Abstract
Purpose
To report a case of B-cell acute lymphocytic leukemia (ALL) relapse presenting as acute retinal necrosis.
Observations
An 11-year old boy with history of B-cell ALL undergoing maintenance therapy presented with a three-month history of intermittent blurry vision and pain in the right eye when a routine lumbar puncture indicated an elevated lymphoblast-predominant white blood cell count. Bone marrow biopsy revealed 42% lymphoblasts, confirming ALL relapse. Ophthalmic imaging demonstrated a hyperemic optic disc, retinal whitening, perivascular sheathing, retinal hemorrhages, and retinal detachment in the right eye. Vitreous fluid biopsy revealed presence of rare atypical lymphoblasts. Chemotherapy, orbital radiation, and systemic prednisone resulted in improvement of visual acuity and retinal hemorrhages, and resolution of retinal detachment.
Conclusions and importance
We have described the clinical features, treatment, and response in a case of B-cell ALL relapse with presenting signs of acute retinal necrosis. The uncommon finding in B-cell ALL highlights the possibility of intraocular involvement and the importance of routine ophthalmologic evaluation in leukemia remission.
Keywords: Acute lymphocytic leukemia, Retinal necrosis
1. Introduction
Acute lymphocytic leukemia (ALL) is the most prevalent cancer among children in the United States, accounting for 20% of all cancers in people under 20 years old.1 It is characterized by the widespread presence of atypical lymphoblasts of either B- or T-cell lineage in the peripheral blood and bone marrow.2 It commonly manifests in children with fatigue, fever, and bruising, and is diagnosed by cell morphology, immunophenotype, and genetics testing.
Acute retinal necrosis (ARN) is an ocular inflammatory condition characterized by peripheral necrotizing retinitis and is most often caused by Varicella Zoster Virus (VZV) and Herpes Simplex Virus (HSV), though it may also be caused by Cytomegalovirus (CMV), Progressive Outer Retinal Necrosis (PORN), Syphilis, Toxoplasmosis, Behcet Disease, or fungal or bacterial endophthalmitis.3 As retinal necrosis occurs, usually from virally induced inflammation, large areas of the retina become exposed and may lead to retinal detachment. ARN commonly occurs in immunocompetent individuals; however, it can also affect immunocompromised patients. ARN often presents in older patient populations as a panuveitis with the patient experiencing rapid-onset ocular pain, decreased or blurred vision, and floaters.
Ocular manifestations may occur in leukemia due to direct infiltration or secondary effects such as anemia and thrombocytopenia, with common manifestations being intraretinal hemorrhages and Roth spots.4 In a cross-sectional observational study on 133 patients in South India, leukemic ophthalmopathy was found in 42% of cases of ALL, with the pediatric population commonly experiencing vitreous hemorrhage, dot/blot hemorrhage, or Roth spots.5 In this report, we describe a case of unilateral ARN in a child presumed to be secondary to relapse of B-cell ALL.
2. Case report
An 11-year-old male presented to our clinic in March 2019 for assessment of his ocular symptoms of intermittent blurring of vision and eye pain in the right eye (OD) for the past 3 months.
Past medical history of the patient revealed a history of CD19 positive B-cell ALL without central nervous system (CNS) involvement diagnosed in January 2017. The patient had been enrolled in a phase 3 Randomized Trial for Newly Diagnosed High Risk B-precursor ALL (AALL1131) and immediately began 2 weeks of 4-drug induction with vincristine, daunorubicin, dexamethasone, and Peg-asparaginase. However, his course was complicated by Charcot-Marie-Tooth disease which resulted in vincristine being withheld for most of treatment duration. During cycle 6 of the maintenance therapy in February 2019 a routine lumbar puncture (LP) was performed which showed high white blood cell (WBC) count (123/μL) with 98% blast cells, and the patient was immediately started on intrathecal (IT) chemotherapy and oral 6-mercaptopurine. A bone marrow biopsy was performed which demonstrated 42% lymphoblasts confirming a relapse of the B-cell ALL with CNS involvement.
