Abstract
Glutaraldehyde (Glut) is an extensively used sterilant and fixative for the crosslinking of natural soft tissue biomaterials like bovine pericardium (BP) to provide stability and is required for its application in vivo. There is plenty of debate around the reaction mechanism of Glut with natural biomaterials. Differential scanning calorimetry (DSC) is a commonly used technique that is typically used to measure the thermal profile of polymers. However, a variation known as quasi-isothermal modulated differential scanning calorimetry (QiMDSC) has been utilised for the analysis of polymorphic transformations in both the pharmaceutical and food industries. This communication will address QiMDSC as a method for analysing soft tissue biomaterials and their crosslinking mechanisms and how it can be applied to other biomaterial applications.
Keywords: Glutaraldehyde, QiMDSC, Bovine pericardium
Highlights
-
•
Calorimetry method used to assess crosslinking reaction of biomaterial.
-
•
Bovine pericardium crosslinked with glutaraldehyde displayed a continuous reaction.
-
•
Bovine pericardium reaction with Glut was completed after 10 min.
1. Introduction
Soft tissue biomaterials like bovine pericardium (BP) are extensively used biomaterials with an array of applications [[1], [2], [3]]. Treatment of tissue is required for it to be used in vivo. These treatments involve the fixation of the tissue using a popular crosslinker and sterilant, glutaraldehyde (Glut) [4,5]. Glutaraldehyde is a five-carbon dialdehyde, with multiple functionalities of sterilant, crosslinker and fixative [6] that make it the “gold standard” [7] over other alternative crosslinkers such as carbodiimides [5], Genipin [8,9] and epoxies [10]. The process for crosslinking involves the immersion of the tissue into a Glut solution of a known concentration, typically 0.6% in phosphate buffer solution (PBS).
The stability of Glut is well documented with its ability to polymerise freely in an aqueous solution [11,12]. The variation of Glut in solution leads to debate over its possible crosslinking mechanisms [13]. Reaction with the primary amine groups in lysyl residues of proteins leads to inter- and intra-molecular crosslinking via covalent bonding, stabilising the tissue against enzymatic degradation [4,14,15]. Numerous crosslinking mechanisms have been reported between Glut and collagen-based materials, with temperature and pH effects of critical importance [11,[16], [17], [18]]. The formation of Schiff bases is the initial mechanism occurring between the ε-amino group of a lysine or hydroxylysine [17,19] present in solution available for reaction with lysine residues [11]. Mechanism of crosslinking post this Schiff base formation is contentious, with the stability of the base key to the debate. Stable Schiff bases from Glut polymers through aldol condensation, to intermediate Schiff bases forming additional crosslinks [5,20] have been reported. The unstable nature under acidic conditions of Schiff bases is known with suggestions that they undergo further reactions during the crosslinking process, Okuda et al. [21] suggested that the Schiff base is the central intermediate where reactions occur before a crosslink is formed. This was also proven by Daminik et al. [5] by monitoring the primary amine groups present with respect to the duration of crosslinking. It was found that only after the hydrolysable Schiff bases were stable that crosslinking of the collagen could occur. The overall effect of the crosslinking is to provide a structure that is biocompatible, non-thrombogenic, prevent against structural deterioration while also maintain the mechanical properties similar to that of a native aortic valve and its hemodynamic properties.
