Abstract
Background
Anemia is a known adverse prognostic factor among patients with cardiovascular diseases. We investigated whether the hemoglobin level was associated with the rhythm outcome after atrial fibrillation (AF) catheter ablation (AFCA).
Methods
We included 2627 patients who underwent AFCA and a guidelines-based rhythm follow-up (age 58 ± 10.9 years, 73% men, 30.6% with persistent AF), and evaluated the association of pre-AFCA anemia (haemoglobin <13 g/dL in men and <12 g/dL in women) and rhythm outcomes. We studied the mechanistic relationship between anemia and AF recurrence using a Mendelian randomization analysis (1775 subjects with genome-wide association study) after reviewing already proven 12 hemoglobin-associated genetic polymorphisms.
Results
The body mass index, paroxysmal AF, warfarin use, and baseline red cell distribution width were independently associated with anemia in patients with AF. During a 23-month follow-up (interval OR 9–48 months), the clinical AF recurrence rate was significantly higher in patients with than without anemia (log-rank p = 0.001; propensity score-matched log-rank p = 0.004). This pattern was more significant in male patients (Log-rank p < 0.001) or patients with paroxysmal AF (Log-rank p < 0.001). Anemia (hazard ratio [HR] 1.45 [1.17–1.80], p = 0.001), left atrial diameter (HR 1.03 [1.01–1.04], p < 0.001), a female sex (HR 1.17 [1.00–1.36], p = 0.047), and persistent AF (HR 1.58 [1.36–1.84], p < 0.001) were independently associated with post-AFCA clinical recurrence. In the Mendelian randomization, we could not find a significant direct causal relationship between anemia and AF recurrence at the genetic level.
Conclusions
Pre-AFCA anemia is an independent predictor of post-AFCA clinical recurrence, especially in male patients, without a genetically direct causal relationship.
Keywords: Atrial fibrillation, Anemia, Catheter ablation, Mendelian randomization
1. Introduction
Atrial fibrillation (AF) is a chronic degenerative rhythm disorder and has been known to be associated with heart failure, strokes, and dementia [1]. AF usually occurs in elderly people with multiple comorbidities, and anemia is potentially likely to be present in patients undergoing anticoagulant therapy for stroke prevention. Anemia is a poor prognostic factor for cardiovascular diseases such as heart failure, coronary artery disease, and arteriosclerosis [2], [3], [4]. However, studies on the effects of anemia on the AF prognosis are limited. Recently, there have been reports of an increased risk of major bleeding and mortality when anemia occurs in association with AF in patients undergoing anticoagulation therapy [5], [6]. However, no study has evaluated the direct link between anemia and the rhythm control outcomes of AF.
Radiofrequency catheter ablation of AF is an effective rhythm control strategy in patients with AF refractory to antiarrhythmic drugs as a guideline-based therapy with a proven efficacy and safety [7], [8]. A few small clinical studies have shown that the red cell distribution width (RDW), another hematological parameter, has a predictive value for the rhythm outcome after cryoballoon ablation of AF; however, its mechanism is unknown [9], [10], [11]. Hemoglobin or anemia, the evaluation of which is included in the pre-procedural routine blood tests, has never been suggested as a surrogate marker of AF recurrence after AF catheter ablation (AFCA). The Mendelian randomization tests the mechanistic causal relationship of a modifiable surrogating parameter among the multiple confounding factors on a clinical outcome by measuring the genetic variations of known function [12], [13]. The purpose of this study was to investigate the effect of pre-procedural anemia on the rhythm outcome in patients with AF after AFCA. We also attempted to prove the mechanistic causal relationship between anemia and the rhythm outcome of AFCA at the genetic level by using the Mendelian randomization method.
