Abstract
Giardia duodenalis is one of the most common causes of diarrhea in humans with about 250–300 million cases per year. It is considered to be a species complex comprising of eight genetic assemblages (A to H), with assemblages A and B being the major causes of human infections. In this study we carried out genotypic characterization of G. duodenalis isolates detected in asymptomatic school-going children aged 3–16 years. Between May and September 2017, a total of 329 fecal samples were collected from school-going children from Chawama compound of Lusaka City and were screened for Giardia by microscopic examination. All microscopically positive fecal samples were analyzed by semi-nested polymerase chain reaction (PCR) targeting the glutamate dehydrogenase (gdh) gene. Genotyping of amplified PCR products was conducted by restriction fragment length polymorphism (RFLP) and DNA sequence analysis. Microscopically, Giardia was found in 10% (33/329) of fecal samples. The PCR-RFLP analysis of the gdh gene revealed assemblages A and B in 27.3% (9/33) and 72.7% (24/33), respectively. Furthermore, analysis with restriction enzymes identified sub-assemblages AII (27.3%, 9/33), BIII (12.1%, 4/33), BIV (51.5%, 17/33) and mixed infections of BIII and BIV (9.1%, 3/33). Phylogenetic analysis showed the clustering of 27.6% (8/29) and 72.4% (21/29) of Zambian Giardia gdh gene sequences into assemblages A and B, respectively. This study has revealed the presence of both assemblage A and B and that spread of G. duodenalis in school-going children appears to be mostly through anthroponotic transmission. To our knowledge, this is the first report of genotypic characterization of G. duodenalis identified in Zambia.
Keywords: Giardia duodenalis, Giardiasis, Genotyping, Glutamate dehydrogenase, Phylogenetic analysis, Zambia
Graphical abstract
Highlights
-
•
Giardia duodenalis infection observed in asymptomatic children
-
•
First report of genotypes circulating in Zambia
-
•
Presence of sub-assemblages AII, BIII and BIV
-
•
Co-infection with sub-assemblages BIII and BIV reported
1. Introduction
Giardia duodenalis is a protozoan parasite which is responsible for 250–300 million symptomatic human infections annually, with less developed communities being the most afflicted (Einarsson et al., 2016). The infection can manifest as acute diarrhea, which may become chronic if not treated, with majority of infections being asymptomatic (Certad et al., 2017). Chronic infection is associated with malabsorption which can lead to subsequent weight loss and wasting in children (Squire and Ryan, 2017). G. duodenalis is also reported to be associated with cognitive impairment in school-age children (Berkman et al., 2002). Transmission of the pathogen occurs either directly (fecal-oral route) or indirectly (through contaminated food and water), with the indirect route being the major route of transmission (Thompson, 2000).
G. duodenalis is considered to be a species complex, with eight genetic assemblages (A-H), whose host range varies widely (A and B – Humans and other mammals, C and D – Canids, E – Hooved animals, F – Cats, G – Rats and H – Marine mammals) (Minetti et al., 2016). However, there have been recent reports of humans being infected with assemblages E and F (Abdel-Moein and Saeed, 2016; Fantinatti et al., 2016; Gelanew et al., 2007; Zahedi et al., 2017). Assemblages A and B are further divided into sub-assemblages (AI, AII, AIII, AIV, BI, BII, BIII and BIV) (Monis et al., 2003). G. duodenalis assemblages can be distinguished based on single nucleotide polymorphism and/or genotypic analysis of the ssu – rRNAbeta, (β)-giardin (bg), triose phosphate isomerase (tpi) and glutamate dehydrogenase (gdh) genes (Caccio and Ryan, 2008).
In Africa, the reported prevalence of Giardia infection varies widely possibly due to use of different diagnostic techniques, with most studies being based on microscopy and serology (Squire and Ryan, 2017). A few molecular studies in Africa have revealed the presence of assemblages A, B, C, E and F in the human population (Squire and Ryan, 2017). Characterization to sub-assemblage level has shown the presence of sub-assemblages AI, AII, BIII, BIV as well as some novel sub-assemblages (Squire and Ryan, 2017). Whilst the occurrence of giardiasis in children has been reported in Zambia (Graczyk et al., 2005; Siwila et al., 2010, Siwila et al., 2011), there has been no information on the genotypes present in the country. The purpose of this study was to determine the prevalence of Giardia infection in asymptomatic children in Lusaka as well as to genetically characterize the isolates infecting these children. Knowledge of the infective genotypes would lead to better understanding of the epidemiology of the disease and assist in designing appropriate control strategies.
2. Materials and methods
2.1. Fecal sample collection and microscopic examination
A cross-sectional study was conducted between May and September 2017, which is the dry season when the area is more accessible and before the beginning of national Grade seven (7) examinations, during which a total of 329 asymptomatic school-going children (146 boys and 183 girls) were sampled from two public (123) and two community (206) schools in Chawama compound of Lusaka District in Zambia. Only children who had not had diarrhea in the month preceding the date of sample collection were considered in this study, whilst those who had at least one bout of diarrhea in the same period were excluded from the study. Chawama is a high-density settlement within the City of Lusaka, whose origin is a result of unplanned settlement which was eventually officially recognized as a squatter settlement. Due to its unplanned settlement status, Chawama is characterized by inadequate sanitation and erratic water supply, with most households lacking adequate income (Ndhlema, 2000).
