Abstract
Ascending aortic pseudoaneurysm is a rare pathology that could have multiple etiologies such as thoracic trauma, infection, and percutaneous or surgical procedures. In patients with aortic pseudoaneurysms, open surgical or endovascular interventions are always indicated, if feasible and independent of size. Several types of endovascular treatment have been reported in the literature, but they have been mostly restricted to those cases when traditional surgery has a prohibitive risk. We report the case of a 75-year-old man with a sinotubular junction pseudoaneurysm, likely developed after coronary artery bypass grafting, which was successfully treated by implanting a multifenestrated cribriform septal occluder, a device already used in few similar cases.
<Learning objective: Aortic pseudoaneurysm could be a consequence of several types of aortic injuries. In frail patients endovascular approach represents a viable option instead of surgical repair. Such percutaneous treatment has to be individualized also according to the lesion location and size, as in our case where a sinotubular junction pseudoaneurysm was successfully treated by implanting a multifenestrated cribriform septal occluder.>
Keywords: Ascending aortic pseudoaneurysm, High surgical risk, Atrial septal occluder
Introduction
Aortic pseudoaneurysm is defined as a dilation of aorta due to disruption of all wall layers, which is only contained by periaortic connective tissue. In addition to fatal rupture, other life-threatening complications – due to the progressive increase of the size of the aortic pseudoaneurysm itself – include fistula formation as well as compression or erosion of surrounding structures. It can present like a noticeable pulsating mass but it can also produce symptoms like shortness of breath and chest pain [1].
Ascending aortic pseudoaneurysms (AAPs) could be a consequence of aortic injury such as surgical cannulation, inflammatory process, blunt trauma, and infection. The incidence is uncertain, although higher rates (up to 13%) were reported in a surveillance imaging series of patients after cardiac aortic surgery [2]. The diagnosis can be easily obtained with contrast-computed tomography (CT) angiography which can define site, dimension, and extension [3]. In high surgical risk patients an endovascular approach represents a viable option instead of surgical repair that is challenging and encumbered by a high mortality rate [3].
We present herein a case of an incidentally found sinotubular junction pseudoaneurysm likely developed after coronary artery bypass grafting (CABG), which was successfully treated by implanting a multifenestrated cribriform septal occluder.
Case report
We report the case of a 75-year-old Caucasian man with hypertension, dyslipidemia, and obesity, who had undergone a CABG in 1999 with right internal mammary artery (RIMA) to left anterior descending, sequential left internal mammary artery to first and second obtuse marginals, saphenous vein graft (SVG) to right coronary artery (RCA) because of acute myocardial infarction. He was electively admitted to our division in April 2013 to clarify the suspicion of severe symptomatic aortic steno-regurgitation in chronic heart failure (CHF) – New York Heart Association functional class III –, after suffering from acute pulmonary edema some weeks before in the absence of recent inflammatory or infective episodes. Transthoracic echocardiography at the admission confirmed the severity of the aortic valvulopathy, showing a dilation of the left ventricle with inferior and inferoseptal segments akinesis, an ejection fraction of 38% and a severe functional mitral regurgitation too.
He underwent pre-surgical diagnostic cardiac catheterization that showed patency of the arterial grafts as well as chronic total occlusion (CTO) of the SVG to RCA, with a good collateralization from left coronary artery (Rentrop and Cohen 3). Ascending aortic angiography revealed an AAP at the level of the sinotubular junction with its neck in the border zone between right coronary and non-coronary sinus (Fig. 1a). A CT (64-slice, SOMATOM Sensation, Siemens, Munich, Germany) angiography (Fig. 2a,b) confirmed our fluoroscopic suspicion, detecting the AAP caudocranial, sagittal and transverse maximum diameters, respectively as 25 × 18 × 11 mm.