The sterilant properties of Glut for medical devices can be the justified reasoning for the longer time periods and variation in this fixation process [4]. However, for general investigative research purposes, where sterilisation is not of a key priority, there is still a wide ranging of times used for the crosslinking of BP using Glut with some fixing between 5 min and 3 h [5,22,23], 24 h [24]to 14 d [25]. A typical method to confirm that the tissue is fully crosslinked is the measurement of its shrinkage temperature using a water or saline bath of increasing temperature of approximately 1 °C, with the sample held in a load cell of a tensometer [26,27]. Another method of assessing the crosslinking efficacy, or denaturation temperature (Td) introduced is that of Differential Scanning Calorimetry (DSC) [28]. This is a common technique that allows the quick assessment of the degree of crosslinking based on an increase of the endothermic peak by approximately 20 °C from the untreated state to the fixed state [5]. Denaturation of tropocollagen occurs as the tissue is heated beyond 60 °C and the helical structure unfolds to produce random chains of gelatin [29,30]. The DSC technique is typically used for the analysis of a polymers thermal profile, providing information on first and second order transitions of polymeric chains like glass transitions (Tg), melting temperatures (Tm) and crystallisation. Further development of the technique in 1993 by Gill et al. established a variation on DSC with the introduction of modulated DSC (mDSC) [31]. The development allowed mDSC to superimpose a sinusoidal modulation over the DSC's standard linear rate [32]. The equation that describes the components of both DSC and mDSC is shown in Eq. (1)
| (1) |
The total heat flow dh/dt as measured by conventional DSC, is equal to the heat capacity Cp (J/C) and its heating rate dT/dt (C/min) and the kinetic component of the heat flow that is a function of T, temperature and t, time.
The overlay of the modulating temperature and the linear rate lets the total heat flow signal be deconvoluted into its reversing element of the specific heat from the Cp and heating rate and its non-reversing element due to the kinetic component of temperature and time [33].
| (2) |
This method produces an increase in both resolution and sensitivity, through the separation of overlapping signals from the baseline [34]. For example, hard to detect Tg, in polymers can be separated to clearly identify other features such as enthalpic recovery beside the Tg that would typically give the inaccurate impression of a melting peak.
The mDSC model adds the additional parameters of the amplitude of the temperature modulation A(T), angular frequency ω and phase shift θ, to those of temperature T, time t, initial temperature To and underlying heating rate β, to produce Eq. (3).
| (3) |
Further evolution of mDSC has seen the development of quasi-isothermal modulated temperature differential scanning calorimetry (QiMDSC). Like mDSC a modulated temperature profile is applied but across a constant underlying temperature or by collecting a series of quasi-isothermal data points by increasing the underlying temperature incrementally [32]. This has found applications in pharmaceutical industry for the analysis of polymorphic transformations through the changes in heat capacity (Cp) [35,36]. The removal of a heating rate creates an increase in the sensitivity to the reverse Cp (Rev Cp) and the removal of melting effects due to the large number of modulations [37].
The measurement of heat capacity in situ is of great benefit in the analysis of the molecular mobility within the crosslinking or curing reactions of a sample [38]. An example of this would be the curing of an epoxy, as it cures the heat capacity will decrease because the crosslinking of molecules reduces their mobility [39]. Also in the crosslinking reaction between biological tissues like BP and Glut there is an increase in stiffness and strength of the tissue due to the bonding between the amino acids.
The goal of this paper is to introduce the QiMDSC technique for the analysis of the crosslinking reaction between a biological biomaterial, BP and Glut. The focus will be on the reaction over time at an isothermal temperature, with the degree of crosslinking measured using conventional DSC. The proof of concept will employ a similar model of crosslinking using an epoxy reacting with a hardener.
2. Materials and methods
2.1. Materials
Bovine pericardium (BP) tissue of an age less than 24 months was acquired from a proprietary vendor defatted and stored in EDTA/PBS solution at 2–8 °C. Samples were washed prior to use twice with saline. Samples of BP were cut using a 3 mm diameter biopsy punch and consumed in the protocols detailed in the methods. A fixation solution of 0.6% GLUT prepared from an electron microscopy grade solution of 25% GLUT (Merck, Darmstadt, Germany) in 0.1 M phosphate buffered solution (PBS) (Sigma Aldrich) was used.
A Loctite M − 31Cl epoxy (Henkel Technologies) consisted of a Part A and Part B components of epoxy and hardener respectively. The ratio of the mix is 2:1 epoxy to hardener. The mix of each sample was tested at different temperatures for 12 h period. Curing was determined when a drop, in heat capacity created a flat baseline. Curing was confirmed using conventional DSC at 10 °C/min from 30 °C to 100 °C of the sample post QiMDSC method with a Tg of 70 °C indicative of a fully cured epoxy.