2. Methods
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
2.1. Study population
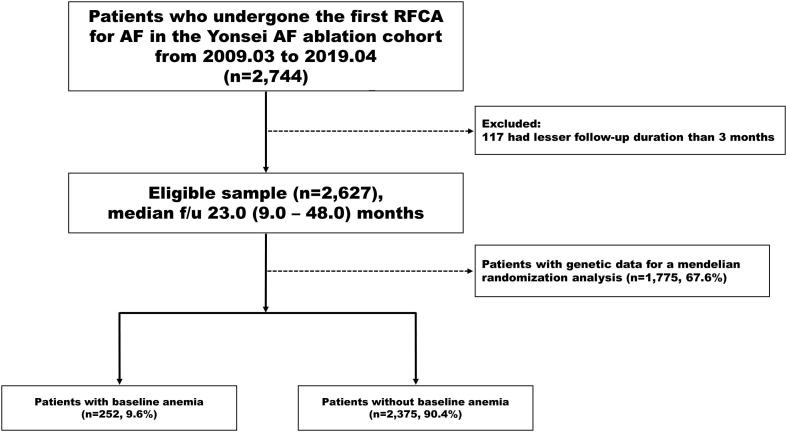
The study population consisted of 2744 patients who underwent AFCA for paroxysmal AF or persistent AF for the first time between January 2009 and January 2019 at Yonsei University Hospital. We included 2627 patients in this study after excluding 117 patients whose follow-up duration was <3 months. The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board at the Yonsei University Health System. Written informed consent was obtained from all patients for inclusion in the Yonsei AF Ablation Cohort Database (ClinicalTrials.gov NCT02138695). The indication for AFCA complied with the latest guidelines. The exclusion criteria were as follows: (1) permanent AF refractory to electrical cardioversion; (2) structural heart disease other than left ventricular hypertrophy, such as significant valvular heart disease of ≥grade 2, hypertrophic/ischemic/dilated cardiomyopathy, and congenital heart diseases; (3) a history of prior AFCA or cardiac surgery; and (4) insufficient examination results at baseline or loss to follow-up within 3 months after the AFCA. All antiarrhythmic drugs were discontinued for at least five half-lives, and amiodarone was stopped at least 4 weeks before the procedure. Anticoagulation therapy was maintained before catheter ablation. All patients underwent transthoracic echocardiography and three-dimensional spiral heart computed tomography for the evaluation of the atrial anatomy and atrioventricular function before the AFCA.
2.2. Radiofrequency catheter ablation
An open-irrigated, 3.5-mm-tip deflectable catheter (Celsius/ Smart Touch SF [Johnson & Johnson Inc., Diamond Bar, CA, USA] or Coolflex/Flexibility [St. Jude Medical Inc., Minnetonka, MN, USA]; 30–35 W, 47 °C) was used for the AFCA. All patients initially underwent a circumferential pulmonary vein isolation and cavo-tricuspid isthmus ablation. We added linear ablation, such as a roof line, posterior inferior line, anterior line, or superior vena cava to the septal line, or a complex fractionated electrogram-guided ablation based on the operator’s discretion, especially in patients with persistent or longstanding persistent AF. The procedure ended when there was no immediate recurrence of AF within 10 min after cardioversion with isoproterenol provocation (5–10 μg/min). Non-pulmonary vein focus triggers under an isoproterenol infusion were also ablated as much as possible, if they were consistent and reproducible.
2.3. Post-ablation management and follow-up
Patients visited the outpatient clinic regularly at 1, 3, 6, and 12 months and then every 6 months thereafter or whenever symptoms occurred after the AFCA. All patients underwent electrocardiography (ECG) during every visit and 24-h Holter recordings at 3 and 6 months and every 6 months thereafter, according to the 2012 HRS/EHRA/ECAS Expert Consensus Statement guidelines [14]. Holter monitoring or event monitor recordings were obtained when the patients reported symptoms of palpitations suggestive of an arrhythmia recurrence. The Holter analysis and adjudication were performed by an individual blinded to the study group assignment. AF recurrence was defined as any episode of AF or atrial tachycardia (AT) of at least 30 s in duration. Any ECG documentation of an AF recurrence within a 3-month blanking period was diagnosed as an early recurrence, and an AF recurrence of more than 3 months after the procedure, was diagnosed as a clinical recurrence.
2.4. Laboratory assessment
Blood samples were drawn within 1 day before the AFCA. Complete blood cell counts were assessed using an XE-2100 automated hematology analyzer (Sysmex Inc., Kobe, Japan). The reference range for a normal hemoglobin level was 13.0–17.4 g/dL. Anemia was defined as a hemoglobin level of <13 g/dL in men and <12 g/dL in women. The plasma blood urea nitrogen, creatinine, and RDW were also evaluated before the procedure. The estimated glomerular filtration ration (eGFR) was calculated using the Cockcroft-Gault equation. The patient baseline characteristics, comorbidities, and therapeutic details were obtained from the hospital medical records.
2.5. DNA genotyping
Among the 2627 patients included in this study, a genome-wide association study (GWAS) was performed in 1775 patients who provided informed consent for a genetic study. The genomic DNA was extracted from peripheral blood samples by using a QuickGene DNA whole blood kit S with QuickGene mini80 (KURABO, Osaka, Japan). The genomic DNA samples were analyzed using the Axiom Precision Medicine Research Array (PMRA) (Thermofisher Scientific, Waltham, MA, USA).
After quality control of the PMRA DNA chip, 403,402 genotyped SNPs were available. Next, for the Mendelian randomization analysis, we chose 12 SNPs, which had been previously proven to be related to anemia, including 2 proxy SNPs (linkage disequilibrium, r2 > 0.8) in 1775 patients.: TF (rs1799852), TF (rs8177240), TF, RAB6B (rs6765093 proxy SNP, r2 = 1 with rs2280673 in 1000 Genome Asians), HFE (rs1799945), CCND3 (rs11970772), HBS1L, MYB (rs4895441), NAT2, PSD3 (rs4921915), ABO, SURF6 (rs651007), ARNTL (rs6486121), FADS2 (rs174577), TEX14 (rs412000 proxy SNP, r2 = 1 with rs411988 in 1000 Genome Asians), and TMPRSS6 (rs855791) [15], [16].