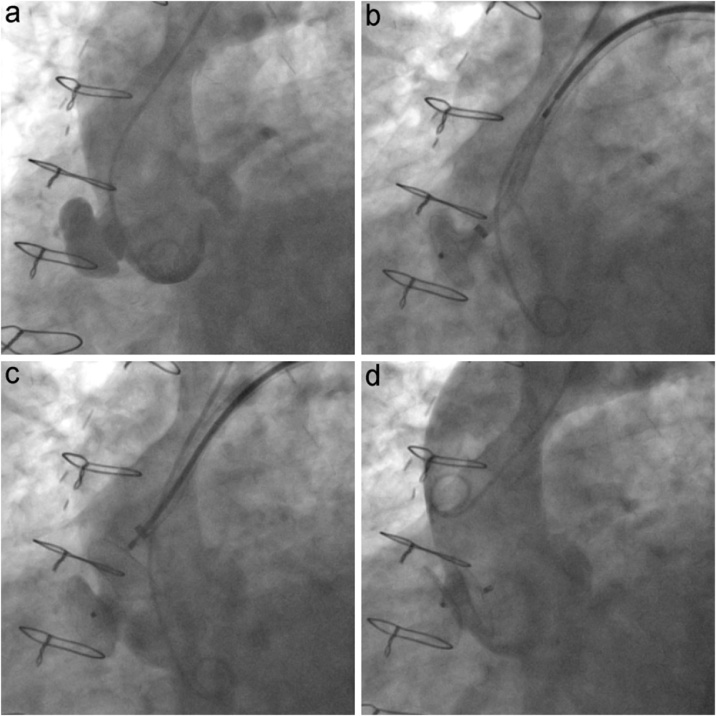
Fig. 1.
Ascending aortic angiography showing pseudoaneurysm of the sinotubular junction before (a), during the delivery of the occluder (b, c), and after its successful exclusion (d).
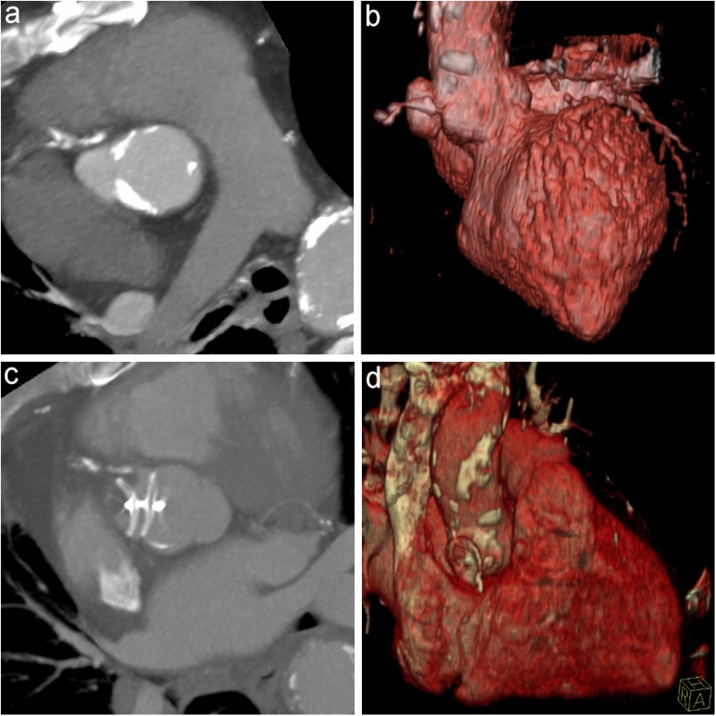
Fig. 2.
Paraxial maximum intensity projection and 3D volume-rendered reconstruction from cardiac computed tomography scan, demonstrating the AAP before (a, b) and after (c, d) the delivery of the device.
The patient had a Logistic and a Standard euroSCORE of 35.85% and 12, respectively, and a euroSCORE II of 24.63%; moreover his RIMA crossed the midline. In view of all these aspects, our “valve team” preferred a totally percutaneous interventional approach to a surgical correction of both valvulopathy and AAP; in fact the AAP exclusion and a transcatheter aortic valve implantation (TAVI) were planned in two subsequent interventional sessions. In order to avoid devices overlap, our first idea was to perform TAVI with a balloon-expandable prosthesis, but its biggest size available (29 mm) was still too small for his aortic annulus (maximum diameter 28.7 mm): consequently implantation of a self-expandable Medtronic CoreValve™ (Medtronic, Santa Rosa, CA, USA) 31 mm was mandatory after AAP exclusion.