2.2. Methods
2.2.1. QiMDSC
The 3 mm diameter BP sections were patted dry, and followed one of two sample preparation methods for QiMDSC, Fig. 1. Method 1 applied a 10 μl volume of 0.6% Glut to the 3 mm BP sample in an aluminium pan and was then hermetically sealed. Method 2 involved immersing the 3 mm BP sample in 10 μl of 0.6% Glut for 30 s. Both timelines for Method 1 and 2 are displayed in Fig. 1. The sample was patted dry and hermetically sealed in an aluminium pan. All QiMDSC and conventional DSC testing were performed using a DSC, (Model Q2000, TA Instruments, Delaware, US). Both method 1 and 2 followed the same protocol of equilibrating the sample at 25 °C and holding isothermally for a selection of isothermal period's from 10 min, 120 min, 240 min, 360 min and 720 min. The epoxy resin samples were also tested over a range of isothermal temperatures consisting of 25 °C, 50 °C, 100 °C and 200 °C for a period of 720 min. All samples were run under a modulation of ± 1 °C every 100 s. The thermograms of the heat capacity (mJ/°C) as a function of temperature were used to measure the total change in heat capacity after 30 min to the end of the time periods.
Fig. 1.
Sample preparation flow for QiMDSC method 1 (A) and method 2 (B) of BP and 0.6% Glut analysis.
2.2.2. Conventional DSC
Directly after each QiMDSC test the samples were tested using a conventional DSC to determine the denaturation temperature (Td). This provides a gauge to the degree of crosslinking within a fixed tissue. The test method followed a heating rate of 5 °C/min as suggested by Loke et al. [28] in a temperature range of 30 °C–100 °C. An empty aluminium pan was used as the reference. Heating was carried out in a nitrogen atmosphere of 50 ml/min. The Td can be calculated from the extrapolated onset or the peak temperature of the endothermic phenomena, here the peak temperature was used to determine the Td [28,40,41]. Calibration of the instrument was carried out using an Indium standard (Melting point, Tm 156.6 °C, Enthalpy 28.74 J/g).
3. Results and discussion
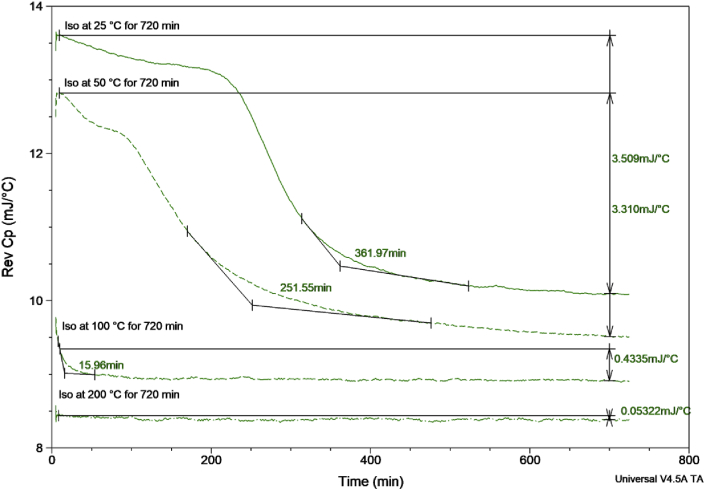
Demonstration of the QiMDSC technique and the proof of concept for its application to the analysis of a crosslinking reaction is illustrated by the thermogram of the epoxy across various isothermal curing temperatures, Fig. 2. The extrapolated onset of the Rev Cp curve was used as an indicator for the start of the epoxy hardener crosslinking reaction. The epoxy samples held isothermally at 25 °C and 50 °C displayed a drop in the Rev Cp after 361.97 min and 251.55 min respectively. While the higher temperature curve of 100 °C produced a drop at 15.96 min. The drop could not be calculated at 200 °C as the baseline appeared flat almost immediately. The total drop in the Rev Cp baseline was also measured between 10 min and 720 min. There was a difference evident across all the cure rates with both the 25 °C and 50 °C cured samples displaying a similar drop of 3.310 and 3.509 mJ/°C respectively. However, these were considerably greater than the values measured at the higher isothermal temperatures with 0.4335 and 0.05322 mJ/°C measured for 100 °C and 200 °C respectively. This would indicate that at the lower curing temperatures there was a greater degree of molecular mobility during curing, resulting in a longer curing time.
