Abstract
Leptospermum petersonii is a native Australian medicinal and aromatic plant. This study was designed to evaluate the influence of solvents and ultrasound-assisted extraction (UAE) parameters including time, temperature, and sonication power on the yield of phenolic compounds and antioxidant capacity from lemon scented tea tree leaves. Extraction efficiency of the optimal UAE conditions were compared with that of shaking water bath technique. The results show that extraction solvents significantly affect extraction yield of phenolic compounds and antioxidant properties, and 50% acetone in water was found to be the most suitable solvent. The UAE optimal conditions were 60 min, 50 °C and sonication power of 200 W. Under these optimal conditions the yields of total phenolics, flavonoids, proanthocyanidins were 98.91 ± 1.20 (mg GAE/g DW), 76.12 ± 0.79 (mg CE/g DW), 117.71 ± 2.18 (mg CE/g DW), respectively. Antioxidant properties from four assays including FRAP, CUPRAC, ABTS and DPPH were 581.29 ± 14.23, 5534.87 ± 19.56, 1636.18 ± 4.11, and 889.29 ± 20.68 (mM TE/g DW) respectively. The UAE extraction technique was found to be more efficient in extraction of total phenolics and antioxidant capacity in comparison with conventional shaking water bath extraction. This study also observed a strong correlation between phenolic compounds and antioxidant capacities. All three phenolic compound groups (TPC, TFC, and Pro.A) were contributed to both free radical scavenging and ion reducing properties in the lemon scented tea tree leaves extract. However, the order of the phenolic groups was TPC > Pro.A > TFC for antioxidant properties.
Keywords: Food science, Lemon scented tea tree, Bioactive compound, Phenolic compound, Flavonoid, Proanthocyanidin, Antioxidant, Response surface methodology, Ultrasound assisted extraction, Extraction solvent, Optimization
Food Science; Lemon scented tea tree; Bioactive compound; Phenolic compound; Flavonoid; Proanthocyanidin; Antioxidant; Response surface methodology; Ultrasound assisted extraction; Extraction solvent; Optimization
1. Introduction
Lemon scented tea tree (Leptospermum petersonii) is a native Australian plant, naturally grown as a tall shrub or small tree in coastal areas of Queensland and New South Wales. The tree is well known for its pleasant lemony aroma and as a medicinal plant (Weiss, 1997). Its leaves contain up to 3.7% essential oil, which has antifungal and antimicrobial activities (Demuner et al., 2011; Kurekci et al., 2013). Recently lemon scented tea tree has been grown in other parts of world such as Brazil, Kenya, Democratic Republic of Congo, Guatemala and South Africa (Van Vuuren et al., 2014). To date, the leaves have predominantly been used for essential oil; though preliminary studies show they have a high quantity of phenolic compounds and strong antioxidant properties, which may contribute to the medicinal properties of the plant.
The techniques applied for extraction of phytochemicals such as phenolics can be divided into three groups: (1) traditional, (2) modern, and (3) a combination of modern and traditional or of two or more techniques. Among the modern techniques, ultrasound assisted extraction (UAE) is a promising and widely used method for extraction of phytochemicals from plant materials because of its simplicity, high extraction yield and quality, is less expensive and easy to scale up (Khoddami et al., 2013; Lee and Lin, 2007; Carrera et al., 2012). Extraction efficiency of phenolic compounds depends on a number of parameters including extraction method, time, temperature, particle size, porosity of sample matrix, solvent type, concentration of solvent, pH, sample to solvent ratio and diffusivity of solvent in the sample (Wang et al., 2008; Irakli et al., 2018; Goltz et al., 2018). For UAE, sonication power also affects extraction efficiency. The effects of extraction parameters on the extraction yield of phytochemicals are reported for various plant materials. For example Vuong et al. (2011), reported the effect of extraction temperature, time, sample to solvent ratio, and pH on the extraction yield of catechins from green tea leaves. The influence of temperature, solvent, and pH on the selective extraction of phenolic compounds from tiger nuts by-products was found by Roselló-Soto et al. (2019).
The selection of suitable extraction solvents is an often overlooked variable for extraction efficiency and end-applications. According to the Food and Drug Administration (FDA) there are a few solvents that are allowed for application in the food and pharmaceutical sectors (FDA, 2017), because of toxic residue of solvent in extract. However, there is limited information about the effects of extraction parameters on extraction yield of total phenolic compounds and antioxidant properties of lemon scented tea tree. Therefore, identification of optimal ranges of extraction parameters is very crucial to get maximum recovery of phytochemicals from a specific type of sample. Response surface methodology (RSM) is a unique, time and cost effective, and mostly use statistical tool for optimising the experimental parameters (Vuong et al., 2015a; Wang et al., 2011). Which provides a functional relationship between a group of extraction parameters and an individual response (Khuri and Mukhopadhyay, 2010).
There have been no previous studies on the effect of solvent and ultrasound assisted extraction parameters on extraction yield of phenolic compound and antioxidant from lemon scented tea tree leaves; and comparison between modern and conventional extraction techniques for lemon scented tea tree leaves. Therefore, the aims of this study were: (i) to investigate the impact of different solvents, which are allowed for application in the food and pharmaceutical sectors; (ii) to determine the effects of UAE extraction parameters, and optimise extraction conditions using UAE technique for maximum recovery of phenolics and antioxidant properties from lemon scented tea tree leaves; (iii) to compare extraction efficiency of UAE technique with conventional shaking water bath; and finally (iv) to investigate the correlation between the phenolic groups and antioxidant properties of lemon scented tea tree leaves extract.
2. Materials and methods
2.1. Plant sample collection and preparation
Lemon scented tea tree leaves were randomly collected from the trees grown in Central Coast, New South Wales, Australia. The trees were authenticated through the herbarium at the University of Newcastle, NSW, Australia (associated number Leptospermum petersonii (10637)). The leaves were transported to the lab immediately after collection. The fresh leaves were dipped in liquid nitrogen and then dried using a freeze dryer (SP Scientific, Bench Top Pro BTP-3ESE0X, Warminster, Philadelphia, USA) for 48 h to minimise degradation. Dried leaves were then ground to reduce particle sizes and homogenised using a commercial blender (John Morris Scientific, Chatswood, NSW, Australia) and then passed through a steel mesh sieve with pore size of 1.4 mm (EFL 2000, Endecotts Ltd., London, England). Finally the ground powdered leaf sample was preserved in an airtight container and store at -18 °C for further extraction and assays.
2.2. Chemical and reagents
The organic solvents acetone, ethanol and methanol were purchased from Merck (Darmstadt, Germany). Folin-Ciocalteu's reagent and other chemicals including anhydrous sodium carbonate (Na2CO3), sodium nitrite (NaNO2), hydrochloric acid (HCl), potassium persulfate (K2S2O8), copper (II) chloride (CuCl2), ferric chloride (FeCl3), Aluminium chloride hexahydrate (AlCl3·6H2O), ammonium acetate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), neocuproine, gallic acid, and catechin were purchased from Sigma-Aldrich Pty Ltd. (Castle Hill, Sydney, Australia). Vanillin and sodium hydroxide (NaOH) were obtained from Merck (Darmstadt, Germany). All the solvents and chemicals used in this study were analytical grade.
