Abstract
Purpose
To describe retinal arterial occlusion and vasculitis following intravitreal brolucizumab administration in a patient with neovascular age-related macular degeneration (nAMD).
Observation
An 88-year-old Caucasian woman with neovascular age-related macular degeneration (nAMD) complained of painless loss of vision with light sensitivity in both eyes (OU) four weeks after bilateral intravitreal brolucizumab. Upon examination, her visual acuity decreased to 20/40 in the right eye (OD) and 20/50 in the left eye (OS). Examination revealed 0.5+ and 1+ anterior chamber cells in OD and OS, respectively. The patient was treated with 1% prednisolone acetate eyedrops in both eyes, and after several weeks, the anterior chamber cells resolved. However, the patient still reported a decline in visual acuity (VA). Fluorescein angiography (FA) revealed retinal arterial occlusion, vasculitis, and optic nerve inflammation in the left eye. Retinal intra-arterial grayish materials were also detected. Laboratory evaluations were performed for common infectious and inflammatory causes and were normal or negative. A delayed inflammatory reaction to brolucizumab was suspected as the cause of the ocular inflammation and retinal vasculitis. An intravitreal dexamethasone implant was inserted into the left eye to treat the inflammation. One week after the dexamethasone implant, VA improved to 20/40 in OU; FA showed improvement, but residual peri-vascular leakage remained.
Conclusion
Medication-associated uveitis is a rare adverse effect that can lead to vision loss. The index report illustrates a case of intraocular inflammation, retinal arterial vaso-occlusion and vasculitis associated with intravitreal brolucizumab. The delay in developing uveitis suggests that the inflammation is due to a delayed hypersensitivity reaction which can occur several days or weeks after administration of the inciting agent. Recently, several cases of uveitis and vasculitis associated with brolucizumab have been presented and those cases have similar features compared to the index case (1). Therapy with steroids (either intraocular or systemic), after infectious etiologies have been excluded, may be beneficial in halting inflammation and preventing further vision loss.
Keywords: Occlusive vasculitis, Retinal vasculitis, Intraocular inflammation, Brolucizumab, Age-related macular degeneration
1. Introduction
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy is the gold standard for the treatment of various retinal vascular diseases including neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), proliferative diabetic retinopathy (PDR) and retinal vein occlusion (RVO). Brolucizumab is the latest FDA-approved anti-VEGF agent that was shown to be non-inferior to aflibercept in visual acuity outcomes in the pivotal HAWK and HARRIER clinical trials.2 In the phase 3 clinical trials of brolucizumab for nAMD, ocular inflammation was reported to be higher in the brolucizumab group compared to aflibercept (1.4–2.2% vs 0–0.3%, respectively), but no cases of retinal vasculitis were reported.1 More recently, there have been reports of inflammation, vasculitis, and arterial occlusions associated with brolucizumab in post-marketing surveillance.1,3
We herein report a case of a patient with neovascular AMD who developed ocular inflammation and occlusive retinal vasculitis in both eyes (OU) one month after intravitreal brolucizumab administration.
2. Case report
An 88-year-old Caucasian woman with active neovascular AMD complained of painless decreased vision and light sensitivity in OU, four weeks after receiving her first intravitreal brolucizumab therapy (6 mg/0.05 ml) in OU. Her medical history was significant for Type 2 diabetes without retinopathy, nephropathy, or neuropathy. She had no medical history of hyperlipidemia or myocardial infarction. Surgical history was significant only for pacemaker placement and cataract extraction with posterior chamber intraocular lenses in OU. Current medications include atenolol, levothyroxine and warfarin. Family history and detailed review of systems were noncontributory. Prior to receiving brolucizumab, the patient had received bevacizumab in right eye (OD) in 2014 and was switched to ranibizumab in 2016. The left eye (OS) progressed to nAMD in 2017 and also received ranibizumab. In total, 26 intravitreal injections of ranibizumab were given in OD and 21 intravitreal injections of ranibizumab in OS. The decision to switch to brolucizumab was made given the persistent subretinal fluid, particularly in OD.