2.6. Statistical analysis
Continuous variables were described as means with standard deviations (normal distribution), and variables with a non-normal distribution were described as medians and interquartile ranges. The Student’s t-test and Mann-Whitney U test were used to compare continuous variables. Categorical variables were compared using the chi-square test or Fisher’s exact test. Univariable Spearman correlation and partial correlation were used to evaluate the magnitude and significance of the relationships among the continuous variables. Multiple Cox proportional hazard analyses were performed to identify the independent predictors of a clinical recurrence of AF after AFCA. The Kaplan-Meier method was used to estimate the event-free survival with a log-rank test. Given the observational nature of our study, patients with anemia and without anemia were not comparable. To reduce the influence of confounding factors, we also performed a propensity score matching analyses adjusted by multiple covariates.
The selected SNPs were evaluated through a Mendelian randomization analysis. The causal effect of hemoglobin on the clinical recurrence of AF was quantified using a two-stage least square regression. We also employed the weighted genetic risk score (wGRS) as an instrument, by using two selected SNPs that were associated with the hemoglobin level in the male population. The wGRS was calculated as the sum of the risk alleles multiplied by the estimated β-coefficient (effect size) for each SNP.
Data analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA), R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 15.1 (StataCorp, College Station, TX, USA) software. Two-tailed p-values of <0.05 were considered to indicate statistical significance.
3. Results
3.1. Patient characteristics related to baseline anemia
A total of 2627 patients who were followed up for >3 months after the AFCA were evaluated in this study. Of those, 252 patients (9.6%) had baseline anemia before the AFCA procedure (Supplementary Fig. 1). Table 1 summarizes the baseline demographic, clinical, echocardiographic, and laboratory characteristics between the baseline anemia and non-anemia groups. Patients with anemia and AF were older (p < 0.001), had higher proportions of women (p < 0.001), individuals with paroxysmal AF (p = 0.006), non-drinkers (p = 0.004), non-smokers (p < 0.001), and warfarin users (p < 0.001), and had a lower body mass index (BMI, p < 0.001) or eGFR (p < 0.001). They also had higher CHA2DS2-VASc scores (p < 0.001), higher prevalence of hypertension (p = 0.008), diabetes (p < 0.001), a prior stroke or transient ischemic attack (TIA; p = 0.009), or vascular disease (p < 0.001), and a higher mean left atrial pressure (p = 0.007) and baseline RDW (p < 0.001) than their counterparts. In a multivariate logistic regression analysis (Supplementary Table 1), the BMI (OR 0.88 [0.83–0.93], p < 0.001), paroxysmal AF (OR 1.95 [1.37–2.79]. p < 0.001), warfarin use (OR 1.42 [1.05–1.92], p = 0.023), and baseline RDW (OR 1.97 [1.70–2.27], p < 0.001) were independently associated with anemia in patients with AF. Iron deficiency anemia (7.1%), malignancy (7.9%), autoimmune disease (2.0%), and chronic kidney disease with a grade ≥3 (34.4%) were suggested as potential causes of anemia. In 48.6% of the patients in whom the exact cause of the anemia could not be confirmed, they did not have any evidence of bleeding or a specific hematologic disorder. Those patients had a mild degree of normocytic anemia (Hb 11.6 ± 1.0 g/dL, MCV 92.7 ± 6.01 fL), and we did not perform a complete hematologic evaluation including the iron kinetics in those patients. Therefore, the cause of the anemia was mostly considered to be an unspecified category. There was no significant difference in the ORBIT-AF bleeding risk score between the patients with and without IDA (Supplementary Table 2).
Table 1.
Baseline characteristics of the clinical non-anemia and anemia groups in patients with AFCA.