From each child, one fecal sample was collected in a 50 mL screw-capped container and processed by the formalin/ethyl acetate concentration method. The collected fecal samples were kept at 4 °C without preservation until processing and examination in the laboratory. Fecal consistency was noted as watery, soft, loose, semi-formed or formed. The fecal samples were examined for parasitic infection using wet smears stained with Lugol's iodine after being processed by the formal ether concentration method (Garcia, 2007). The intensity of infection was estimated as the average number of cysts count per high power field (HPF/ X40) of light microscope. The grading of Giardia infection was determined as described by Almeida et al. (2006). In brief, those with 1–2 cysts were recorded as 1+ (very low), 3–10 cysts recorded as 2+ (low), 11–30 cysts recorded as 3+ (medium or moderate), >30 cysts recorded as 4+ (high).
2.2. DNA extraction
All fecal samples that were Giardia positive by microscopic examination were stored at 2 to 8 °C prior to DNA extraction. Extraction of genomic DNA was performed directly from all positive stool samples using Fecal DNA MiniPrep (Zymo Research, USA) kit in accordance with the manufacturer's protocol. The extracted DNA samples were stored at −20 °C for PCR assays and other downward applications.
2.3. PCR amplification
Amplification of the 432 base pairs (bp) region of the gdh gene was carried out in a semi-nested PCR assay using primers and specific conditions described by Read et al. (2004). Briefly, in the first reaction, amplification was performed using the primer pair GDHeF: 5′-TCA ACG TYA AYC GYC GYT TCC GT-3′ and GDHiR: 5′-GTT RTC CTT GCA CAT CTC C-3′. The PCR product of this reaction was used in the second reaction with the primer set GDHiF: 5′-CAG TAC AAC TCY GCT CTC GG-3′ and GDHiR: 5′-GTT RTC CTT GCA CAT CTC C-3′. After electrophoresis, the final PCR product was visualized under ultraviolet (UV) light on 1.5% agarose gel stained with ethidium bromide.
2.4. PCR-RFLP analysis
The RFLP analysis was performed and interpreted as described by Read et al. (2004). Ten microliters (10 μL) of PCR product was added to 1 X enzyme buffer and 2 μL (10 U/μL) BspLI (NIaIV) or 2 μL (10 U / μL) RsaI enzymes (New England Biolabs, USA) to make final volumes of 30 μL and 25 μL, respectively. The reaction mixture was incubated for a period of 16 h at 37 °C. The NlaIV enzyme digestion was used to distinguish assemblages A, B, C, D and sub-assemblages AI and AII. RsaI enzyme digestion was used to distinguish sub-assemblages BIII and BIV. Restriction fragments were separated on 3% agarose gel stained with ethidium bromide and visualized under UV light.
2.5. Sequence and phylogenetic analysis
Purification of PCR products, sequencing, sequence assembly and editing was conducted as previously described (Simulundu et al., 2018). The primer pair GDHiF/GDHiR was used for sequencing. The sequences generated in this study were deposited in GenBank (LC430549 – LC430577). Phylogenetic analysis was performed in MEGA V6.06 software using the Maximum Likelihood method and the Tamura-Nei evolutionary model (Tamura and Nei, 1993; Tamura et al., 2013). Model selection was conducted using MEGA V6.06 software (Tamura et al., 2013). Phylogenetic tree topological reliability was determined using the bootstrap method, with 1000 replicates.
2.6. Data analysis
Demographic data (sex, age, type of school) and presence of pathogen after stool microscopic examination was collected and entered into an Excel data spreadsheet for each child. The age of children ranged from 3 years to 16 years. Age was categorized into three groups of 3–6 years old, 7–9 years old and 10–16 years old, in line with the average age of children in pre-school, lower primary and upper primary grades, respectively. Prevalence was presented as percentage for all children as well as for the different categories (age, sex, type of school). Association of Giardia infection as a dependent variable with demographic factors as independent variables was assessed by Pearson's Chi Square test or Fischer's exact test were the number of outcomes was less than five. A P value of <0.05 (P < 0.05) was considered as the level of statistical significance for all tests. Data analysis was performed using Statistix version 9 (Analytical software).
2.7. Ethics statement
Ethical approval was granted by the University of Zambia Biomedical Research Ethics Committee (Reference no: 007-06-16). Fecal samples were collected only after written informed consent was obtained from parents and legal guardians for children to participate in the study. Permission to conduct the study from the various schools was obtained from the Lusaka District Education Board Secretary, Ministry of General Education in Zambia and Head teachers of the respective schools.