Fig. 2.
Comparison of curing behaviour at different isothermal temperatures for an epoxy adhesive. The Rev Cp was taken as the drop in the baseline from 10 min to 720 min. Also extrapolated onset was used to determine the drop in the baseline as an indicator of crosslinking reaction occurring between epoxy and hardener.
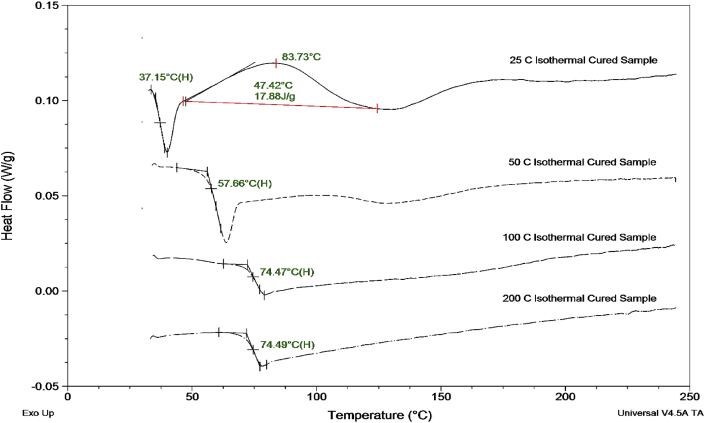
The measurement of the Tg of each epoxy post curing are shown in Fig. 3. The lowest curing temperature of 25 °C displayed a Tg value of 37.15 °C, while also displaying an exothermic peak at 83.73 °C suggesting some unreacted components present within the epoxy. The epoxy cured at 50 °C showed an increase in the Tg to 57.66 °C. However, this temperature is significantly lower than those for the higher cure temperatures of 100 °C and 200 °C with both displaying temperatures above 74 °C. Both these temperatures are above the reported value of the typical physical properties of the cured epoxy with a Tg of 70 °C.
Fig. 3.
Measurement of Tg for each epoxy sample after their respective isothermal curing temperature.
The curves in Fig. 4, display the BP and 0.6% Glut reaction of respective samples after 10 min, 360 min and 720 min following method 1. A continuous drop in the curves and Rev Cp is evident after 360 min and is also present with a sample after 720 min. Applying the same principle, from the epoxy model, this continuous drop in the curve indicates that the reaction is an ongoing process.
Fig. 4.
QiMDSC thermograms after 10, 360 and 720 min for BP and 0.6% Glut following method 1.
However, on testing the samples once the QiMDSC method is complete using conventional DSC and analysing the Td, it is evident that all samples are fully crosslinked even after 10 min, Fig. 5.
Fig. 5.
Measurement of Td post QiMDSC method that suggests BP and Glut reaction produced a fully crosslinked material, even after 10 min.
This suggests that while the Glut reaction with the amide bonds is swift and occurs before 10 min, there is still a continuing reaction taking place. This quick reaction was also reported by Damink et al. who reported that free amine groups in collagen of dermal sheep react quickly with Glut to form Schiff bases [5]. When looking at the control samples of Glut only, the continuous drop in the baseline is present Fig. 6.
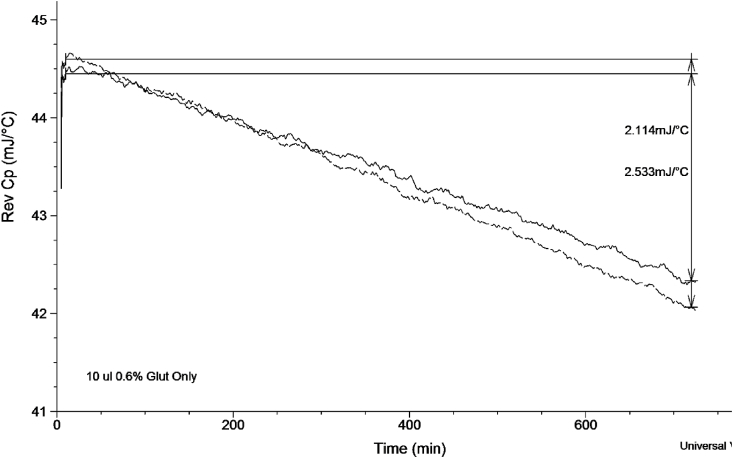
Fig. 6.