During the patient's admission for the management of CNS recurrence, he was also seen by the on-call ophthalmologist in February 2019. The ocular examination showed presence of optic nerve hyperemia and retinal thickening in OD. B-scan demonstrated retinal thickening and optic nerve edema with a small area of retinal detachment surrounding the optic nerve head in OD. The examination of the left eye (OS) was unremarkable. A vitreous fluid biopsy was performed and sent for infectious analysis including viral culture and polymerase chain reaction (PCR). Based on the clinical picture, the patient was empirically started on prophylactic therapy with intravitreal injection of foscavir and a tapering dose of oral prednisone. Brain magnetic resonance imaging (MRI) was also performed which demonstrated asymmetric thickening and mild enhancement along the right globe retinal surface, and a diffusely abnormal bone marrow signal, but no intracranial abnormalities.
Three days later, the vitreous biopsy results showed presence of rare atypical lymphocytes with blast cell morphology. The patient was started on intravenous cytarabine and etoposide, and intrathecal triple chemotherapy (methotrexate, cytarabine, hydrocortisone) for the CNS recurrence. The decision was made also to administer 24-Gy of radiation to the right orbit daily for 3 weeks to prepare for chimeric antigen receptor T-cell (CART) therapy.
On the fourth day of radiation therapy on March 8, 2019, the patient presented to ophthalmology clinic for ancillary testing and ophthalmic imaging. The patient's visual acuity was 20/125 (pinhole to 20/60) and 20/20 in OD and OS, respectively. On fundus photography the optic disc appeared hyperemic OD. There was an area of retinal whitening along the inferior arcade with extensive perivascular sheathing and sclerotic vessels in the peripheral retina of OD. Additionally, there were large areas of preretinal and retinal hemorrhage along the inferior periphery (Fig. 1A), which is an atypical feature of ARN, which usually does not exhibit very prominent retinal hemorrhage, if at all.3 Wide-angled fluorescein angiography showed optic disc hyperfluorescence in OD with extensive perivascular leakage in the temporal, inferior, and nasal periphery (Fig. 1C). Spectral domain optical coherence tomography (SD-OCT) showed atypical optic nerve infiltration and elevation of the optic nerve head along with the presence of retinal detachment in OD (Fig. 2). The ocular imaging did not demonstrate any abnormal findings in OS (Fig. 1B and D).
Fig. 1.
A) Ultra-widefield color fundus photograph of the right eye (March 8, 2019) shows optic disc hyperemia along with an area of retinal whitening along the inferior arcade, with extensive perivascular sheathing and sclerotic vessels in the peripheral retina. Additionally, large areas of preretinal and retinal hemorrhage can be seen along the inferior periphery. B) Ultra-widefield color fundus photograph of the left eye showing normal optic disc, macula, vessels, and periphery. C) Fluorescein angiography of the right eye demonstrates optic disc hyperfluorescence and extensive peripheral perivascular leakage. D) Fluorescein angiography of the left eye showing normal vascular blood flow. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
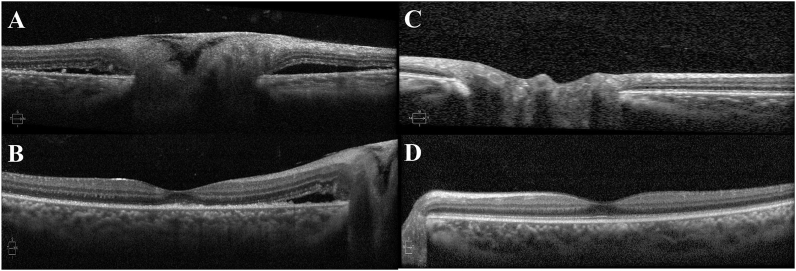
Fig. 2.
A and B) Spectral domain optical coherence tomography (SD-OCT) of the patient's right eye (March 8, 2019) shows presence of optic nerve infiltration and optic nerve edema with surrounding areas of serous retinal detachment. The foveal contour is preserved; however, there is a small area of disruption of the photoreceptor layer with retinal pigment epithelium thickening. C and D) SD-OCT of the left eye shows normal optic nerve and fovea.