| Variables | Overall n = 2627 |
No anemia n = 2375 |
Baseline anemia n = 252 |
pvalue |
|---|---|---|---|---|
| Age, (years) | 59 (51, 66) | 58 (51, 66) | 65 (57, 72) | <0.001 |
| Female, n (%) | 708 (27.0) | 604 (25.4) | 104 (41.3) | <0.001 |
| BMI, (kg/m2) | 24.8 (23.0, 26.8) | 24.9 (23.2, 26.9) | 23.4 (21.9, 25.6) | <0.001 |
| Duration of AF, (day) | 23 (9, 48) | 24 (9, 50) | 19 (8, 36) | 0.147 |
| Paroxysmal AF, n (%) | 1823 (69.4) | 1629 (68.6) | 194 (77.0) | 0.006 |
| Clinical recurrence, n (%) | 870 (33.1) | 757 (31.9) | 113 (44.8) | <0.001 |
| Smoking, n (%) | <0.001 | |||
| Never | 1608 (61.2) | 1429 (60.2) | 179 (71.0) | |
| Former | 758 (28.9) | 696 (29.3) | 62 (24.6) | |
| Current | 259 (9.9) | 248 (10.5) | 11 (4.4) | |
| Alcohol, n (%) | 0.004 | |||
| Never | 1298 (49.4) | 1152 (48.5) | 146 (57.9) | |
| Former | 533 (20.3) | 482 (20.3) | 51 (20.2) | |
| Current | 795 (30.3) | 740 (31.2) | 55 (21.8) | |
| Comorbidities | ||||
| Hypertension, n (%) | 1204 (45.8) | 1068 (45.0) | 136 (54.0) | 0.008 |
| Diabetes, n (%) | 394 (15.0) | 336 (14.2) | 58 (23.0) | <0.001 |
| Prior stroke/TIA, n (%) | 298 (11.3) | 256 (10.8) | 42 (16.7) | 0.009 |
| Vascular disease, n (%) | 302 (11.5) | 253 (10.7) | 49 (19.4) | <0.001 |
| CHA2DS2-VASc score | 1 (0, 3) | 1 (0, 2) | 2 (1, 4) | <0.001 |
| *Anticoagulation, n (%) | 0.001 | |||
| Warfarin | 1359 (51.7) | 1203 (51.3) | 156 (63.2) | |
| NOAC | 1231 (46.9) | 1140 (48.7) | 91 (36.8) | |
| †Anticoagulation, n (%) | 0.007 | |||
| Warfarin | 634 (53.2) | 529 (51.7) | 105 (62.9) | |
| NOAC | 557 (46.8) | 495 (48.3) | 62 (37.1) | |
| Laboratory data, baseline | ||||
| BUN, (mg/dL) | 15.7 (13.0, 18.8) | 15.7 (13.1, 18.7) | 16.9 (12.9, 21.4) | 0.004 |
| Creatinine, (mg/dL) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.7, 1.1) | 0.646 |
| eGFR, (mL/min/1.73 m2) | 84.7 (69.0, 103.8) | 87.1 (71.3, 106.4) | 70.8 (52.6, 89.7) | <0.001 |
| Hemoglobin, (g/dL) | 14.4 (13.4, 15.4) | 14.6 (13.7, 15.6) | 11.8 (11.1, 12.5) | <0.001 |
| RDW, (%) | 12.9 (12.5, 13.4) | 12.9 (12.5, 13.3) | 13.5 (12.9, 14.3) | <0.001 |
| Echocardiographic data | ||||
| LA diameter, (mm) | 41 (37, 45) | 41 (37, 45) | 42 (38, 46) | 0.063 |
| LVEF, (%) | 64 (59, 68) | 64 (59, 68) | 63 (59, 69) | 0.874 |
| Mean LA pressure, (mmHg) | 12 (9, 17) | 12 (9, 16) | 14 (9, 19) | 0.007 |
The data are presented as the number (%) and median (interquartile). Non-parametric continuous variables which were evaluated by Kolmogorov-Smimov method, were analysed through a Mann-whitney u test.
Abbreviations: AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; LA, left atrial; LVEF, left ventricular ejection fraction; NOAC, non-vitamin K oral anticoagulants; RDW, red blood cell distribution widths; TIA, transient ischemic attack.
All patients were analysed regardless of the CHA2DS2-VASc score.
Patients with a CHA2DS2-VASc score of 0 and 1 were excluded, n = 1191.
