1993;2:2063–2067. doi: 10.1093/hmg/2.12.2063. [DOI] [PubMed] [Google Scholar]
- 5.Soong BW, Morrison PJ. Spinocerebellar ataxias. Handb Clin Neurol. 2018;155:143–174. doi: 10.1016/B978-0-444-64189-2.00010-X. [DOI] [PubMed] [Google Scholar]
- 6.Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia a systematic review of prevalence studies. Neuroepidemiology. 2014;42:174–183. doi: 10.1159/000358801. [DOI] [PubMed] [Google Scholar]
- 7.Sermon K, Goossens V, Seneca S, Lissens W, Vos AD, Vandervorst M, et al. Preimplantation diagnosis for Huntington’s disease (HD) clinical application and analysis of the HD expansion in affected embryos. Prenat Diagn. 1998;18:1427–1436. doi: 10.1002/(SICI)1097-0223(199812)18:13<1427::AID-PD493>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Sermon K, Seneca S, Vanderfaeillie A, Lissens W. Preimplantation diagnosis for fragile X syndrome based on the detection of the non-expanded paternal and maternal CGG. Prenat Diagn. 1999;19:1223–1230. doi: 10.1002/(SICI)1097-0223(199912)19:13<1223::AID-PD724>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Sermon K, Seneca S, Rycke MD, Goossens V. PGD in the lab for triplet repeat diseases-myotonic dystrophy, Huntington’s disease and fragile-X syndrome. Mol Cell Endocrinol. 2001;183:S77–S85. doi: 10.1016/S0303-7207(01)00572-X. [DOI] [PubMed] [Google Scholar]
- 10.Moutou C, Gardes N, Viville S. New tools for preimplantation genetic diagnosis of Huntington’s disease and their clinical applications. Eur J Hum Genet. 2004;12:1007–1014. doi: 10.1038/sj.ejhg.5201291. [DOI] [PubMed] [Google Scholar]
- 11.Van Rij MC, De Rademaeker M, Moutou C, Dreesen JC, De Rycke M, Liebaers I, et al. Preimplantation genetic diagnosis (PGD) for Huntington’s disease: the experience of three European centres. Eur J Hum Genet. 2012;20:368–375. doi: 10.1038/ejhg.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pecina A, Lozano Arana MD, Garcia-Lozano JC, Borrego S, Antinolo G. One-step multiplex polymerase chain reaction for preimplantation genetic diagnosis of Huntington disease. Fertil Steril. 2010;93:2411–2412. doi: 10.1016/j.fertnstert.2009.01.120. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer E, Nicod JC, Gardes N, Kastner C, Becker N, Celebi C, Pirrello O, Rongières C, Koscinski I, Gosset P, Moutou C. Improving preimplantation genetic diagnosis for fragile X syndrome: two new powerful single-round multiplex indirect and direct tests. Eur J Hum Genet. 2016;24:221–227. doi: 10.1038/ejhg.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moutou C, Nicod JC, Gardes N, Viville S. Birth after pre-implantation genetic diagnosis (PGD) of spinocerebellar ataxia 2 (Sca2) Prenat Diagn. 2008;28:126–130. doi: 10.1002/pd.1909. [DOI] [PubMed] [Google Scholar]
- 15.Lasken RS. Genomic DNA amplification by the multiple displacement amplification (MDA) method. Biochem Soc Trans. 2009;37:450–453. doi: 10.1042/BST0370450. [DOI] [PubMed] [Google Scholar]
- 16.Buijsen RAM, Toonen LJA, Gardiner SL, van Roon-Mom WMC. Genetics, mechanisms, and therapeutic progress in polyglutamine spinocerebellar ataxias. Neurotherapeutics. 2019;16:263–286. doi: 10.1007/s13311-018-00696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handyside AH, Harton GL, Mariani B, Thornhill AR, Affara N, Shaw MA, Griffin DK. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J Med Genet. 2010;47:651–658. doi: 10.1136/jmg.2009.069971. [DOI] [PubMed] [Google Scholar]
- 18.Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C, et al. Chromosome instability is common in human cleavage-stage embryos. Nat Med. 2009;15:577–583. doi: 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- 19.Fragouli E, Alfarawati S, Spath K, Jaroudi S, Sarasa J, Enciso M, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132:1001–1013. doi: 10.1007/s00439-013-1309-0. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102. doi: 10.1146/annurev-genom-090413-025352. [DOI] [PubMed] [Google Scholar]
- 21.Yan L, Huang L, Xu L, Huang J, Ma F, Zhu X, et al. Live births after simultaneous avoidance of monogenic diseases and chromosome abnormality by next-generation sequencing with linkage analyses. Proc Natl Acad Sci U S A. 2015;112:15964–15949. doi: 10.1073/pnas.1523297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natesan SA, Bladon AJ, Coskun S, Qubbaj W, Prates R, Munne S, Coonen E, Dreesen JC, Stevens SJ, Paulussen AD, Stock-Myer SE, Wilton LJ, Jaroudi S, Wells D, Brown AP, Handyside AH. Genome-wide karyomapping accurately identifies the inheritance of single-gene defects in human preimplantation embryos in vitro. Genet Med. 2014;16:838–845. doi: 10.1038/gim.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstantinidis M, Prates R, Goodall NN, Fischer J, Tecson V, Lemma T, Chu B, Jordan A, Armenti E, Wells D, Munné S. Live births following karyomapping of human blastocysts: experience from clinical application of the method. Reprod BioMed Online. 2015;31:394–403. doi: 10.1016/j.rbmo.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Natesan SA, Handyside AH, Thornhill AR, Ottolini CS, Sage K, Summers MC, Konstantinidis M, Wells D, Griffin DK. Live birth after PGD with confirmation by a comprehensive approach (karyomapping) for simultaneous detection of monogenic and chromosomal disorders. Reprod BioMed Online. 2014;29:600–605. doi: 10.1016/j.rbmo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Nagi J, Wells D, Doye K, Loutradi K, Exeter H, Drew E, Alfarawati S, Naja R, Serhal P. Karyomapping: a single centre’s experience from application of methodology to ongoing pregnancy and live-birth rates. Reprod BioMed Online. 2017;35:264–271. doi: 10.1016/j.rbmo.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Sermon K. Novel technologies emerging for preimplantation genetic diagnosis and preimplantation genetic testing for aneuploidy. Expert Rev Mol Diagn. 2017;17:71–82. doi: 10.1080/14737159.2017.1262261. [DOI] [PubMed] [Google Scholar]
- 27.Treff NR, Fedick A, Tao X, Devkota B, Taylor D, Scott RT., Jr Evaluation of targeted next-generation sequencing-based preimplantation genetic diagnosis of monogenic disease. Fertil Steril. 2013;99:1377–1384. doi: 10.1016/j.fertnstert.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C. The third revolution in sequencing technology. Trends Genet. 2018;34:666–681. doi: 10.1016/j.tig.2018.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Copy number variations (CNVs) of case 2 and case 3. The CNVs of case 2 are shown by the B-allele and Log R charts in a and b: a. Del(5)(pter-p13.1) in embryo E7; b. Transferred euploid embryo E3. The CNVs of case 3 are displayed in c-g: c. Del(22)(q13.1-q13.2) in embryo E1; d. 47, XXY in embryo E2; e. Monosomy of the 14th chromosome in embryo E4; f. Multiple trisomy of the chromosomes 2, 5, 10, 11, 14, 15 and 16 in embryo E5; g. Transferred euploid embryo E11. (PNG 3305 kb)
(DOCX 34 kb)