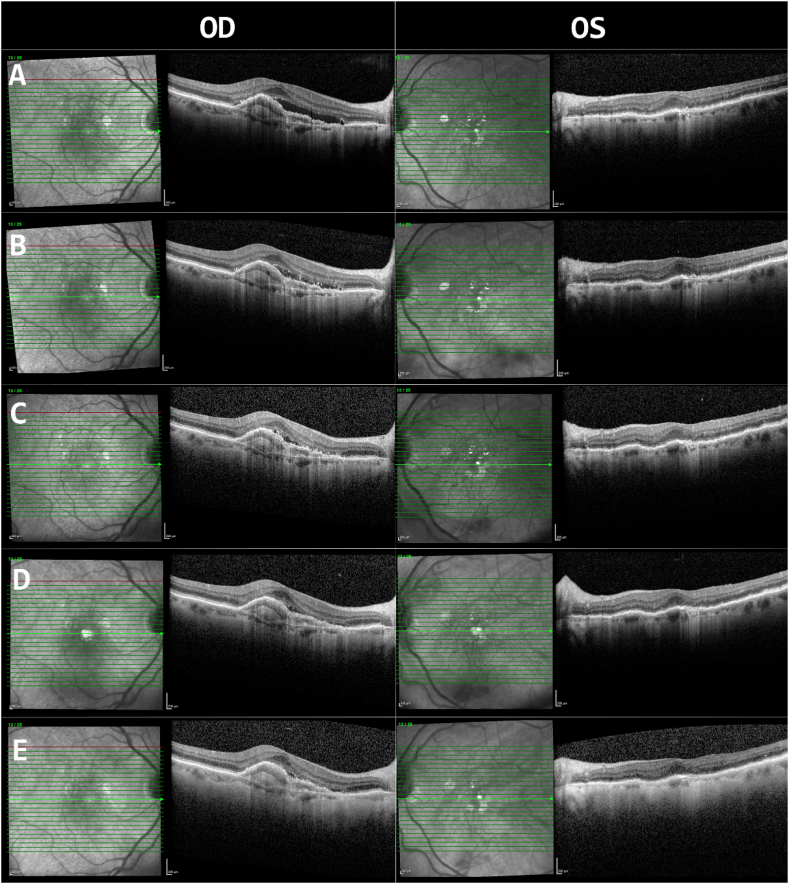
On examination four weeks after brolucizumab injection, visual acuity (VA) had decreased from 20/30 to 20/40 in OD and from 20/25 to 20/50 in OS. Intraocular pressure (IOP) was within normal limits in OU. Anterior chamber evaluation revealed 0.5+ and 1+ cells in OD and OS, respectively. Posterior examination revealed drusen and retina pigment epithelial (RPE) changes in OU, with patchy atrophy in OD but no evidence of posterior inflammation or vitritis. Comparing OCT images from before brolucizumab injection to images at current presentation, there was a reduction in subretinal fluid, OD > OS (Fig. 1A and B). The patient was started on prednisolone acetate 1% drops four times daily for the anterior inflammation in OU. Over the next two weekly follow-up visits, the anterior chamber cells resolved and the subretinal fluid continued to improve in OD (Fig. 1C). However, the patient reported persistent decline in VA and started to experience flashing lights in OS. Examination revealed supratemporal retinal vessel sheathing in OD (Fig. 2A) and temporal retinal vessel sheathing and superior optic nerve edema in OS (Fig. 3A); there were discrete intra-arterial grayish materials in OS. Fluorescein angiography was performed and revealed arteriovenous occlusion and vasculitis with vascular and superior optic nerve leakage in OS (Fig. 3C). A delayed inflammatory reaction to brolucizumab was suspected. An extensive work up including complete blood counts, C-reactive protein, erythrocyte sedimentation rate, c-ANCA, p-ANCA, angiotensin converting enzyme, lysozyme, Quantiferon Gold, syphilis screen, herpes simplex and varicella zoster IgG and IgM were conducted and all were negative or within normal limits. Hemoglobin A1C was 6.0%. Chest X-ray and urinalysis were also within normal limits. Intravitreal dexamethasone implant was inserted in OS to decrease the intraocular inflammation. One week after dexamethasone implant placement, VA improved to 20/40 in OU and repeated FA showed improved but persistent peri-vascular and optic disc leakage (Fig. 3F). At two weeks, repeated FA showed resolved peri-vascular and optic disc leakage; however, the temporal retinal artery occlusion remained (Fig. 3H).