Overlay of two runs of 10 μl 0.6% Glut only using QiMDSC for 720 min. Both samples display good reproducibility with similar Rev Cp decreasing value of over 2 mJ/°C.
This suggests that the Glut polymerisation is a continuous process over time. When the BP was dipped into 0.6% Glut the similar drop in the Rev Cp was again present, however the Td analysis post-test showed a gradually increasing Td of value with increase in time with a levelling off at 720 min Fig. 7.
Fig. 7.
Td measurements of BP + Glut post QiMDSC using method 2. Displays an increase of over 10 °C from a native untreated BP sample to BP + Glut tested after 720 min (n = 3) for all but 720 min which due to time constraints (n = 1). (p < 0.05).
The overlay of the 0.6% Glut only and BP + PBS controls were overlaid with BP + Glut, Fig. 8. There is a difference across the sample's slopes. The 0.6% Glut slope is dropping, suggesting that there is a continuous reaction occurring with the Glut solution itself, which is indicative of its reported behaviour of continuous monomer transformations in aqueous solutions [4,18]. The drop-in slope of Rev Cp in BP + PBS, 2.114 mJ/°C is greater than that of the Glut solution alone 1.239 mJ/°C but half that of the BP + Glut 5.406 mJ/°C. This change in BP + PBS indicates that the reaction mechanism between the Hydrogen bonds while continuous is not as great as the molecular mobility and the continuous reaction between BP + Glut.
Fig. 8.
Overlay of individual Glut component, BP + Glut and BP + PBS. The BP and Glut drop in reverse Cp is double than that of Glut only and multiple times greater than the BP + PBS interaction. All display a drop in the Rev Cp indicating reactions ongoing.
4. Conclusion
The crosslinking of BP and the use of Glut as a fixative for numerous biological materials is a common occurrence, with the analysis of the resultant denaturation temperature of BP a critical step in its quality assessment. Despite the commonality of this form of crosslinking, the time parameter is still wide ranging. The use of QiMDSC can prove a useful tool in the analysis of the time of the crosslinking reaction. The changing monomeric chemistry of Glut is well documented, with this research providing further evidence of this. The proof of concept using the crosslinking reaction of an epoxy resin showed that the reaction kinetics over time could be measured using the technique and the resultant thermal properties measured immediately post-test. This method too was applied to a biological material of BP and Glut and showed that the reaction is a continually ongoing process, and the measurement of the Td possible directly after the experiment. Here, QiMDSC showed that the BP and Glut is crosslinked after approximately 10 min, with the volume of the solution important.
The technique utilised here was for determining any changes evident within the Rev Cp of the Glut and BP crosslinking reaction and for comparison purposes with control groups. To get absolute Cp values for the crosslinking reaction, a full Rev Cp calibration under the conditions of the test, would have to be carried out on the QiMDSC system. Also, the technique would be limited for the analysis of fast crosslinking reactions as the system requires approximately 10 min for the modulation to equilibrate.
For lower and higher concentrations, a follow-on study is warranted. The authors believe that a lower crosslinking density would be at the lower tolerances of the method. However, at higher concentrations such as the 2.5% Glut traditionally used for the crosslinking and preparation of biological samples electron microscopy samples, the continuous and ongoing crosslinking reaction would be present.
The technique has been utilised in pharmaceuticals and the food industry, and this paper demonstrates its benefits when applied to soft tissue biomaterials and their crosslinking mechanisms with further development into other biomaterials such as hydrogels and polymers worth exploring.
CRediT authorship contribution statement
K. Joyce: Conceptualization, Data curation, Formal analysis. S. Rahmani: Project administration. Y. Rochev: Investigation, Methodology.
Declaration of competing interest
The author(s) confirm that this article content has no conflicts of interest.
Acknowledgements
This author thanks all the members of CURÁM. This research was supported by both Boston Scientific Galway [grant number: Higher Education].