2.3. Experimental design
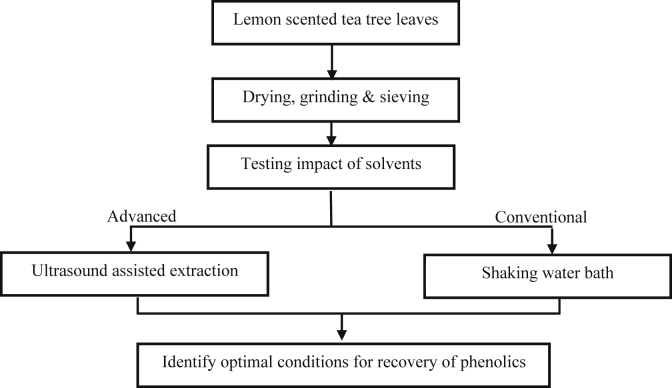
In this study, different solvents were tested to determine the best extraction solvent for recovery of phenolic compounds from lemon scented tea tree leaves. The best solvent was then applied for further investigation to determine the effect of extraction parameters in two extraction techniques on recovery yields of phenolics. A conventional technique using a shaking water bath, and advanced technique using ultrasound, were applied. Levels of phenolic compounds and antioxidant capacity after extraction were compared for identification of suitable conditions for maximum recovery of phenolic compounds from lemon scented tea tree leaves. Figure 1 shows the experimental design.
Figure 1.
Experimental design.
2.4. Extraction of phenolic compounds using different solvents
Five solvents were used in this study to investigate the effect of solvent selection on extraction yields of phenolic compounds and antioxidant properties of lemon scented tea tree leaves. The solvents were deionized water, acetone, ethanol, 50% acetone in deionized water and 50% ethanol in deionized water. For extraction, 25 mL preheated solvent was added with 0.25 g of the freeze-dried ground leaves in 50 mL plastic centrifuge tube. The tubes containing sample and solvent were closed with screw cap to prevent solvent loss during extraction; followed by sonication using a preheated ultrasonic bath (Soniclean, 220 V, 50 Hz and 250 W, Soniclean Pty LTD, Thebarton, SA, Australia); the extraction was carried out at 30 °C and 150 W power level for 15 min. During sonication the tubes were vortexed for 1–3 s in every 5 min to facilitate better extraction. After extraction the tubes were immediately cooled down in an ice bath followed by filtration using 0.45 μm cellulose syringe filter (Phenomenex Australia Pty. Ltd., Australia). Finally the extracts were preserved at -18 °C for further analysis of phenolic compounds and antioxidant capacity.
2.5. Optimisation of UAE extraction conditions using Response Surface Methodology (RSM)
Response Surface Methodology (RSM) was applied for optimisation of UAE extraction conditions. RSM design and analysis were conducted based on JMP software (version 14). A three level, three factor Box–Behnken design was applied with three central point replicates for designing experimental conditions to investigate the individual and interaction effects of extraction parameters on extraction yield of phenolics and antioxidant properties. The individual UAE parameters for RSM were extraction time (30, 45 and 60 min), temperature (30, 40 and 50 °C) and power (150 W (60%), 200 W (80%), 250 W (100%)). As the boiling point of acetone is 56 °C, in order to prevent excessive pressure build up in tubes during extraction in this study, 50 °C was selected as the highest temperature range for optimization. The individual independent variables were coded at three levels (-, 0, +) (Table 1). The extraction operation was performed according to the RSM design using UAE. The relationship between the response and independent variables was postulated by following the second order polynomial model equation (Eq. (1)).
| (1) |
where, Y is predicted response for phytochemicals or antioxidant; Xi and Xj are the independent variables, β0 is coefficient for intercept, βi, βii and βij represent the regression coefficients of the linear, quadratic and interaction effects respectively; n is the number of variables. The Eq. (1) is written in simplified form using coding of individual variables as
| (2) |
Table 1.
Box–Behnken design of experimental conditions and observed responses for TPC, TFC, Pro.A content and antioxidant capacity of lemon scented tea tree leaves extracted using ultrasonic assisted extraction and 50% acetone.
| Run | Experimental conditions (Independent variables) |
Observed responses (dependent variables) (n=3) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern | X1 | X2 | X3 | Phytochemicals |
Antioxidant capacity |
||||||
| TPC | TFC | Pro. A | FRAP | CUPRAC | ABTS | DPPH | |||||
| 1 | +0+ | 60 | 40 | 250 | 86.56961 | 68.88355 | 104.86 | 518.1493 | 4907.362 | 1442.833 | 739.8215 |
| 2 | 000 | 45 | 40 | 200 | 89.90219 | 66.25174 | 108.0865 | 538.0879 | 5062.372 | 1464.213 | 800.6135 |
| 3 | 0−+ | 45 | 30 | 250 | 75.40548 | 60.09899 | 95.43791 | 462.0825 | 4240.286 | 1285.927 | 700.7808 |
| 4 | −0− | 30 | 40 | 150 | 75.72089 | 62.9679 | 95.83176 | 460.6339 | 4371.779 | 1279.885 | 728.9459 |
| 5 | −+0 | 30 | 50 | 200 | 86.68863 | 69.48223 | 102.0122 | 592.5358 | 4825.153 | 1422.197 | 775.5159 |
| 6 | 0+− | 45 | 50 | 150 | 95.22241 | 74.44356 | 110.5961 | 601.9087 | 5395.706 | 1490.333 | 853.0396 |
| 7 | 0 | 60 | 50 | 200 | 97.73374 | 77.30654 | 114.4942 | 669.9046 | 5524.949 | 1660.067 | 971.5561 |
| 8 | 0++ | 45 | 50 | 250 | 94.58565 | 70.91076 | 112.227 | 654.2263 | 5459.305 | 1594.348 | 925.8226 |
| 9 | −0+ | 30 | 40 | 250 | 72.98936 | 63.75626 | 95.75602 | 469.4104 | 4388.957 | 1288.994 | 738.1484 |
| 10 | 000 | 45 | 40 | 200 | 81.24344 | 72.20296 | 106.6323 | 542.5187 | 5015.746 | 1510.969 | 820.4127 |
| 11 | 000 | 45 | 40 | 200 | 84.57007 | 69.0436 | 105.4558 | 556.2372 | 4755.419 | 1423.871 | 808.7005 |
| 12 | +−0 | 60 | 30 | 200 | 80.51741 | 64.2601 | 107.6876 | 508.9468 | 4459.714 | 1410.485 | 783.324 |
| 13 | 0−− | 45 | 30 | 150 | 75.38168 | 62.84935 | 95.66513 | 437.7982 | 4354.397 | 1329.708 | 693.8093 |
| 14 | −−0 | 30 | 30 | 200 | 72.84059 | 65.6175 | 94.20081 | 437.9686 | 4251.943 | 1272.82 | 710.8199 |
| 15 | +0− | 60 | 40 | 150 | 89.04524 | 80.50147 | 105.678 | 556.4076 | 4975.256 | 1500.837 | 805.633 |
X1 (extraction time, min), X2 (extraction temperature, °C), and X3 (ultrasonic power, W), TPC (mg GAE/g DW), TFC (mg CE/g DW), Proanthocyanidins (mg CE/g DW), FRAP (mM TE/g DW), ABTS (mM TE/g DW), DPPH (mM TE/g DW), CUPRAC (mM TE/g DW).
2.6. Conventional extraction
Shaking water bath extraction of phenolic compounds was carried out under the extraction conditions of sample to solvent ratio 1 g/100 mL for 60 min at 50 °C. Solvent selection was based on the previous experiment, in which 50% acetone gave the highest yields and thus selected for extraction. The shaking water bath was set at an agitation rate of 200 rpm. For the experiment, 0.25 g of the freeze-dried ground leaves were put into 50 mL plastic centrifuge tubes with 25 mL solvent. The tube was sealed using a screw cap and put into the preheated 50 °C shaking water bath. The tube was vortex 1–2 s in every 5 min. At the end of extraction time the tube was immediately cooled down in an ice bath. Finally, the solution was filtered using 0.45 μm cellulose syringe filter (Phenomenex Australia Pty. Ltd., Australia) and the filtrate (extract) stored at -18 °C for further analysis of phenolic compounds and antioxidant capacity.