3. Results
3.1. Microscopic examination and genotyping of Giardia isolates by PCR-RFLP analysis
Giardia isolates were detected microscopically in 10.0% (33/329) of fecal samples collected from asymptomatic school-going children. Upon grading of infection level, the majority of the children (51.5%; 17/33) were found to have high infection levels (4+), with 21.2% (7/33), 15.2% (5/33) and 12.1% (4/33) having moderate (3+), low (2+) and very low (1+) infection levels, respectively. The prevalence amongst girls and boys was 12% (22/183) and 7.5% (11/146), respectively. By age group, the prevalence amongst the 3–6 year olds, 7–9 year olds and 10–16 year olds was 5.6%, 9.5% and 11.2%, respectively. The differences in prevalence between sexes and age group were not statistically significant (P > 0.05). However, statistically significant (P < 0.05) difference was observed in prevalence of infection between children attending public schools (15.4%; 19/123) and those attending community schools (6.8%; 14/206). Also, no statistically significant association was observed between Giardia assemblage and either sex or age group (P > 0.05).
The 432 bp fragment of the gdh gene was successfully amplified in all the 33 DNA samples (Table 1). The RFLP analysis of the gdh gene revealed assemblage A (27.3%, 9/33) and B (72.7%, 24/33) (Table 1). Furthermore, analysis with restriction enzymes identified sub-assemblages AII (27.3%, 9/33), BIII (12.1%, 4/33), BIV, (51.5%, 17/33) and mixed infections of BIII and BIV (9.1%, 3/33) (Table 1).
Table 1.
Genotyping by PCR-RFLP and Phylogenetic analysis of gdh gene of G. duodenalis detected in school-going children in Zambia.
| Isolate name | PCR-RFLP genotype |
Phylogenetic clustering (assemblage) | GenBank accession no. | |
|---|---|---|---|---|
| Assemblage | Sub-assemblage | |||
| ZAM-01-2017 | B | BIV | B | LC430549 |
| ZAM-02-2017 | B | BIV | B | LC430550 |
| ZAM-03-2017 | B | BIV | B | LC430551 |
| ZAM-04-2017 | A | AII | A | LC430552 |
| ZAM-05-2017 | B | BIII | B | LC430553 |
| ZAM-06-2017 | B | BIV | B | LC430554 |
| ZAM-07-2017 | B | BIV | B | LC430555 |
| ZAM-08-2017 | A | AII | A | LC430556 |
| ZAM-09-2017 | B | BIII/BIV | NS | NS |
| ZAM-10-2017 | B | BIV | B | LC430557 |
| ZAM-11-2017 | B | BIV | B | LC430558 |
| ZAM-12-2017 | B | BIV | B | LC430559 |
| ZAM-13-2017 | A | AII | NS | NS |
| ZAM-14-2017 | B | BIII | B | LC430560 |
| ZAM-15-2017 | B | BIV | B | LC430561 |
| ZAM-16-2017 | B | BIV | NS | NS |
| ZAM-17-2017 | A | AII | A | LC430562 |
| ZAM-18-2017 | B | BIV | B | LC430563 |
| ZAM-19-2017 | A | AII | A | LC430564 |
| ZAM-20-2017 | B | BIII/BIV | B | LC430565 |
| ZAM-21-2017 | B | BIV | B | LC430566 |
| ZAM-22-2017 | B | BIII/BIV | B | LC430567 |
| ZAM-23-2017 | A | AII | A | LC430568 |
| ZAM-24-2017 | B | BIV | B | LC430569 |
| ZAM-25-2017 | B | BIV | NS | NS |
| ZAM-26-2017 | A | AII | A | LC430570 |
| ZAM-27-2017 | B | BIV | B | LC430571 |
| ZAM-28-2017 | B | BIV | B | LC430572 |
| ZAM-29-2017 | A | AII | A | LC430573 |
| ZAM-30-2017 | A | AII | A | LC430574 |
| ZAM-31-2017 | B | BIII | B | LC430575 |
| ZAM-32-2017 | B | BIV | B | LC430576 |
| ZAM-33-2017 | B | BIII | B | LC430577 |
NS - Not sequenced.