3.2. Higher AF recurrence in patients with pre-AFCA anemia
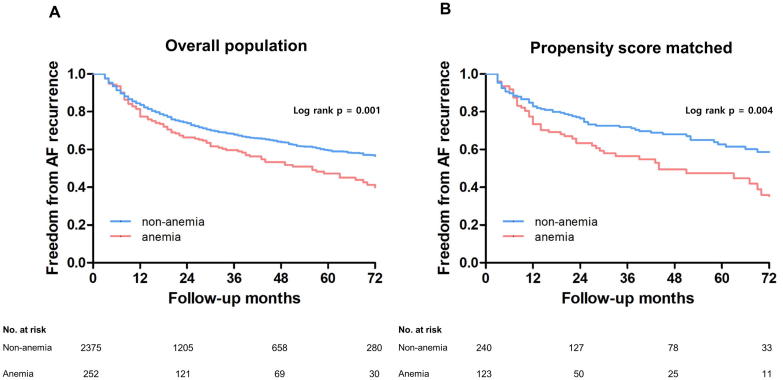
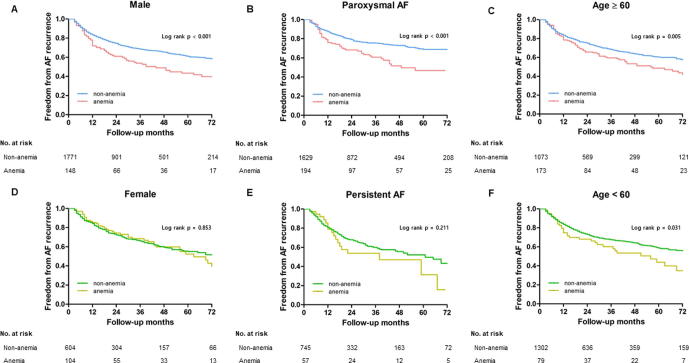
During a follow-up of 23 months (IQR 9–48 months), the rate of an AF clinical recurrence was significantly higher in the patients with anemia than in those without (log-rank p = 0.001; Fig. 1A). Even after propensity score-matching with respect to the age, BMI, sex, AF type, anticoagulation, history of smoking, history of alcohol consumption, hypertension, diabetes, prior stroke/TIA, vascular disease, CHA2DS2-VASc score, left atrial diameter, left ventricular ejection fraction, and baseline eGFR (Supplementary Table 3), this finding was consistent (log-rank p = 0.004; Fig. 1B). This pattern was more significant in male patients (log-rank p < 0.001), patients with paroxysmal AF (log-rank p < 0.001), and the patients both younger than 60 years (log rank p = 0.005) and older than 60 years (log rank p = 0.031; Supplementary Fig. 2).
Fig. 1.
The Kaplan-Meier analysis of AF-free survival rates in patients with and without baseline anemia within the overall population (A) and the propensity score matched population (B).
The AF recurrence group had a longer AF duration (p = 0.008), lower prevalence of paroxysmal AF (p < 0.001) and prior stroke or TIA (p = 0.036), and higher proportion of warfarin users (p < 0.001) than those remained in sinus rhythm (Supplementary Table 4). The left atrial diameter (p < 0.001) and mean left atrial pressure (p = 0.004) were greater in the clinical recurrence group. The pre-AFCA hemoglobin level was significantly lower (p < 0.001), RDW higher (p < 0.001), and anemia more commonly found (p < 0.001) in patients with clinical recurrence than in those who remained in sinus rhythm. In a multivariate Cox regression analysis, paroxysmal AF (HR 0.63 [0.54–0.73], p < 0.001), the left atrial diameter (HR 1.03 [1.01–1.04], p = 0.001), a female sex (HR 1.17 [1.00–1.36], p = 0.047, and anemia (HR 1.45 [1.17–1.80], p = 0.001) were independently associated with the clinical recurrence of AF after AFCA (Table 2).
Table 2.
Cox regression analysis for the predictors of a clinical recurrence of AF after AFCA.
| Univariable analysis |
Multivariable model 1* |
Multivariable model 2† |
||||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p value | Adjusted HR (95%CI) | p value | Adjusted HR (95% CI) | p value | |
| Age | 1.00 (0.99–1.01) | 0.722 | 0.99 (0.99–1.00) | 0.104 | 0.99 (0.99–1.00) | 0.061 |
| BMI | 1.01 (0.99–1.04) | 0.227 | 1.00 (0.98–1.03) | 0.864 | 1.00 (0.98–1.03) | 0.753 |
| Female | 1.13 (0.97–1.30) | 0.114 | 1.17 (1.00–1.36) | 0.047 | 1.02 (0.86–1.21) | 0.800 |
| Paroxysmal AF | 0.57 (0.50–0.66) | <0.001 | 0.69 (0.58–0.82) | <0.001 | 0.67 (0.57–0.80) | <0.001 |
| Warfarin vs. NOAC | 1.19 (1.03–1.39) | 0.020 | 1.10 (0.94–1.28) | 0.240 | 1.09 (0.94–1.28) | 0.263 |
| Smoking (vs. Never) | ||||||
| Former | 0.93 (0.80–1.09) | 0.374 | ||||
| Current | 1.07 (0.86–1.34) | 0.555 | ||||
| Alcohol (vs. Never) | ||||||
| Former | 0.90 (0.75–1.07) | 0.239 | ||||
| Current | 0.90 (0.78–1.05) | 0.197 | ||||
| Hypertension | 1.03 (0.90–1.17) | 0.695 | ||||
| Diabetes | 1.04 (0.87–1.25) | 0.659 | ||||
| Prior stroke/TIA | 1.15 (0.94–1.40) | 0.167 | ||||
| Vascular disease | 1.02 (0.84–1.25) | 0.812 | ||||
| CHA2DS2-VASc score | 1.03 (0.99–1.07) | 0.170 | ||||
| LA diameter | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.01–1.04) | <0.001 | 1.03 (1.01–1.04) | 0.001 |
| LVEF | 0.99 (0.99–1.00) | 0.102 | ||||
| Extra-PV LA ablation | 1.55 (1.36–1.77) | <0.001 | 1.17 (0.98–1.38) | 0.076 | 1.16 (0.97–1.37) | 0.098 |
| CTI ablation | 0.87 (0.69–1.09) | 0.221 | ||||
| Baseline eGFR | 1.00 (0.99–1.00) | 0.722 | ||||
| Baseline hemoglobin | 0.93 (0.89–0.97) | 0.001 | 0.90 (0.86–0.95) | <0.001 | ||
| Baseline RDW | 1.09 (1.02–1.16) | 0.014 | 1.01 (0.94–1.09) | 0.804 | 1.00 (0.93–1.08) | 0.913 |
| Baseline anemia | 1.41 (1.16–1.72) | 0.001 | 1.45 (1.17–1.80) | 0.001 | ||
CI = confidence interval, HR = Hazard ratio. The other abbreviations are defined in Table 1.