2.7. Phenolic compound assays
The level of total phenolic, total flavonoid and proanthocyanidin content in the extracts were assessed using spectrophotometric methods as described in previous studies by Škerget et al. (2005), Zhishen et al. (1999) and Li et al. (2006), respectively. A UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia) set to 765 nm, 510 nm and 500 nm was used to measure absorbance for total phenolic content (TPC), total flavonoid content (TFC) and proanthocyanidin content (Pro.A), respectively. Gallic acid was used to prepare standard curve for TPC and the results expressed as mg of gallic acid equivalent per g of sample dry weight (mg GAE/g DW). Catechin was used to build standard curve for TFC and Pro.A and the results are expressed as mg of catechin equivalent per gram of sample dry weight (mg CE/g DW).
2.8. Antioxidant assays
The antioxidant capacity of the extract was evaluated through measuring ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), ABTS free radical scavenging activities and DPPH free radical scavenging activities. FRAP, ABTS and DPPH were measured according to the method described by Thaipong et al. (2006) and CUPRAC was measured by following the method described by Apak et al. (2004). A UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia) was used to measure the absorbance at 593 nm, 450 nm, 734 nm, 515 nm for FRAP, CUPRAC, ABTS and DPPH, respectively. Trolox was used to prepare standard curve for all four types of assays and the results are expressed as mM of trolox equivalents per g of sample dry weight (mM TE/g DW).
2.9. Statistical analysis
To compare the effects of extraction solvents on extraction yield of phenolic compounds and antioxidant properties of a one-way analysis of variance (ANOVA), all pair mean comparison Tukey-Kramer HSD hoc test was performed using JMP software (Version 14.1, SAS Institute Inc., Cary, USA). All the experiments were conducted in triplicates and the results are expressed as means ± standard deviation (n = 3). RSM model fitting analysis, predicted polynomial formula generation for each response, comparison of experimental and predicted response values by paired comparison student's t-test were also carried out by using same JMP software. The p-value less than 0.05 (p < 0.05) is considered as statistically significant. To compare UAE and shaking water bath methods, a paired comparison student's t-test was performed. The Pearson correlation analysis was carried out using JMP software to evaluate the correlation between different phenolic compound groups and antioxidant capacities in the extract.
3. Result and discussion
3.1. Effects of solvent on extraction yield of phenolic compounds and antioxidant capacity
In the field of extraction chemistry, the selection of suitable solvents is crucial for extracting maximum amount of desired chemical compounds. In this study, solvents which have various polarities and are safe for food and pharmaceutical uses were applied for extraction. The results (Table 2) show that the extraction solvents have a significant influence on the extraction yield of phenolic compounds and antioxidant properties of lemon scented tea tree leaves. The binary solvents (mixture of water and organic solvent) gave higher extraction efficiency as compared to single solvents. Among the combined solvent mixtures, 50% acetone in water gave the highest extraction yields of TPC (60.24 ± 6.21 mg GAE/g DW), TFC (33.87 ± 0.69 mg CE/g DW), and Pro.A (45.99 ± 0.88 mg CE/g DW). Results from the four antioxidant assays show that this solvent mixture obtained the most potent antioxidant properties with FRAP (304.77 ± 19.99 mM TE/g DW), CUPRAC (3542.43 ± 317.95 mM TE/g DW), ABTS (1078.84 ± 16.28 mM TE/g DW) and DPPH (556.63 ± 34.72 mM TE/g DW). Similar findings were reported by Moo-Huchin et al. (2019) and Irakli et al. (2018), who found that solvents have significant impact on the extraction yields of TPC, TFC and antioxidant capacity, and organic solvents containing 50% water gave higher yields of phenolic and antioxidant capacity in comparison with single solvents. The difference in extraction yields can be explained by variation of polarity of the extraction solvents (Vuong et al., 2013). Acetone has been grouped in Class 3 by the U.S Food and Drug Administration and it is regarded as less toxic and of lower risk to human health and is normally accepted in pharmaceuticals (FDA, 2017). Therefore, a mixture of acetone: water (50:50 v/v) was selected as the best solvent for further extraction of phenolic compounds.
Table 2.
Effect of solvent on TPC, TFC, proanthocyanidin (Pro.A) content, and antioxidant activity from lemon scented tea tree leaves using ultrasound assisted extraction.
| Solvent | TPC (mg GAE/g DW) | TFC (mg CE/g DW) | Pro. A (mg CE/g DW) | FRAP (mM TE/g DW) | DPPH (mM TE/g DW) | ABTS (mM TE/g DW) | CUPRAC (mM TE/g DW) |
|---|---|---|---|---|---|---|---|
| Water | 22.46 ± 3.68c | 13.05 ± 1.95c | 18.97 ± 2.83c | 153.75 ± 19.43b | 259.09 ± 22.36c | 432.18 ± 33.78c | 1426.21 ± 70.85c |
| Acetone | 7.99 ± 0.08d | 4.84 ± 0.09d | 23.89 ± 0.93b | 25.93 ± 0.31c | 503.69 ± 9.82ab | 215.07 ± 24.91d | 348.67 ± 13.56d |
| Ethanol | 7.35 ± 1.03d | 5.27 ± 0.57d | 3.79 ± 0.24d | 25.77 ± 7.36c | 34.88 ± 7.79d | 132.29 ± 14.36e | 397.80 ± 39.71d |
| 50% Acetone | 60.24 ± 6.21a | 33.87 ± 0.69a | 45.99 ± 0.88a | 304.77 ± 19.99a | 556.63 ± 34.72a | 1078.84 ± 16.28a | 3542.43 ± 317.95a |
| 50% Ethanol | 35.11 ± 2.84b | 19.98 ± 2.01b | 27.34 ± 2.54b | 160.34 ± 35.43b | 485.77 ± 12.80b | 859.71 ± 17.83b | 2016.48 ± 249.61b |
The values are the mean ± standard deviation for at least triplicate experiments and those in the same row not sharing the same superscript letter are significantly different from each other at p < 0.05. mg GAE/g DW = mg gallic acid equivalent per gram of sample dry weight, mg CE/g DW = milligram catechin equivalent per gram of sample dry weight, mM TE/g DW = mM trolox equivalent per gram of sample dry weight.
3.2. Optimisation of UAE extraction conditions
3.2.1. Testing the models
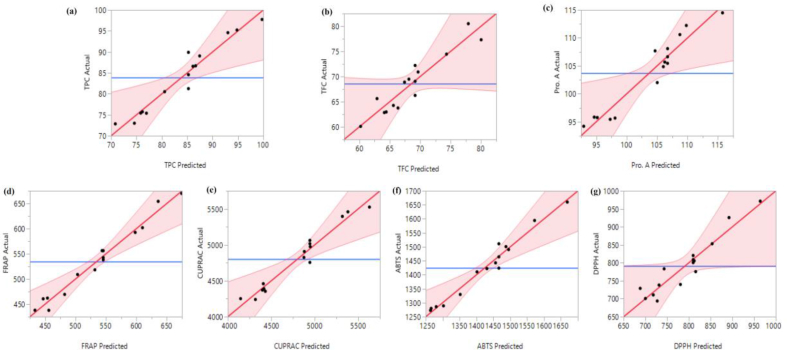
Fitting of models represents how precisely the RSM mathematical models can predict the optimal variance and the relationship between the selected parameters. The reliability of the model's analysis of variance results are presented in Table 3 and Figure 2. The coefficient of determination (R2) is a measure of the degree of fit (Wang et al., 2013). This study found that R2 for TPC, TFC and Pro.A were 0.94, 0.88 and 0.93, respectively, reflecting that 94%, 0.88%, and 93% of the actual TPC, TFC, and Pro.A levels could be matched with those predicted by the models accordingly. Fitting of the models was further supported by low RMSE values and insignificant lack of fit (p > 0.05) values (Table 3). RMSE value is a measure of standard deviation of prediction errors and lack of fit is an indicator of null hypothesis. F-values of the models for TPC, TFC and Pro.A were 9.01, 3.98 and 7.03, respectively. In addition, p-values, which represents confidence level of models were significant, as p-value of models was less than 0.05, which these further confirm the reliability of the models for the prediction of the responses in the current study (Mota et al., 2012).