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Li X. Current usage and future directions for the bovine pericardial patch. Ann. Vasc. Surg. 2011;25(4):561–568. doi: 10.1016/j.avsg.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding K. Acellular matrices for the treatment of wounds. Int. Consens. Group. 2011:1–13. [Google Scholar]
- 3.Sinning J.-M. Next-generation transcatheter heart valves: current trials in Europe and the USA. Methodist DeBakey Cardiovasc. J. 2012;8(2):9. doi: 10.14797/mdcj-8-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayakrishnan A., Jameela S. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996;17(5):471–484. doi: 10.1016/0142-9612(96)82721-9. [DOI] [PubMed] [Google Scholar]
- 5.Damink L.O. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995;6(8):460–472. [Google Scholar]
- 6.Schmidt C.E., Baier J.M. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 7.Jorge-Herrero E. Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials. 1999;20(6):539–545. doi: 10.1016/s0142-9612(98)90205-8. [DOI] [PubMed] [Google Scholar]
- 8.Sung H.W. Crosslinking of biological tissues using genipin and/or carbodiimide. J. Biomed. Mater. Res. 2003;64(3):427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 9.Tsai C.C. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J. Biomed. Mater. Res. 2000;52(1):58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Sung H.W. Degradation potential of biological tissues fixed with various fixatives: an in vitro study. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997;35(2):147–155. doi: 10.1002/(sici)1097-4636(199705)35:2<147::aid-jbm2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Migneault I. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004;37(5):790–806. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- 12.Hardy P., Nicholls A., Rydon H. The nature of glutaraldehyde in aqueous solution. J. Chem. Soc. D Chem. Commun. 1969;(10):565–566. [Google Scholar]
- 13.Zilla P. Glutaraldehyde detoxification of aortic wall tissue: a promising perspective for emerging bioprosthetic valve concepts. J. Heart Valve Dis. 1997;6(5):510–520. [PubMed] [Google Scholar]
- 14.Golomb G. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am. J. Pathol. 1987;127(1):122. [PMC free article] [PubMed] [Google Scholar]
- 15.Nimni M.E. Chemically modified collagen: a natural biomaterial for tissue replacement. J. Biomed. Mater. Res. 1987;21(6):741–771. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp R.O. A critical review of the toxicology of glutaraldehyde. Crit. Rev. Toxicol. 1992;22(3–4):143–174. doi: 10.3109/10408449209145322. [DOI] [PubMed] [Google Scholar]
- 17.Farris S., Song J., Huang Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010;58(2):998–1003. doi: 10.1021/jf9031603. [DOI] [PubMed] [Google Scholar]
- 18.Kuznetsova N. Reactions of glutaraldehyde with dipolar ions of amino acids and proteins. Russ. Chem. Bull. 2013;62(4):918–927. [Google Scholar]
- 19.Barbosa O. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014;4(4):1583–1600. [Google Scholar]
- 20.Cheung D.T., Nimni M.E. Mechanism of crosslinking of proteins by glutaraldehyde II. Reaction with monomeric and polymeric collagen. Connect. Tissue Res. 1982;10(2):201–216. doi: 10.3109/03008208209034419. [DOI] [PubMed] [Google Scholar]
- 21.Okuda K. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991;71(2):100–105. [Google Scholar]