Fig. 1.
Optical coherence tomography (Spectralis; Heidelberg Engineering, Heidelberg, Germany) of the right (OD) and left (OS) eyes. Before intravitreal brolucizumab injection in both eyes (row A), after 4 weeks (row B), after 6 weeks (row C), after 8 weeks and before intravitreal dexamethasone implant in OS (row D), two weeks post-intravitreal dexamethasone implant (row E).
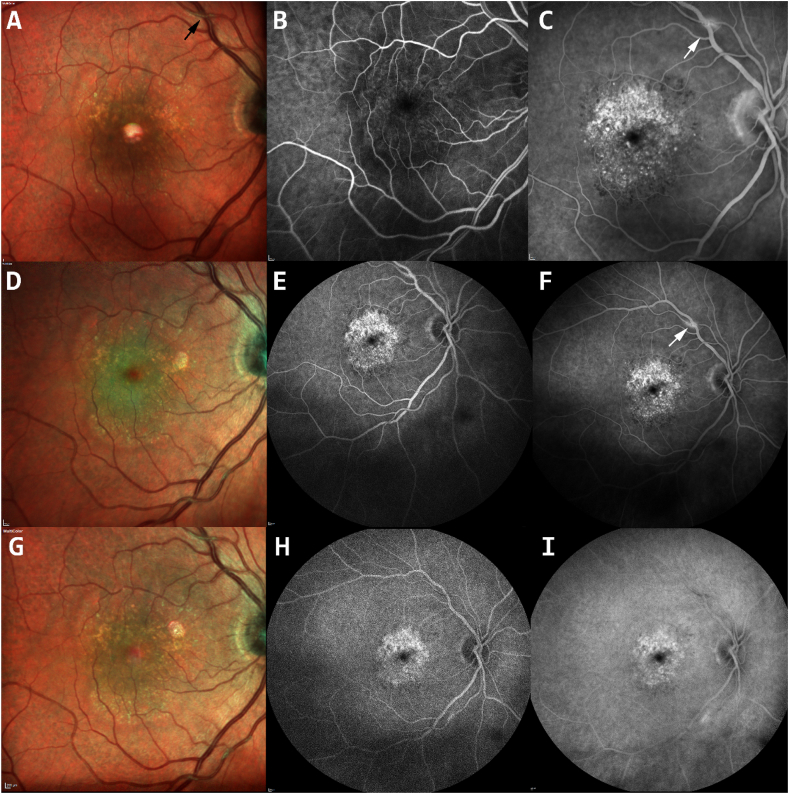
Fig. 2.
Fundus photos and fluorescein angiography of the right eye (RE) 8 weeks after intravitreal brolucizumab (top row), one weeks later (middle row) and two weeks later (bottom row) showing area of pigmentary changes (A and C), supratemporal retinal vessel sheathing (black arrow-A). Staining of a segment of the superior arcade retinal arterial (white arrow) is noted in the late phase, (C and F) which resolved spontaneously after 2 weeks (I).