Table 3.
Analysis of variance for determination of optimisation model fit.
| Parameters | Phytochemicals |
Antioxidant capacity |
|||||
|---|---|---|---|---|---|---|---|
| TPC | TFC | Pro. A | FRAP | CUPRAC | ABTS | DPPH | |
| R2 | 0.94 | 0.88 | 0.93 | 0.98 | 0.96 | 0.97 | 0.90 |
| Adjusted R2 | 0.84 | 0.66 | 0.79 | 0.93 | 0.89 | 0.92 | 0.71 |
| RMSE | 3.38 | 3.37 | 3.06 | 18.94 | 147.52 | 34.36 | 42.89 |
| Lack of fit | 0.809 | 0.429 | 0.109 | 0.145 | 0.649 | 0.787 | 0.032 |
| F ratio of model | 9.01 | 3.98 | 7.03 | 23.00 | 13.51 | 17.75 | 4.86 |
| P of model > F | 0.013 | 0.07 | 0.02 | 0.001 | 0.005 | 0.002 | 0.048 |
Figure 2.
Correlation between the predicted and experimental values for phenolic compounds, and antioxidant capacities: (a) TPC, (b) TFC, (c) Pro.A, (d) FRAP, (e) CUPRAC, (f) ABTS, (g) DPPH.
In case of antioxidant properties, For the FRAP assay, analysis of variance results show that the coefficient of determination (R2) value, RMSE, lack of fit, F value and p-value of the model were 0.98, 18.94, 0.145, 23.00 and 0.001, respectively, revealing the RSM model for FRAP was fitted well with the experimental data. For CUPRAC, values of R2, p-value, F value and RMSE (0.96, 0.01, 13.51 and 147.52, respectively) confirmed that the model for CUPRAC is reliable. Similarly, for ABTS and DPPH, values of R2, RMSE, lack of fit, F-ratio of model and p-value clearly indicated that models for ABTS and DPPH were fitted for analysis and prediction (Table 3, Figure 2). Overall, Analysis of variance revealed that the RSM models were reliable for analysing the effects of the UAE parameters on recovery of phenolic compounds and for prediction of the optimal conditions. RSM models for phenolic compounds and antioxidant properties is expressed by the following second-order polynomial Eqs. (3), (4), (5), (6), (7), (8), and (9):
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
3.2.2. Effects of different extraction parameters on phenolic compounds
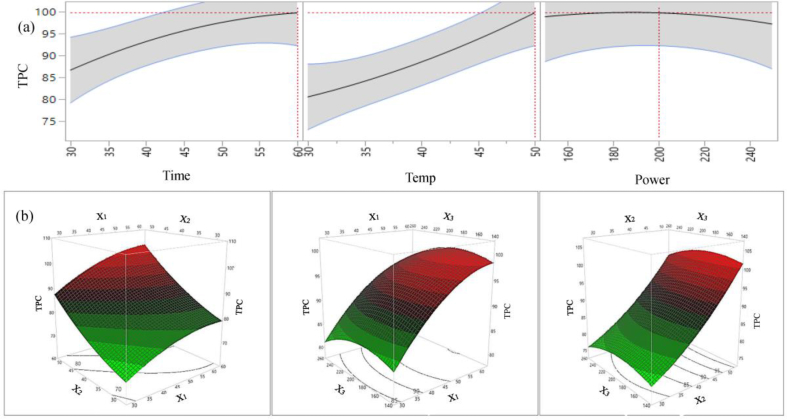
The individual, interactions and quadratic effects of UAE time, temperature and sonication power level on the yield of TPC, TFC and Pro.A are presented in Table 4. Figure 3 (a) and (b) shows the 2D counter plot and 3D surface plot of the parameters effects on extraction yield phenolic compounds respectively. From the results it can be seen that there is a linear positive significant effect of extraction time and temperature on extraction yield of TPC (p < 0.05); however, the effect of sonication power on TPC yield is insignificant. The interaction and quadratic effects of the extraction variables on UAE extraction yield of TPC are also insignificant. The TPC yield increased with increasing extraction time and temperature and it is maximised at extraction time 60 min and temperature 50 °C (Figure 3a). Nevertheless, the effect of sonication power on TPC extraction yield is negligible. Similar results are observed for a number of different plant materials; for example Santos et al. (2016) studied the effects of extraction time and temperature on extractable phenolic compounds from Aspalathus linearis leaves and they found extraction time and temperature significantly and positively influence the total phenolic compound and flavonoid yield. Papoutsis et al. (2016) and Dang et al. (2017) also reported positive significant effect of extraction time and temperature on extractable total phenolic compounds during extraction and optimization of UAE parameters for lemon pomace peel and brown alga respectively.
Table 4.
Analysis of variance for the experimental results for extraction parameters.
| Model Parameter | DF | Phytochemicals |
Antioxidant capacity |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC |
TFC |
Pro. A |
FRAP |
CUPRAC |
ABTS |
DPPH |
|||||||||
| Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | Estimate | Prob>|t| | ||
| Intercept | |||||||||||||||
|
β0 |
1 |
85.24 |
<.0001 |
69.16 |
<.0001 |
106.72 |
<.0001 |
545.61 |
<.0001 |
4944.5 |
<.0001 |
1466.35 |
<.0001 |
809.91 |
<.0001 |
| Linear term | |||||||||||||||
| β | 1 | 5.70 | 0.0050∗∗ | 3.64 | 0.0283∗ | 5.61 | 0.0035∗∗ | 36.61 | 0.0028∗∗ | 253.68 | 0.0046∗∗ | 93.79 | 0.0006∗∗∗ | 43.36 | 0.0354∗ |
| β2 | 1 | 8.76 | 0.0007∗∗∗ | 4.91 | 0.0092∗∗ | 5.79 | 0.0031∗∗ | 83.97 | 0.0001∗∗∗ | 487.35 | 0.0002∗∗∗ | 108.50 | 0.0003∗∗∗ | 79.65 | 0.0033∗∗ |
| β3 | 1 | -0.727 | 0.5691 | -2.14 | 0.1328 | 0.063 | 0.9553 | 5.89 | 0.4195 | -12.65 | 0.8179 | 1.42 | 0.9116 | 2.89 | 0.8562 |
| Interactions | |||||||||||||||
| β12 | 1 | 0.842 | 0.6393 | 2.29 | 0.2316 | -0.251 | 0.8761 | 1.59 | 0.8727 | 123.00 | 0.1562 | 25.05 | 0.2046 | 30.88 | 0.2094 |
| β13 | 1 | 0.064 | 0.9713 | -3.10 | 0.1253 | -0.186 | 0.9082 | -11.76 | 0.2696 | -21.27 | 0.7847 | -16.78 | 0.3736 | -18.75 | 0.4219 |
|
β23 |
1 |
-0.165 |
0.9259 |
-0.195 |
0.9122 |
0.465 |
0.7737 |
7.00 |
0.4927 |
44.43 |
0.5732 |
36.95 |
0.0842 |
16.45 |
0.4777 |
| Quadratic | |||||||||||||||
| β11 | 1 | -2.43 | 0.2254 | 0.976 | 0.6021 | -2.538 | 0.1720 | -15.56 | 0.1753 | -190.33 | 0.0559∗ | -35.95 | 0.1006 | -19.92 | 0.4132 |
| β22 | 1 | 1.63 | 0.3946 | -0.976 | 0.6023 | 0.4119 | 0.8063 | 22.29 | 0.0733 | 11.26 | 0.8892 | 10.99 | 0.5656 | 20.31 | 0.4046 |
| β33 | 1 | -1.73 | 0.3712 | -1.11 | 0.5532 | -3.655 | 0.0702 | -28.89 | 0.0326∗ | -93.34 | 0.2783 | -52.26 | 0.0329∗ | -36.85 | 0.1597 |
Significantly different at ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < .001; β0: intercept; β1, β2, and β3: linear regression coefficients for time, temperature, and power; β12, β13, and β23: regression coefficients for interaction between time × temperature, time × power, temperature × power; β11, β22, and β33: quadratic regression coefficients for time × time, temperature × temperature, and power × power.