After a careful evaluation of all the potential devices, we identified an AMPLATZER™ Multi-Fenestrated Septal Occluder — “Cribriform”™ 25–25 mm (AGA Medical Corporation, Plymouth, MN, USA) as the best option for such lesion exclusion. It is a self-expandable double-disc device (matched disc diameter with a narrow waist) composed of nitinol wire mesh and polyester fabric, commonly used to close multifenestrated atrial septal defects (ASDs) ostium secundum type. In this case the fact that the “aortic” disc would have covered the RCA ostium should have not represented a problem because its already known CTO; according to our AAP, the narrow low-profile disc is the most useful feature of this device, because of two reasons:
-
1
the slow endothelization of such disc improved by its closeness to a high-flow – and consequently high thrombotic risk – zone like the valvular plane;
-
2
the fact that a self-expandable aortic bioprosthesis would have covered and stabilized it.
A narrow low-profile disc is a key component of one of the most diffuse model of left atrial appendage (LAA) occlusion devices too, but we excluded the possibility to use it because its stabilizing wires (hooks) could damage an AAP wall made of an adventitial layer only.
Considering the AAP site, we needed a long angled at least 8 French sheath like an AMPLATZER™ TorqVue™ 45° × 45° (AGA Medical Corporation). In our case, such a sheath, commonly accommodating the LAA occlusion device, was the best option because its soft tip would have been the most coaxial to the AAP neck, compared to other sheaths; indeed it allowed a slow and precise rotation, suitable for a safe delivery of the septal occluder in the AAP (Fig. 1b,c).
The final fluoroscopic control – performed via radial artery – showed the complete exclusion of the AAP (Fig. 1d); it was almost entirely thrombosed also at the 10-day CT angiography control (Fig. 2c,d).
In view of the absence of periprocedural complications, the patient was discharged with optimal antithrombotic therapy prescription, i.e. double antiplatelet therapy with acetylsalicylic acid and clopidogrel; moreover we planned to readmit him for elective TAVI in a few weeks, in order to allow the complete AAP thrombosis as well as the endothelialization of the “aortic” disc. Unfortunately, some days after the discharge, he was referred to another heart center where he had been implanted with an implantable cardioverter defibrillator for primary prevention, irrespective of the valvular etiology of his CHF. He died a few days later because of infectious complications connected to this last implantation.
Discussion
The percutaneous exclusion of a pseudoaneurysm could be performed using different interventional techniques, with low risks and great results. Considering the features as well as the site of our AAP, other previously reported interventional treatments such as coil embolization, thrombin injections, stent grafts, vascular plugs, septal occluder devices, and patent foramen ovale (PFO) occluder device, were inadvisable. A LAA occluder has never been used for such purpose.
Coil embolization and thrombin injection are considered hazardous maneuvers, because of the high risk of thrombus embolization to the cerebral circulation. Stent grafting is commonly ruled out due to the closeness of the AAP to the aortic bulb. Indeed a prerequisite of stent grafting of the ascending aorta is the presence of sufficient proximal and distal landing zones: an appropriate distance to the aortic valve and the coronary arteries is required proximally, as well as an appropriate distance to the head vessels is required distally [4]. An occluder belonging to the AMPLATZER™ Vascular Plug (AVP) family was not suitable for our AAP because of their shape but also the absence of a connecting waist, and consequently the high risk of dislocation or aortic protrusion. The current literature contains only one case of AAP exclusion with a type II AVP [5]. Even though ASD and ventricular septal defect as well as duct occluder devices were already used for this purpose [6], [7], [8], we avoid to use them because their connecting waist is too big and it would have been too heavy for the AAP’s neck, with a consequent high risk of rupture and hemorrhagic complications. Despite a case of AAP exclusion implanting a PFO occluder device had already been described [9], the convex shape of its discs – as well as ASD occluder device ones – was one more reason to rule out this occluder.