3.2. Phylogenetic analysis of gdh gene
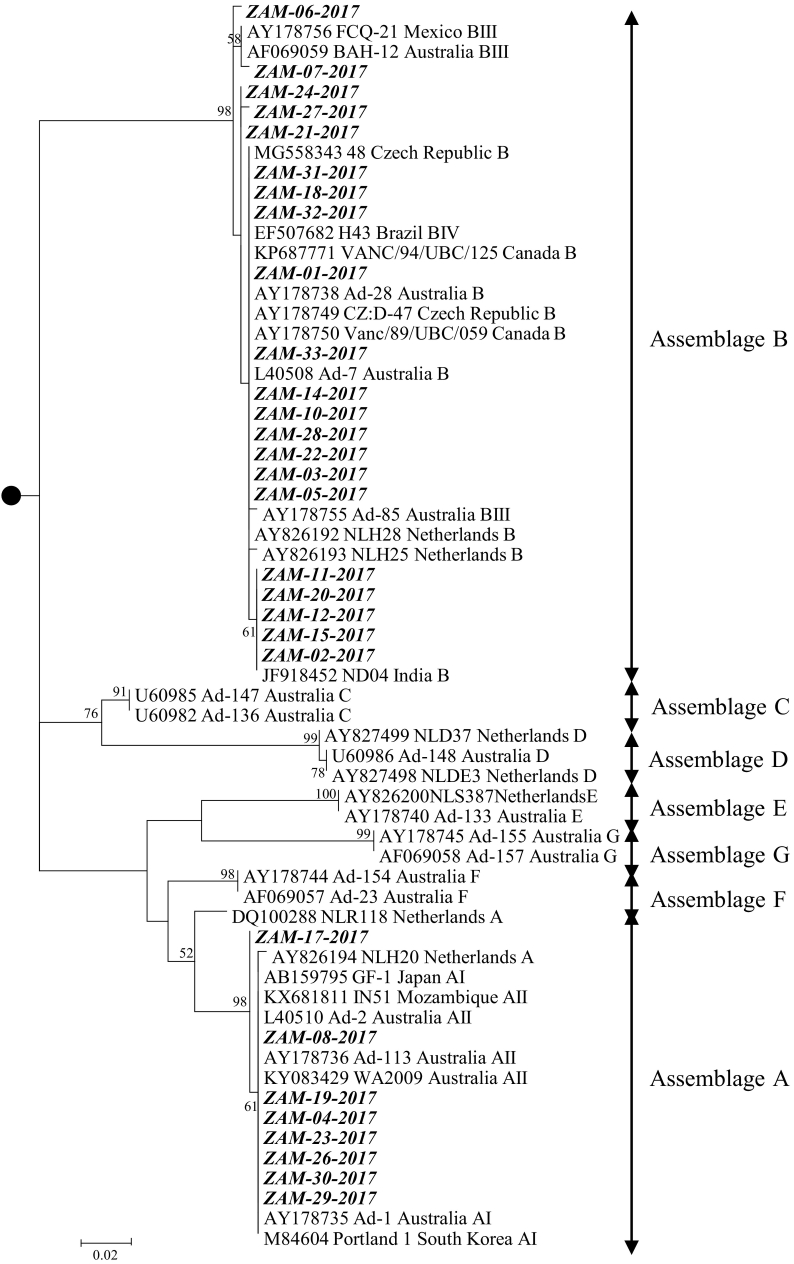
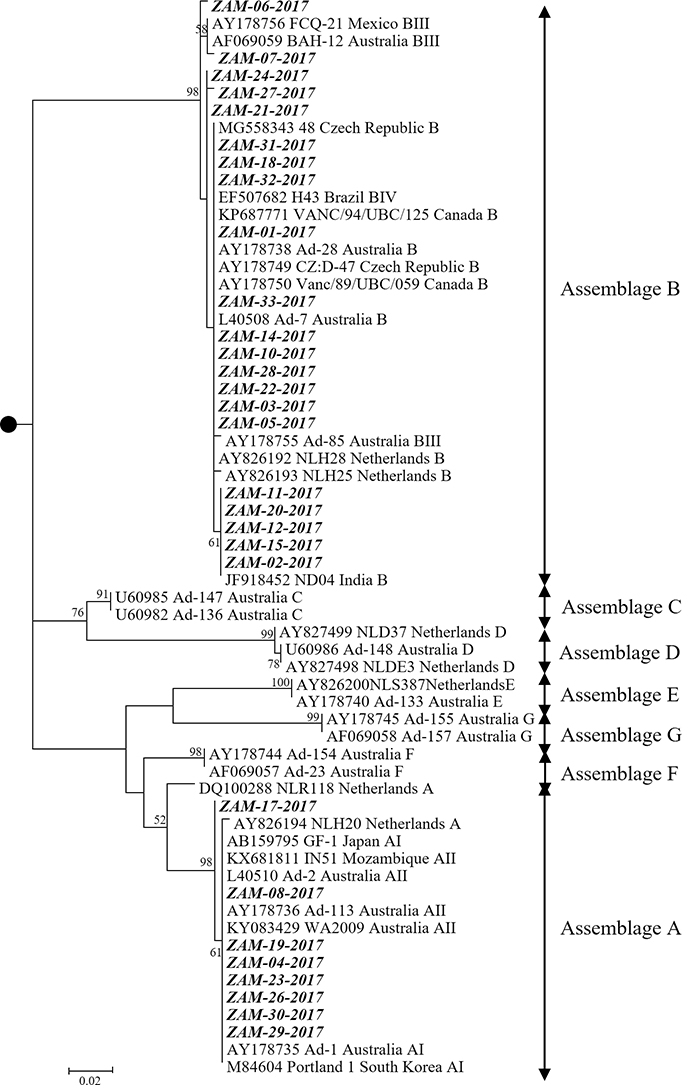
From the 33 DNA samples, 29 (87.9%) isolates were successfully sequenced (Table 1). At the nucleotide level, the gdh gene sequences of G. duodenalis isolates detected in Zambia shared 89.8% to 100% similarity whilst the predicted amino acid sequences showed 97.2% to 100% sequence identity. By the Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) analysis, the nucleotide sequences of the gdh gene of G. duodenalis of Zambian assemblage A (as determined by RFLP analysis) strains were highly similar (99–100%) to isolate Ad-2 (accession no. L40510) isolated from humans in Australia (Monis et al., 1996). Also, Zambian assemblage B gdh sequences were highly similar (99–100%) to isolate NLH25 (accession no. AY826193) found in humans in the Netherlands (van der Giessen et al., 2006). Phylogenetically, the gdh gene sequences of G. duodenalis were separated into various assemblages (Fig. 1). Phylogenetic analysis of isolates examined in this study revealed that 27.6% (8/29) clustered with assemblage A isolates, whilst 72.4% (21/29) belonged to assemblage B (Fig. 1).