Figure 3.
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of TPC.
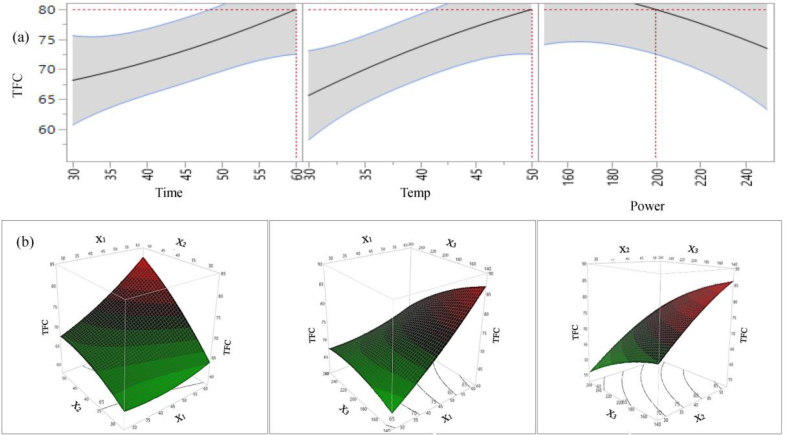
In case of TFC, the effect of extraction time and temperature on extractable TFC is positively significant. However, the linear effects of sonication power, interactions, and quadratic effect of all parameters are statistically non-significant (p > 0.05). The extractable TFC is increased with increasing time, and conversely it is decreased with increasing sonication power (Figure 4a). The maximum TFC yield is found at the highest range of time (60 min) and temperature (50 °C), similar to TPC. Our findings are supported by Zhong et al. (2019) where they optimized UAE condition for extracting flavonoids from Dendranthema indicum var. aromaticum and found optimized condition for total flavonoid was the highest limit of time and temperature in their experimental RSM design.
Figure 4.
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of TFC.
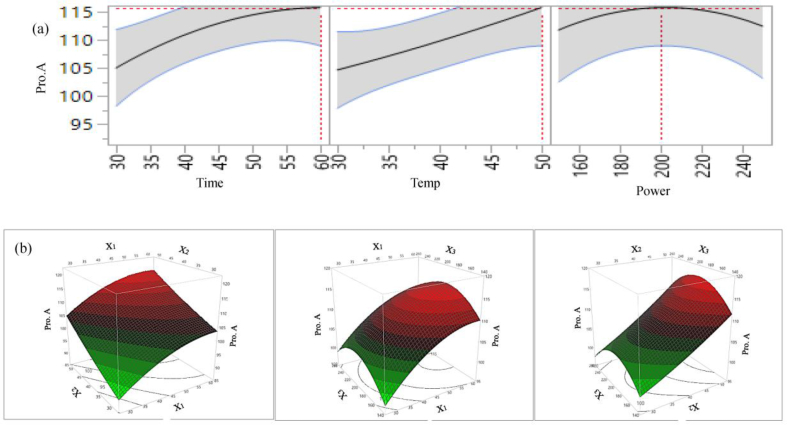
For Pro.A, the results (Table 4) show that the effect of extraction parameters time, temperature and sonication power on the extraction yield of Pro.A is similar to two other phenolic groups (TPC and TFC). There is a significant linear positive effect of extraction time and temperature on extractable Pro.A. However, the influence of sonication power on Pro.A is insignificant. From the 2D counter plot of extraction parameter (Figure 5a) it can be seen that Pro.A yield is higher with increasing time and temperature, with maximum Pro.A at 60 min and 50 °C. On the other hand, Pro.A yield initially increased with increasing sonication power and continued up to 200 W followed by decline again with sonication power above 200 W.
Figure 5.
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of Pro.A.
Temperature plays an important role during extraction which increases extraction yields of phenolic compounds. Temperature does this by: (i) softening the plant tissues to increase the rate of solvent penetration into the sample (Al-Farsi and Lee, 2008), (ii) reducing the viscosity and density of solvents, (iii) breaking down certain chemical bonds and help to release compounds from the sample, (iv) increasing the diffusion coefficient and mass transfer rate (Setford et al., 2017; Cacace and Mazza, 2003; Bucić-Kojić et al., 2007), and (v) increasing the solubility of chemical compounds (Dai and Mumper, 2010). However, certain compounds such as heat sensitive compounds may degrade when exposed to higher temperatures for a longer time (Cacace and Mazza, 2003). In this study, extraction with the maximum temperature and time range gave higher extraction yields of phenolic compounds and antioxidant properties than extraction with short times and lower temperatures.
Ultrasonic power, which represents wave frequency and distribution, also influences extraction yield of certain phenolic compounds (Khoddami et al., 2013). In this study, we found a negative linear effect of sonication power on TPC and TFC, a negative interaction effect of (time and power) on TFC and Pro.A, a negative interaction effect of temperature and power on TPC and TFC, a negative quadratic effect of time on TPC and Pro.A, a negative quadratic effect of temperature on TFC and a negative quadratic effect of sonication power on different phenolic groups and antioxidant properties (Table 4). However, all these negative linear and quadratic effects are not significant. From Figure 3 (b) and 5 (b) it can be seen that TPC and Pro.A yield in the extract was gradually increased with increasing extraction time up to certain time and start declining at around time 60 min.