Abbreviations: PV, pulmonary vein; CTI, cavo-tricuspid isthmus.
Model 1; Adjusted for the age, BMI, sex, AF types, anticoagulation, LA diameter, Extra-PV LA ablation, CTI ablation, baseline RDW, and anemia.
Model 2; Adjusted for the age, BMI, sex, AF types, anticoagulation, LA diameter, Extra-PV LA ablation, CTI ablation, baseline RDW, and haemoglobin.
3.3. Indirect causal relationship between anemia and AF recurrence
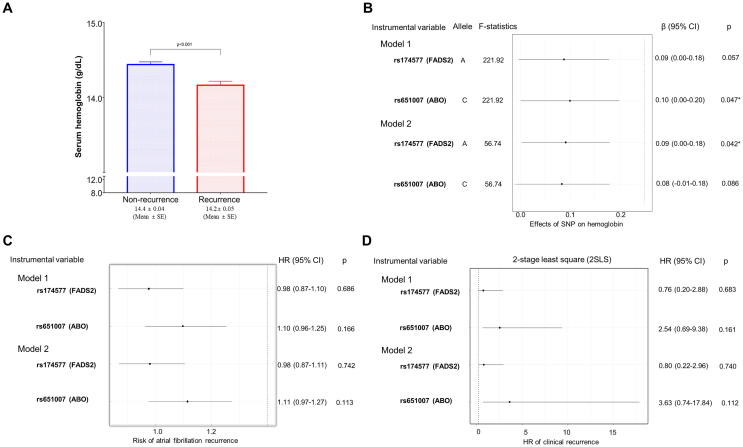
Among the 2627 total patients, GWAS data were available in 1775 (67.6%) patients (Supplementary Fig. 1). Before the Mendelian randomization, an association between the hemoglobin level and clinical recurrence was consistently observed in this group with genetic data (Fig. 2A). Of the 12 SNPs previously reported to be associated with anemia, rs651007 (ABO gene at 9q34.2) was associated with the hemoglobin level in model 1 (adjusted for the age and sex, F-statistics = 221.9, p = 0.047) and rs174577 (FADS2 gene at 11q12.2) was associated with the hemoglobin level in model 2 (adjusted for the age, BMI, sex, AF type, history of smoking and alcohol consumption, hypertension, diabetes, prior stroke/TIA, CHA2DS2-VASc score, left atrial diameter, left ventricular ejection fraction, baseline eGFR, and RDW; F-statistics = 56.7, p = 0.042; Fig. 2B). None of the 12 SNPs exhibited a significant odds ratio between the anemia and non-anemia (Data not shown). However, a single instrument variable analysis using a two-stage least square regression did not show an association between the hemoglobin level and AF clinical recurrence (Fig. 2C and D).
Fig. 2.
Comparison of the baseline hemoglobin level between the clinical recurrence and non-recurrence groups in the overall population (A). A Mendelian randomization instrumental variable analysis with a two-stage least square estimation in the overall population (B–D).
4. Discussion
4.1. Main findings
In this study, we explored the relationship between pre-procedural anemia and the rhythm outcome of AFCA. We found that 9.6% of the included patients had anemia, which was associated with a low BMI, paroxysmal AF, warfarin use, and high RDW. During the median 23 month follow-up, anemia was independently associated with a clinical recurrence of AF, especially in male patients and patients with paroxysmal AF. However, we did not find a direct causal relationship between anemia and a post-AFCA recurrence at the genetic level by using the Mendelian randomization.