Various laboratory tests were performed including complete blood counts, peripheral blood smears, and electrolytes, which showed normal electrolytes and WBC count but abnormal WBC morphology. Infectious disease workup was negative, including viral culture, PCR, and testing for herpes family antibodies (HSV-1 and 2/VZV/CMV), toxoplasma IgG/IgM antibodies, and syphilis antibody.
Given the absence of an infectious etiology and presence of blast cells in the vitreous cavity, B-cell ALL with CNS involvement was considered to be the etiology of his atypical retinal necrosis instead of infection.
At the conclusion of ocular radiation therapy on March 20, 2019, the patient followed up in ophthalmology clinic. His visual acuity improved to 20/60 (pinhole to 20/50) and 20/20 in OD and OS, respectively. Slit-lamp examination demonstrated punctate inner keratopathy OD and was unremarkable in OS. The perivascular sheathing and retinal whitening demonstrated stability on dilated ocular examination compared to the previous visit OD (Fig. 3A and B). There was improvement in optic disc hyperemia and preretinal and retinal hemorrhages along the inferior arcade. The examination findings in OS were unremarkable. Repeat B-scan exam showed resolution of the area of retinal detachment OD with increased thickness in the posterior pole and mild elevation of the optic nerve head that was improved compared to the initial visit in February 2019 (Fig. 4B). No chemotherapy was delivered intravitreally due to stabilization of his ocular condition. The patient was scheduled for future frequent follow up visits in uveitis and retina clinic.
Fig. 3.
Ultra-widefield color fundus photograph of the patient's right eye after radiation therapy on March 20, 2019 (B) showing mild improvement in the optic nerve hyperemia along with the preretinal and retinal hemorrhages in the inferior periphery compared to March 8, 2019 (A). The perivascular sheathing and retinal whitening noticed on March 8, 2019 (A) remained stable at the follow up visit on March 20, 2019 (B). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
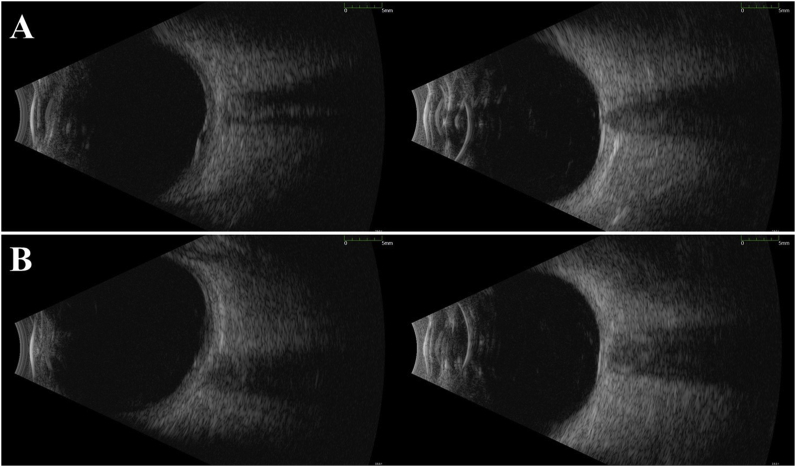
Fig. 4.
A) The B-scan performed at the time of patient's admission for the central nervous system relapse in February 2019 shows retinal thickening and optic nerve edema with a small area of retinal detachment surrounding the optic nerve head. B) The B-scan on the follow up visit after the radiation therapy in March 2019, shows resolution of the retinal detachment. It also demonstrated thickness in the posterior pole and mild elevation of the optic nerve head that has improved compared to the initial visit in February 2019 (A).
3. Discussion
Leukemias are malignant proliferative disorders that can present with ocular involvement. The incidence of ocular involvement in acute versus chronic leukemia cases seems to be dependent upon the prevalence of the disease in the geographic area.6,7 The most common ocular manifestations include hemorrhagic retinal lesions and Roth spots.4 The retinal involvement seen in leukemia cases may be secondary to direct invasion of leukemic cells, to systemic complications of leukemia such as thrombocytopenia, anemia, or because of the secondary infections.