Fig. 1.
Phylogenetic analysis of G. duodenalis detected from human fecal samples in Zambia. The analysis was based on the gdh gene and involved 63 nucleotide sequences, with a total of 318 positions in the final dataset. The phylogenetic tree was rooted (black circle) to G. ardeae (GenBank accession number AF069060). The numbers at branch nodes represent bootstrap values ≥50%. Reference sequences included in the analysis are shown with their respective GenBank accession numbers, strain name, country of origin and genotype. G. duodenalis strains characterized in this study are shown in bold italic text. Bar, number of substitutions per site.
4. Discussion
In the present study, we report a prevalence of 10% (33/329) in the sampled population, which is lower when compared to previously reported studies in Zambia. Contrary to the study by Siwila et al. (2010) which focused on children living in a peri-urban area and that by Graczyk et al. (2005) which focused on children in a rural setting, our study focused on children in an urban area and this could explain the differences in prevalence reported. Urban settings tend to have better infrastructural development than in peri-urban and rural settings. Indeed, the level of development of a community and its education (especially the level of education of mothers) has been reported to be risk factors in the epidemiology of giardiasis (Al-Mekhlafi, 2017; Karan et al., 2012). The higher prevalence observed by Graczyk et al. (2005) could also be due to the fact that they sampled children who were exhibiting diarrheal symptoms, contrary to those from our study, considering that Giardia is considered to be a common cause of diarrhea (Torgerson et al., 2015). It is also possible that the prevalence reported in our study is lower than those reported by Siwila et al. (2010) and Graczyk et al. (2005) due to the difference in techniques used. Our study used simple microscopy whilst the other two studies used the more sensitive fluorescence microscopy, thus our study could have under-reported the prevalence. The overall prevalence in our study could also have been underestimated because we used a single stool sample which has been reported to have a significantly low sensitivity (46%) when compared to the recommended 3 stool samples over 3–5 days (94% sensitivity).
In this study, sampling was conducted only during the dry season and this could have resulted in an underestimation of the overall prevalence of infection in this population as it has previously been reported that there is a positive correlation between wet weather conditions and increased prevalence of Giardia infection in populations (Britton et al., 2010; Siwila et al., 2011; Lai et al., 2013). It is postulated that the increased runoff during rainy season leads to increased contamination of water bodies, with consequent increased transmission of pathogens to humans (Galway et al., 2015). Also, the increased turbidity of water during rainy season, due to runoff, reduces the effectiveness of water treatment using chlorine (Schwartz et al., 2000), leading to increased pathogen transmission. Considering that our study site is characterized by inadequate sanitation and water supply, resulting in many households relying on shallow boreholes for drinking water, it is possible that there could be increased transmission in the rainy season than the dry season. Therefore, it is important to conduct a similar study covering the wet season in order to better understand the epidemiology of Giardiasis in this community.
The finding of Giardia in non-diarrheal children in our study further adds to observations by other authors that Giardia could be a commensal in children from high prevalence settings (Bartelt and Platts-Mills, 2016). Indeed, some studies have reported comparable prevalence in both symptomatic and asymptomatic children (Anim-Baidoo et al., 2016; Jerez Puebla et al., 2017). Such asymptomatic cases are important as possible sources of infection for people in close proximity and pose a risk of infecting other children in areas such as schools (Oliveira-Arbex et al., 2016).
Whilst some studies (Damitie et al., 2018; Julio et al., 2012) have reported that younger children have higher prevalence of infection with Giardia, this was not the case in our study. In fact, though not statistically significant, we observed an increase in prevalence with increased age. This finding of increased prevalence with increased age has also been reported in other studies (Al-Mekhlafi et al., 2013; Anim-Baidoo et al., 2016).
Contrary to the findings by Siwila et al. (2010) that sex/gender was a significant risk factor for Giardia infection with more girls infected than boys, our study did not find a significant difference in infection rates between sexes. Our findings are in agreement with those of other authors (Damitie et al., 2018; Julio et al., 2012) who found no association between sex and Giardia infection. It is possible that the effect of sex on infection could be context-specific and is not a general phenomenon, given the variation in reports.