3.2.3. Effects of extraction parameters on antioxidant properties
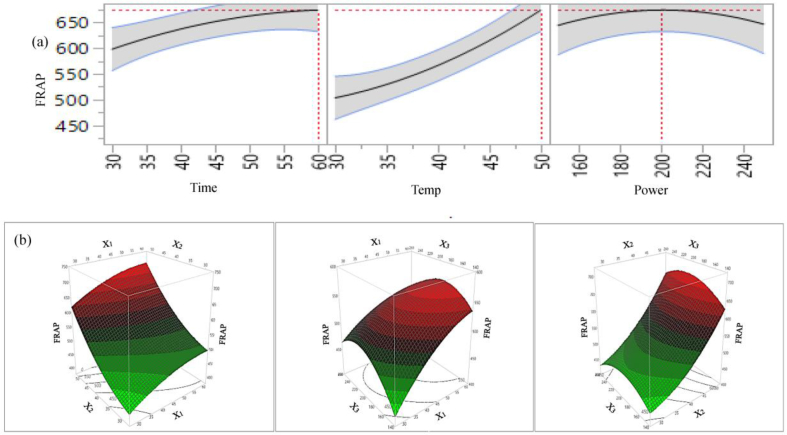
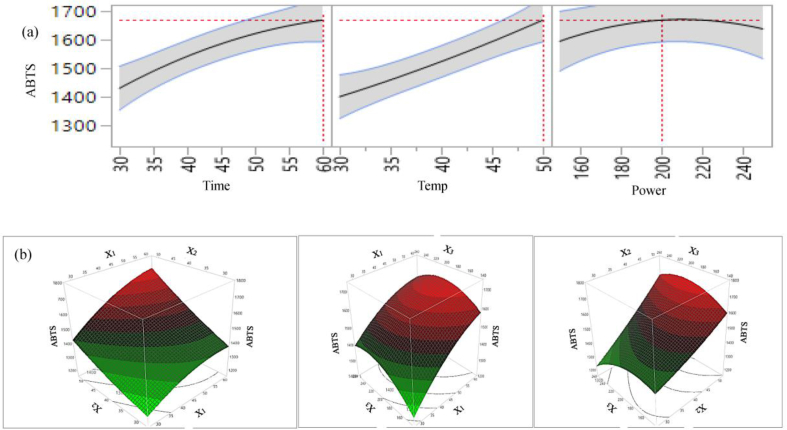
The effects of individual parameters and their interaction on antioxidant capacity of lemon scented tea tree leaves extract are presented in Table 4, and 2D counter plot and 3D surface plot in Figures 6 (a & b), 7 (a & b), 8 (a & b), and 9 (a & b). For FRAP and ABTS the individual parameters including extraction time, temperature, sonication power; and the interaction of time × temperature, temperature × power and quadratic effects of temperature × temperature positively influence antioxidant capacity. On the other hand, interaction of time × power and quadratic effects of time × time, and power × power have a negative influence on FRAP and ABTS capacity. Among the different parameters, the effects of time, temperature, and power × power on FRAP and ABTS is significant (p < 0.01 or 0.05) and conversely, other parameters including individual sonication power, all interactions, and quadratic time × time, temperature × temperature effects are not statistically significant (p > 0.05).
Figure 6.
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of FRAP.
Figure 7.
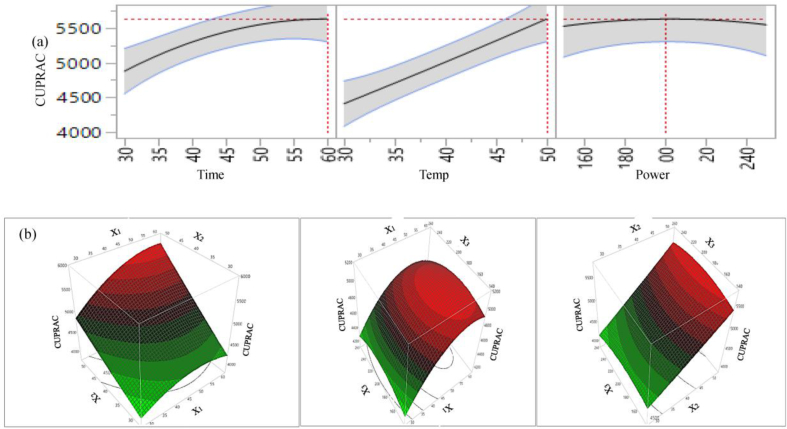
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of CUPRAC.
Figure 8.
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of ABTS.
Figure 9.
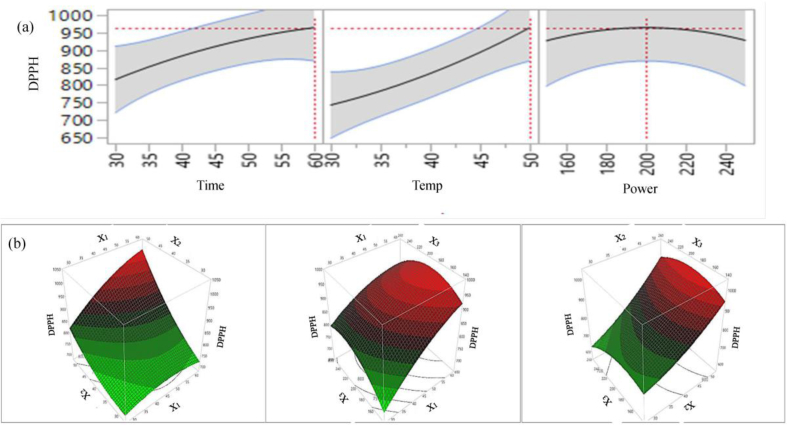
2D counter plot (a) and 3D surface plot (b) of the effects of extraction parameters on extraction yield of DPPH.
For CUPRAC, extraction time and temperature has a positive significant effect (p < 0.01) and quadratic time × time has a negative significant effect on antioxidant capacity. The interaction: time × temperature, temperature × power and quadratic: power × power positively influence; and sonication power, time × power, power × power negatively influence the CUPRAC capacity. However, their influence on CUPRAC capacity of the extract is not significant. For DPPH, all individual parameters, interaction of time × temperature, and temperature × power; quadratic: temperature × temperature positively influence the DPPH capacity. However, among the extraction parameters, only extraction time and temperature have significant (p < 0.05 and p < 0.01, respectively) effect on DPPH capacity in lemon scented tea tree leaf extracts. 2D Figures (6a, 7a, 8a, 9a) revealed the positive influence of time and temperature on antioxidant capacities. The highest FRAP, CUPRAC, ABTS and DPPH values are observed at 60 min, 50 °C and 200 W. Our findings are in agreement with Tabaraki et al. (2012) who reported extraction temperature considerably influences the FRAP activity of pomegranate extracts, however in their temperature effects on DPPH value was not significant. For all four antioxidant assays, from figures 6(b), 7(b), 8(b), 9(b) it can be see that free radical scavenging and ion reducing properties of extract were gradually increased with increasing extraction time up to certain time and start declining at around time 60 min.
3.3. Optimization and validation of the models
Optimal extraction conditions for maximum yield of phenolic compounds and antioxidant capacities from lemon scented tea tree leaves were further predicted using Box-Behnken RSM mathematical models. The optimal UAE conditions were extraction time: 60 min, temperature: 50 °C, and sonication power: 200 W. At these extraction conditions 94.88% of TFC and 100% of TPC, Pro.A, FRAP, CUPRAC, ABTS and DPPH could be obtained under their individual ideal conditions. To confirm these predicted conditions were in line with the actual conditions, validation of the optimal condition was performed through experimental extraction process by using same extraction conditions (60 min, 50 °C, 200 W and 50% acetone in water as solvent). The predicted and experimental values are presented in Table 5. Student's t-test was performed to determine the significant difference between the predicted and experimental extraction yields of phenolic compounds and antioxidant properties. It can be seen that there is no significant difference between predicted and experimental values of phenolic compounds and antioxidant capacities. Therefore, it can be concluded that the predicted optimal UAE extraction is validated with experimental results.
Table 5.
Validation of the predicted value for phytochemical content, antioxidant capacity and individual compounds.
| Phenolic compounds and antioxidant properties | Values (n = 3) |
|
|---|---|---|
| Predicted | Experimental | |
| TPC (mg GAE/g DW) | 99.75 ± 7.52a | 98.91 ± 1.20a |
| TFC (mg CE/g DW) | 80.02 ± 7.51a | 76.12 ± 0.79a |
| Proanthocyanidins (mg CE/g DW) | 115.75 ± 6.81a | 117.71 ± 2.18a |
| FRAP (mM TE/g DW) | 674.52 ± 42.18a | 581.29 ± 14.23a |
| CUPRAC (mM TE/g DW) | 5629.47 ± 328.4a | 5534.87 ± 19.56a |
| ABTS (mM TE/g DW) | 1668.74 ± 76.48a | 1636.18 ± 4.11a |
| DPPH (mM TE/g DW) | 964.20 ± 95.49a | 889.29 ± 20.68a |
All the values are means ± standard deviations and those in the same row sharing the same superscript letter are not significantly different from each other (p < 0.05).