- 22.Duncan A.C., Boughner D. Effect of dynamic glutaraldehyde fixation on the viscoelastic properties of bovine pericardial tissue. Biomaterials. 1998;19(7–9):777–783. doi: 10.1016/s0142-9612(97)00215-9. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P. Effects of glutaraldehyde concentration, pretreatment time, and type of tissue (porcine versus bovine) on postimplantation calcification. J. Thorac. Cardiovasc. Surg. 2012;143(1):224–227. doi: 10.1016/j.jtcvs.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Arevalo F.M. The micromechanical behavior of lyophilized glutaraldehyde-treated bovine pericardium under uniaxial tension. J. Mech. Behav. Biomed. Mater. 2010;3(8):640–646. doi: 10.1016/j.jmbbm.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Lee C. High-concentration glutaraldehyde fixation of bovine pericardium in organic solvent and post-fixation glycine treatment: in vitro material assessment and in vivo anticalcification effect. Eur. J. Cardio. Thorac. Surg. 2011;39(3):381–387. doi: 10.1016/j.ejcts.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Witnauer L.P., Wisnewski A. Absolute measurement of shrinkage temperature of leather by different thermal analysis. J. Am. Leather Chem. Assoc. 1964;59:598–612. [Google Scholar]
- 27.McClain P. Application of differential thermal analysis to the study of hydrothermal shrinkage in epimysial and corium collagen. Biochim. Biophys. Acta Protein Struct. 1968;168(1):143–149. doi: 10.1016/0005-2795(68)90243-2. [DOI] [PubMed] [Google Scholar]
- 28.Loke W.K., Khor E. Validation of the shrinkage temperature of animal tissue for bioprosthetic heart valve application by differential scanning calorimetry. Biomaterials. 1995;16(3):251–258. doi: 10.1016/0142-9612(95)92125-p. [DOI] [PubMed] [Google Scholar]
- 29.F2027-16, A., Standard Guide for Characterization and Testing of Raw or Starting Materials for Tissue-Engineered Medical Products. ASTM International; West Conshohocken, PA: 2016. [Google Scholar]
- 30.Harrington W.F., Von Hippel P.H. Advances in Protein Chemistry. Elsevier; 1962. The structure of collagen and gelatin; pp. 1–138. [DOI] [PubMed] [Google Scholar]
- 31.Gill P., Sauerbrunn S., Reading M. Modulated differential scanning calorimetry. J. Therm. Anal. 1993;40(3):931–939. [Google Scholar]
- 32.Verdonck E., Schaap K., Thomas L.C. A discussion of the principles and applications of modulated temperature DSC (MTDSC) Int. J. Pharm. 1999;192(1):3–20. doi: 10.1016/s0378-5173(99)00267-7. [DOI] [PubMed] [Google Scholar]
- 33.Knopp M.M. Recent advances and potential applications of modulated differential scanning calorimetry (mDSC) in drug development. Eur. J. Pharmaceut. Sci. 2016;87:164–173. doi: 10.1016/j.ejps.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Simon S.L. Temperature-modulated differential scanning calorimetry: theory and application. Thermochim. Acta. 2001;374(1):55–71. [Google Scholar]
- 35.Qi S., Craig D.Q. The development of modulated, quasi-isothermal and ultraslow thermal methods as a means of characterizing the α to γ indomethacin polymorphic transformation. Mol. Pharm. 2012;9(5):1087–1099. doi: 10.1021/mp2003412. [DOI] [PubMed] [Google Scholar]
- 36.Manduva R. Calorimetric and spatial characterization of polymorphic transitions in caffeine using quasi‐isothermal MTDSC and localized thermomechanical analysis. J. Pharmaceut. Sci. 2008;97(3):1285–1300. doi: 10.1002/jps.21048. [DOI] [PubMed] [Google Scholar]
- 37.Chen W. Isotropization of nematic liquid crystals by TMDSC. Thermochim. Acta. 1998;324(1–2):87–94. [Google Scholar]
- 38.Swier S., Van Mele B. Reaction thermodynamics of amine‐cured epoxy systems: validation of the enthalpy and heat capacity of reaction as determined by modulated temperature differential scanning calorimetry. J. Polym. Sci. B Polym. Phys. 2003;41(6):594–608. [Google Scholar]
- 39.McHugh J. Determination and review of specific heat capacity measurements during isothermal cure of an epoxy using TM-DSC and standard DSC techniques. Polym. Test. 2010;29(6):759–765. [Google Scholar]
- 40.Oswal D. Biomechanical characterization of decellularized and cross-linked bovine pericardium. J. Heart Valve Dis. 2007;16(2):165. [PubMed] [Google Scholar]
- 41.Tattini V., Jr. Evaluation of shrinkage temperature of bovine pericardium tissue for bioprosthetic heart valve application by differential scanning calorimetry and freeze-drying microscopy. Mater. Res. 2007;10(1):1–4. [Google Scholar]