In view of all these aspects we opted to deliver a multifenestrated septal occluder through a long-angled sheath. Such a carefully planned procedure was perfectly successful for our AAP occlusion, considering the curvature of the aorta, the closeness of the lesion to the aortic bulb but also the fact that the dimension and the structure of the device fit entirely the AAP, reducing the rate of any possible complication. As far as we know this is the third case presented [6], [10], where a multifenestrated ASD occluder device has been used to successfully exclude an AAP in the same location.
Conclusion
According to current European guidelines [1], the choice of treatment for aortic pseudoaneurysms is individualized, based on anatomical features, clinical presentation, and comorbidities. Considering the increasing number of high surgical risk patients as well as the evolution of techniques and engineering, randomized studies should be considered to evaluate efficacy, durability, and complication rate of endovascular treatment compared to surgical one.
In this case the patient frailty as well as the location and the size of the AAP imposed a tailored interventional strategy, in order to reduce the risk of procedural complications such as dislocation of the device, thrombosis, and rupture of the lesion. Hence multifenestrated ASD occluders could be a valid alternative for AAP exclusion, especially if located so close to the aortic bulb.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgments
We would like to thank Dr. Vito Alberotanza for his precious help in the acquisition, elaboration, and reconstruction of CT images.
References
- 1.Erbel R., Aboyans V., Boileau C., Bossone E., Di Bartolomeo R., Eggebrecht H. ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 2.Mesana T.G., Caus T., Gaubert J., Collart F., Ayari R., Bartoli J. Late complications after prosthetic replacement of the ascending aorta: what did we learn from routine magnetic resonance imaging follow-up? Eur J Cardiothorac Surg. 2000;18:313–320. doi: 10.1016/s1010-7940(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 3.Quevedo H.C., Alonso A. Endovascular therapy for ascending aorta pseudoaneurysm. Cardiovasc Revasc Med. 2016;17:586–588. doi: 10.1016/j.carrev.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Ruge H., Herold U., Lange R. Stent-graft treatment of an ascending aortic false aneurysm after surgical aortic valve replacement. J Thorac Cardiovasc Surg. 2018;1256:183–185. doi: 10.1016/j.jtcvs.2018.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Scholtz W., Jategaonkar S., Haas N.A. Successful interventional treatment of a retrosternal pseudoaneurysm of the ascending aorta with an Amplatzer vascular plug II. J Invasive Cardiol. 2010;22:44–46. [PubMed] [Google Scholar]
- 6.Hussein J., Strumpf R., Wheatley G., Dientrich E. Percutaneous closure of aortic pseudoaneurysm by Amplatzer occluder device — case series of six patients. Catheter Cardiovasc Interv. 2009;73:521–529. doi: 10.1002/ccd.21833. [DOI] [PubMed] [Google Scholar]
- 7.Kanani R.S., Neilan T.G., Palacios I.F., Garasic J.M. Novel use of the Amplatzer septal occluder device in the percutaneous closure of ascending aortic pseudoaneurysms: a case series. Catheter Cardiovasc Interv. 2007;69:146–153. doi: 10.1002/ccd.20794. [DOI] [PubMed] [Google Scholar]
- 8.Tipaldi M.A., Orgera G., Krokidis M.E., Laurino F., Capuano F., Rossi M. Postoperative ascending aortic gigantic pseudoaneurysm: endovascular treatment with the use of a septal occluder plug. Interv Med Appl Sci. 2018;10:213–215. doi: 10.1556/1646.10.2018.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cawley P.J., Gill E.A., Goldberg S. Successful percutaneous closure of an aortic graft pseudo-aneurysm with a patent foramen ovale occluder device. J Invasive Cardiol. 2008;20:19–22. [PubMed] [Google Scholar]
- 10.Stabenow L., Byers M., Malik A., Ali F. Endovascular repair of a pseudoaneurysm adjacent to the ascending thoracic aorta using a 25-mm Amplatzer multi-fenestrated septal occluder — cribriform. Vasc Dis Manag. 2018;15:39–41. [Google Scholar]