Considering that most community schools lack the basic amenities, it would be expected that children attending these schools would have higher infection levels than those attending public schools which have better amenities. This is because lack of basic sanitary conditions is one of the main risk factors for Giardia infection. It was therefore surprising that in our study, children attending community schools had significantly lower infection levels than those from public schools. The observed high prevalence in children from public schools could be explained by the high enrolment levels in these schools, which would put a burden on the available amenities. This would possibly increase contact, reduce hygiene in the school environment and enhance transmission. As such, construction of more amenities at public schools could help reduce the transmission. Alternatively, construction of more schools, so as to reduce the student population per school, would result in reduced contact between children, ease the burden on amenities and improve hygiene at schools thus consequently reduce disease transmission.
Phylogenetic analysis, based on the gdh gene, showed that our isolates clustered with isolates of assemblages A and B (Fig. 1) and this is not surprising considering that these assemblages are well-known to infect humans (Caccio et al., 2017). There was a predomination of assemblage B in our study (73%) and this is in agreement with most studies conducted across the world (Ryan and Caccio, 2013). In comparison to other assemblages that have been reported to be infective to humans, assemblage B transmission has mainly been associated with human hosts, who serve as a source of infection (Sprong et al., 2009) and this possibly explains its predominance in areas of limited hygiene and sanitation. In our study, we did not find any statistically significant association between infecting Giardia assemblage and either of sex or age group, a finding which is in agreement with other studies (Jerez Puebla et al., 2017; Rostaminia et al., 2017).
Further characterization of the isolates in our study based on RFLP of the gdh gene showed the presence of sub-assemblages AII, BIII and BIV. Finding of only sub-assemblage AII was not surprising considering that within assemblage A, this particular sub-assemblage (AII) has mostly been identified in humans whereas sub-assemblage AI has been found mostly in animals (Caccio and Ryan, 2008). We also found sub-assemblages BIII and BIV, which are also normally found in humans (Monis et al., 2003), with some mixed infections of sub-assemblages BIII and BIV. Contrary to findings by Sprong et al. (2009) that sub-assemblage BIII was more prevalent in Africa, sub-assemblage BIV was more prevalent in our study. Predominance of sub-assemblage BIV in our study is in agreement with findings from studies in Ethiopia (de Lucio et al., 2016), Kenya (Mbae et al., 2016) and Tanzania (Di Cristanziano et al., 2014). Assemblage and sub-assemblage co-infections have been reported before in other studies (Faria et al., 2016; Gelanew et al., 2007; Hussein et al., 2017; Mbae et al., 2016), and their occurrence has been hypothesized to be due to repeated and cumulative infection in an area (Roointan et al., 2013) as well as human exposure to multiple sources of infection (Damitie et al., 2018).
Whilst multilocus typing is recommended for genetic characterization (Broglia et al., 2013), single locus typing using the gdh gene has been widely used (Reviewed by Caccio and Ryan, 2008). The advantage of multilocus typing is that it increases the chances of successful PCR amplification as there may be nucleotide mismatches between the PCR primers and the genomic sequences, which may result in non-amplification of some isolates (Broglia et al., 2013). However, in our study, the gdh gene of all the microscopically positive samples was successfully amplified.
In conclusion, to our knowledge, this is the first report of genotypic characterization of G. duodenalis circulating in Zambia, revealing the circulation of assemblages A and B in school-going children in Lusaka District. The predominance of assemblages AII, BIII and BIV, which are mostly associated with human infections, suggests that anthroponotic transmission plays a major role in the epidemiology of giardiasis within the studied community. As such, control measures aimed at improving hygiene in this community, along with education campaigns could go a long way in curbing giardiasis.
Declaration of competing interest
All the authors declare that there is no conflict of interest.
Acknowledgements
This work was partly supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) and the Japan Agency for Medical Research and Development (AMED)/Japan International Cooperation Agency (JICA) within the framework of the Science and Technology Research Partnership for Sustainable Development (SATREPS); The National Institutes of Health [UNZA sub-award 18/119/100684/010 (PI: Chitanga) of Grant number 1R01AI136035 (PI: Gaff)]. We are grateful to the Lusaka District Education Board Secretary under the Ministry of Education, for granting us permission to conduct this study in the selected schools. We are also grateful to the parents and guardians for allowing their children to participate in the study.