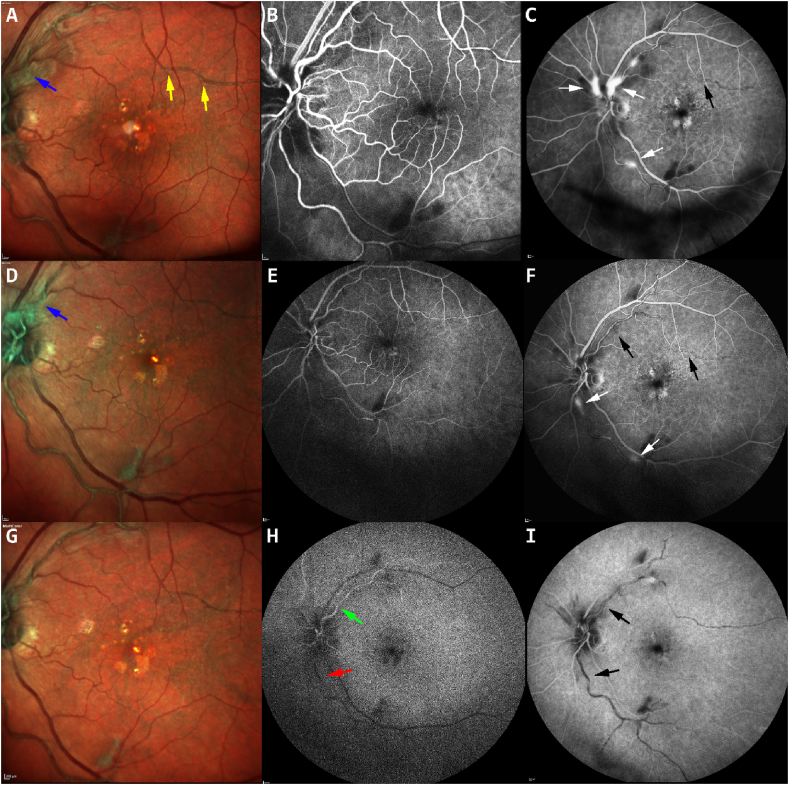
Fig. 3.
Fundus photos and fluorescein angiography of the left eye 8 weeks after intravitreal brolucizumab and before intravitreal dexamethasone implant (top row), showing discrete intraarterial grayish materials (gold arrows-A), retinal vessel sheathing and superior optic nerve edema (blue arrow-A) and vasculitis with vascular and superior optic nerve leakage (white arrow-C). One week after dexamethasone implant (middle row), sheathing of temporal retinal arcade and superior optic nerve edema (blue arrow-D) were still present. Much improved but persistent peri-vascular and optic disc leakage, and temporal retinal vaso-obliteration in the late phase (white arrow-F) with visible intraarterial grayish materials (black arrow-F). At two weeks (bottom row) the inferior temporal retinal artery showed delayed filling (red arrow-H) compared to the superior temporal retinal artery (green arrow-H) and intraarterial blockages in late phase (black arrow-I). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
3.1. Generating a diagnosis
We have presented a case of an 88-year-old female with nAMD who developed bilateral intraocular inflammation after intravitreal brolucizumab. Initially, both eyes presented with mild anterior uveitis without posterior involvement. Subsequently, within two weeks, the left eye displayed posterior involvement with retinal intra-arterial deposition of grayish materials and vasculitis which progressed to vascular occlusion. The differential diagnosis for occlusive retinal vasculitis includes systemic vasculitis and connective tissue diseases such as Adamantiades-Behçet's disease, multiple sclerosis, granulomatosis with polyangiitis, and systemic lupus erythematosus, among others. There are also common infectious etiologies which can lead to occlusive vasculitis including tuberculosis, herpes, and syphilis.
In this index case, there were neither retinochoroidal lesions, systemic manifestation, nor abnormalities in blood evaluation to suggest a systemic or infectious etiology. The excellent response to steroidal implant further excluded infectious causes. Another possibility is Eales disease which is a bilateral idiopathic obliterative vasculopathy that usually involves the peripheral retina of young male adults. Middle-age female patients diagnosed with unilateral Eales disease have been reported to have occlusive disease4; however, in our patient there was no evidence of retinal vein involvement and peripheral neovascularization. Given the timely manner of the occurrence and exclusion of other possibilities, this index patient was diagnosed with possible delayed or type IV hypersensitivity to brolucizumab. The patient had received multiple intravitreal ranibizumab treatments previously in both eyes without any sequelae.
3.2. Delayed-type hypersensitivity
Hypersensitivity reactions after anti-VEGF therapy have been reported, mostly type I hypersensitivity reactions due to substances used during intravitreal injection5,6 and type IV or delayed-type hypersensitivity reactions to the anti-VEGF agent itself.7,8 It is important to recognize type IV reaction as it usually happens later than other types of reactions and can become more severe during re-challenge.