3.4. Comparison of novel and traditional extraction techniques
The influence of different extraction methods on extraction yield of phenolic compounds and their antioxidant properties for various plant materials has been studied widely (Uzel, 2018; Das et al., 2019). There are different advantages and limitations in the use of advanced and conventional techniques in the extraction of phenolic compounds from plant materials. UAE is known as a common advanced extraction technique. It is efficient with only a short time required for extraction; however, it is costly for setting up. In contrast, conventional extraction with only temperature control and agitation is inexpensive to establish as compared to advanced techniques, but has varied efficiency and can be time consuming. In this study, UAE and extraction using a shaking water bath were compared for their efficiency on extraction yields of TPC, TFC, Pro.A, and antioxidant properties of lemon scented tea tree and the results are shown in Table 6. Paired student's t-test was performed to statistically compare the difference between the yields from the two extraction methods. The TPC value using UAE is significantly higher (15.27%) than the value obtained for shaking water bath extraction. However, there is no significant difference between the two methods for TFC and Pro. A value.
Table 6.
Comparison of ultrasound assisted extraction (UAE), shaking water bath (SWB) extraction method.
| Phenolic compounds | UAE | SWB |
|---|---|---|
| TPC (mg GAE/g DW) | 98.91 ± 1.20a | 85.81 ± 3.35b |
| TFC (mg CE/g DW) | 76.12 ± 0.79a | 77.61 ± 4.26a |
| Pro.A (mg CE/g DW) | 117.71 ± 2.18a | 119.81 ± 7.77a |
| Antioxidant capacities | ||
| FRAP (mM TE/g DW) | 581.29 ± 14.23a | 639.19 ± 20.76a |
| CUPRAC (mM TE/g DW) | 5534.87 ± 19.56a | 4149.61 ± 195.41b |
| ABTS (mM TE/g DW) | 1636.18 ± 4.11a | 1544.65 ± 33.47b |
| DPPH (mM TE/g DW) | 889.29 ± 20.68a | 935.42 ± 10.66a |
Ultrasound assisted extraction (UAE), Shaking water bath (SWB). The same row sharing the same superscript letter are not significantly different from each other (p < 0.05).
Results from CUPRAC and ABTS assays show that antioxidant capacity of the extract prepared using UAE technique is significantly higher as compared to that extract prepared from shaking water bath. However, results from FRAP and DPPH assays indicate that antioxidant capacity of the extract prepared using UAE is insignificantly different as compared to that of shaking water bath. The differences in antioxidant capacity with different assays can be explained by factors that may affect the reactions in the assays. For example, ABTS assay can work well in different pH and in various solvents, but it is challenging to select the right reaction time for this assay. Whereas, for the DPPH assay many phytochemicals can react quickly with peroxyl radicals, but react slowly or even be inert to DPPH due to steric inaccessibility (Vuong et al., 2015b). This is why overall antioxidant capacity should be evaluated, using multiple antioxidant assays. From the results, it can be concluded that the UAE extract has higher antioxidant properties in comparison with the conventional shaking water bath method of extraction.
3.5. Correlation between phenolic compounds and antioxidant capacities
Antioxidant property of an individual sample may vary according to assay used (Thaipong et al., 2006), it is due to the reactions of individual phytochemical with chemicals in various assays were different. In addition, antioxidant capacity of plant extracts prepared from different extraction conditions is also varied because of different soluble phytochemicals under different extraction conditions. It should be noted that antioxidant capacity can be contributed by different groups of phytochemicals, including phenolic compounds. Furthermore, different sub-groups of phenolic compounds, such as flavonoids and proanthocyanidins can possess different antioxidant power. Therefore, this study further investigated the correlations between TPC and their second metabolites (TFC and Pro.A), and different antioxidant assays. The Pearson's correlation analysis was applied to evaluate the correlations and the results are shown in Table 7. Correlation analysis data (Table 7) revealed that there was a significant correlation between phenolic groups (TPC, TFC, and Pro.A), and antioxidant capacity measured by all four antioxidant assays (FRAP, CUPRAC, ABTS and DPPH). The correlation coefficient r value was ranging from 0.726 to 0.957 and probability value p was 0.0022 - <0.0001. The highest correlation was observed for TPC and CUPRAC with r value of 0.957 and p < 0.0001. On the other hand, the lowest correlation (r = 0.726 and p = 0.0022) was found in between TFC and DPPH activity. These findings indicated that antioxidant capacity of lemon scented tea tree leaves is mainly contributed by phenolic compounds, other phytochemicals or vitamins only contributed a minor part. These also further elucidate why the effects of extraction conditions on phenolics were similar tend to those on antioxidant capacity in this study. Previous studies also found a high correlation between phenolic compounds and antioxidant properties in a plant extracts (Sulaiman et al., 2011; Nguyen et al., 2018, and Vu et al., 2017).
Table 7.
Correlation between phenolic compounds and antioxidant capacity.
| TPC |
TFC |
Pro.A |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| FRAP | 0.933 | <0.0001 | 0.755 | >0.001 | 0.894 | <0.0001 |
| CUPRAC | 0.957 | <0.0001 | 0.794 | >0.001 | 0.914 | <0.0001 |
| ABTS | 0.913 | <0.0001 | 0.806 | 0.0003 | 0.946 | <0.0001 |
| DPPH | 0.865 | <0.0001 | 0.726 | 0.0022 | 0.726 | 0.0022 |
4. Conclusion
The influence of solvent on extraction yield of TPC, TFC, Pro.A, and antioxidant capacity was investigated, and 50% acetone in water was found to be the most efficient solvent for lemon scented tea tree leaves. Other UAE parameters including time, temperature and sonication power was optimized using RSM and the optimized UAE conditions for the maximum yield of phenolic compounds and antioxidant properties were time of 60 min, temperature of 50 °C and sonication power of 200 W. The predicted optimized condition was found to be validated through further experimental investigation and the RSM prediction model was also reliable for individual phenolic compound groups and antioxidant capacities. The individual UAE parameter time and temperature have positive significant effects on TPC, TFC, Pro.A, and antioxidant capacities measured by FRAP, CUPRAC, DPPH and ABTS; however, the effect of sonication power was not significant. The UAE was found to be more efficient to extract phenolic compounds and antioxidant capacities from lemon scented tea tree leaves in comparison with a shaking water bath extraction system. Further a strong correlation between different phenolic groups and antioxidant capacities elucidate phenolic compounds are major antioxidant contributor in lemon scented tea tree leaf extract. Finally, this study recommends applying these UAE optimal conditions as a sustainable green technique for extraction of phenolic compounds from lemon scented tea tree leaves. This study also recommends further research on the identification, quantification and isolation of individual phenolic compounds from this plant leaves extract; which may indicate the importance of this plant leaf extract in food and pharmaceutical sectors.
Declarations
Author contribution statement
Md Saifullah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rebecca McCullum: Analyzed and interpreted the data.
Adam McCluskey: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Quan Vuong: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Md Saifullah was supported by the Australian Government Research Training Program (RTP) scholarship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Md Saifullah, Email: md.saifullah@uon.edu.au.
Quan Vuong, Email: vanquan.vuong@newcastle.edu.au.