References
- Abdel-Moein K.A., Saeed H. The zoonotic potential of Giardia intestinalis assemblage E in rural settings. Parasitol. Res. 2016;115:3197–3202. doi: 10.1007/s00436-016-5081-7. [DOI] [PubMed] [Google Scholar]
- Almeida A.A., Delgado M.L., Soares S.C., Castro A.O., Moreira M.J., Mendonca C.M., Canada N.B., Da Costa J.M. Genotype analysis of Giardia isolated from asymptomatic children in northern Portugal. J. Eukaryot. Microbiol. 2006;53(Suppl. 1):S177–S178. doi: 10.1111/j.1550-7408.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Al-Mekhlafi H.M. Giardia duodenalis infection among rural communities in Yemen: a community-based assessment of the prevalence and associated risk factors. Asian Pac J Trop Med. 2017;10:987–995. doi: 10.1016/j.apjtm.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Al-Mekhlafi H.M., Al-Maktari M.T., Jani R., Ahmed A., Anuar T.S., Moktar Burden of Giardia duodenalis infection and its adverse effects on growth of school children in rural Malaysia. PloS Negl. Trop. Dis. 2013;7(10) doi: 10.1371/journal.pntd.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anim-Baidoo I., Narh C.A., Oddei D., Brown C.A., Enweronu-Laryea C., Bandoh B. Giardia lamblia infections in children in Ghana. Pan Afr. Med. J. 2016;24:217. doi: 10.11604/pamj.2016.24.217.8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt L.A., Platts-Mills J.A. Giardia: a pathogen or commensal for children in high-prevalence settings? Curr. Opin. Infect. Dis. 2016;29(5):502–507. doi: 10.1097/QCO.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman D.S., Lescano A.G., Gilman R.H., Lopez S.L., Black M.M. Effects of stunting, diarrhoeal disease and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- Britton E., Hales S., Venugopal K., Baker M.G. The impact of climate variability and change in cryptosporidiosis and giardiasis rates in New Zealand. J. Water Health. 2010;8(3):561–571. doi: 10.2166/wh.2010.049. [DOI] [PubMed] [Google Scholar]
- Broglia A., Weitzel T., Harms G., Caccio S.M., Nockler K. Molecular typing of Giardia duodenalis isolates from German travellers. Parasitol. Res. 2013;112:3449–3456. doi: 10.1007/s00436-013-3524-y. [DOI] [PubMed] [Google Scholar]
- Caccio S.M., Ryan U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008;160(2):75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Caccio S.M., Lalle M., Svard S.G. Host specificity in the Giardia duodenalis species complex. Infect. Genet. Evol. 2017;S1567-1348(17):30418. doi: 10.1016/j.meegid.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Certad G., Viscogliosi E., Chabe M., Caccio S.M. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017;33:561–576. doi: 10.1016/j.pt.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Damitie M., Mekonnen Z., Getahun T., Santiago D., Leyns L. Molecular epidemiology of Giardia duodenalis infection in humans in Southern Ethiopia: a triosephosphate isomerase gene-targeted analysis. Infect. Dis. Poverty. 2018;7:17. doi: 10.1186/s40249-018-0397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucio A., Amor-Aramendia A., Bailo B., Saugar J.M., Anegagrie M., Arroyo A. Prevalence and diveristy of Giardia duodenalis and Cryptosporidium spp. among school children in a rural area of the Amhara region, North-West Ethiopia. PloS One. 2016;11(7) doi: 10.1371/journal.pone.0159992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristanziano V., Santoro M., Parisi F., Albonico M., Shaali M.A., Cave D., Berrilli F. Genetic characterization of Giardia duodenalis by sequence analysis in humans and animals in Pemba Island. Tanzania. Parasitol. Int. 2014;63(2):438–441. doi: 10.1016/j.parint.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Einarsson E., Ma'ayeh S., Svard S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016;34:47–52. doi: 10.1016/j.mib.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Fantinatti M., Bello A.R., Fernandes O., Da-Cruz A.M. Identification of Giardia lamblia assemblage E in humans points to a new anthropozoonotic cycle. J. Infect. Dis. 2016;214:1256–1259. doi: 10.1093/infdis/jiw361. [DOI] [PubMed] [Google Scholar]
- Faria C.P., Zanini G.M., Dias G.S., da Silva S., Sousa Mdo C. Molecular characterization of Giardia lamblia: first report of assemblage B in human isolates from Rio de Janeiro (Brazil) PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway L.P., Allen D.M., Parkes M.W., Li L., Takaro T.K. Hydroclimatic variables and acute gastro-intestinal illness in British Columbia, Canada: a time-series analysis. Water Resour. Res. 2015;51(2):885–895. [Google Scholar]
- Garcia L.S. American Society for Microbiology Press; Washington DC: 2007. Diagnostic Medical Parasitology. [Google Scholar]
- Gelanew T., Lalle M., Hailu A., Pozio E., Caccio S.M. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K., Shiff C.K., Tamang L., Munsaka F., Beitin A.M., Moss W.J. The association of Blastocystis hominis and Endolimax nana with diarrheal stools in Zambian school-age children. Parasitol. Res. 2005;98:38–43. doi: 10.1007/s00436-005-0003-0. [DOI] [PubMed] [Google Scholar]
- Hussein E.M., Ismail O.A., Mokhtar A.B., Mohammed S.E., Saad R.M. Nested PCR targeting intergenic spacer (IGS) in genotyping of Giardia duodenalis isolated from symptomatic and asymptomatic infected Egyptian school children. Parasitol. Res. 2017;116(2):763–771. doi: 10.1007/s00436-016-5347-0. [DOI] [PubMed] [Google Scholar]