The direct infiltration of leukemic cells may be evident by grayish white nodules surrounded by hemorrhage, perivascular sheathing, and rarely pale swelling of the optic nerve head,8 of which this patient had perivascular sheathing and extensive retinal and pre-retinal hemorrhages.
The retinal involvement in leukemia could also occur secondary to infectious cases because of immunosuppression associated with the underlying disease and/or the management associated with it. ARN, although commonly seen in immunocompetent individuals, can also be secondary to immunosuppression. However, ARN is an unusual presentation in cases of ALL and is also an uncommon finding in the pediatric population. Such nature makes the finding of ARN noteworthy in this patient, with atypical features raising additional concern. Furthermore, the absence of infectious markers in vitreous and blood tests as well as presence of blast cells in the vitreous cavity of our patient increases the likelihood of his ARN being related to his ALL relapse as opposed to another etiology. As his CSF was positive for WBCs with 98% lymphoblasts, the potential mechanism of his ARN is direct inoculation of the retinal tissue with neoplastic cells.
The most frequently affected extramedullary site of ALL relapse is the CNS, at up to 30–40% of cases.9 With the retina being an anatomic and developmental extension of the CNS, it is not uncommon for ocular findings to be present in neurological disorders such as multiple sclerosis and stroke. The index patient's three-month history of blurry vision prior to biopsy-proven relapse suggests early ocular involvement in the CNS relapse of his disease, and it is possible that earlier ophthalmologic examination could have revealed relapse and provided the opportunity for earlier intervention. Therefore, visual symptoms in a patient with history of ALL may be an indicator of relapse and should be investigated with a thorough ophthalmological examination.
4. Conclusion
While relapsed ALL does not commonly present with ocular symptoms, this case report highlights the potential for intraocular involvement to be an early presentation. This report emphasizes the importance of considering intraocular involvement and performing routine ophthalmological examination in patients otherwise in leukemic remission.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
IRB approval
Institutional review board approval is not required because this is a single case report and no intervention has been made for research.
Acknowledgments
No funding or grant support. The following authors have no financial disclosures: KLC, MH, DVD, QDN. All authors attest that they meet the current ICMJE criteria for Authorship. Acknowledgements: None.
References
- 1.Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics. CA Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman J.W., Thiele J., Arber D.A. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom R., Tripathy K. Acute retinal necrosis. In: Bergstrom R., Tripathy K., editors. StatPearls. StatPearls Publishing; Treasure Island (FL): 2019. [Internet] [Google Scholar]
- 4.Dhasmana R., Prakash A., Gupta N., Verma S.K. Ocular manifestations in leukemia and myeloproliferative disorders and their association with hematological parameters. Ann Afr Med. 2016;15(3):97–103. doi: 10.4103/1596-3519.188887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soman S., Kasturi N., Srinivasan R., Vinod K.V. Ocular manifestations in leukemias and their correlation with hematologic parameters at a tertiary care setting in south India. Ophthalmology Retina. 2018;2(1):17–23. doi: 10.1016/j.oret.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Eze B.I., Ibegbulam G.O., Ocheni S. Ophthalmic manisfestations of leukemia in a tertiary hospital population of adult Nigerian africans. Middle East Afr J Ophthalmol. 2010;17(4):325–329. doi: 10.4103/0974-9233.71599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy S.C., Jackson N., Menon B.S. Ocular involvement in leukemia—a study of 288 cases. Ophthalmologica. 2003;217(6):441–445. doi: 10.1159/000073077. [DOI] [PubMed] [Google Scholar]
- 8.Nagpal M.P., Mehrotra N.S., Mehta R.C., Shukla C.K. Leukemic optic nerve infiltration in a patient with acute lymphoblastic leukemia. Retin Cases Brief Rep. 2016;10(2):127–130. doi: 10.1097/ICB.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 9.Pui C.H., Howard S.C. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]