References
- Al-Farsi M.A., Lee C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108:977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Apak R., Güçlü K., Özyürek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of Neocuproine: CUPRAC method. J. Agr. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Bucić-Kojić A., Planinić M., Tomas S., Bilić M., Velić D. Study of solid–liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007;81:236–242. [Google Scholar]
- Cacace J.E., Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003;59:379–389. [Google Scholar]
- Carrera C., Ruiz-Rodríguez A., Palma M., Barroso C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta. 2012;732:100–104. doi: 10.1016/j.aca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang T.T., Van Vuong Q., Schreider M.J., Bowyer M.C., Van Altena I.A., Scarlett C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phys. 2017;29:3161–3173. [Google Scholar]
- Das S., Ray A., Nasim N., Nayak S., Mohanty S. Effect of different extraction techniques on total phenolic and flavonoid contents, and antioxidant activity of betelvine and quantification of its phenolic constituents by validated HPTLC method. 3 Biotech. 2019;9:37. doi: 10.1007/s13205-018-1565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuner A.J., Almeida Barbosa L.C., Gonçalves Magalhaes C., Da Silva C.J., Alvares Maltha C.R., Lelis Pinheiro A. Seasonal variation in the chemical composition and antimicrobial activity of volatile oils of three species of Leptospermum (myrtaceae) grown in Brazil. Molecules. 2011;16:1181. doi: 10.3390/molecules16021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . 2017. Q3C —Tables and List Guidance for Industry. [Google Scholar]
- Goltz C., Ávila S., Barbieri J.B., Igarashi-Mafra L., Mafra M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crop. Prod. 2018;115:227–234. [Google Scholar]
- Irakli M., Chatzopoulou P., Ekateriniadou L. Optimization of ultrasound-assisted extraction of phenolic compounds: oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2018;124:382–388. [Google Scholar]
- Khoddami A., Wilkes M., Roberts T. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuri A.I., Mukhopadhyay S. Response surface methodology. Wiley Interd. Reviews: Comput. Stat. 2010;2:128–149. [Google Scholar]
- Kurekci C., Padmanabha J., Bishop-Hurley S.L., Hassan E., Al Jassim R.A.M., McSweeney C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Micr. 2013;166:450–457. doi: 10.1016/j.ijfoodmicro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Lin C.C. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: ultrasonic and pressurized solvent extractions. Food Chem. 2007;105:223–228. [Google Scholar]
- Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. [Google Scholar]
- Moo-Huchin V.M., Canto-Pinto J.C., Cuevas-Glory L.F., Sauri-Duch E., Pérez-Pacheco E., Betancur-Ancona D. Effect of extraction solvent on the phenolic compounds content and antioxidant activity of Ramon nut (Brosimum alicastrum) Chem. Pap. 2019;73:1647–1657. [Google Scholar]
- Mota I., Rodrigues Pinto P.C., Novo C., Sousa G., Guerreiro O., Guerra Â.R., Duarte M.F., Rodrigues A.E. Extraction of polyphenolic compounds from Eucalyptus globulus bark: process optimization and screening for biological activity. Ind. Eng. Chem. Res. 2012;51:6991–7000. [Google Scholar]
- Nguyen K.Q., Vuong Q.V., Nguyen M.H., Roach P.D. The effects of drying conditions on bioactive compounds and antioxidant activity of the Australian maroon bush, Scaevola spinescens. J. Food Process. Preserv. 2018;42 [Google Scholar]
- Papoutsis K., Pristijono P., Golding J.B., Stathopoulos C.E., Bowyer M.C., Scarlett C.J., Vuong Q.V. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int. J. Food Sci. Technol. 2016;51:2009–2018. [Google Scholar]
- Roselló-Soto E., Marti-Quijal F., Cilla A., Munekata P., Remize F., Barba F. Influence of temperature, solvent and pH on the selective extraction of phenolic compounds from tiger nuts by-products: triple-TOF-LC-MS-MS characterization. Molecules. 2019;24:797. doi: 10.3390/molecules24040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.S., Deolindo C.T.P., Esmerino L.A., Genovese M.I., Fujita A., Marques M.B., Rosso N.D., Daguer H., Valese A.C., Granato D. Effects of time and extraction temperature on phenolic composition and functional properties of red rooibos (Aspalathus linearis) Food Res. Int. 2016;89:476–487. doi: 10.1016/j.foodres.2016.08.041. [DOI] [PubMed] [Google Scholar]
- Setford P.C., Jeffery D.W., Grbin P.R., Muhlack R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017;69:106–117. [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A.R., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- Sulaiman S., Nor Adlin Y., Eldeen I.M.S., Seow E.M., Abu Bakar A., Supriatno, Ooi K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.) J. Food Compos. Anal. 2011;24:1–10. [Google Scholar]
- Tabaraki R., Heidarizadi E., Benvidi A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Separ. Purif. Technol. 2012;98:16–23. [Google Scholar]
- Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. [Google Scholar]
- Uzel R.A. Effect of extraction method and extraction solvent on recovery of phenolic compounds from olive leaves in Kemalpaşa-İzmir (Turkey): oleuropein recovery as a case example. Separ. Sci. Technol. 2018;53:1531–1539. [Google Scholar]
- Van Vuuren S.F., Docrat Y., Kamatou G.P.P., Viljoen A.M. Essential oil composition and antimicrobial interactions of understudied tea tree species. South Afr. J. Bot. 2014;92:7–14. [Google Scholar]
- Vu H.T., Scarlett C.J., Vuong Q.V. Optimization of ultrasound-assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J. Food Process. Preserv. 2017;41 [Google Scholar]
- Vuong Q.V., Golding J.B., Stathopoulos C.E., Nguyen M.H., Roach P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Separ. Sci. 2011;34:3099–3106. doi: 10.1002/jssc.201000863. [DOI] [PubMed] [Google Scholar]
- Vuong Q.V., Hirun S., Roach P.D., Bowyer M.C., Phillips P.A., Scarlett C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Her. Med. 2013;3:104–111. [Google Scholar]
- Vuong Q.V., Nguyen V.T., Thanh D.T., Bhuyan D.J., Goldsmith C.D., Sadeqzadeh E., Scarlett C.J., Bowyer M.C. Optimization of ultrasound-assisted extraction conditions for euphol from the medicinal plant, Euphorbia tirucalli, using response surface methodology. Ind. Crop. Prod. 2015;63:197–202. [Google Scholar]
- Vuong Q.V., Zammit N., Munro B.R., Murchie S., Bowyer M.C., Scarlett C.J. Effect of drying conditions on physicochemical and antioxidant properties of vitex agnus-castus leaves. J. Food Process. Preserv. 2015;39:2562–2571. [Google Scholar]
- Wang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. [Google Scholar]
- Wang L., Wang Z., Li X. Optimization of ultrasonic-assisted extraction of phenolic antioxidants from Malus baccata (Linn.) Borkh. using response surface methodology. J. Sep. Sci. 2013;36:1652–1658. doi: 10.1002/jssc.201300062. [DOI] [PubMed] [Google Scholar]
- Wang T., Liang H., Yuan Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica Juncea L.) using response surface methodology. Phyt. Anal. 2011;22:205–213. doi: 10.1002/pca.1266. [DOI] [PubMed] [Google Scholar]
- Weiss E.A. CAB INTERNATIONAL; Wallingford: 1997. Essential Oil Crops; p. xi + 600. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- Zhong L., Liu Y., Xiong B., Chen L., Zhang Y., Li C. Optimization of ultrasound-assisted extraction of total flavonoids from Dendranthema indicum var. aromaticum by response surface methodology. J. Anal. Met. Chem. 2019;10 doi: 10.1155/2019/1648782. [DOI] [PMC free article] [PubMed] [Google Scholar]