In the CATT trial, the percentage of ocular adverse events including uveitis, scleritis, and anterior chamber inflammation were 0.3%–0.7% in ranibizumab and bevacizumab groups, respectively.9 In the HAWK and HARRIER studies, uveitis was noted in 5 (1.4%), 8 (2.2%) and 1 (0.3%) cases in brolucizumab 3 mg, brolucizumab 6 mg and aflibercept 2 mg group, respectively.2 In addition to commonly seen adverse events (AEs), the frequency of intraocular inflammation noted was relatively high in those clinical trials compared to what has been reported for ranibizumab and aflibercept.2,9,10 As described in the studies, intraocular inflammation included anterior chamber flare, anterior chamber inflammation, iritis, iridocyclitis, vitreous haze, vitritis, and choroiditis.2,10, 11, 12 Most of these intraocular inflammations were categorized as mild to moderate and were treated with a course of topical corticosteroid/anti-infective agents.2 Based on the reports of phase 2 and 3 clinical trials, brolucizumab was well tolerated and the safety profile of ocular and non-ocular AEs were comparable to ranibizumab and aflibercept(2, 10–12). To date, the most frequently reported ocular AEs related to brolucizumab treatment are conjunctival hemorrhage, vitreous floaters, reduced visual acuity and eye pain.2,10,11
3.3. Why does ocular inflammation happen with intravitreal brolucizumab?
The present case is the first published report of arteriovenous occlusion with vasculitis, possible type IV hypersensitivity reaction, after intravitreal brolucizumab for the treatment of nAMD. Brolucizumab is a humanized, single-chain variable fragment that is no longer dependent on a heavy molecular support structure but still retains full binding capacity to its target. These molecules are composed of the monoclonal antibody's variable light and heavy chain domains tethered by a flexible linker, resulting in a small protein fragment of ~26 kDa,11,13,14 which is the smallest of the anti-VEGF antibodies evaluated in humans. The brolucizumab molecule is substantially smaller than aflibercept and ranibizumab, which have molecular masses of 97–115 kDa and 48 kDa, respectively. Such a size difference gives brolucizumab theoretically better target-tissue penetration and therefore higher concentration which allows up to 6 mg of brolucizumab in a single 50-μL intravitreal injection, which is considered 11 to 22 times greater than what can be clinically administered for aflibercept and ranibizumab, respectively.11
To protect visual function, the eye has special mechanisms to prevent invasion of infectious agents and inflammation through anatomical mechanisms and cytokines responses.15 Anatomical mechanisms, such as lack of efferent lymphatics and the presence of the blood–retinal barrier, protect the eye from toxic substances. In addition, oral and intravenous drugs achieve therapeutic levels in intraocular tissues16 whereas mechanisms involving cytokines, such as upregulation of tumor growth factor-beta (TGF-β) and alpha-neuropeptide [alpha-melanocyte stimulating hormone (α-MSH)], prevent inflammation by various mechanisms. It suppresses IFN-γ production,17 activates regulatory T cells and suppresses delayed-type hypersensitivity-mediating T cells.18 Furthermore, it suppresses the innate immunity and the interface between innate and adaptive immunity19 and also promotes immune privilege in the posterior segment.20 The ocular immune privilege has been known for more than 100 years and was first noted by Van Dooremal. In 1873, he observed the growth of tumor cells implanted into the anterior chamber of rabbit's eyes. However, when such tumor cells were placed elsewhere in a rabbit's body, they showed significantly less or even no signs of growth. The finding suggested a different immune response in the eye compared to other regions of the body.21 Seventy five years later, ocular immune privilege was first defined experimentally by Medawar and his colleagues by placing skin and/or other types of grafts in the anterior chamber of the eye and the brain22,23. They defined immune privilege as a tissue site that supports prolong survival of histo-incompatible tissue grafts. Subsequently, this phenomenon was termed Anterior Chamber–Associated Immune Deviation (ACAID). High doses of intravitreal foreign protein may not be a concern if these defense mechanisms are intact. The index patient had diabetes (concurrent with AMD) which is one of known causes of inner blood–retinal barrier breakdown and increase in inflammatory cytokines.24 It can be hypothesized that the combination of intravitreal high concentration of protein with diseases resulting in an increase of inflammatory markers such as diabetes, in particular, and even in systemic vasculitic diseases, connective tissue diseases, or any preceding intraocular inflammation may outweigh the protective mechanisms, which ultimately leads to an inflammatory response inside the vessels (such as accumulation of immune complexes) along the vessel wall where the plasma is in contact with the foreign protein. In addition, pericytes and endothelial cells are likely the earliest cells to die in diabetic retinopathy with vascular occlusion.25 Therefore, vasculitis in such condition may lead to severe vascular occlusion.