4.2. Anemia and cardiovascular disease
Anemia is an independent predictor of adverse cardiovascular outcomes in various cardiovascular diseases, including heart failure, coronary artery disease, and atherosclerosis [2], [3], [4]. The presence of anemia has been known to increase the heart failure mortality and hospitalization rates [3], [17], [18], [19], [20], [21], [22], the risk of acute coronary syndrome [2], [23], and the incidence of thromboembolic or bleeding events in patients with AF under anticoagulation [5], [6], [24]. Multiple mechanisms, such as neurohormonal, hemodynamic, and renal alterations, seem to contribute to poor outcomes in anemic patients with cardiovascular disease. Anemia has been shown to have adverse effects on the myocardial and large arterial hypertrophic remodeling that occurs owing to sustained increases in the cardiac output [25], [26], [27]. However, it is not clear whether anemia has a causal relationship with poor cardiovascular outcomes or if it is a simple marker of more advanced disease.
4.3. Anemia as a prognostic marker of AF
Studies about the prognosis of anemia in association with AF are still limited. In the ARISTOTLE sub-study, Westenbrink et al. [5] reported that anemia is associated with bleeding and a high mortality, and not with a stroke risk, in patients under anticoagulation for AF. Bonde et al. [6] performed a Danish registry analysis and reported that moderate to severe anemia (hemoglobin < 6.83 mmol/L [11.0 g/dL]) was associated with major bleeding and a lower time in a therapeutic range in patients with AF. In this anemia group, oral anticoagulation was associated with major bleeding but did not lower the stroke or systemic thromboembolic risk.
Although there have been small-scale studies on the association of the RDW with the rhythm outcome after cryoballoon ablation of AF [9], [10], [11], to the best of our knowledge, this study was the first to report, with the largest number of patients, on the independent association of anemia with the AFCA outcome. The RDW is known to be a prognostic parameter in patients with heart failure, and this study also confirmed its association with the AF rhythm outcome after AFCA. However, the RDW is a parameter influenced by various hematologic confounding factors, and hemoglobin was more predictive of the rhythm outcome than the RDW in this study.
4.4. Potential mechanisms and the Mendelian randomization
In this study, anemia was more frequently found in patients with multiple comorbidities and had statistically significant associations with a low BMI, paroxysmal AF, and warfarin use. However, in 252 patients with anemia, the prevalence of iron deficiency anemia was only 7.1% and chronic diseases such as chronic kidney disease ≥grade 3, cancer, or inflammatory disease were observed in 44% of patients with anemia (Supplementary Table 2). Moreover, no other relevant factors were found other than the hematologic parameters after a propensity score matching for comorbidities associated with anemia (Supplementary Table 3). It was difficult to determine why the co-occurrence of anemia increased the recurrence rate of AF after AFCA, especially in male patients or patients with paroxysmal AF. Therefore, a Mendelian randomization was performed to determine the causal relationship between anemia and AF recurrence through a genetic study.
The Mendelian randomization is a method of using measured variations in genes of known function to examine the causal effect of a modifiable exposure on a disease in observational studies [12], [13]. In this study, we confirmed the existence of a significant relationship between anemia and AF recurrence, and 2 of the 12 already proven anemia-associated genes showed a statistically significant β-coefficient for anemia among the included patient group. However, those two significant genes did not have a significant relationship with AF recurrence in the two-stage least square regression analysis. Therefore, the direct causal relationship between anemia and AF recurrence has not been proven statistically and is presumed to be the result of indirect effects. Further observational studies with more patients and more genes, or clinical intervention studies, will be needed.
4.5. Limitations
The present study should be interpreted in the context of its limitations. First, our study had an observational design and was based on a single-center prospective cohort registry. Second, a patient with anemia may seek more medical attention or even be more symptomatic during AF, which leads to more patients being classified into the recurrence group. Third, the exact cause of the anemia was unknown in 48.6% of 252 patients with clinical anemia. The baseline characteristics rather differed between the anemia group and non-anemia group. Thereby, we used a propensity score matching to attenuate the impact of the confounding factors, and the results were consistent with the findings from the overall population. Although we attempted to explain the contributing mechanism to the causal relationship of anemia and AF recurrence, we could not find a direct association.
5. Conclusion
Pre-AFCA anemia was independently associated with a clinical recurrence of AF, especially in male patients and patients with paroxysmal AF. However, we did not find a direct causal relationship between anemia and post-AFCA recurrence at the genetic level using the Mendelian randomization.
Acknowledgments
Acknowledgement
We would like to thank Mr. John Martin for his linguistic assistance.
Sources of Funding
This work was supported by grants (HI18C0070 and HI19C0114) from the Ministry of Health and Welfare and a grant (NRF-2017R1A2B4003983) from the Basic Science Research Program of the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, & Future Planning (MSIP).