- Jerez Puebla L.E., Nunez F.A., Santos L.P., Rivero L.R., Silva I.M., Valdes L.A. Molecular analysis of Giardia duodenalis isolates from symptomatic and asymptomatic children from La Habana, Cuba. Parasite Epidemiol. Control. 2017;2(3):105–113. doi: 10.1016/j.parepi.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio C., Vilares A., Oleastro M., Ferreira I., Gomes S., Monteiro L. Prevalence and risk factors for Giardia duodenalis infection among children: a case study in Portugal. Parasit. Vectors. 2012;5:22. doi: 10.1186/1756-3305-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan A., Chapman G.B., Galvani A. The influence of poverty and culture on the transmission of parasitic infections in rural Nicaraguan villages. J. Parasitol Res. 2012;2012:478292. doi: 10.1155/2012/478292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A., Baker M.G., Hales S., French N.P. Potential effects of global environmental changes on cryptosporidiosis and giardiasis transmission. Trends Parasitol. 2013;29(2):83–90. doi: 10.1016/j.pt.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Mbae C., Mulinge E., Guleid F., Wainaina J., Waruru A., Njiru Z.K., Kariuki S. Molecular characterization of Giardia duodenalis in children in Kenya. BMC Infect. Dis. 2016;16:135. doi: 10.1186/s12879-016-1436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C., Chalmers R.M., Beeching N.J., Probert C., Lamden K. Giardiasis. BMJ. 2016;355:i5369. doi: 10.1136/bmj.i5369. [DOI] [PubMed] [Google Scholar]
- Monis P.T., Mayrhofer G., Andrews R.H., Homan W.L., Limper L., Ey P.L. Molecular genetic analysis of Giardia intestinalis isolates at the glutamate dehydrogenase locus. Parasitology. 1996;112(Pt 1):1–12. doi: 10.1017/s0031182000065021. [DOI] [PubMed] [Google Scholar]
- Monis P.T., Andrews R.H., Mayrhofer G., Ey P.L. Genetic diversity within the morphological species Giardia intestinalis and its relationship to host origin. Infect. Genet. Evol. 2003;3(1):29–38. doi: 10.1016/s1567-1348(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Ndhlema D. University of Zambia; BSc Dissertation: 2000. An Environmental Profile of an Urban Squatter Settlement, Chawama Compound in Lusaka. [Google Scholar]
- Oliveira-Arbex A.P., David E.B., Oliveira-Sequeira T.C., Bittencourt G.N., Guimaraes S. Genotyping of Giardia duodenalis isolates in asymptomatic children attending daycare centre: evidence of high risk for anthroponotic transmission. Epidemiol. Infect. 2016;144:1418–1428. doi: 10.1017/S0950268815002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read C.M., Monis P.T., Thompson R.C. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 2004;4:125–130. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Roointan E.S., Rafiei A., Samarbaf-Zadeh A.R., Shayesteh A.A., Shamsizadeh A., Borujeni M.P. Molecular identification of Giardia lamblia siolates from adult human cases in southwest of Iran. Afr. J. Biotechnol. 2013;12(9):901–906. [Google Scholar]
- Rostaminia A., Ebrahimzadeh A., Shahrakipoor M. The assemblage B of Giardia duodenalis is the most common assemblage in isolates from southeast of Iran. Asian Pac. J. Trop. Dis. 2017;7(12):715–718. [Google Scholar]
- Ryan U., Caccio S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013;43(12−13):943–956. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Levin R., Goldstein R. Drinking water turbidity and gastrointestinal illness in the elderly of Philadelphia. J. Epidemiol. Community Health. 2000;54(1):45–51. doi: 10.1136/jech.54.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simulundu E., Chambaro H.M., Sinkala Y., Kajihara M., Ogawa H., Mori A. Co-circulation of multiple genotypes of African swine fever viruses among domestic pigs in Zambia (2013-2015) Transbound. Emerg. Dis. 2018;65:114–122. doi: 10.1111/tbed.12635. [DOI] [PubMed] [Google Scholar]
- Siwila J., Phiri I.G., Enemark H.L., Nchito M., Olsen A. Intestinal helminths and protozoa in children in pre-schools in Kafue district, Zambia. Trans. R. Soc. Trop. Med Hyg. 2010;104:122–128. doi: 10.1016/j.trstmh.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Siwila J., Phiri I.G., Enemark H.L., Nchito M., Olsen A. Seasonal prevalence and incidence of Cryptosporidium spp. and Giardia duodenalis and associated diarrhoea in children attending pre-school in Kafue, Zambia. Trans. R. Soc. Trop. Med. Hyg. 2011;105:102–108. doi: 10.1016/j.trstmh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Sprong H., Caccio S.M., van der Giessen J.W. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire S.A., Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasit. Vectors. 2017;10:195. doi: 10.1186/s13071-017-2111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6 molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 2000;30:1259–1267. doi: 10.1016/s0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giessen J.W., De V.A., Roos M., Wielinga P., Kortbeek L.M., Mank T.G. Genotyping of Giardia in Dutch patients and animals: a phylogenetic analysis of human and animal isolates. Int. J. Parasitol. 2006;36:849–858. doi: 10.1016/j.ijpara.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Zahedi A., Field D., Ryan U. Molecular typing of Giardia duodenalis in humans in Queensland - first report of Assemblage E. Parasitology. 2017;144:1154–1161. doi: 10.1017/S0031182017000439. [DOI] [PubMed] [Google Scholar]