On February 23, 2020, the American Society of Retina Specialists (ASRS) provided a statement to its members on reported cases of ocular inflammation since the FDA approval of brolucizumab on October 7, 2019. Up to that date, the ASRS has received reports of inflammation following brolucizumab administration. In addition to cases of mild-moderate intraocular inflammation, these reports have included 14 cases of vasculitis, of which 11 were designated as occlusive retinal vasculitis by the reporting providers. The ASRS indicated that “some cases of occlusive vasculitis may initially be subtle or present in a delayed fashion.” Novartis, the manufacturer of brolucizumab, is compiling reported cases of inflammation post brolucizumab administration in the real world and is providing safety updates on www.brolucizumab.info.
3.4. Treatment
It is important to detect early type IV hypersensitivity reactions. They are distinguished from other hypersensitivity reactions by the lag time from exposure to the antigen until the response is evident (1–3 days) or in some cases it can be up to 6 weeks before symptoms and clinical findings present.7 In this case, visual disturbances and anterior reactions were noted and persisted four weeks after injections in OU. Vaso-occlusion and vasculitis OS mainly involved retinal arteries and foci of vasculitis detected on FA after eight weeks. Given the potential for severe vision loss in the left eye, immediate treatment while waiting for evaluation was warranted. Local steroid seemed appropriate in this situation. There are several options including periocular and intravitreal corticosteroids with triamcinolone acetate, corticosteroid implants with or dexamethasone or fluocinolone acetonide. In cases of bilateral involvements or persistence after dexamethasone intravitreal implants, systemic steroid (if diabetic control is not a concern) may be administered and pars plana vitrectomy may be considered to reduce the anti-VEGF load and increase clearance.
However, there remains a clinically important question: if the patient has recurrent choroidal neovascularization (CNV) activity, should the patient be treated again with brolucizumab or with another anti-VEFG or PDT? Currently, Novartis recommends withholding additional brolucizumab if intraocular inflammation has occurred after its use. If CNV activity has responded to therapy with another anti-VEGF agent without any observed intraocular inflammation, then one can consider resuming that agent. In addition, the patient can elect to undergo formal allergy evaluation with intradermal or other testing.
4. Conclusion
In conclusion, medication-associated uveitis is a rare adverse effect of drug administration that can typically induce mild to severe intraocular inflammation that can lead to different severity of visual loss. Being vigilant to the potential ocular inflammation that can occur with anti-VEGF therapy, evaluating patients urgently if they develop new symptoms post injection, early evaluation of other underlying causes of inflammation, immediate cessation of the offending agent, and prompt employment of topical, local and/or systemic corticosteroids to control the inflammation may lead to stabilization and prevention of further visual loss.
Patient consent
Verbal and written consents have been obtained from the patient.
Funding
Research to Prevent Blindness Departmental Challenge Award and National Eye Institute of the National Institutes of Health P30 Grant (EY026877) have been awarded to the Byers Eye Institute at Stanford University.
Authorship
All authors attest that they met the current ICMJE criteria.