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100507.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnak M.J., Tighiouart H., Manjunath G., MacLeod B., Griffith J., Salem D. Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (aric) study. J. Am. Coll. Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 3.Groenveld H.F., Januzzi J.L., Damman K. Anemia and mortality in heart failure patients: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Brown D.W., Giles W.H., Croft J.B. Hematocrit and the risk of coronary heart disease mortality. Am. Heart J. 2001;142:657–663. doi: 10.1067/mhj.2001.118467. [DOI] [PubMed] [Google Scholar]
- 5.Westenbrink B.D., Alings M., Granger C.B. Anemia is associated with bleeding and mortality, but not stroke, in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am. Heart J. 2017;185:140–149. doi: 10.1016/j.ahj.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Bonde A.N., Blanche P., Staerk L. Oral anticoagulation among atrial fibrillation patients with anaemia: an observational cohort study. Eur. Heart J. 2019:ehz155. doi: 10.1093/eurheartj/ehz155. (Epub 2019 April 1) [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 8.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J. Am. Coll. Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Li H., Liu T., Xu G., Liu E., Jiao Z., Li G. Red blood cell distribution width and the recurrence of atrial fibrillation after ablation in patients with paroxysmal non-valvular symptomatic atrial fibrillation. Int. J. Cardiol. 2016;203:834–836. doi: 10.1016/j.ijcard.2015.11.077. [DOI] [PubMed] [Google Scholar]
- 10.Gurses K.M., Yalcin M.U., Kocyigit D. Red blood cell distribution width predicts outcome of cryoballoon-based atrial fibrillation ablation. J. Interv. Card. Electrophysiol. 2015;42:51–58. doi: 10.1007/s10840-014-9959-y. [DOI] [PubMed] [Google Scholar]
- 11.Aksu T., Guler T., Golcuk E., Erden I., Ozcan K., Baysal E. Predictors of atrial fibrillation recurrence after cryoballoon ablation. J. Blood Med. 2015;6:211–217. doi: 10.2147/JBM.S81551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes M.V., Ala-Korpela M., Smith G.D. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat. Rev. Cardiol. 2017;14:577–590. doi: 10.1038/nrcardio.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett D.A., Holmes M.V. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart. 2017;103:1400–1407. doi: 10.1136/heartjnl-2016-310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calkins H., Kuck K.H., Cappato R. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9 doi: 10.1016/j.hrthm.2011.12.016. 632-96.e621. [DOI] [PubMed] [Google Scholar]
- 15.Benyamin B., McRae A.F., Zhu G. Variants in TF and HFE explain ~40% of genetic variant in serum transferrin levels. Am. J. Hum. Genet. 2009;84:60–65. doi: 10.1016/j.ajhg.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesh S.K., Zakai N.A., van Rooij F.J. Multiple loci influence erythrocyte phenotype in the CHARGE Consortium. Nat. Genet. 2009;41:1191–1198. doi: 10.1038/ng.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand I.S. Anemia and chronic heart failure. J. Am. Coll. Cardiol. 2008;52:501–511. doi: 10.1016/j.jacc.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Formiga F., Chivite D., Castañer O., Manito N., Ramón J.M., Pujol R. Anemia in new-onset congestive heart failure inpatients admitted for acute decompensation. Eur. J. Intern. Med. 2006;17:179–184. doi: 10.1016/j.ejim.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Gardner R.S., Chong K.S., Morton J.J., Mcdonagh T.A. N-terminal brain natriuretic peptide, but not anemia, is a powerful predictor of mortality in advanced heart failure. J. Card. Fail. 2005;11:S47–S53. doi: 10.1016/j.cardfail.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.-H., Kim M.-S., Kim E.J. KSHF guidelines for the management of acute heart failure: Part I. Definition, epidemiology and diagnosis of acute heart failure. Korean Circ. J. 2019;49:1–21. doi: 10.4070/kcj.2018.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y.-D., Katz S.D. The prevalence of anemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail. Rev. 2008;13:387–392. doi: 10.1007/s10741-008-9089-7. [DOI] [PubMed] [Google Scholar]
- 22.O’Meara E., Clayton T., McEntegart M.B. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation. 2006;113:986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 23.Lawler P.R., Filion K.B., Dourian T., Atallah R., Garfinkle M., Eisenberg M.J. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am. Heart J. 2013;165:143–153. doi: 10.1016/j.ahj.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Takabayashi K., Unoki T., Ogawa H., Esato M., Chun Y.H., Tsuji H., Wada H., Hasegawa K., Abe M., Akao M. Clinical characteristics of atrial fibrillation patients with anemia: from the Fushimi AF registry. Eur. Heart J. 2013;34(suppl 1):P389. [Google Scholar]
- 25.Varat M.A., Adolph R.J., Fowler N.O. Cardiovascular effects of anemia. Am. Heart J. 1972;83:415–426. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons G.H., Dzau V.J. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 27.London G.M., Parfrey P.S. Cardiac disease in chronic uremia: pathogenesis. Adv. Ren. Replace. Ther. 1997;4:194–211. doi: 10.1016/s1073-4449(97)70029-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.