Declaration of competing of interest
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Dugel P.U. Presented at the 2020 Meeting of the Macula Society, March 19 to 22, San Diego, California. 2017. Expanded week 96 safety outcomes: analysis of pooled data from HAWK & HARRIER studies. [Google Scholar]
- 2.Dugel P.U., Koh A., Ogura Y. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen Q.D., Das A., Do D.V. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration [published online ahead of print, 2020 Jan 17] Ophthalmology. 2020 doi: 10.1016/j.ophtha.2019.12.031. S0161-6420(20)30041-5. [DOI] [PubMed] [Google Scholar]
- 4.Nicolcescu A., Mocanu C., Dinu L., Olaru A., Ionete M., Stefanescu D.A. Unilateral Eales' disease a case report. Rom J Ophthalmol. 2017;61(2):144–149. doi: 10.22336/rjo.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veramme J., de Zaeytijd J., Lambert J., Lapeere H. Contact dermatitis in patients undergoing serial intravitreal injections. Contact Dermatitis. 2016;74(1):18–21. doi: 10.1111/cod.12478. [DOI] [PubMed] [Google Scholar]
- 6.Kleris R.S., Keswani A., Lugar P. The eyes have it: eyelid swelling and rash in a 79-year-old woman with macular degeneration. Allergy & rhinology (Providence, RI) 2018;9 doi: 10.1177/2152656718763385. 2152656718763385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai N., Ibuki M., Shinoda H., Kameyama K., Tsubota K., Ozawa Y. Maculopapular rash after intravitreal injection of an antivascular endothelial growth factor, aflibercept, for treating age-related macular degeneration: a case report. Medicine (Baltim) 2017;96(21) doi: 10.1097/MD.0000000000006965. e6965-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meredith G.G., Schkade P.A., Joondeph B.C. Allergic reaction UPON intravitreal administration OF anti-vascular endothelial growth factor Agents. Retin Cases Brief Rep. 2019;13(3):287–289. doi: 10.1097/ICB.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 9.Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dugel P.U., Jaffe G.J., Sallstig P. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124(9):1296–1304. doi: 10.1016/j.ophtha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 11.Holz F.G., Dugel P.U., Weissgerber G. Single-chain antibody fragment vegf inhibitor RTH258 for neovascular age-related macular degeneration: a randomized controlled study. Ophthalmology. 2016;123(5):1080–1089. doi: 10.1016/j.ophtha.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Semeraro F., Morescalchi F., Duse S., Parmeggiani F., Gambicorti E., Costagliola C. Aflibercept in wet AMD: specific role and optimal use. Drug Des Dev Ther. 2013;7:711–722. doi: 10.2147/DDDT.S40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auf der Maur A., Escher D., Barberis A. Antigen-independent selection of stable intracellular single-chain antibodies. FEBS Lett. 2001;508(3):407–412. doi: 10.1016/s0014-5793(01)03101-5. [DOI] [PubMed] [Google Scholar]
- 14.Thiel M.A., Coster D.J., Standfield S.D. Penetration of engineered antibody fragments into the eye. Clin Exp Immunol. 2002;128(1):67–74. doi: 10.1046/j.1365-2249.2002.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Andrade F.A.F.S., Benchimol E.I., Fiorot S.H., Provenzano J., Martins V.J., Levy R.A. The autoimmune diseases of the eyes. Autoimmun Rev. 2016;15:258–271. doi: 10.1016/j.autrev.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Smith R.S., Rudt L.A. Ocular vascular and epithelial barriers to microperoxidase. Invest Ophthalmol Vis Sci. 1975;14(7):556–560. [PubMed] [Google Scholar]
- 17.Taylor A.W., Streilein J.W., Cousins S.W. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. 1994;1(3):188–194. doi: 10.1159/000097167. [DOI] [PubMed] [Google Scholar]
- 18.Taylor A.W. Modulation of regulatory T cell immunity by the neuropeptide alpha-melanocyte stimulating hormone. Cell Mol Biol. 2003;49(2):143–149. [PubMed] [Google Scholar]
- 19.Nishida T., Miyata S., Itoh Y. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int Immunopharm. 2004;4(8):1059–1066. doi: 10.1016/j.intimp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Taylor A.W. Alpha-melanocyte stimulating hormone (alpha-MSH) is a post-caspase suppressor of apoptosis in RAW 264.7 macrophages. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0074488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dooremall J.C. Die entwicklung der in fremden grund versetzten lebenden gewebe. Albrecht Von Graefes Arch Ophthalmol. 1873;19:358–373. [Google Scholar]
- 22.Medawar P.B. A second study of the behaviour and fate of skin homografts in rabbits: a Report to the War Wounds Committee of the Medical Research Council. J Anat. 1945;79(Pt 4):157–1576.4. [PubMed] [Google Scholar]
- 23.Medawar P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon J.-W., Jee D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PloS One. 2018;13(9) doi: 10.1371/journal.pone.0203408. e0203408-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin E.S., Sorenson C.M., Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res. 2014;9(3):362–373. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]